Abstract
Reaching movements in the real world have typically a direction and a depth component. Despite numerous behavioral studies, there is no consensus on whether reach coordinates are processed in separate or common visuomotor channels. Furthermore, the neural substrates of reach depth in parietal cortex have been ignored in most neurophysiological studies. In the medial posterior parietal area V6A, we recently demonstrated the strong presence of depth signals and the extensive convergence of depth and direction information on single neurons during all phases of a fixate-to-reach task in 3-dimensional (3D) space. Using the same task, in the present work we examined the processing of direction and depth information in area PEc of the caudal superior parietal lobule (SPL) in three Macaca fascicularis monkeys. Across the task, depth and direction had a similar, high incidence of modulatory effect. The effect of direction was stronger than depth during the initial fixation period. As the task progressed toward arm movement execution, depth tuning became more prominent than directional tuning and the number of cells modulated by both depth and direction increased significantly. Neurons tuned by depth showed a small bias for far peripersonal space. Cells with directional modulations were more frequently tuned toward contralateral spatial locations, but ipsilateral space was also represented. These findings, combined with results from neighboring areas V6A and PE, support a rostral-to-caudal gradient of overlapping representations for reach depth and direction in SPL. These findings also support a progressive change from visuospatial (vergence angle) to somatomotor representations of 3D space in SPL.
Keywords: eye-hand coordination, reaching, sensorimotor, distance, vergence
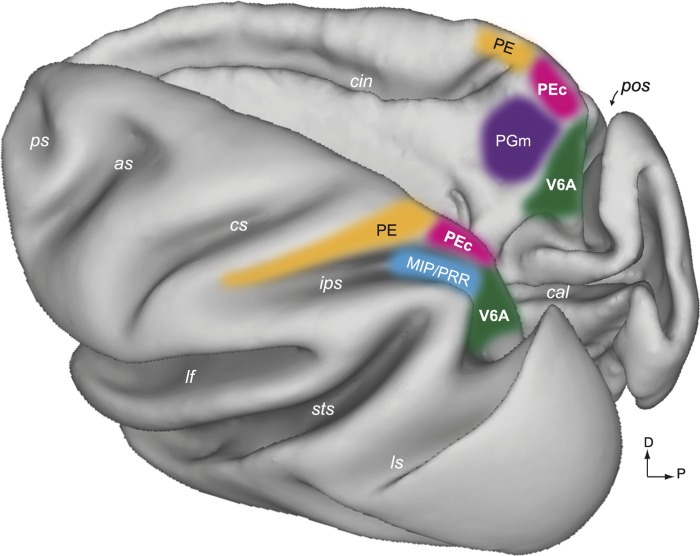
the medial sector of the posterior parietal cortex (PPC) in macaques encompasses several anatomically and physiologically defined areas that combine visual and somatosensory information with motor commands to guide body movements. As shown in Fig. 1, the medial PPC includes areas V6A, medial intraparietal (MIP), PE, PEc, and PGm (Bakola et al. 2010, 2013; Cavada and Goldman-Rakic 1989a, 1989b; Colby and Duhamel 1991; Galletti et al. 1999; Pandya and Seltzer 1982). All these areas form the reaching network of the superior parietal lobule (SPL) (Battaglia-Mayer et al. 2001; Fattori et al. 2001, 2005; Ferraina et al. 1997; McGuire and Sabes 2011; Snyder et al. 1997).
Fig. 1.
Areas of the medial posterior parietal cortex (PPC). A dorsal view of the left hemisphere (left) and a medial view of the right hemisphere (right) of a Macaca fascicularis brain reconstructed in three dimensions using Caret software (http://brainvis.wustl.edu/wiki/index.php/Caret:About) show the location and extent of areas PEc (pink) and V6A (green). The other medial PPC areas are also shown: orange, PE (Pandya and Seltzer 1982); blue, medial intraparietal area/parietal reach region (MIP/PRR; Colby and Duhamel 1991; Snyder et al. 1997); magenta, PGm (Pandya and Seltzer 1982). as, Arcuate sulcus; cal, calcarine sulcus; cin, cingulate sulcus; cs, central sulcus; ips, intraparietal sulcus; lf, lateral fissure; ls, lunate sulcus; pos, parieto-occipital sulcus; ps, principal sulcus; sts, superior temporal sulcus; D, dorsal; P, posterior.
Based on functional and anatomical evidence, caudal SPL areas such as V6A are thought to rely primarily on visual input, whereas rostral SPL areas such as PE are thought to mainly process proprioceptive input (Bakola et al. 2013; Ferraina et al. 2009; Gamberini et al. 2009; McGuire and Sabes 2011; Passarelli et al. 2011; Shi et al. 2013). These differences might be important if we consider that natural arm movements are usually performed in three-dimensional (3D) space, and there is behavioral evidence suggesting that movement in depth relies more on proprioceptive than visual input, whereas vision is more crucial for controlling the direction of movement (Monaco et al. 2010; Sainburg et al. 2003; van Beers et al. 1998, 2002, 2004). There is also conflicting psychophysical evidence on the specification of reach direction and depth. Many works support the possibility that reach coordinates are processed in separate channels (Bagesteiro et al. 2006; Gordon et al. 1994; Sainburg et al. 2003; Soechting and Flanders 1989; Tramper and Gielen 2011; Van Pelt and Medendorp 2008; Vindras et al. 2005). Differently, other studies suggest that the processing of movement depth and direction is not independent (Bhat and Sanes 1998; Medendorp et al. 2003; Sarlegna and Blouin 2010; Wijdenes et al. 2013).
In contrast to the evidence from behavioral studies, the neural correlates of depth and direction in the medial PPC have been rarely addressed. Most single-unit studies have employed center-out reaching tasks, thus neglecting depth; a few have studied only the depth, leaving out direction (Bhattacharyya et al. 2009; Ferraina et al. 2009; Hadjidimitrakis et al. 2014). Until now, in only two works was depth tuning directly compared with direction tuning. The first work showed that the spatial coordinates (azimuth, distance, and elevation) of reaching targets were encoded by distinct subpopulations in area PE (Lacquaniti et al. 1995), thus supporting the prevailing view from the psychophysical studies. The second work reported that in V6A, in contrast to PE, information about distance and direction was jointly encoded in many neurons (Hadjidimitrakis et al. 2014). Another issue relates to the type of activity influenced by depth information in SPL. Whereas in V6A, both eye position- and arm movement-related activity were strongly affected by depth information (Hadjidimitrakis et al. 2014), in PE, depth tuning occurred mostly during arm movement (Ferraina et al. 2009). These findings raise two questions. 1) Is the convergence of depth and direction signals on single cells present in other SPL areas apart from V6A? 2) Is there a rostrocaudal trend of increased eye vergence sensitivity within the SPL?
To address these issues, we studied the activity of single neurons in area PEc, another medial PPC area located between V6A and PE (Fig. 1). Area V6A belongs to Brodmann area 19, area PE is part of Brodmann area 5, and area PEc, differently from the common idea, is not the caudal part of Brodmann area 5 but is part of Brodmann area 7 (Brodmann 1909). PEc has a distinct set of anatomical connections (Bakola et al. 2010) and contains cells that respond to passive somatosensory stimulation (Breveglieri et al. 2006) and neurons with visual (Squatrito et al. 2001) and combined visual and somatosensory sensitivity (Breveglieri et al. 2008). PEc studies with oculomotor and reaching tasks in 2D space (Battaglia-Mayer et al. 2000, 2001; Ferraina et al. 2001; Raffi et al. 2008) have shown the directional tuning of eye- and hand-related activity, whereas the neural correlates of fixating and reaching in 3D space have not been addressed so far.
In the present work, we recorded neural activity while monkeys performed the same fixate-to-reach task in 3D space that was previously used to study area V6A (Hadjidimitrakis et al. 2014). In PEc, just after the target was initially fixated, the effect of direction was more prominent than that of depth. The influence of depth information became gradually stronger than direction, and peaked during movement execution. The convergence of direction and depth signals on single cells was rather limited in the beginning of the task and became more extensive during the arm movement and holding the target phases. These findings show that the target direction and depth are represented in overlapping populations of PEc cells. Comparison of the PEc results with findings from V6A and PE supports a rostral-to-caudal gradient in the visuospatial processing of depth information in SPL.
MATERIALS AND METHODS
Experimental Procedures
Three male macaque monkeys (Macaca fascicularis) with a weight ranging between 3.8 and 4.4 kg were used. Experiments were performed in accordance with national laws on care and use of laboratory animals and with the European Communities Council Directive of September 22, 2010 (2010/63/EU). The Bioethical Committee of the University of Bologna approved all the experimental protocols.
General Procedures
Initially, the animals got used to sitting in a primate chair and to interacting with the experimenters. A head-restraint system and the recording chamber were then surgically implanted under general anesthesia (thiopental sodium, 8 mg·kg−1·h−1 iv) following the procedures reported by Galletti et al. (1995). A full program of postoperative analgesia (ketorolac tromethamine, 1 mg/kg im immediately after surgery and 1.6 mg/kg im on the following days) and antibiotic care (Ritardomicina: benzatinic benzylpenicillin + dihydrostreptomycin + streptomycin, 1–1.4 ml/10 kg every 5–6 days) followed surgery.
Extracellular recordings from area PEc were daily performed using a five-channel multielectrode recording system (5-channel MiniMatrix; Thomas Recording). The electrode signals were amplified (at a gain of 10,000) and filtered (bandpass between 0.5 and 5 kHz). Action potentials in each channel were isolated with a waveform discriminator (Multi Spike Detector; Alpha Omega Engineering) and were sampled at 100 kHz. During the initial recordings, the approximate location of the parieto-occipital sulcus (POS) in the recording chamber's coordinates was estimated on the basis of whether several electrode penetrations passed through the occipital pole or not. Once this landmark was established, it could be assumed that penetrations located >2–3 mm anterior to POS were almost certain to be in PEc.
Histological Reconstruction of Recording Sites
During the last week of recording, electrolytic lesions (cathodal current 40–50 μA for 30 s) were made at different depths along single penetrations carried out at different coordinates within the recording chamber. After the last recording session, the animals were anesthetized with ketamine hydrochloride(15 mg kg im) followed by an intravenous lethal injection of thiopental sodium and perfusion through the left cardiac ventricle with 0.9% sodium chloride followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and by 5% glycerol in the same buffer. The brains were then removed from the skull, photographed, placed in 10% buffered glycerol for 3 days, then placed in 20% glycerol and cut at 60 μm along the parasagittal plane. Electrode tracks and location of each recording site were reconstructed on Nissl-stained sections of the brain on the basis of several cues: 1) marking electrolytic lesions, 2) the coordinates of penetrations within the recording chamber, and 3) whether the electrode passed through another cortical area before reaching the region of interest. The reconstructed recording sites were assigned to area PEc according to the cytoarchitectural criteria of Pandya and Seltzer (1982) and Luppino et al. (2005).
Behavioral Task
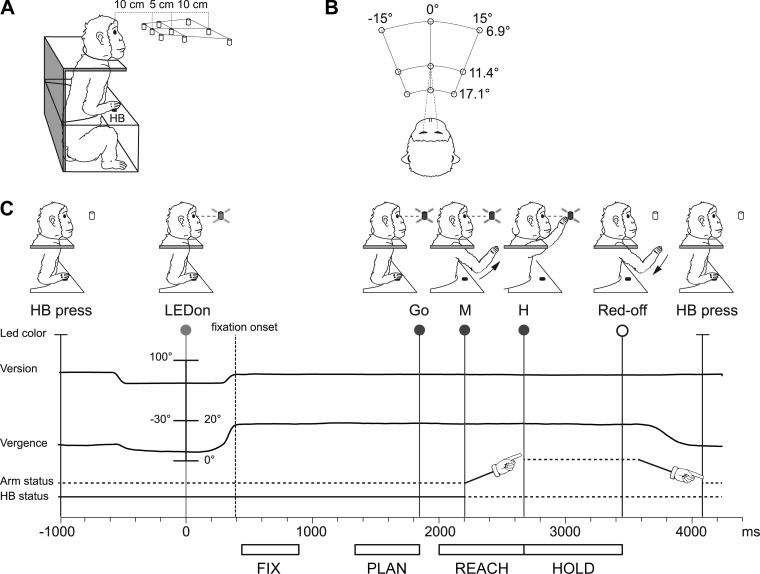
Electrophysiological data were collected while monkeys were performing a fixation-to-reach task. The animal performed arm movement with the contralateral limb (with respect to the recording hemisphere), with the head restrained, in darkness, while maintaining steady fixation of the target. Before starting the arm movement, the monkey kept its hand on a home button (HB; 2.5 cm in diameter) located next to its trunk (Fig. 2A). Reaches were performed to one of nine light-emitting diodes (LEDs; 6 mm in diameter). The LEDs were mounted on a panel located in front of the animal, at different distances and directions with respect to the eyes but always at eye level. Target LEDs were arranged in three rows: one central, along the sagittal midline, and two lateral, at version angles of −15° and +15°, respectively (Fig. 2B). Along each row, three LEDs were located at vergence angles of 17.1°, 11.4°, and 6.9°. The nearest targets were located at 10 cm from the eyes, whereas the LEDs placed at intermediate and far positions were at a distance of 15 and 25 cm, respectively. The range of vergence angles was selected to include most of the peripersonal space in front of the animal, from the very near space (10 cm, vergence angle ∼17°) up to the farthest distances reachable by the monkeys (25 cm, vergence angle ∼7°).
Fig. 2.
Experimental setup and task sequence. A: scheme of the setup used for the task. Nine light-emitting diodes (LEDs) that were used as fixation and reaching targets were located at eye level. The distances of the 3 targets of the central row from mid-eye level are shown. HB, home button. B: top view of the target configuration showing the values of version and vergence angles. C: time sequence of task events with LED status, the eye's vergence and version traces, arm status, and HB status. From left to right, vertical lines indicate, respectively, trial start (HB press), target appearance (LEDon), fixation onset (dashed line, end of saccade movement), go signal (Go), start of the arm movement (M), holding phase of the target (H), turning off of the LED (Red-off), and trial end (HB press). Arm drawings indicate the forward and backward arm movement. White bars below the time axis illustrate the time intervals (epochs) used for the analysis of neural activity, from left to right: Fix, from 50 ms after fixation onset till 450 ms after it; Plan, the last 500 ms before the Go signal; Reach, from 200 ms before the start of the arm movement (M) to the pressing of the LED; Hold, from LED pressing till Red-off.
The time sequence of the task is shown in Fig. 2C. A trial began when the monkey pressed the button near its chest (HB press). After 1 s, one of the nine LEDs was switched on to green. The monkey had to fixate the LED while keeping the HB button pressed. The monkey then had to wait 1.7–2.5 s for a change in the color of the LED (from green to red) without performing any eye or arm movement. The color change was the go signal (Go) for the animal to release the HB and to start an arm movement (M) toward the target. The monkey then reached the target (H) and held its hand on the target for 0.8–1.2 s. The switching off of the target (Red-off) cued the monkey to release it and to return to the HB (HB press), which ended the trial and allowed the monkey to receive its reward. The presentation of stimuli and the animal's performance were monitored using custom software written in LabVIEW (National Instruments), as described previously (Kutz et al. 2005). Eye position signals were sampled with two cameras (1 for each eye) of an infrared oculometer system (ISCAN) at 100 Hz and were controlled by an electronic window (4° × 4°) centered on the fixation target. If the monkey fixated outside this window, the trial was aborted. The task was performed in darkness, in blocks of 90 randomized trials, 10 for each target position. The luminance of LEDs was adjusted to compensate for difference in retinal size between LEDs located at different distances. The background light was switched on between blocks to avoid dark adaptation.
At the beginning of each recording session, the monkey was required to perform a calibration task gazing at targets on a frontal panel placed at a distance of 15 cm from the eyes. For each eye, signals to be used for calibration were extracted during fixation of five LEDs arranged in the shape of a cross, one centrally aligned with the eye's straight-ahead position and four peripherally placed at an angle of ±15° (distance 4 cm) in both the horizontal and vertical directions. From the two individual calibrated eye position signals, we derived the mean of the two eyes (conjugate or version signal) and the difference between the two eyes (disconjugate or vergence signal) using the following equations: version = (R + L)/2 and vergence = R − L, where R and L are the gaze direction of the right and left eye, respectively, expressed in degrees of visual angle from the straight-ahead direction. The version and vergence values were also used by the LabVIEW software to control the 3D eye position.
Neural Data Analysis
The effect on neural activity of gazing at different target positions was analyzed in different epochs during the task. The task epochs taken into account for the analysis are indicated in Fig. 2C, bottom, and were 1) the early fixation epoch (Fix), from 50 ms after the end of the saccade performed to catch the LED till 450 ms after it; 2) the preparation epoch (Plan), the last 500 ms of fixation before the GO signal; 3) the reach epoch (Reach), from 200 ms before the start of the arm movement (M) till the end of it, signaled by the pressing of the LED target (H), and 4) the hold epoch (Hold), from the pressing of the LED target till the target offset; this epoch lasted either 800 or 1,200 ms, depending on the trial length.
Rasters of spiking activity were aligned on specific events of the task sequence, depending on the epoch analyzed. The effect of target depth and direction on epoch activity was analyzed only in those units that had a mean firing rate higher than 3 spikes/s in at least one spatial position. To be included in the analysis, neurons had to be tested in at least seven trials for each spatial position. The reasons for this conservative choice are connected to the implicit high variability of biological responses and are explained in detail in a previous work (Kutz et al. 2003).
Significant modulation of neural activity relative to different target locations was studied using a two-way analysis of variance (ANOVA) performed separately for each epoch with factors being the target's depth and direction. Target depth was defined as the distance of the target from the animal (near, intermediate, far), and target direction as its position with respect to the recording hemisphere (contralateral, central, ipsilateral). Neurons were considered modulated by a given factor only when the factor's main effect was significant (P < 0.01). To find whether the incidence of each of the main effects differed significantly between two epochs, a two-proportion z-test (Zar 1999) was applied, as detailed in Fluet et al. (2010).
To perform this test, the SE of the sampling distribution difference between two proportions was computed as
with p = [(n1 × p1) + (n2 × p2)]÷(n1 + n2) representing the pooled sample proportion and n1/p1 and n2/p2 representing the size and proportion, respectively, of each sample. Subsequently, the z score was calculated as z = (p1 − p2)/SE, and its corresponding P value was obtained from the (cumulative) normal distribution.
To quantify the magnitude of the effect of depth and direction signals in each epoch, we calculated the η2 index (Zar 1999), which is an estimate of the proportion of the explained variance. The η2 index was calculated using values from the ANOVA table and by applying the following formula: η2 = SSeffect/SStotal, where SSeffect is the deviance of the main effect and SStotal is the total deviance.
To analyze the spatial tuning of activity, a stepwise multilinear regression model was applied in each epoch considered. Regression methods quantify relationship between dependent (neural activity) and independent (target depth and direction) variables. Given that the monkeys fixated the target in all epochs of interest, its depth and direction in space were equal to the vergence and version angles of the eyes, respectively. That being said, in the rest of this article, when we refer to spatial tuning analysis and data, the terms depth and vergence, as well as direction and version, are interchangeable.
In the multiple linear regression model relating the neural activity in the epochs of interest to the different target positions, we used the following equation for the firing rate:
where A is the neural activity in spikes per second for the ith trials; Xi and Yi are the positions of the target, defined as vergence and version angles, respectively, of the eyes during target fixation; b1 and b2 are regression coefficients; and b0 is the intercept. After being tested for their significance, the vergence and version coefficients were normalized by calculating their ratio with the standard deviation of vergence and version, correspondingly. This procedure was performed because vergence and version had a different angle range (10.2° vs. 30°), and thus their corresponding coefficients could not be compared directly The standardized coefficients allow a comparison among the independent variables and provide information about its relative influence in the regression equation. The regression coefficients were selected using a backward stepwise algorithm (Matlab function “stepwise”) that determined whether the coefficients were significantly different from zero. At the conclusion of the stepwise algorithm, only the coefficients that were significantly different from zero remained (P < 0.05). These coefficients were then used to determine the spatial preference only in the cells with a significant main effect (ANOVA, P < 0.01) in a certain epoch. The linear regression model was used because few neurons displayed their maximal firing rates for intermediate and central positions. In each neuron, the sign of the linear correlation coefficients (standardized) were used to determine the spatial preference in a certain epoch. In modulated neurons without significant linear coefficients, a Bonferroni post hoc test (P < 0.05) was applied to define the preferred position.
Additional analysis was performed to remove the influences of gaze and arm position signals from the neural activity during the reach planning and executions periods. Because the animal had both its eyes and hand on the target during the hold period after the reach, activity in this period reflected both gaze- and arm-related signals and was compared with planning- and movement-related activity. In the cells ANOVA modulated during the Plan and Reach epochs, the firing rates of the Hold period were subtracted trial per trial from the Plan and Reach activities. Subsequently, the effects of target depth and direction were tested on the residual Plan and Reach discharge with a two-way ANOVA (P < 0.01).
Population analysis.
For each cell modulated by target depth and/or direction in the epochs of interest, a spike density function (SDF; Gaussian kernel, half width at half maximum 40 ms) was calculated for each trial and averaged across all the trials of the preferred and the null depths and directions as defined by the linear regression analysis. The peak discharge of the preferred condition was used to normalize the SDFs. Population SDF curves representing the activity of the preferred and nonpreferred target positions were constructed by averaging the individual SDFs of the cells (Marzocchi et al. 2008), aligned at the behavioral event of interest. SDFs curves of preferred and null positions were statistically compared pairwise with a permutation test with 10,000 iterations comparing the sum of squared errors of the actual and randomly permuted data (P < 0.05). The intervals of the curve we compared were different according to the epoch considered: for cells modulated by depth/direction during Fix, the interval was from 50 to 450 ms after saccade offset; for cells modulated during Reach, the interval was from 200 ms before the movement onset (M) to 400 ms after it. To describe the time course of the activity of the different functional categories of cells, we performed a sliding window permutation test (width 100 ms). The sliding window was placed before the SDF alignment event (1,000 and 2,000 ms before saccade offset and movement onset, respectively) and was shifted in sequential 20-ms steps. The difference between the preferred and nonpreferred SDF was calculated in a window and was compared against a bootstrap distribution of differences after the trials were shuffled. The onset of difference in the activity between the two SDF curves was determined as the time of the first of five consecutive windows where comparisons were statistically significant (P < 0.05).
All the analyses were performed using custom scripts written in MATLAB (The MathWorks, Natick, MA).
RESULTS
We recorded the neuronal activity of 200 single neurons from area PEc in 3 monkeys (total/left hemisphere: monkey A, 119/59; monkey B, 68/47; monkey C, 13/5). The monkeys were required to execute reaches to foveated targets located at different depths and directions while the targets' elevation was kept constant at eye level (Fig. 2). No significant differences between monkeys were found, and therefore the results were pooled together.
Depth and Direction Tuning in the Population
To quantify the effect of depth and direction on the activity of PEc neurons, a two-way ANOVA (P < 0.01) was performed with factors target depth (near, intermediate, far) and target direction (contralateral, center, ipsilateral with respect to the recording hemisphere) for each task epoch. Across all epochs, 66% and 68% of all PEc neurons were modulated by target depth and direction, respectively (Table 1). For each effect, we observed any possible combination between epochs. Although many depth-tuned cells could be modulated only in one epoch, similar proportions of neurons showed depth tuning during the whole task or in the last three epochs. In contrast, directionally tuned cells tended to be modulated during distinct epochs. Comparing the incidence of depth/direction effect in the different epochs, the number of depth-tuned cells was moderate during the early fixation (Fix, 21%), increased significantly during the late delay (Plan, 29%), reached its peak just before and during the movement execution (Reach, 47%), and remained high during the subsequent holding period (Hold, 41%). Differently, the percentage of cells with directional modulations was more constant (range 28–36%) across the task epochs.
Table 1.
Cell grouping by tuning in task epoch
| Fix | Plan | Reach | Hold | Depth, % | Direction, % |
|---|---|---|---|---|---|
| + | + | − | − | 0.5 | 3 |
| + | − | + | − | 2.5 | 4.5 |
| + | − | − | + | 1 | 4.5 |
| + | + | + | − | 2.5 | 5 |
| + | + | − | + | 1 | 2.5 |
| + | − | + | + | 2 | 3 |
| + | + | + | + | 7.5 | 4 |
| + | − | − | − | 4 | 9 |
| − | + | − | − | 3.5 | 3.5 |
| − | − | + | − | 7.5 | 6 |
| − | − | − | + | 8.5 | 9 |
| − | + | + | − | 5 | 4 |
| − | + | − | + | 1 | 3 |
| − | − | + | + | 11 | 4 |
| − | + | + | + | 8.5 | 3 |
| − | − | − | − | 34 | 32 |
Cell groups based on the presence (+) or absence (−) of modulation (2-way ANOVA, P < 0.01) by depth and direction in each epoch.
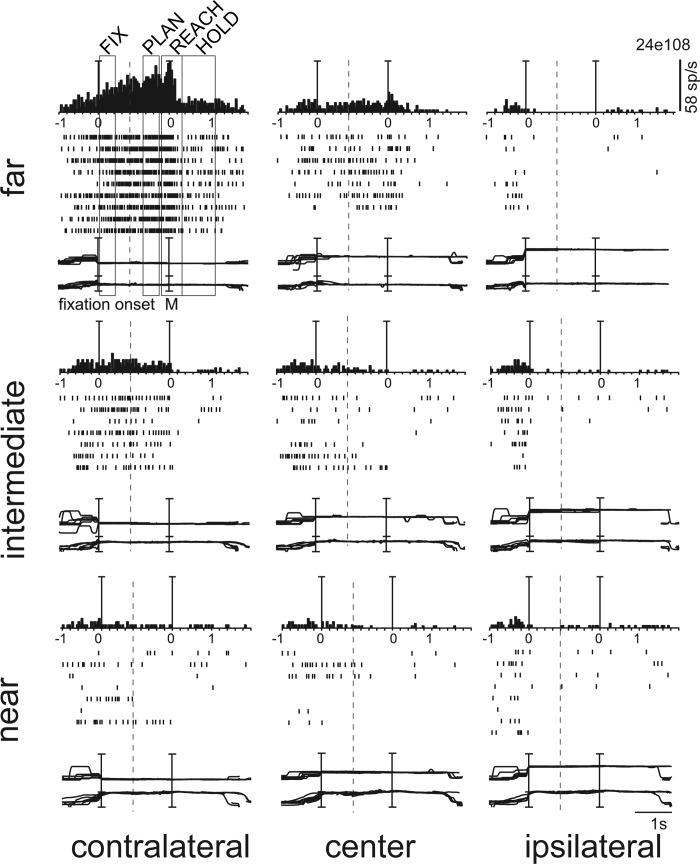
Figure 3 shows the tuning of activity of an example PEc neuron. When the animal looked at the contralateral far target (Fig. 3, top left), the neuron started to respond and continued to fire tonically. The activity slightly increased during Plan, reached its peak in the Reach epoch, and strongly decreased in Hold. It is very clear that this activity pattern occurred only when the monkey performed the task for the far, contralateral target, with the neuron's firing being much weaker or absent for the other target locations. The preference for the far contralateral space was evident in all four epochs of analysis, including the Hold epoch, where the activity was inhibited with respect to Fix.
Fig. 3.
Depth and direction tuning in several epochs of the task in an example PEc neuron. Spike histograms (top), rasters (middle), and version (upper) and vergence (lower) eye position traces (bottom) are shown for the 9 target positions. Rows represent the 3 depths (far, intermediate, near) and columns the 3 directions (contralateral, center, ipsilateral). Vertical lines indicate the alignment of activity and eye position traces at the onset of fixation and at the onset of arm movement. Trial cut is evidenced with a vertical dashed line. This neuron showed a consistent preference in all epochs for far and contralateral space. The epoch's duration is indicated at top left. The scale for version and vergence traces is 100° and 20°, respectively.
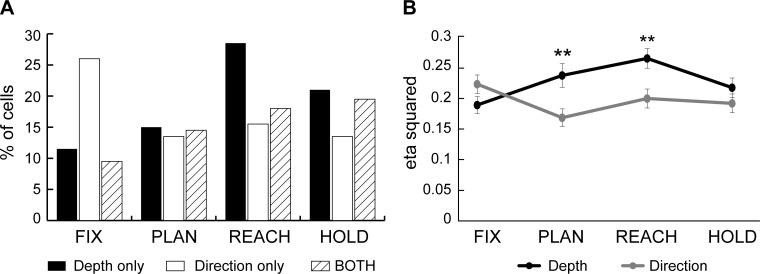
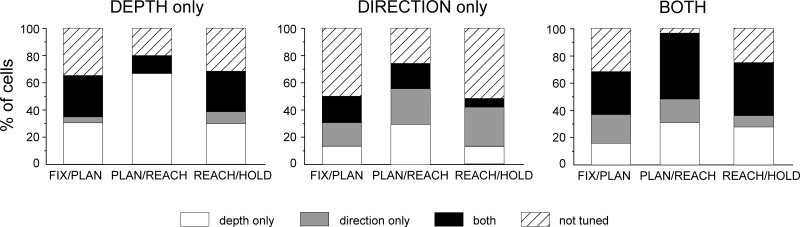
In the example of Fig. 3, depth and direction were processed jointly. In other cases, cell activity was modulated mostly, and in some cases only, by one of the two parameters. To study these effects at the population level, we calculated the percentage of PEc cells that encoded both spatial parameters and that of cells encoding only one of the two (Fig. 4A). The proportion of neurons that showed only depth modulations consistently increased as the task progressed from Fix to Reach epoch. In contrast, the percentage of cells showing only an effect of direction was highest in FIX and decreased to constant levels in the following epochs. A two-proportion z-test showed that the depth-only tuned cells increased significantly between the initial fixation and reaching epoch (Fix to Reach, P < 0.0001), whereas the number of direction-only cells dropped (Fix to Reach, P < 0.01). Interestingly, an important fraction of cells (9.5–19.5%) were modulated by both signals in all epochs. Their percentage increased gradually as the task progressed and was significantly larger in the epochs of arm movement and target holding with respect to the beginning of task (Fix to Reach, P = 0.014; Fix to Hold, P < 0.01). In summary, we found a different temporal pattern in the processing of depth and direction information in PEc. Shortly after the target was fixated, the directional modulations were stronger than the depth ones. As the task progressed, the number of neurons carrying depth signals increased significantly and outnumbered those containing directional information. Moreover, around the arm movement there was a clear increase in the number of neurons coding for both signals.
Fig. 4.
Depth, direction, and combined tuning during each task epoch. A: percentage of cells in the population of PEc (n = 200) with tuning for depth only (solid), direction only (open), and both signals (hatched) during different task epochs (Fix, Plan, Reach, and Hold). B: strength of depth and directional tuning. Depth (black) and direction (gray) effect size calculated as mean ± SE of the eta-squared (η2) index for the population of neurons modulated in each epoch. Tuning strength for depth increased after the fixation epoch and reached a maximum at the movement epoch. Direction effect remained was highest in Fix and decreased to relatively constant levels in the subsequent epochs. Asterisks indicate a significant (Student's t-test, P < 0.01) difference between the depth and direction effects.
We quantified the magnitude of depth and direction effects on individual cells as detailed in materials and methods. To compute the population average effect of each parameter, all neurons modulated by depth/direction (i.e., only and both cells) were included. Figure 4B shows the average η2 of depth and direction effects of the tuned neurons in each epoch. In the Fix epoch, the size of the two effects was comparable, but as the task progressed (i.e., in Plan and Reach), the size of the depth effect was significantly higher (t-test, P < 0.01). The analysis of η2 index, together with the ANOVA results, showed that the difference in the temporal evolution of depth and direction signals was evident not only through the incidence of modulated cells but also through an increase in the strength of tuning.
Time Course of Depth and Direction Modulations in Single Cells
The analyses presented so far show that directional modulations of activity in PEc are predominant during the Fix epoch and that depth tuning is prevalent during Reach and Hold. An important question is whether cells that are directionally tuned early in the task lose this tuning and become modulated by depth (temporal parcellation of spatial information) or whether they combine directional with depth tuning in the subsequent epochs. Alternatively, cells with early directional tuning could become completely untuned, and new subpopulations of depth tuned cells could be recruited in later task stages to account for the increased depth effect. Conversely, some of the directional modulations in the late epochs could be due to cells that were depth tuned early in the task. To address these issues we examined the temporal evolution of depth and direction tuning in single neurons. Cells with depth or direction tuning in one epoch could maintain the same tuning, change and represent only the other parameter, encode both parameters, or become completely untuned in the next epoch. The incidence of these categories are reported in Fig. 5. For depth-only tuning (Fig. 5, left) very few cells changed to encode direction-only information. The majority of cells either showed the same tuning (Fig. 5, left, open bars) or combined depth with direction tuning (Fig. 5, left, solid bars). About one-third of the depth-only cells became not modulated (Fig. 5, left, hatched bars). Differently, direction-only cells (Fig. 5, middle) showed a strong tendency to become not tuned, and only about 40% maintained the same tuning from one epoch to the next or encoded both spatial parameters A smaller but significant fraction (∼25%) shifted from direction-only to depth-only tuning. We also looked at the cells that combined depth and direction tuning (Fig. 5, right) and found that they were most likely to remain stable or to become depth only. In summary, the above analysis demonstrated that a relatively small fraction of cells that were directionally tuned early in the task contributed to depth tuning at the later stages. As a result, the increase of depth modulations was in large part due to new subpopulations of cells being recruited.
Fig. 5.
Time course of the depth and direction tuning in single cells. Histograms illustrate the temporal evolution of tuning for the cells tuned in depth only (left), direction only (middle), and both (right) across pairs of consecutive epochs. Each histogram shows the percentage of cells that were depth only (open), direction only (shaded), or both (solid) or that lost their tuning (hatched) in the second of 2 consecutive epochs. Note 1) the high proportion of direction-only cells that lost their tuning and 2) the smaller but significant proportion of direction-only cells that become depth-only in later epochs.
Spatial Preference Across Epochs
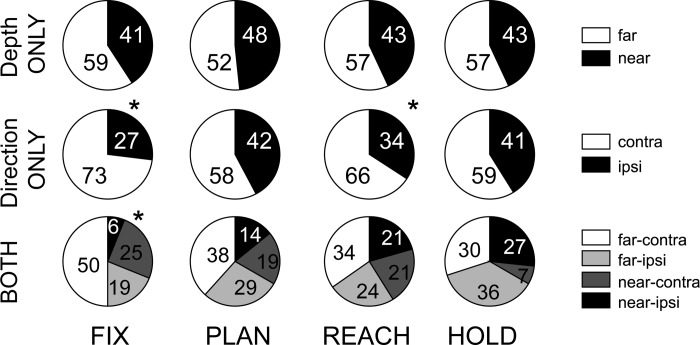
To define the spatial preference of modulated neurons, a linear regression analysis was performed with target depth and direction as independent variables. The vast majority (89.5%) of neurons with a depth and/or direction effect showed a monotonic increase of activity for changes of target position in depth and/or in direction. A small number of cells gave their maximum response for intermediate positions. Averaging across all epochs showed that this occurred in 15% and 13% of the cases for depth and direction, respectively. In a small number of these cases the linear regression still produced significant coefficients. Neurons tuned in depth were classified as “near” or “far,” and neurons with directional tuning were classified as “contra” or “ipsi.” Figure 6 shows the percentage of PEc cells falling into the above categories and their combinations for cells that were tuned by both depth and direction. Neurons tuned only in depth (Fig. 6, top) showed a preference for far space; however, cells tuned for near space were also present. Cells with only directional tuning (Fig. 6, middle) showed a bias for contralateral space (Fix: χ2, P < 0.05). In neurons modulated by both depth and direction (Fig. 6, bottom), the group of “far-contra” cells was the most represented before the movement. In summary, area PEc showed bias of the contralateral space. This representational bias for contralateral space has not been reported previously for medial PPC but is consistent with findings from the lateral PPC areas LIP (lateral intraparietal) and 7a (Battaglia-Mayer et al. 2005; Kagan et al. 2010).
Fig. 6.
Spatial preference in single epochs. Classification of PEc neurons with monotonic tuning by depth and direction signals. Top, percentage of neurons that preferred far (open) and near space (solid) in each epoch. Middle, percentage of the neurons that preferred contralateral (open) and ipsilateral space (solid) in each epoch. Bottom, percentage of the neurons belonging to the combination of classes in cells linearly modulated by both depth and direction. Asterisk indicates a statistically significant (χ2, P < 0.05) spatial preference.
Relationship Between Eye Position and Arm Movement Signals
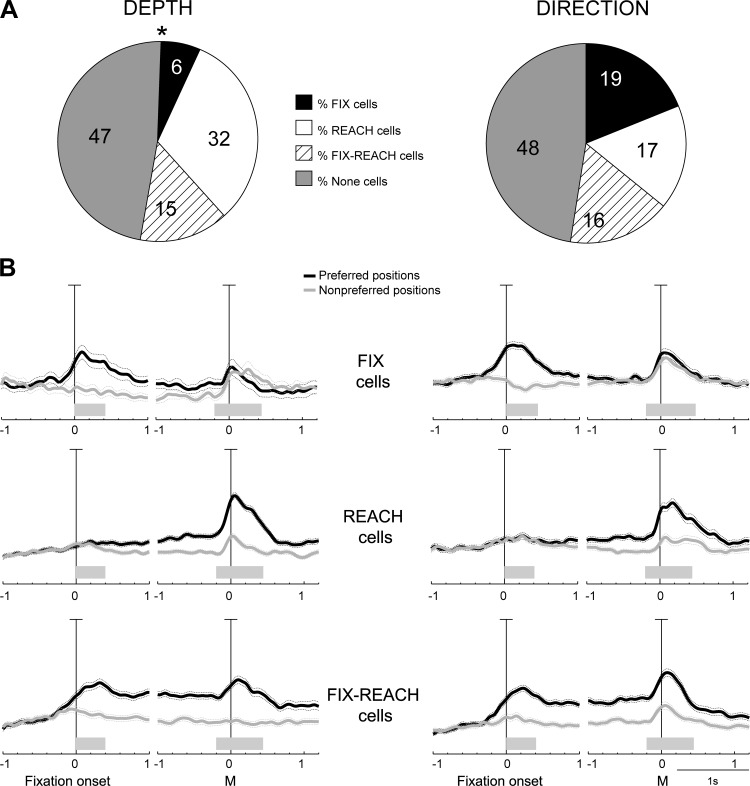
According to the relationship between eye- and hand-related tuning, we divided PEc cells into three main categories: 1) “Fix” cells when they showed spatial tuning in Fix but not in Reach, 2) “Reach” cells when the opposite condition occurred, and 3) “Fix-Reach” cells when the neurons were spatially tuned in both epochs. The percentage of PEc cells belonging to each category is reported in Fig. 7A. Neurons modulated by depth (Fig. 7A, left) fell mostly in the Reach category (32%; χ2, P < 0.05), whereas those affected by direction (Fig. 7A, right) were almost equally divided among the three categories (χ2, P > 0.05). Thus a much smaller fraction of PEc neurons were found to process information about the gaze depth (vergence angle) compared with gaze direction (Fig. 7A, Fix cells, 6% vs 19%).
Fig. 7.
Main cell categories in PEc. A: percentage of PEc neurons tuned in depth (left) and direction (right) during both Fix and Reach epochs (Fix-Reach cells; hatched), in Fix but not in Reach (Fix cells; solid), and vice versa (Reach cells; open) or in none of them (shaded).The Asterisk indicates that depth modulations were not observed with the same frequency in the 3 categories in PEc (χ2, P < 0.05). B: population activity of the main categories of cells, represented as average normalized spike density functions (SDF) of Fix (top), Reach (middle), and Fix-Reach cells (bottom) modulated by depth (left) and direction (right), doubly aligned (vertical lines) at the beginning of fixation and at movement onset. For each cell category and type of modulation, the average SDF for the preferred (black) and null positions (gray) are plotted. In Fix-Reach cells, the preferred condition was defined using the spatial preference of the Reach epoch, which was the same in most cases (>90%) with the preferred condition in Fix. Scale bar in all SDF plots: 100% of normalized activity. Shaded bars above the time axis indicate the duration of the Fix and Reach epochs. Sliding permutation tests (P < 0.05; see materials and methods) were performed for each category to calculate the time point when the population activity was different in the preferred and nonpreferred conditions: PEc Fix cells, 80 (depth) and 200 ms (direction) before fixation onset; PEc Reach cells, 880 and 1,210 ms after fixation onset for depth and for direction; PEc Fix-Reach cells, 20 ms before fixation onset for depth and 180 ms for direction.
We also investigated the temporal pattern of population activity in the three main categories of cells. Solid curves in Fig. 7B are the SDFs illustrating the average population activity of each category of PEc cells for depth and direction modulations (black) and for preferred and null conditions (gray). All in all, there was a similar trend and time course between depth and direction modulations. Regarding the specific categories, the SDFs of preferred and nonpreferred conditions in Fix cells (Fig. 7B, top) diverged slightly before fixation onset (because many cells showed spatially congruent perisaccadic responses), and the difference between preferred and null conditions was more pronounced during the first part (about 500 ms) of fixation. Interestingly, Fix cells showed also small arm movement related responses, but these responses had similar magnitude in the preferred and the nonpreferred conditions. In other words, Fix cells showed spatially tuned fixation activity and received information about the occurrence of an arm movement, regardless of its amplitude and/or direction. This latter behavior is reminiscent of the “pandirectional cells” described in area PE by Acuna et al. (1990) that showed changes in activity during arm movements that was independent of the target's direction. In Reach cells (Fig. 7B, middle), the modulation of activity during early fixation was negligible in both the preferred and null conditions. During the execution of arm movement, there was a robust increase of the preferred activity and a moderate increment of the null activity. It is interesting to note that the preferred activity started to progressively increase well before the arm movement, just after the beginning of fixation. This type of activity modulation is identical to the classic set-related activity that is observed in parietal, premotor, and motor areas (Alexander and Crutcher, 1990; Crammond and Kalaska 1989). The difference between the preferred and nonpreferred activity was very clear in the Plan epoch, in particular for movements in depth. Interestingly, also the spatial tuning during arm movement was stronger in depth than in direction. The behavior of Fix-Reach cells (Fig. 7B, bottom) resembled that of Fix cells at the beginning of the task and that of Reach cells during the arm movement. In this type of cells there was constantly a much higher activity for the preferred position during the whole delay period that is likely to reflect the effect of reaching preparation as it was observed in V6A (Breveglieri et al. 2014; Hadjidimitrakis et al. 2014). Fix-Reach cells showed a strong tuning during movement execution not only for movements in depth, like the Reach cells, but also for movements toward different directions. This finding suggests that neurons carrying both eye and hand signals are equally engaged in the control of reach direction and depth, whereas neurons with only arm signals are more involved in reaches at different depths.
In a further step of analysis, we examined the effector selectivity of the main cell categories. As shown previously in the population SDF curves (Fig. 7B), Fix and Fix-Reach cells showed increased activity in the preferred depth/direction during the saccade that preceded target fixation. To explore further this observation, we quantified the activity of the population of recorded cells during a time interval around the saccade occurrence (i.e., from 50 ms before saccade onset till 50 ms after the saccade end). We subsequently tested for the effects of depth and direction during that period with a two-way ANOVA (P < 0.01), and the modulated neurons were further classified with respect to the main cell categories. As shown in Table 2, the modulations of perisaccadic activity occurred almost exclusively in Fix and Fix-Reach categories. Only occasional Reach cells were found to be modulated during the perisaccadic period. As a result, effector selectivity defined by the presence/absence of activity tuning during the saccade is a good predictor of whether neurons are Fix, Fix-Reach, or Reach cells. It should be also mentioned that only two cells (Table 2, None) were found to be tuned during the saccade but not during the subsequent initial fixation period. This tight coupling between saccadic and fixation modulations was further confirmed when we examined the spatial tuning with linear regression analysis. Of 17 and 37 cells with linear tuning by depth and direction, respectively, during the perisaccadic epoch, 15 and 35 had the same tuning during the Fix epoch. Effector selectivity has been studied recently in parietal areas MIP and LIP and was shown to be related to information coding and decision making (Dean et al. 2012; de Lafuente et al. 2015). Our data show that PEc processes reach either independently from or together with saccades.
Table 2.
Tuning of perisaccadic activity by depth and direction in the cell categories
| Depth | Direction | |
|---|---|---|
| Fix | 6/13 (46%) | 17/38 (45%) |
| Reach | 0/64 (0%) | 3/34 (9%) |
| Fix-Reach | 13/29 (45%) | 19/33 (58%) |
| None | 1/94 (1%) | 1/95 (1%) |
Values are no. of cells (with percentage in parentheses) with depth and direction tuning in each cell category.
We subsequently looked in the main cell categories whether the activity during Reach epoch was related to behavioral parameters of reaches, in particular to the reaction time (RT). Significant correlation of Reach activity with RT could reflect a more direct involvement of a cell to the motor performance. To test for this correlation, we considered only the Reach activity that was above the levels of Plan activity. The results of this analysis are reported in Table 3. Of 148 observations (each cell tested for either depth, direction, or both), only in 11 cases (7.4%) did cells show a significant correlation (P < 0.05) of residual Reach activity with RT. In two of these cases, higher activity was significantly (P < 0.05) correlated with faster RTs. Our results are in agreement with findings from the parietal reach region (PRR; Snyder et al. 2006) that neighbors V6A. In that study, numerous cells with significant correlations between RTs and preparatory activity were found when the location of the reach target was specified simultaneously with the Go signal to start the reach. However, these correlations were not present when the reach target location was cued early in the task, i.e., in the standard delayed reach task that was also employed in our study.
Table 3.
Mean correlation coefficient between Reach firing rate above Plan activity and RT in main cell categories
| No. of Cells | P < 0.05 | Mean rValue | Mean PValue | |
|---|---|---|---|---|
| Depth | ||||
| Fix | 5 (13) | 1 | 0.04 | 0.29 |
| Reach | 52 (64) | 2 | 0.01 | 0.46 |
| Fix-Reach | 17 (29) | 1 | 0.01 | 0.50 |
| Direction | ||||
| Fix | 24 (38) | 3 | 0.01 | 0.57 |
| Reach | 27 (34) | 1 | 0.01 | 0.55 |
| Fix-Reach | 23 (33) | 3 | 0.02 | 0.48 |
Values are no. of cells included in this analysis (with total no. of cells of a given main category in parentheses). These cells had at least 15 of 30 trials with a Reach > Plan firing rate in the preferred depth/direction. RT, reaction time.
Pure Reach Planning and Execution Signals
In our task design the reaching target was fixated during most phases, including the reach planning, execution, and holding of the target epochs. As a result, it is conceivable that spatial modulations of activity during these epochs could reflect gaze signals. Moreover, activity during reaching execution might be attributed not only to motor commands but also to somatosensory feedback from the moving arm and/or a forward estimate of the final arm position (Mulliken et al. 2008). Neural activity during Hold could be reasonably interpreted as reflecting either gaze and/or arm position influences on firing rate, and no longer an influence of reach plans or reach motor commands, since the reach had already occurred. On the basis of this assumption, we tested for the effects of depth and direction in the Plan and Reach epochs (2-way ANOVA, P < 0.01) after subtracting trial-wise the firing rates of the Hold from Plan and Reach discharges. As reported in Table 4, the vast majority of cells modulated in Plan and Reach continued to be modulated by depth and direction signals even when the activity above the levels of Hold activity was considered. This finding argues for the strong presence in PEc of neurons with pure arm movement planning and execution-related, signals, not attributable either to gaze angle or arm position.
Table 4.
Tuning of Reach and Plan activity above Hold levels
| Cells | Plan minus Hold | Reach minus Hold |
|---|---|---|
| Depth only | 28/30 | 40/57 |
| Direction only | 19/27 | 28/31 |
| Both | 19/29 | 17/36 |
Values are no. of cells with Plan and Reach activity > Hold level in each tuning category.
DISCUSSION
We investigated the tuning of activity in PEc during the several phases of a fixate-to-reach task with a systematic variation of target depth and direction in 3D peripersonal space. The modulations of neural activity by depth and direction had on average a similar, high incidence across the task. Nevertheless, the effects of the two parameters showed a different time course. Directional tuning prevailed early in the task, i.e., when the target was initially fixated. Depth tuning became much stronger during and after movement execution. The difference in the temporal evolution of depth and direction signals was not only evident in the incidence of modulated cells but also was manifested in terms of tuning strength. Convergence of direction and depth information on single neurons was limited in the initial fixation period, but it increased significantly during the arm reaching and holding phases. As the task progressed, individual cells frequently lost their tuning and different neurons were recruited. At the population level, the majority of PEc neurons preferred far and contralateral space, with a slight preference for far-peripersonal space in depth only cells, a clear preference for the contralateral space in directionally tuned cells, and a preference for far and contralateral space in the neurons that combined depth and direction tuning. Many individual PEc cells showed tuning of the arm movement-related activity, or of both the eye position- and arm movement-related activity, whereas neurons carrying only eye position information, especially in depth, were a minority (Fig. 7A).
Role of PEc in Arm Movements in 3D Space
Area PEc has been studied so far only during center-out reaching tasks (Battaglia-Mayer et al. 2001; Ferraina et al. 2001; Sayegh et al. 2014) and was reported to have many neurons modulated by the direction of arm movements and/or by the direction of gaze. In the present study, we compared the effects of direction and depth information on PEc neuronal activity and found the former to be predominant in the early task epochs. The stronger effect of direction vs. depth well before the onset of arm movement is reminiscent of findings in the dorsal premotor cortex (PMd) (Fu et al. 1993, 1995; Messier and Kalaska 2000). Similarly to PEc, the encoding of direction in PMd appeared early, i.e., during the target cue or movement planning period, whereas movement distance exerted its effect mostly during movement execution. Given the well-established anatomic connection between PEc and PMd (Bakola et al. 2010; Johnson et al. 1996; Marconi et al. 2001; Matelli et al. 1998), signals about the target direction could be transmitted directly, i.e., without interacting with vergence signals, to PMd to first specify the movement direction that is more pivotal in the initial stages of movement planning and execution (Fu et al. 1995; Messier and Kalaska 2000).
Another similarity between PEc and PMd is the temporal evolution of the convergence of direction and depth signals. As was reported for PMd (Fu et al. 1993, 1995; Messier and Kalaska 2000), we found in the present study that the convergence of direction and depth signals in the activity of individual PEc neurons increased as the task progressed. This convergence on single neurons is in contrast with the view that the depth and direction of reaching targets are processed by separate visuomotor channels (Crawford et al. 2011; Flanders et al. 1992), a view supported by many behavioral studies (Bagesteiro et al. 2006; Flanders and Soechting 1990; Gordon et al. 1994; Sainburg et al. 2003; Soechting and Flanders 1989; Van Pelt and Medendorp 2008; Vindras et al. 2005). However, in PEc we have also observed a different temporal course of depth and direction processing, with sizeable populations of cells coding for only one spatial parameter, even in the late stages of the task (e.g., depth-only cells in Reach). The difference in the degree of convergence of depth and direction information between the early and late task phases might be related to the different representations of movement (Crawford et al. 2011; Flanders et al. 1992). Before the onset of movement, depth and direction are expected to be defined mostly in extrinsic reference frames, so they are more likely to be independent. However, during and after the movement, depth and direction signals are also referred to the intrinsic coordinates of the elbow and shoulder joint angles and thus are expected to be more tightly coupled. Consistent with this view is the fact that the maximum degree of convergence in PEc was observed during the Hold target epoch (Fig. 4A), i.e., when the coding of depth and direction signals in intrinsic coordinates most likely occurs.
Visuospatial and Arm Movement Coding in SPL
Vergence angle information has been reported to have a strong influence on the activity of a large fraction (∼60%) of neurons in the medial posterior parietal areas V6A (Breveglieri et al. 2012) and a similar (∼70%) number of cells in PRR (Bhattacharyya et al. 2009). The present results show a weaker depth tuning in PEc, compared with V6A and PRR, during fixation. This is a new finding since no studies have investigated vergence signals in PEc to date. In area PE, vergence angle has an even weaker modulatory effect on neural activity (Ferraina et al. 2009). Taken together, these findings hint at the existence of a rostral-to-caudal gradient of increased eye vergence sensitivity in medial PPC.
During the initial target fixation, a much larger number of PEc neurons processed gaze direction compared with gaze depth, suggesting a bias of visuospatial activity for 2D space. As a result, few PEc neurons showed convergence of depth and direction information just after the target was fixated. This implies that PEc is not strongly involved in encoding the 3D location of the reaching target in space. This finding adds to other evidence suggesting that caudal SPL areas, such as V6A and PRR, encode the goal of the reaching movement, whereas more rostral areas, such as PE, are more related to the implementation of the movement plan (Breveglieri et al. 2014; Cui and Andersen 2011; Li and Cui 2013). The difference with V6A may be indicative of a genuine trend between the two caudal SPL areas, since the task used was identical in both studies (Hadjidimitrakis et al. 2014). V6A cells that carried depth information from the beginning of the task were more numerous compared with PEc, and they only slightly increased in the later stages (see Hadjidimitrakis et al. 2014, Fig. 4A). As the task progressed toward arm movement execution, the two SPL areas showed a very similar pattern of spatial encoding, with depth information becoming more influential than direction and with increased convergence of depth and direction signals on single cells. Overall, the differences in spatial processing between PEc and V6A, combined with the similarities between PEc and PMd mentioned in the previous section, place PEc closer to the premotor circuit compared with V6A.
In the movement period, a large proportion of PEc neurons tuned only in depth was recruited, and this was more pronounced compared with V6A (compare Fig. 4A in present study with Fig. 4A in Hadjidimitrakis et al. 2014). This difference might be related to the fact that PEc receives much more somatosensory input compared with V6A (Bakola et al. 2010; Breveglieri et al. 2002, 2006). Recently, we proposed a conceptual framework for the processing of depth and direction signals in SPL reaching areas (Hadjidimitrakis et al. 2014). This framework is based on behavioral and computational evidence suggesting that movement in depth relies on proprioceptive information, whereas vision is more important for the specification of reach direction (Monaco et al. 2010; Sainburg et al. 2003; van Beers et al. 1998, 2002, 2004). Further support to this evidence stems from neurophysiological studies in PE and in the somatosensory cortex (Lacquaniti et al. 1995; Tillery et al. 1996) reporting increased numbers of neurons modulated during active or passive hand displacements in depth compared with the azimuth and elevation. On the basis of all the above-mentioned evidence, we suggested that the relative proportion of visual vs. proprioceptive inputs of a given SPL area could be critical for its contribution to the specification of the reach direction and depth. PEc primarily processes somatosensory information about the movement and static posture of the hand (Bakola et al. 2010; Breveglieri et al. 2006; Ferraina et al. 2001), and in the SPL circuitry it occupies a position closer to PE than to V6A. As visual sensitivity increases toward area V6A and somatosensory sensitivity increases toward area PE, in the opposite direction, PEc was expected to show a pattern of increased depth modulations during the arm movement and static posture. Our findings are consistent with this framework and provide further neurophysiological support to the link between proprioception and movement in depth suggested by other lines of evidence.
Task Advantages and Limitations
In our study reaches were always performed toward foveated targets. As a result, in our paradigm reaches were always made from the periphery of the visual field to the center of gaze. This configuration that imitates the natural behavior adds, to our view, a particular strength to our results. When humans reach to grasp objects, they look at the target first and then bring the hand to the center of gaze as the object is grasped (Hayhoe et al., 2002; Johansson et al. 2001; Land et al. 1999). However, our task configuration also has a limitation, because it cannot dissociate between several reference frames. For example, depth can be defined both in a motor perspective (i.e., wherein depth is the endpoint of reach) and also in a visuospatial context (i.e., vergence at which the LED is presented). It should be noted that these two definitions are overlapping in the course of the visuomotor transformations required in our task. Further experiments are needed to disambiguate between the two definitions.
Another limitation of the present study is that a small range of directions was tested compared with the center-out configuration employed in most studies of the reach network. Given the limited directional space, the tuning of neural activity by direction was not fit with nonlinear models (e.g., cosine, Gaussian) used in many previous studies but with a linear model. Nevertheless, our regression analysis showed that the linear model gave significant fits in the vast majority of tuned cells.
Neural Substrates of Reaching in Depth in Human Parietal Cortex
The involvement of SPL in the processing of distance was first demonstrated in a human case study (Holmes and Horrax 1919). Additional studies on patients with lesions in SPL reported a stronger deficit in depth than in direction during reaching (Baylis and Baylis 2001) and pointing movements (Danckert et al. 2009). Differently, in a recent transcranial magnetic stimulation study where the putative homolog of the macaque MIP was inactivated, only directional errors were observed (Davare et al. 2012). Regarding the convergence of depth and direction signals, a recent functional MRI study in humans (Fabbri et al. 2012) found that directionally tuned parietal regions were also sensitive to movement depth and that the degree of this sensitivity increased in an anterior-to-posterior fashion. These observations are in good agreement with present findings in PEc and previous ones in areas V6A (Hadjidimitrakis et al. 2014) and PE (Lacquaniti et al. 1995) and suggest a common framework of depth and direction processing in human and nonhuman primates.
GRANTS
This work was supported by the European Commission FP7-ICT-217077 EYESHOTS Project, Ministero dell'Universita' e della Ricerca (Italy), Fondazione del Monte di Bologna e Ravenna (Italy), FIRB (Basic Research Investment Fund) 2013 N. RBFR132BKP (Italy), and National Health and Medical Research Council Grants APP1020839 and APP1082144 (Australia).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.H., G.D.B., and R.B. performed experiments; K.H. and G.D.B. analyzed data; K.H., R.B., C.G., and P.F. interpreted results of experiments; K.H., G.D.B., C.G., and P.F. drafted manuscript; K.H., G.D.B., R.B., C.G., and P.F. edited and revised manuscript; K.H., G.D.B., R.B., C.G., and P.F. approved final version of manuscript; G.D.B. prepared figures; R.B., C.G., and P.F. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. N. Marzocchi and G. Placenti for setting up the experimental apparatus, Dr. L. Passarelli and Dr. M. Gamberini for valuable assistance in the reconstruction of the penetrations, and Dr. F. Bertozzi for help during recordings.
REFERENCES
- Acuna C, Cudeiro J, Gonzalez F, Alonso JM, Perez R. Lateral-posterior and pulvinar reaching cells–comparison with parietal area 5a: a study in behaving Macaca nemestrina monkeys. Exp Brain Res 82: 158–166, 1990. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Preparation for movement: neural representation of intended direction in three motor areas of the monkey. J Neurophysiol 64: 133–150, 1990. [DOI] [PubMed] [Google Scholar]
- Bagesteiro L, Sarlegna F, Sainburg R. Differential influence of vision and proprioception on control of movement distance. Exp Brain Res 171: 358–370, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakola S, Gamberini M, Passarelli L, Fattori P, Galletti C. Cortical connections of parietal field PEc in the macaque: linking vision and somatic sensation for the control of limb action. Cereb Cortex 20: 2592–2604, 2010. [DOI] [PubMed] [Google Scholar]
- Bakola S, Passarelli L, Gamberini M, Fattori P, Galletti C. Cortical connectivity suggests a role in limb coordination for macaque area PE of the superior parietal cortex. J Neurosci 33: 6648–6658, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Ferraina S, Mitsuda T, Marconi B, Genovesio A, Onorati P, Lacquaniti F, Caminiti R. Early coding of reaching in the parietooccipital cortex. J Neurophysiol 83: 2374–2391, 2000. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Ferraina S, Genovesio A, Marconi B, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb Cortex 11: 528–544, 2001. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Mascaro M, Brunamonti E, Caminiti R. The over-representation of contralateral space in parietal cortex: a positive image of directional motor components of neglect? Cereb Cortex 15: 514–525, 2005. [DOI] [PubMed] [Google Scholar]
- Baylis GC, Baylis LL. Visually misguided reaching in Balint's syndrome. Neuropsychologia 39: 865–875, 2001. [DOI] [PubMed] [Google Scholar]
- Bhat RB, Sanes JN. Cognitive channels computing action distance and direction. J Neurosci 18: 7566–7580, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya R, Musallam S, Andersen RA. Parietal reach region encodes reach depth using retinal disparity and vergence angle signals. J Neurophysiol 102: 805–816, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breveglieri R, Galletti C, Dal Bo' G, Hadjidimitrakis K, Fattori P. Multiple aspects of neural activity during reaching preparation in the medial posterior parietal area V6A. J Cogn Neurosci 26: 878–895, 2014. [DOI] [PubMed] [Google Scholar]
- Breveglieri R, Galletti C, Monaco S, Fattori P. Visual, somatosensory, and bimodal activities in the macaque parietal area PEc. Cereb Cortex 18: 806–816, 2008. [DOI] [PubMed] [Google Scholar]
- Breveglieri R, Galletti C, Gamberini M, Passarelli L, Fattori P. Somatosensory cells in area PEc of macaque posterior parietal cortex. J Neurosci 26: 3679–3684, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breveglieri R, Kutz DF, Fattori P, Gamberini M, Galletti C. Somatosensory cells in the parieto-occipital area V6A of the macaque. Neuroreport 13: 2113–2116, 2002. [DOI] [PubMed] [Google Scholar]
- Breveglieri R, Hadjidimitrakis K, Bosco A, Sabatini SP, Galletti C, Fattori P. Eye position encoding in three-dimensional space: integration of version and vergence signals in the medial posterior parietal cortex. J Neurosci 32: 159–169, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien Dargestellt auf Grund des Zellenbaues. Leipzig, Germany: Barth, 1909. [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol 287: 422–445, 1989a. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol 287: 393–421, 1989b. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia 29: 517–537, 1991. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Neuronal activity in primate parietal cortex area 5 varies with intended movement direction during an instructed-delay period. Exp Brain Res 76: 458–462, 1989. [DOI] [PubMed] [Google Scholar]
- Crawford JD, Henriques DY, Medendorp WP. Three-dimensional transformations for goal-directed action. Annu Rev Neurosci 34: 309–331, 2011. [DOI] [PubMed] [Google Scholar]
- Cui H, Andersen RA. Different representations of potential and selected motor plans by distinct parietal areas. J Neurosci 31: 18130–18136, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckert J, Goldberg L, Broderick C. Damage to superior parietal cortex impairs pointing in the sagittal plane. Exp Brain Res 195: 183–191, 2009. [DOI] [PubMed] [Google Scholar]
- Davare M, Zenon A, Pourtois G, Desmurget M, Olivier E. Role of the medial part of the intraparietal sulcus in implementing movement direction. Cereb Cortex 22: 1382–1394, 2012. [DOI] [PubMed] [Google Scholar]
- Dean HL, Hagan MA, Pesaran B. Only coherent spiking in posterior parietal cortex coordinates looking and reaching. Neuron 73: 829–841, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lafuente V, Jazayeri M, Shadlen MN. Representation of accumulating evidence for a decision in two parietal areas. J Neurosci 35: 4306–4318, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri S, Caramazza A, Lingnau A. Distributed sensitivity for movement amplitude in directionally tuned neuronal populations. J Neurophysiol 107: 1845–1856, 2012. [DOI] [PubMed] [Google Scholar]
- Fattori P, Gamberini M, Kutz DF, Galletti C. ‘Arm-reaching’ neurons in the parietal area V6A of the macaque monkey. Eur J Neurosci 13: 2309–2313, 2001. [DOI] [PubMed] [Google Scholar]
- Fattori P, Kutz DF, Breveglieri R, Marzocchi N, Galletti C. Spatial tuning of reaching activity in the medial parieto-occipital cortex (area V6A) of macaque monkey. Eur J Neurosci 22: 956–972, 2005. [DOI] [PubMed] [Google Scholar]
- Ferraina S, Battaglia-Mayer A, Genovesio A, Marconi B, Onorati P, Caminiti R. Early coding of visuomanual coordination during reaching in parietal area PEc. J Neurophysiol 85: 462–467, 2001. [DOI] [PubMed] [Google Scholar]
- Ferraina S, Brunamonti E, Giusti MA, Costa S, Genovesio A, Caminiti R. Reaching in depth: hand position dominates over binocular eye position in the rostral superior parietal lobule. J Neurosci 29: 11461–11470, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraina S, Garasto MR, Battaglia-Mayer A, Ferraresi P, Johnson PB, Lacquaniti F, Carniniti R. Visual control of hand-reaching movement: activity in parietal area 7m. Eur J Neurosci 9: 1090–1095, 1997. [DOI] [PubMed] [Google Scholar]
- Flanders M, Soechting JF. Parcellation of sensorimotor transformations for arm movements. J Neurosci 10: 2420–2427, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders M, Helms Tillery SI, Soechting JF. Early stages in a sensorimotor transformation. Behav Brain Sci 15: 309–362, 1992. [Google Scholar]
- Fluet MC, Baumann MA, Scherberger HR. Context-specific grasp movement representation in macaque ventral premotor cortex. J Neurosci 30: 15175–15184, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QG, Suarez JI, Ebner TJ. Neuronal specification of direction and distance during reaching movements in the superior precentral premotor area and primary motor cortex of monkeys. J Neurophysiol 70: 2097–2116, 1993. [DOI] [PubMed] [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Temporal encoding of movement kinematics in the discharge of primate primary motor and premotor neurons. J Neurophysiol 73: 836–854, 1995. [DOI] [PubMed] [Google Scholar]
- Galletti C, Battaglini PP, Fattori P. Eye position influence on the parieto-occipital area PO (V6) of the macaque monkey. Eur J Neurosci 7: 2486–2501, 1995. [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P, Kutz DF, Gamberini M. Brain location and visual topography of cortical area V6A in the macaque monkey. Eur J Neurosci 11: 575–582, 1999. [DOI] [PubMed] [Google Scholar]
- Gamberini M, Passarelli L, Fattori P, Zucchelli M, Bakola S, Luppino G, Galletti C. Cortical connections of the visuomotor parietooccipital area V6Ad of the macaque monkey. J Comp Neurol 513: 622–642, 2009. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C. Accuracy of planar reaching movements. Exp Brain Res 99: 97–111, 1994. [DOI] [PubMed] [Google Scholar]
- Hadjidimitrakis K, Bertozzi F, Breveglieri R, Bosco A, Galletti C, Fattori P. Common neural substrate for processing depth and direction signals for reaching in the monkey medial posterior parietal cortex. Cereb Cortex 24: 1645–1657, 2014. [DOI] [PubMed] [Google Scholar]
- Hayhoe M, Aivar P, Shrivastavah A, Mruczek R. Visual short-term memory and motor planning. Prog Brain Res 140: 349–363, 2002. [DOI] [PubMed] [Google Scholar]
- Holmes G, Horrax G. Disturbances of spatial orientation and visual attention, with loss of stereoscopic vision. Arch Neurol Psychiatry 1: 385–407, 1919. [Google Scholar]
- Johansson RS, Westling G, Backstrom A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci 21: 6917–6932, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6: 102–119, 1996. [DOI] [PubMed] [Google Scholar]
- Kagan I, Iyer A, Lindner A, Andersen RA. Space representation for eye movements is more contralateral in monkeys than in humans. Proc Natl Acad Sci USA 107: 7933–7938, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz DF, Fattori P, Gamberini M, Breveglieri R, Galletti C. Early- and late-responding cells to saccadic eye movements in the cortical area V6A of macaque monkey. Exp Brain Res 149: 83–95, 2003. [DOI] [PubMed] [Google Scholar]
- Kutz DF, Marzocchi N, Fattori P, Cavalcanti S, Galletti C. Real-time supervisor system based on trinary logic to control experiments with behaving animals and humans. J Neurophysiol 93: 3674–3686, 2005. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Guigon E, Bianchi L, Ferraina S, Caminiti R. Representing spatial information for limb movement: role of area 5 in the monkey. Cereb Cortex 5: 391–409, 1995. [DOI] [PubMed] [Google Scholar]
- Land M, Mennie N, Rusted J. The roles of vision and eye movements in the control of activities of daily living. Perception 28: 1311–1328, 1999. [DOI] [PubMed] [Google Scholar]
- Li Y, Cui H. Dorsal parietal area 5 encodes immediate reach in sequential arm movements. J Neurosci 33: 14455–14465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Ben Hamed S, Gamberini M, Matelli M, Galletti C. Occipital (V6) and parietal (V6A) areas in the anterior wall of the parieto-occipital sulcus of the macaque: a cytoarchitectonic study. Eur J Neurosci 21: 3056–3076, 2005. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex 11: 513–527, 2001. [DOI] [PubMed] [Google Scholar]
- Marzocchi N, Breveglieri R, Galletti C, Fattori P. Reaching activity in parietal area V6A of macaque: eye influence on arm activity or retinocentric coding of reaching movements? Eur J Neurosci 27: 775–789, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matelli M, Govoni P, Galletti C, Kutz DF, Luppino G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J Comp Neurol 402: 327–352, 1998. [PubMed] [Google Scholar]
- McGuire LM, Sabes PN. Heterogeneous representations in the superior parietal lobule are common across reaches to visual and proprioceptive targets. J Neurosci 31: 6661–6673, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp WP, Tweed DB, Crawford JD. Motion parallax is computed in the updating of human spatial memory. J Neurosci 23: 8135–8142, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier J, Kalaska JF. Covariation of primate dorsal premotor cell activity with direction and amplitude during a memorized-delay reaching task. J Neurophysiol 84: 152–165, 2000. [DOI] [PubMed] [Google Scholar]
- Monaco S, Kroliczak G, Quinlan DJ, Fattori P, Galletti C, Goodale MA, Culham JC. Contribution of visual and proprioceptive information to the precision of reaching movements. Exp Brain Res 202: 15–32, 2010. [DOI] [PubMed] [Google Scholar]
- Mulliken GH, Musallam S, Andersen RA. Forward estimation of movement state in posterior parietal cortex. Proc Natl Acad Sci USA 105: 8170–8177, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J Comp Neurol 204: 196–210, 1982. [DOI] [PubMed] [Google Scholar]
- Passarelli L, Rosa MG, Gamberini M, Bakola S, Burman KJ, Fattori P, Galletti C. Cortical connections of area V6Av in the macaque: a visual-input node to the eye/hand coordination system. J Neurosci 31: 1790–1801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffi M, Ballabeni A, Maioli MG, Squatrito S. Neuronal responses in macaque area PEc to saccades and eye position. Neuroscience 156: 413–424, 2008. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Lateiner JE, Latash ML, Bagesteiro LB. Effects of altering initial position on movement direction and extent. J Neurophysiol 89: 401–415, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlegna FR, Blouin J. Visual guidance of arm reaching: online adjustments of movement direction are impaired by amplitude control. J Vis 10: 24, 2010. [DOI] [PubMed] [Google Scholar]
- Sayegh PF, Hawkins KM, Neagu B, Crawford JD, Hoffman KL, Sergio LE. Decoupling the actions of the eyes from the hand alters beta and gamma synchrony within SPL. J Neurophysiol 111: 2210–2221, 2014. [DOI] [PubMed] [Google Scholar]
- Shi Y, Apker G, Buneo CA. Multimodal representation of limb endpoint position in the posterior parietal cortex. J Neurophysiol 109: 2097–2107, 2013. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature 386: 167–170, 1997. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Dickinson AR, Calton JL. Preparatory delay activity in the monkey parietal reach region predicts reach reaction times. J Neurosci 26: 10091–10099, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechting JF, Flanders M. Sensorimotor representations for pointing to targets in three-dimensional space. J Neurophysiol 62: 582–594, 1989. [DOI] [PubMed] [Google Scholar]
- Squatrito S, Raffi M, Maioli MG, Battaglia-Mayer A. Visual motion responses of neurons in the caudal area PE of macaque monkeys. J Neurosci 21: RC130, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillery SI, Soechting JF, Ebner TJ. Somatosensory cortical activity in relation to arm posture: nonuniform spatial tuning. J Neurophysiol 76: 2423–2438, 1996. [DOI] [PubMed] [Google Scholar]
- Tramper JJ, Gielen CC. Visuomotor coordination is different for different directions in three-dimensional space. J Neurosci 31: 7857–7866, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers RJ, Haggard P, Wolpert DM. The role of execution noise in movement variability. J Neurophysiol 91: 1050–1063, 2004. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Sittig AC, Denier van der Gon JJ. The precision of proprioceptive position sense. Exp Brain Res 122: 367–377, 1998. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Wolpert DM, Haggard P. When feeling is more important than seeing in sensorimotor adaptation. Curr Biol 12: 834–837, 2002. [DOI] [PubMed] [Google Scholar]
- Van Pelt S, Medendorp WP. Updating target distance across eye movements in depth. J Neurophysiol 99: 2281–2290, 2008. [DOI] [PubMed] [Google Scholar]
- Vindras P, Desmurget M, Viviani P. Error parsing in visuomotor pointing reveals independent processing of amplitude and direction. J Neurophysiol 94: 1212–1224, 2005. [DOI] [PubMed] [Google Scholar]
- Wijdenes LO, Brenner E, Smeets JB. Comparing online adjustments to distance and direction in fast pointing movements. J Mot Behav 45: 395–404, 2013. [DOI] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall, 1999. [Google Scholar]