Abstract
Transcranial magnetic stimulation (TMS) of the motor cortex shows that hand action observation (AO) modulates corticospinal excitability (CSE). CSE modulation alternatively maps low-level kinematic characteristics or higher-level features, like object-directed action goals. However, action execution is achieved through the control of muscle synergies, consisting of coordinated patterns of muscular activity during natural movements, rather than single muscles or object-directed goals. This synergistic organization of action execution also underlies the ability to produce the same functional output (i.e., grasping an object) using different effectors. We hypothesize that motor system activation during AO may rely on similar principles. To investigate this issue, we recorded both hand CSE and TMS-evoked finger movements which provide a much more complete description of coordinated patterns of muscular activity. Subjects passively watched hand, mouth and eyelid opening or closing, which are performing non-object-directed (intransitive) actions. Hand and mouth share the same potential to grasp objects, whereas eyelid does not allow object-directed (transitive) actions. Hand CSE modulation generalized to all effectors, while TMS evoked finger movements only to mouth AO. Such dissociation suggests that the two techniques may have different sensitivities to fine motor modulations induced by AO. Differently from evoked movements, which are sensitive to the possibility to achieve object-directed action, CSE is generically modulated by “opening” vs. “closing” movements, independently of which effector was observed. We propose that motor activities during AO might exploit the same synergistic mechanisms shown for the neural control of movement and organized around a limited set of motor primitives.
Keywords: transcranial magnetic stimulation, action observation network, corticospinal excitability, finger kinematics, motor generalization
action observation (ao) induces corticospinal excitability (CSE) modulations (Fadiga et al. 1995, 2005) similar to those of action production. CSE is modulated by low-level kinematic features of the observed action (i.e., finger aperture during reach-to-grasp actions; Gangitano et al. 2001), as well as the amplitude of muscle activities and forces applied when lifting objects of different weights (Alaerts et al. 2009; Senot et al. 2011). Indeed, CSE modulations were shown to closely match the pattern of muscle activities in the observed action, in terms of both muscle somatotopy and temporal evolution of such activities (Borroni et al. 2005). Moreover, besides these low-level movement descriptors, CSE is also modulated for higher-level features, like object-directed action goals. This mechanism was shown for grasping AO with pliers dissociating goals and movements (Cattaneo et al. 2009; but see Cavallo et al. 2012 and Cattaneo et al. 2013). Analogously, bilateral hand CSE is modulated during one-hand AO (Borroni et al. 2008) and by object-directed actions performed by different body parts (Senna et al. 2014). These latter studies demonstrate that CSE modulations may be independent from the kinematic features and muscle activities in the observed action.
Therefore, the use of CSE to investigate the motor activities during AO yielded some contrasting results. Thus it is not clear whether CSE maps the low-level motor implementation details of the observed action or rather higher-level features of actions, such as the grasping of a specific object, regardless of how that goal is achieved. Based on these inconsistent data, it has been proposed that different features could alternatively be extracted from the observed action, in relation to the task. CSE modulations during AO could switch between different levels, depending on task constraints and/or prior knowledge provided to the subject (Mc Cabe et al. 2015).
Alternatively, we propose these conflicting results to come from both a methodological and a theoretical issue. From a methodological standpoint, most of the studies measure CSE from a few muscles (Naish et al. 2014), thus displaying only a small fraction of the ongoing motor activities during AO. Hand action emerges from the composition of several intrinsic hand and forearm muscle activities (Santello et al. 2013), and activity in one muscle is hardly sufficient to distinguish two goal-directed actions. Rather, the complexity of these effects is better characterized by exploring transcranial magnetic stimulation (TMS)-evoked movements (Gentner and Classen 2006; Gentner et al. 2010). Hence, movements triggered by TMS better reflect the rich pattern of motor activities induced by AO (Barchiesi and Cattaneo 2013). Additionally, from a theoretical point of view, motor and premotor activations during AO should be at least in part similar to those in action execution. Action execution is centered on the possibility of producing the same functional output (i.e., grasping an object) by using different effectors, starting from different postures and targeting objects in different locations (Graziano et al. 2002). If we assume that AO deals with the same methodological and theoretical constraints than action execution, AO should reflect functional outputs only through measures capturing the whole complexity of muscle synergies (D'Ausilio et al. 2015).
To test these hypotheses, we measured the output of the corticospinal system during AO. This was achieved by recording TMS-evoked hand movements (Bartoli et al. 2014; Classen et al. 1998; Gentner and Classen 2006; Gentner et al. 2010). In parallel, we monitored CSE of the flexor digitorum superficialis (FDS), a muscle fundamentally recruited in finger flexion. Subjects watched hand, mouth or eyelid performing opening or closing non-object-oriented actions (intransitive action). Intransitive actions were used to investigate effector-specific AO modulations, independently of object (goal) presence. These effectors were selected to test generalization across effectors, as recently described by Senna and colleagues (2014), with an additional and important difference. Hand and mouth movements allow object-directed action (transitive action), whereas eyelid movements do not. This is a critical distinction since eyelid movements do not share the same set of motor primitives with hand and mouth.
We predict dissociation between CSE and TMS-evoked movements, potentially accounting for some of the previously reported inconsistent results. Furthermore, we expect that CSE might not be able to discriminate between effectors during AO, due to the rather low-action-specificity of single-muscle CSE. On the other hand, TMS-evoked movements could show reduced generalization, limited to the transitive effectors (hand and mouth, not the eyelid action). This latter finding might result from the greater efficacy by which TMS-evoked movements describe motor output synergies. In this sense, and in agreement with the synergistic organization of action execution, we postulate that observation of effectors enabling object-directed action activate a shared set of motor primitives.
MATERIALS AND METHODS
Subjects
A total of 35 healthy subjects (20 men, aged 24.47 ± 3.80 yr) participated in this study. The participants were randomly assigned to two experimental groups, one of them composed of 18 subjects (10 men, aged 24.36 ± 3.02 yr) and the second of 17 subjects (10 men, aged 24.48 ± 4.52 yr). Due to technical problems during data acquisitions, we had to exclude one subject of the first group from the analysis of kinematic data and four participants of the second group from the analyses of CSE. Thus the following evaluations were carried out on data obtained on 31 subjects for CSE measure (18 subjects from the first group, 10 men, and 13 subjects from the second group, 7 men) and 30 subjects for the kinematic one (17 subjects from the first group, 10 men, and 13 subjects from the second group, 7 men). The experimental protocol was reviewed and approved by the local ethics committee (internal review board) prior to starting the study. Participants gave informed, written consent for participation in the study according to the Declaration of Helsinki of 1975, as revised in 1983. All subjects were naive to the purpose of the study and received an attendance fee at the end of the experiment. All had normal or corrected-to-normal vision and were right-handed, as assessed by an adapted Italian version of the Edinburgh handedness inventory (Oldfield 1971). None of the subjects had contraindications to TMS.
Stimuli
The experimental stimuli consisted in short video clips, each of 3,000-ms duration. The videos showed a female actor who executed opening or closing actions performed by the hand, mouth or eyelid (6 different video clips). The effectors were always presented in a lateral view (Figure 1B). The stimuli were recorded using a camera Legria HF (Canon, Tokyo, Japan) and edited with Adobe After Effects (CS5 version). Stimuli dimensions were 720 × 576 pixels, and they were displayed in the center of a 17-in. computer screen through E-Prime Software (Psychology Software Tools, version 2.0.8.22). In addition to these experimental stimuli, other unrelated video clip fillers were introduced in the baseline recordings. These movies were extracted from a nature documentary and thus showed animals and environmental events, such as clouds, rivers, etc. In all cases, we included no stimulus showing or implying grasp-related actions, and no animals with human-like hands (i.e., penguins, dolphins, birds).
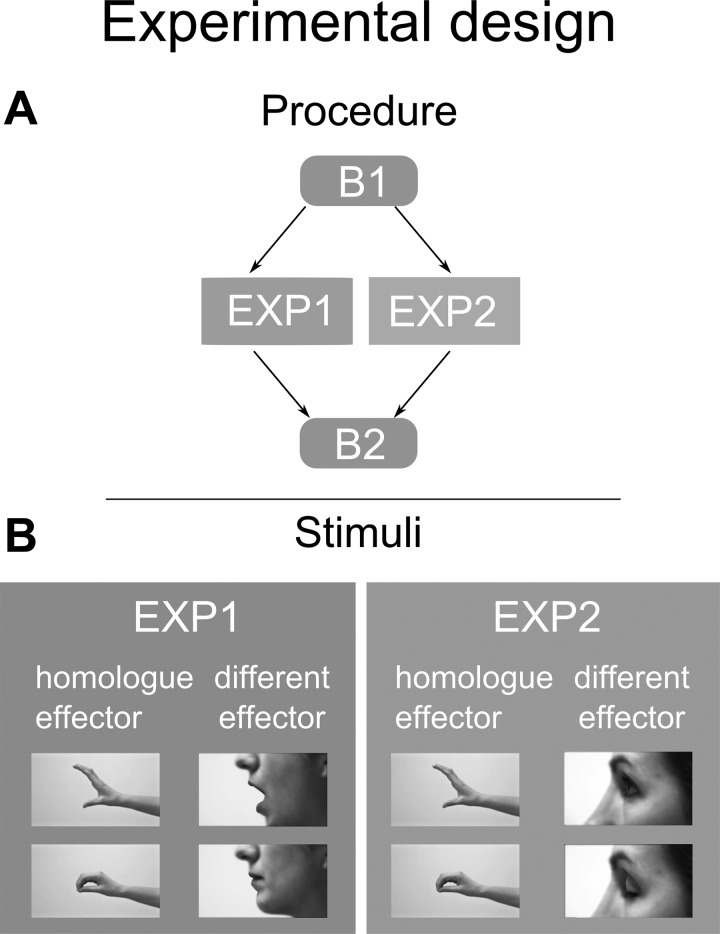
Fig. 1.
Experimental design. A: the procedure of the two experiments is detailed. In both experiments, a first baseline recording (B1) during which subjects were at rest was followed by an experimental session (EXP) during which participants observed videos clips, and by a second baseline recording (B2). B: the pictures show frames from each video clip representing the experimental stimuli for the first (left) and the second experiment (right). Opening and closing actions performed by the homologue (hand for both groups) and different effectors (mouth for group 1 and eyelid for group 2) are shown.
Procedure
Subjects were seated in a comfortable chair with their right forearm resting on a molded arm rest. They were instructed to keep their hands still and as relaxed as possible with the wrist flexed and the fingers pointed toward the floor but entirely unconstrained. We first set up the devices for the electromyography (EMG) and for the movement acceleration recording, then we proceeded to the muscle hotspot location and resting motor threshold (rMT) definition. The experiment consisted of a baseline recording (B1), followed by the experimental session (EXP) and then again another baseline block (B2) (Fig. 1A). Both baselines included 20 trials during which subjects observed nature-related stimuli. For each trial, motor evoked potentials (MEPs) from the target muscle and the acceleration of the target finger movements evoked by the same TMS pulse were evaluated at B1, B2 and during EXP.
The EXP sessions differed between the two experiments. In experiment 1, subjects were presented with hand video clips and mouth video clips. In experiment 2, subjects were presented with hand video clips and eyelid video clips. Although we were fully aware that a better design should include all of the three effectors in the same session, we decided to split the recording into two experiments (groups) to avoid possible confounding carry-over effects and to reduce fatigue in the subjects.
Therefore, in each experimental setting, we presented four video clips, showing the closing or opening of the homologue effector (hand) and of a different effector (experiment 1: mouth; experiment 2: eyelid) (Fig. 1B). The order of video clips appearance was random. In both experiments, 20 repetitions for each of the 4 video clips were shown, thus leading to a total of 80 trials. Five trials without TMS pulse were randomly presented in each condition, thus leading to 60 TMS trials out of total 80.
TMS was delivered before the end of the movement, at 90% of the whole duration of the movement. Movement length in all three video clips was the same. Subjects were requested to carefully observe the video clips, then, in 9% of trials randomly distributed for the whole duration of the task, they were asked to answer if the last presented video was the same as of the previous one, in terms of observed effector and type of movement. To avoid any contaminations between our measurements and this control task, the answers had to be given pressing one of the two buttons on a response pad by their left hand. No time-out for the response was assigned, and no TMS pulses were administered during these trials. The task was devised to keep high level of attention throughout the experiment.
TMS and EMG
TMS was delivered through a figure-eight coil (70 mm) and a Magstim Rapid stimulator (Magstim, Whitland, UK). For each subject, the left primary motor cortex was first functionally localized by means of visual inspection of MEPs recorded through EMG on the right arm from the FDS muscle (Fig. 2A). We determined the optimal position for activation of the right FDS muscle (i.e., the scalp position from which maximal amplitude MEPs were elicited) by moving the coil in 0.5-cm steps around the presumed motor hand area by using a slightly suprathreshold stimulus. The optimal position of the coil was then marked on a cap placed on the scalp to ensure the correct coil placement through the experiment. The FDS has been chosen because it is necessary for hand closing movement. EMG was recorded through a wireless EMG system (Zerowire EMG, Aurion, Italy) with a tendon-belly montage. EMG signals were sampled at 2 kHz, filtered and digitized with a data acquisition interface (Power1401, Cambridge Electronics Designs) and data were displayed and stored for offline analysis using the Signal software version 4. A prestimulus recording of 300 ms was acquired to check for the presence of EMG activity before TMS pulse. Trials with EMG background activity were excluded from the analysis. EMG data were collected for 1,000 ms after the TMS pulse. The TMS coil was held tangentially to the scalp with the handle pointing backward and laterally to form a 45° angle with the midline, and it was fastened to an articulated mechanical arm held by a heavy tripod. The rMT was established as the lowest stimulation intensity capable of evoking at least 5 MEPs out of 10 consecutive pulses with 50-μV peak-to-peak amplitude (Rossini et al. 1994). During the experiment, single-pulse TMS was applied to the identified hotspot, with an intensity of stimulation corresponding to 130% of the rMT. TMS was triggered through the parallel port.
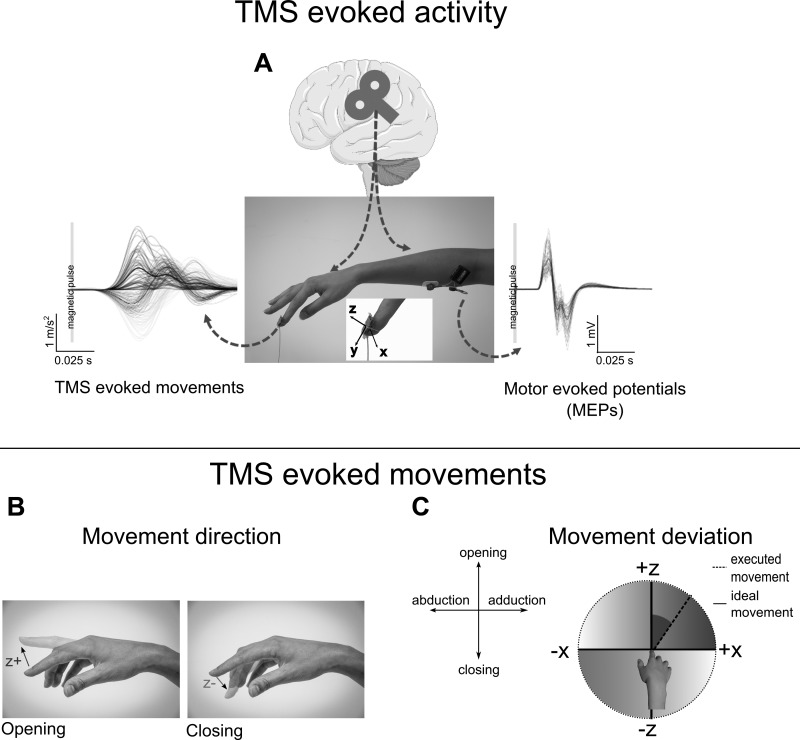
Fig. 2.
Experimental recording of the transcranial magnetic stimulation (TMS) evoked effects and dependent variables. A: the experimental setup used to measure the TMS-evoked movement and the motor evoked potentials (MEPs). The acceleration components recorded with the accelerometer in a representative subject are depicted for each trial (thin lines), and for the averaged value (thick line) for the z, x components and for the module. Single trials (thin lines) and averaged (thick line) values of the MEPs are instead represented on the right part of the same panel. The middle picture shows the setup to record accelerometer data and electromyography of the right flexor digitorum superficialis muscle. Bottom: the TMS-evoked movement parameters. B: opening or closing movement direction is derived from the positive or negative values, respectively, of the z-component. C: movement deviation expresses the angular displacement of the finger on the frontal axis (abduction/adduction movement) during the opening or closing motion (dashed line). This angle is expressed with respect to the ideal opening or closing movement (solid line). Higher movement deviation results in greater contribution of the abduction/adduction component.
Accelerometer
Beside the measurement of the MEPs, for each trial the movements of the right index finger evoked by the TMS pulse were considered. This movement was recorded by means of a custom-made three-dimensional accelerometer fixed over the distal index phalanx. The muscle twitch induced by TMS is typically characterized by short excursions and large acceleration peaks, and for such a reason it is critical that the measuring device does not interfere. To reduce this problem, our departmental electronic laboratory developed a small (8 × 8 × 1 mm) and very light (<20 g) high-precision device (Fig. 2A). This strategy allowed the return of accelerometric analog data for the three separate axes that are fed to the A/D board (Power1401) and acquired in parallel with the EMG data, at the same sampling frequency.
Our hand AO task contemplated the presentation of videos of opening and closing of the index and thumb. For this reason, recording index finger three-axis accelerations capture the critical movement feature required for this study (i.e., Classen et al. 1998). Although techniques for whole hand kinematics tracking have been employed in the past (Bartoli et al. 2014; Gentner and Classen 2006), in this case accelerometers offer some critical advantages. One of them is the simplicity of use and the little amount of preprocessing and data analyses needed. Conversely, whole hand motion capture requires either the selection of few specific movement features (Bartoli et al. 2014) or the use of complex dimensionality reduction techniques (Gentner and Classen 2006).
Data Analysis
MEPs preanalyses.
EMG data were analyzed via a custom-made Matlab script. The script enabled trial-by-trial visual verification of pre-TMS activity. Trials with EMG preactivation (background EMG > 0.05 mV) were excluded from the analyses. After that, we extracted peak-to-peak amplitude data (expressed in mV) for all trials, in a temporal window ranging from 15 to 35 ms after TMS delivery. MEPs exceeding 2 standard deviations (SD) from the mean peak-to-peak amplitude, at the single subject level, were excluded from the data set. The remaining MEPs (97.22%, SD = 6.18% for experiment 1, and 96.15%, SD = 1.5% for experiment 2) were then averaged for every experimental condition separately for each subject and used for further analysis. CSE measures were normalized by dividing MEPs data, in all of the conditions, by the average baseline CSE (both B1 and B2), separately for each subject.
TMS-evoked movements preanalyses.
The accelerometer outputs continuous data in the form of voltage changes over time for each of the three axes. Data are later converted to g-forces values after calibration and filtered with a low-pass set at 25 Hz. The accelerometer was fixed on the index nail with the Z-component normal to the nail, the Y-component along the phalanx axis and the X-component on the transversal plane (Fig. 2A). Z-component was then sensitive to finger opening/closing movement, whereas the X-component, to abduction/adduction. The acceleration modulus was first computed for a 200-ms window, starting from TMS delivery. We then calculated the acceleration onset as the time when 5% of the peak acceleration was detected. Trials were included in the analyses if peak acceleration appeared between 15 and 55 ms after the TMS pulse and amplitude reached at least 0.05 g (mean remaining trials: 96.56 ± 0.90% for experiment 1, and 95.38 ± 1.26% for experiment 2). X-, Y- and Z-component values at peak acceleration were then extracted for the successive analyses.
It is important to note that TMS-evoked movements likely reflect muscle activity that last longer than MEPs. Indeed, there may be other reflexes or descending systems contributing to the TMS-evoked movements, whereas MEPs assess only the first corticospinal descending volley. In light of this, it is critical to evaluate only the first portion of a TMS-evoked movement that presumably would be more representative of the initial descending drive.
Considering that each movement is always computed starting from a static position, the acceleration represents the direction of the movement so that positive Z values indicate an opening movement, while a negative Z indicates a closing one (Fig. 2B). Movement direction was the first variable that we considered, and it corresponds to the percentage of closing (evoked) movements.
We then extracted movement deviation data. This second variable refers to the angles of the movement vector elicited by the TMS (considering both Z- and X-components). The angle is then expressed with respect to an ideal, geometrically straight (i.e., without any lateral deviation, that is with X-component equal to 0) opening or closing movement (Fig. 2C).
Statistical Analysis
Statistical analyses were run separately on MEPs, direction of movement and movement deviation datasets from the two experimental groups. Repeated-measures analysis of variance (RM-ANOVA) was first used on the data recorded on the two baselines to check for significant changes within the experiment and between experiments. We employed 2 × 2 RM-ANOVA with a within-factor time (B1, B2) and between-factor experiment (experiment 1 and experiment 2). As dependent variable, raw MEPs amplitude, movement direction and movement deviation data were used. For this last variable, the analysis was divided into two separate 2 × 2 RM-ANOVAs for opening and closing evoked movements. Once we verified that no changes in both CSE and in TMS evoked movements occurred within the experiment and across experiments, we moved to a second step to analyze the AO data.
For MEPs, the 2 × 2 × 2 design consisted of within-factors observed movement (opening, closing) and observed effector (homologue, different), as well as a between-factor experiment (1, 2). In this study, our aim was to assess if the modulation of our dependent variable was due to the observation of an opening vs. closing action performed either by the homologue (observed and recorded effector are the same) or a different effector (the observed effector, mouth or eyelid, is different from the recorded one).
For movement direction, we used a RM-ANOVA on the percentage of closing movements as a dependent variable. The 2 × 2 × 2 design consisted of within-factors observed movement (opening, closing) and observed effector (homologue, different), as well as a between-factor experiment (1, 2). Here we intended to evaluate if the frequency of index finger opening vs. closing was modulated by AO conditions.
For movement deviation data, we separated the design into two separate RM-ANOVAs for opening and closing evoked movements. The 2 × 2 × 2 design consisted of within-factors observed movement (opening, closing) and observed effector (homologue, different), as well as a between-factor experiment (1, 2). Noteworthy, for this variable, the number of trials could not be determined a priori by our design, since it depends on the intersubjects variability in evoking opening and closing movements. Considering that each subject may have a different number of “opening” and “closing” trials, we repeated the same analyses, adding, as a covariate, the number of trials used for each subject in each condition. Our purpose was to investigate whether AO influences movement deviation, by controlling for the residual effect of the different number of trials across our conditions.
As a third step, we separated all of the designs, on all three dependent variables (MEPs, movement direction and movement deviation), in homologue and different effectors. In fact, the homologue effector consisted of a classic hand AO task, common to both experiments. Hence, we can additionally control for differences across groups, in the classic hand AO task (Fadiga et al. 1995). In this case, we used a RM-ANOVA with a within-factor observed movement (opening, closing) and a between-factor experiment (1, 2) on the homologue effector data. On the other hand, the comparison of the different effectors across experiments verifies whether mouth (transitive effector) or eyelid (intransitive effector) stimuli were differently modulating our dependent variables. In this other case, we used a RM-ANOVA with a within-factor observed movement (opening, closing) and a between-factor experiment (1, 2) on the different effector data. Additional Newman-Keuls post hoc tests were used when the associated main effect or interaction was significant (statistical significance threshold: P < 0.05). Data were preprocessed in Matlab (Mathworks,) and analyzed using R statistical package (https://www.r-project.org).
RESULTS
MEPs
No difference was found between MEP values recorded at B1 (before the experiment) and B2 (after the experiment) [time: F(1,29) = 1.94, P = nonsignificant (ns)], without any difference between experiment 1 and experiment 2 [experiments: F(1,29) = 0.07, P = ns, experiments × time: F(1,29) = 0.51, P = ns]. Consequently, motor excitability during the experiment and between the groups did not differ significantly.
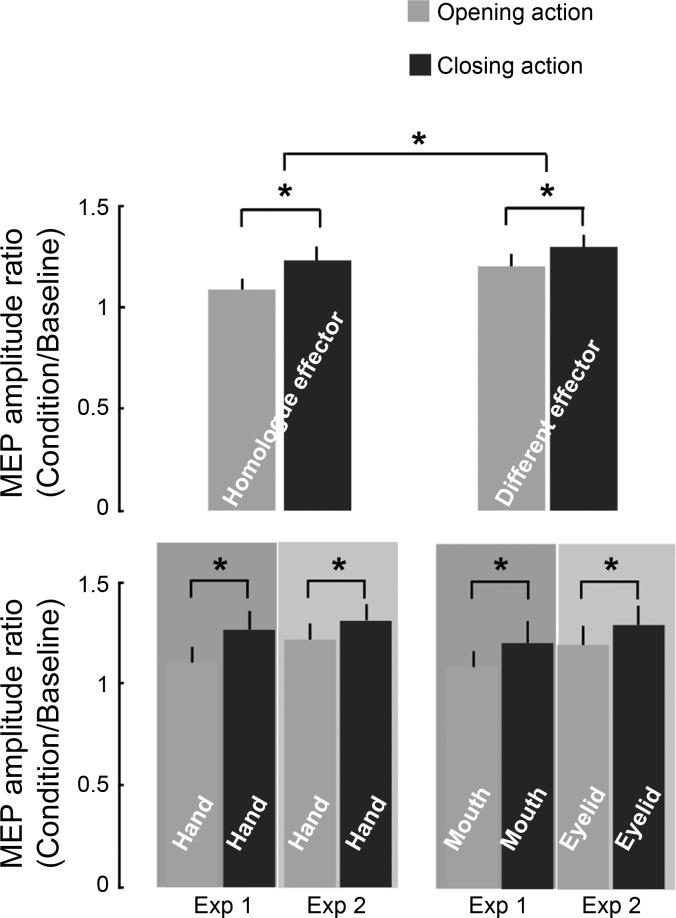
RM-ANOVA on MEPs collected in the two experiments revealed a main effect of the observed movement [observed movement: F(1,29) = 29.28, P < 0.0001]. Specifically, MEP amplitude increased when participants observed closing rather than opening actions, regardless of the displayed effector and the experiments [observed effector × observed movement: F(1,29) = 1.06, P = ns; observed effector × observed movement × experiment: F(1,29) = 0.17, P = ns; observed movement × experiment; F(1,29) = 0.11, P = ns]. Additionally, a main effect of effector was found [observed effector: F(1,29) = 10.33, P < 0.005], indicating that, independently of the observed action [observed effector × observed movement: F(1,29) = 1.06, P = ns], MEP amplitude was greater when mouth or eyelid rather than hand was displayed (Fig. 3). This shows a generally higher modulation for the different effectors that is not specific to opening and closing and thus can be recognized as a general habituation effect. Indeed, subjects wore sensors and electrodes on their hand, and, consequently, there was an implicit bias toward that body location. Spatial orienting of attention to specific body locations is able to affect motor and somatosensory processing (Carson and Ruddy 2012). The repeated (random) visual presentation of actions executed by the hand (for which there was an implicit bias) and the other effector (experiment 1: mouth, experiment 2: eyelid), might have induced a differential modulation between the two situations. Larger attenuation of CSE to hand stimuli can explain the larger modulations for the different effector.
Fig. 3.
Effect of opening and closing action observation (AO) on MEPs. Top: the modulation of the MEP amplitude ratio (condition/average baseline) due to the observation of opening (gray) and closing (black) movements of the homologue effector and the different effector. Bottom: the same data when the homologue and different effectors of the two experiments are separated. Values are means ± SE. *Significant comparisons.
Moreover, considering only those conditions in which the observed movement were performed by the homologue effector, the same effect of increased MEP amplitude during closing AO rather than opening AO was detected [observed movement: F(1,29) = 15.44, P < 0.001], with no differences between the two groups of participants [observed movement × experiment: F(1,29) = 0.22, P = ns]. Therefore, the subjects in the two experimental groups, when presented with hand AO, showed the same amount of corticospinal modulation and comparable movement specificity. An analogous result was also found considering the different effector [observed movement: F(1,29) = 12.04, P < 0.005] in both of the experiments [observed movement × experiment: F(1,29) = 0.01, P = ns], meaning that such modulation of MEP amplitude is not influenced if the observed movement is performed by the mouth (experiment 1) or by the eyelid (experiment 2). Such supplementary analyses confirmed that MEP amplitude recorded at the flexor muscle was enhanced only when a closing AO was observed, independently of the employed effector.
TMS-evoked Movement Direction
Closing movements comprised 56.60% (SD = 31.24%) of all evoked movements at B1 and 66.22% (SD = 28.71%) at B2, for experiment 1. Closing movements comprised 66.51% (SD = 34.00%) of all evoked movements at B1 and 58.88% (SD = 26.99%) at B2, for experiment 2. The remaining evoked movements were the opening ones. Opening and closing movements were equally distributed during baseline trials [experiment 1: t(16) = 0.87, P = ns at B1 and t(16) = 2.33, P = ns at B2; experiment 2: t(12) = 1.75, P = ns at B1 and t(12) = 1.19, P = ns at B2].
The same percentage of closing movements among all TMS evoked movements was present at B1 and B2 [time: F(1,28) = 0.03, P = ns] in the two experiments [experiment: F(1,28) = 0.02, P = ns; experiment × time: F(1,28) = 1.94, P = ns]. Consequently, there was no difference in the TMS-evoked movement direction before and after the experiment and between the two groups.
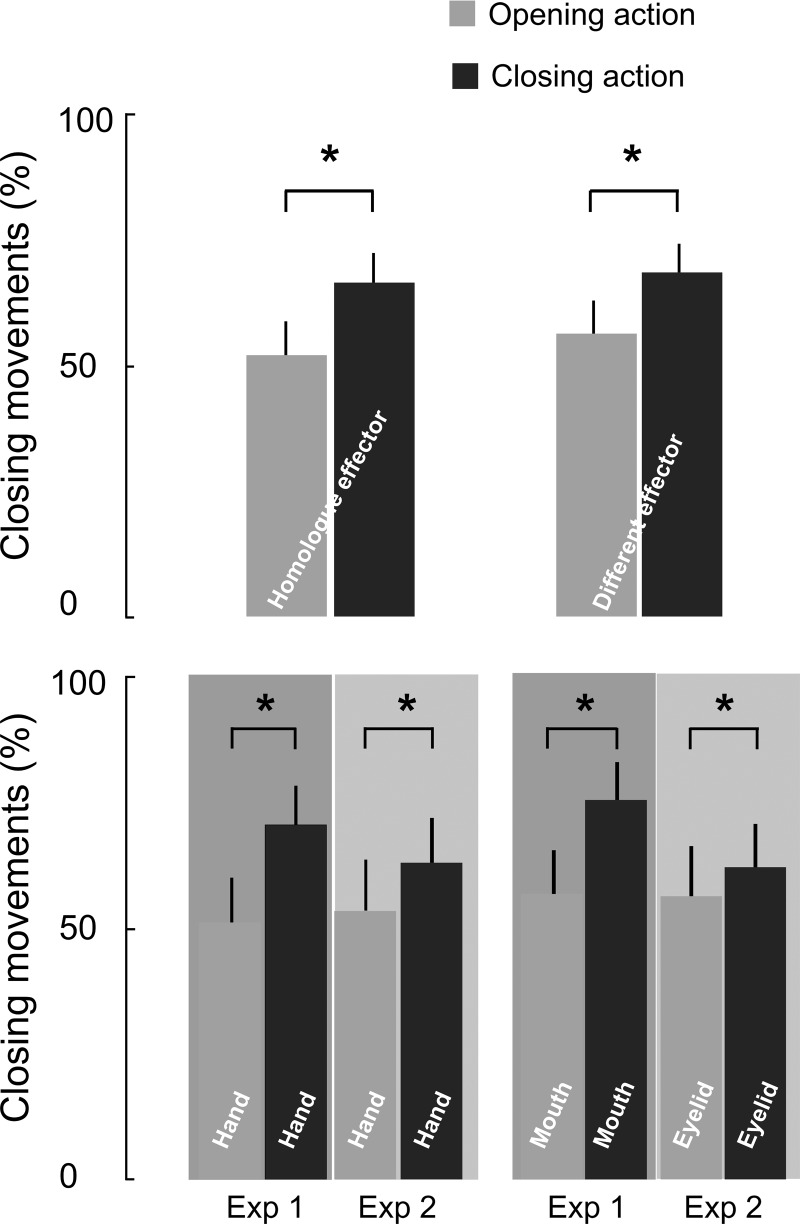
RM-ANOVA run on the TMS-evoked movement direction in the two experiments for both homologue and different effector revealed a main effect of observed movement [observed movement: F(1,28) = 19.93, P < 0.0005]. Post hoc comparisons showed that, similar to the MEP data, the observed movement influenced the evoked ones (Fig. 4). Specifically, the percentage of closing movements decreased when participants observed opening actions rather than closing ones, regardless of the displayed effector and the experiments [effector × observed movement: F(1,28) = 0.41636, P = ns; effector × observed movement × experiment: F(1,28) = 0.19745, P = ns; observed movement × experiment; F(1,28) = 0.41636, P = ns].
Fig. 4.
Effect of AO on the movement direction. Changes in movement direction (%closing movements) for opening and closing AO, considering the homologue and different effectors (top) and by separating the homologue and different effectors of the two experiments (bottom). Values are mean angles ± SE of the movement deviation. *Significant comparisons.
Moreover, the following RM-ANOVA, run exclusively on the homologue effector, indicated that the percentage reduction of closing movements during observation of opening action was equally present in the two experiments [observed movement: F(1,28) = 15.066, P < 0.001; observed movement × experiment: F(1,28) = 1.7237, P = ns]. Accordingly, a partially similar result was found for the different effector in the two experiments [observed movement: F(1,28) = 14.792, P < 0.001; observed movement × experiment: F(1,28) = 4.1256, P = 0.052]. This nearly significant tendency observed in the interaction for the different effector has been clarified in the following analyses on TMS-evoked movement deviation.
TMS-evoked Movement Deviation
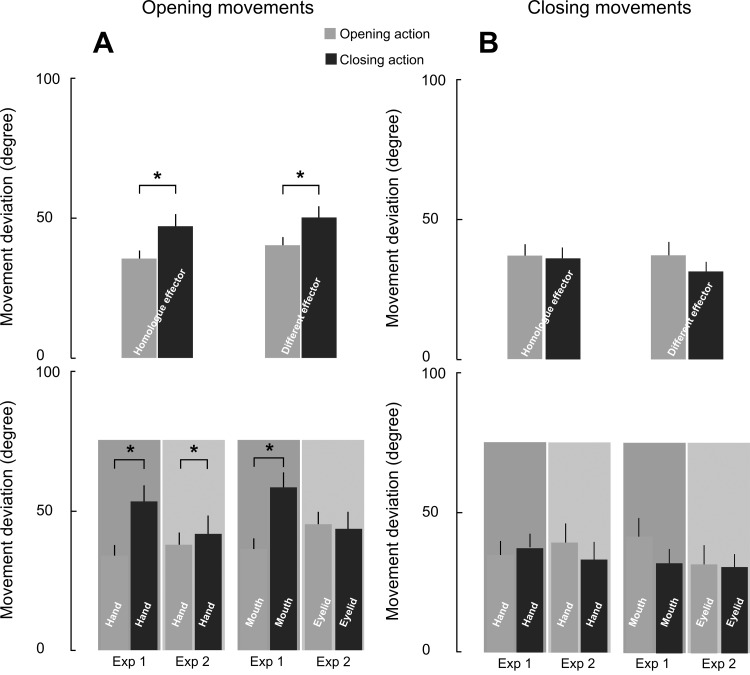
Movement deviation of evoked closing and opening movements did not change significantly at B1 and B2 in both experiments [time: F(1,28) = 0.074, P = ns; experiment × time: F(1,28) = 0.008, P = ns for opening movements; time: F(1,28) = 1.795, P = ns; experiment × time: F(1,28) = 0.298, P = ns for closing movements]. Consequently, there was no difference during the experiment and between groups.
The 2 × 2 × 2 ANOVA on evoked closing movements did not highlight any significant effect or interactions (all P = ns; Fig. 5B). Importantly, the analyses on the evoked opening movement revealed a main effect of the observed movement [F(1,28) = 16.308, P < 0.0005] and a significant interaction of observed movement × experiment: [F(1,28) = 13.100, P < 0.005], regardless of the observed effectors [effector × observed movement × experiment: F(1,28) = 0.32894, P = ns]. Newman-Keuls tests proved that movement deviation was higher during the observation of closing action in experiment 1 (P < 0.0005) but not in experiment 2. The same pattern of results was obtained after including the number of trials for each condition as covariate (number of evoked opening movement: homologue effector observed opening = 6.9 ± 5.1; homologue effectors observed closing = 4.7 ± 4.5; different effector observed opening = 6.3 ± 5.1; different effector observed closing = 4.4 ± 4.3; number of evoked closing movement: homologue effector observed opening = 7.4 ± 5.2; homologue effectors observed closing = 9.6 ± 4.6; different effector observed opening = 8.2 ± 5.2; different effector observed closing = 10.2 ± 4.6). The main effect of the observed movement [F(1,24) = 15.235, P < 0.001] and the significant interaction of observed movement × experiment [F(1,24) = 10.04, P < 0.005], which was independent of the observed effectors [effector × observed movement × experiment: F(1,24) = 0.95854, P = ns] did not further interact with our covariates (all P = ns). Crucially, these analyses ruled out the possibility that our results might be biased by the different sample sizes of the trials.
Fig. 5.
Effect of AO on the movement deviation. Changes in movement deviation for opening (A) and closing (B) motion during AO, considering the homologue and different effectors (top) and by separating the homologue and different effectors of the two experiments (bottom). Values are mean angles ± SE of the movement deviation. *Significant comparisons.
The RM-ANOVA run on the homologue effector showed that an increase of opening movement deviation during closing AO was equally present in the two experiments [observed movement: F(1,28) = 9.02, P < 0.01; and no interaction observed movement × experiment: F(1,28) = 4.02, P = ns]. The qualitatively larger effect found in the hand in experiment 1 as opposed to hand in experiment 2 might be explained by the fact that two different groups participated in each experiment. More importantly, the significantly consistent result across two independent groups adds strength to these measurements. In contrast, the same analysis run on the different effectors revealed a significant interaction between observed movement and experiment [F(1,28) = 6.18, P < 0.05], indicating that AO was able to influence evoked movement deviation only when the observed effector was the mouth (P < 0.05), but not the eyelid (P = ns; Fig. 5A).
DISCUSSION
The present study suggests that the CSE of a muscle involved in hand closing is able to replicate the pattern of muscle contraction in the observed action, in agreement with previous studies (Naish et al. 2014). Remarkably, the specific motor resonance effect generalized to other effectors (the mouth and the eyelid), supporting the claim that motor activations, during AO, map complex action features like “opening” or “closing”, regardless of the involved effector. An important point is that the TMS-evoked movement data displayed a different pattern. TMS-evoked movement deviation (Classen et al. 1998; Stefan et al. 2005) showed no generalization to the eyelid. A more extensive discussion of these findings is given below in the frame of their potential implication in the debate on the level of detail simulated by the human mirror mechanism.
Different AO Generalization between CSE and TMS-evoked Movements
A first result regards the effector-independent enhancement of flexor muscle CSE. Our data indicate specific cross-effector generalization for the observation of an intransitive closing movement as opposed to the opening one. CSE generalization across effectors was previously shown between hand and foot for transitive AO (Cattaneo et al. 2010; Senna et al. 2014). Accordingly, a neuroimaging study reported greater generalization of the putative mirror neuron brain network, to transitive robotic grasping actions (Gazzola et al. 2007). Analogous results were described in a behavioral study investigating the automatic imitation transfer across hand and mouth. During the execution of hand or mouth opening or closing actions, a movement compatibility effect, due to task irrelevant hand or mouth action images, was observed for both effectors. However, the transfer effect was smaller when the task-irrelevant stimulus and the response effectors were incompatible (Leighton and Heyes 2010).
Our investigation extends these findings to intransitive actions and to effectors that do not allow transitive actions. Indeed, eyelid action is critically different from that allowed by the hand or mouth, as it cannot achieve any grasping, displacing or tapping of any object (as is the case for hand and foot action used by Senna et al. 2014). Motor activations elicited by mouth or eyelid AO might have exerted a facilitatory drive toward hand motoneurons. Interestingly, this cross-effector functional connectivity maintains its specificity, suggesting that it is mediated by lateral connections, which preserve the same pattern of agonist-antagonist mapping. Therefore, our results show that CSE maps general features such as “opening” vs. “closing” of any effector.
A second important finding is that, when considering TMS-evoked movement deviation, we noticed differences among observed effectors, which were not present in CSE modulation. This result suggests that the motor system would extract various levels of action representation. Accordingly, recent studies suggest that, during AO, two interacting processes extract kinematic or object-directed action features, following a temporal gradient (Cavallo et al. 2013), or depending on task constraint (Mc Cabe et al. 2015). However, the dissociation found between CSE and TMS-evoked movements also suggests that the very existence of two processes may actually depend on the specific limitations associated with evaluating CSE from a few muscles. In fact, we demonstrate here that two classical measurements, of the same corticospinal phenomenon, can dissociate in this respect. Notably, MEPs were pooled together as it is typically done in all other studies investigating CSE during AO, notwithstanding the fact that MEPs could be associated with very different evoked movements, as it has been shown here.
Difference between CSE and TMS-evoked Movements
Movement emerges from the spatiotemporal composition of several muscle activities. Opening and closing hand action is accomplished by a combination of intrinsic and forearm muscles activities. However, forearm muscles have higher thresholds, and their representations are not necessarily colocated with intrinsic hand muscles. Thus, even if measuring CSE from all of these muscles is in principle the same as measuring TMS-evoked movements, it is impractical. It is known that, to assess CSE from several muscles, it is necessary to raise TMS intensities. However, high TMS intensities may saturate CSE for some muscles (Devanne et al. 1997), and, consequently, in this case, mirror-like modulations may disappear (Loporto et al. 2013).
In addition, the same stimulation can be suprathreshold for some units and subthreshold for others. Particularly, subthreshold TMS still have an important effect on local facilitatory and inhibitory circuits, even though this is not visible in the EMG responses via single-pulse TMS (Kujirai et al. 1993). Thus CSE modulations from few selected muscles cannot account for the complexity of local intracortical and corticospinal effects, triggered by AO.
TMS-evoked movement direction, instead, shows the composition of a variable amount of EMG activity induced in several muscles, as well as the local subthreshold effects (Classen et al. 1998). Significant TMS-evoked movement rotations highlight the balance of excitatory/inhibitory interactions of all synergistic muscles for which the same TMS pulse may be supra- or subthreshold. Indeed, TMS-evoked movements are a very compact description of how the complex synergistic intracortical interactions are modulated by specific experimental manipulations.
Synergies are invariant patterns of activation across muscles that could be linearly summed, with specific amplitude and timing coefficients, to generate hand functions (Overduin et al. 2008; Santello et al. 2013). Convergent validation of this idea came also from the electrical stimulation of the monkey motor cortex (Overduin et al. 2012), as well as the magnetic stimulation of the human motor areas (Gentner and Classen 2006; Gentner et al. 2010). Therefore, CSE from a limited set of muscles may show only part of the complexity of these motor activities during AO (D'Ausilio et al. 2015). Rather, the complex synergistic pattern of muscle activities, triggered by AO, could be better investigated via the recording of the TMS-evoked hand kinematics (Barchiesi and Cattaneo 2013; Bartoli et al. 2014). However, we should keep in mind that AO modulate TMS-evoked movement deviations less than actual physical practice (Stefan et al. 2005). In our case, we were interested in the instantaneous changes induced by the observation of a single action event, to investigate the properties of the motor coding of AO, rather than its plasticity.
Anatomo-Functional Differences between the Actions of Different Effectors
Our findings indicate a different degree of generalization among effectors during AO, suggesting a stronger functional similarity between hand and mouth compared with hand and eyelid. Indeed, hand and mouth movements can be synergistically programmed to achieve similar object-directed actions (e.g., grasping an object), in contrast with eyelid movements. Such a functional connection is supported by the presence of a class of neurons representing both hand and mouth actions in monkey's ventral premotor areas (Rizzolatti et al. 1988). Furthermore, human behavioral (Castiello 1997; Gentilucci et al. 2001) and neurophysiological data (Castiello et al. 2000) showed that mouth and hand action might share similar motor synergies.
The difference between mouth and eyelid effects could also be ascribed to their different neural organization and/or distance to the hand motor representation. The motor representation of the mouth lies ventrally to the hand and is well characterized. Tongue and lips (Orbicularis oris) corticobulbar excitability threshold is much higher than that of the hand (>60% of the stimulator output instead of 30–46% for the hand), and, consequently, it is very unlikely that stimulation of the hand area could directly affect the lower face area (D'Ausilio et al. 2011, 2014; Fadiga et al. 2002; Paradiso et al. 2005).
Eyelid voluntary control is less known. Conditioned eyelid responses are mediated by a cerebellum, red nucleus, facial motoneuron pathway (Morcuende et al. 2002). Clinical studies on humans revealed a dissociation between voluntary and involuntary eyelid control, and effectively only the former is impaired with lesions of frontal cortical areas and/or the corticospinal system (Esteban et al. 2004). In agreement with that, TMS stimulation of the motor cortex demonstrated projection to the contralateral orbicularis oculi muscle, which is recruited during eyelid closing (Paradiso et al. 2005). Data obtained by positron emission tomography imaging have also shown that voluntary eyelid control activates the right supplementary motor area, left pre-supplementary motor area, right angular gyrus and the left primary motor cortex (Suzuki et al. 2010).
Brain metabolic activity found in the primary motor strip seems to be located very close to that of the hand (eyelid MNI coordinates: −44, −12, 50; Suzuki et al. 2010). Intrinsic hand muscles are located on average (first dorsal interosseous MNI coordinates: −37, −25, 58; Niyazov et al. 2005) closer to the eyelid than to the face area (Orbicularis oris MNI coordinates: −55.4, −9.2, 43.9; tongue MNI coordinates: −59.4, −7.4, 22.8; Schomers et al. 2014). This might potentially exclude the pattern of effects observed on TMS-evoked movements to be explained by the distance on the lateral cortical surface. This would argue in favor of a differential cross-effector wiring of agonist-antagonist synergies, depending on the action potentiality of the effector. Eyelid action does not allow transitive action and thus may not share the same pattern of lateral synergistic connectivity. To the best of our knowledge, current available literature on voluntary control of eyelid movements is too limited, and we cannot exclude that the eyelid motor representation is smaller or less excitable than the mouth area.
Anatomo-Functional Differences between the Opening and Closing Actions
An additional difference between opening and closing action emerged in the movement deviation data. Hand closing movements were not affected by AO, whereas opening movements were significantly deviated during the observation of closing actions. Although TMS-evoked hand opening and closing showed equal probability at rest (in line with Gentner and Classen 2006), the two movements revealed a different degree of robustness to AO modulations.
This could be due to the differences in the neural control of opening and closing hand actions. Flexion-based movements, like grasping objects, are more frequently executed and require finer force control and independence than extension movements (Oliveira et al. 2008; Schieber 1991; Yu et al. 2010). The corticospinal control of each muscle group is also quite different. Human TMS studies suggest stronger monosynaptic connections to wrist and finger extensors than flexors (de Noordhout et al. 1999), in agreement with nonhuman primate models reporting differences in the cortico-motoneuronal connections to forearm muscles. These differences include stronger facilitation in extensors and stronger suppression in wrist flexors (Park et al. 2004). However, stronger facilitatory drive does not imply greater descending control of extensors. Indeed, in line with this evidence, Fetz and Cheney (1980) proposed that flexor motoneurons might receive more important contribution from other descending systems, to achieve greater cortical inhibitory control of hand closing than opening.
Differences in the neural control of hand opening and closing may have been interacting with other important and yet unexplored aspects. One of them refers to the difference between AO and action execution. Indeed, it is still a matter of debate whether the AO brain network shares only part of the action execution network, or the same network but activated to a lower extent (Waldert et al. 2015). As a consequence, it is difficult to ascertain whether AO modulates only the CSE, or its effects extend to other descending paths.
In parallel, it is essential to note that TMS activates exclusively the corticospinal tract, and the latency of our movement data suggest a fundamental corticospinal origin. In consequence, the specific pattern of hand movement deviation that we found could be driven by the specificity of the AO network and/or the limited capability of TMS to measure all contributions to movement organization. However, further investigations are needed to confirm each of these hypotheses.
Conclusions
In conclusion, classical CSE on a few muscles alone is not sufficient to show the whole hand synergistic motor resonance, which was instead better quantified in TMS-evoked movements. In fact, our results provide some initial support for the hypothesis that the observation of actions may elicit subthreshold activation of motor cortical synergies. According to a synergistic organization of action, the potentiality for goal-directedness of a given effector seems to be critical in modulating cortical activities during AO.
GRANTS
This work was supported by European Community grants SIEMPRE [Information and Communication Technologies (ICT)-Future and Emerging Technologies project no. 250026], POETICON++ (Specific Targeted Research Project ICT-288382), CAPES-COFECUB (project no. 819-14), and La Fondation Motrice with the “Pace for CP program”.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.F. and L.M. performed experiments; A.F., L.M., M.B., and M.J. analyzed data; A.F., L.M., and M.J. prepared figures; A.F., L.M., M.B., M.J., T.P., and A.D. approved final version of manuscript; M.B., T.P., and A.D. conception and design of research; M.B., T.P., and A.D. interpreted results of experiments; M.B., T.P., and A.D. edited and revised manuscript; A.D. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Laura Taverna for help with experimental stimuli and figures.
REFERENCES
- Alaerts K, Swinnen SP, Wenderoth N. Is the human primary motor cortex activated by muscular or direction-dependent features of observed movements? Cortex 45: 1148–1155, 2009. [DOI] [PubMed] [Google Scholar]
- Barchiesi G, Cattaneo L. Early and late motor responses to action observation. Soc Cogn Affect Neurosci 8: 711–719, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli E, Maffongelli L, Jacono M, D'Ausilio A. Representing tools as hand movements: early and somatotopic visuomotor transformations. Neuropsychologia 61: 335–44, 2014. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. Bilateral motor resonance evoked by observation of a one-hand movement: role of the primary motor cortex. Eur J Neurosci 28: 1427–1435, 2008. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. Cyclic time course of motor excitability modulation during the observation of a cyclic hand movement. Brain Res 1065: 115–124, 2005. [DOI] [PubMed] [Google Scholar]
- Carson RG, Ruddy K. Vision modulates corticospinal suppression in a functionally specific manner during movement of the opposite limb. J Neurosci 32: 646–652, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello U. Arm and mouth coordination during the eating action in humans: a kinematic analysis. Exp Brain Res 115: 552–556, 1997. [DOI] [PubMed] [Google Scholar]
- Castiello U, Bennet KM, Egan GF, Tochon-Danguy HJ, Kritikos A, Dunai J. Human inferior parietal cortex “programs” the action class of grasping. Cogn Syst Res 1: 89–97, 2000. [Google Scholar]
- Cattaneo L, Maule F, Barchiesi G, Rizzolatti G. The motor system resonates to the distal goal of observed actions: testing the inverse pliers paradigm in an ecological setting. Exp Brain Res 231: 37–49, 2013. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Sandrini M, Schwarzbach J. State-dependent TMS reveals a hierarchical representation of observed acts in the temporal, parietal, and premotor cortices. Cereb Cortex 20: 2252–2258, 2010. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Caruana F, Jezzini A, Rizzolatti G. Representation of goal and movements without overt motor behavior in the human motor cortex: a transcranial magnetic stimulation study. J Neurosci 29: 11134–11138, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo A, Bucchioni G, Castiello U, Becchio C. Goal or movement? Action representation within the primary motor cortex. Eur J Neurosci 38: 3507–3512, 2013. [DOI] [PubMed] [Google Scholar]
- Cavallo A, Becchio C, Sartori L, Bucchioni G, Castiello U. Grasping with tools: corticospinal excitability reflects observed hand movements. Cereb Cortex 22: 710–716, 2012. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79: 1117–23, 1998. [DOI] [PubMed] [Google Scholar]
- D'Ausilio A, Jarmolowska J, Busan P, Bufalari I, Craighero L. Tongue corticospinal modulation during attended verbal stimuli: priming and coarticulation effects. Neuropsychologia 49: 3670–3676, 2011. [DOI] [PubMed] [Google Scholar]
- D'Ausilio A, Maffongelli L, Bartoli E, Campanella M, Ferrari E, Berry J, Fadiga L. Listening to speech recruits specific tongue motor synergies as revealed by transcranial magnetic stimulation and tissue-Doppler ultrasound imaging. Philos Trans R Soc Lond B Biol Sci 369: 20130418, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ausilio A, Bartoli E, Maffongelli L. Grasping synergies: a motor-control approach to the mirror neuron mechanism. Phys Life Rev 12: 91–103, 2015. [DOI] [PubMed] [Google Scholar]
- de Noordhout AM, Rapisarda G, Bogacz D, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ. Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain 122: 1327–1340, 1999. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114: 329–338, 1997. [DOI] [PubMed] [Google Scholar]
- Esteban A, Traba A, Prieto J. Eyelid movements in health and disease. The supranuclear impairment of the palpebral motility. Neurophysiol Clin 34: 3–15, 2004. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Olivier E. Human motor cortex excitability during the perception of others' action. Curr Opin Neurobiol 15: 213–218, 2005. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Buccino G, Rizzolatti G. Speech listening specifically modulates the excitability of tongue muscles: a TMS study. Eur J Neurosci 15: 399–402, 2002. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol 73: 2608–2611, 1995. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol 44: 751–772, 1980. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport 12: 1489–1492, 2001. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage 35: 1674–1684, 2007. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Benuzzi F, Gangitano M, Grimaldi S. Grasp with hand and mouth: a kinematic study on healthy subjects. J Neurophysiol 86: 1685–1699, 2001. [DOI] [PubMed] [Google Scholar]
- Gentner R, Gorges S, Weise D, aufm Kampe K, Buttmann M, Classen J. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol 20: 1869–74, 2010. [DOI] [PubMed] [Google Scholar]
- Gentner R, Classen J. Modular organization of finger movements by the human central nervous system. Neuron 52: 731–742, 2006. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851, 2002. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton J, Heyes C. Hand to mouth: automatic imitation across effector systems. J Exp Psychol Hum Percept Perform 36: 1174–1183, 2010. [DOI] [PubMed] [Google Scholar]
- Loporto M, Holmes PS, Wright DJ, McAllister CJ. Reflecting on mirror mechanisms: motor resonance effects during action observation only present with low-intensity transcranial magnetic stimulation. PLoS One 8: e64911, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Cabe SI, Villalta JI, Saunier G, Grafton ST, Della-Maggiore V. The relative influence of goal and kinematics on corticospinal excitability depends on the information provided to the observer. Cereb Cortex 25: 2229–2237, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende S, Delgado-Garcia JM, Ugolini G. Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat. J Neurosci 22: 8808–8818, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naish KR, Houston-Price C, Bremner AJ, Holmes NP. Effects of action observation on corticospinal excitability: muscle specificity, direction, and timing of the mirror response. Neuropsychologia 64C: 331–348, 2014. [DOI] [PubMed] [Google Scholar]
- Niyazov DM, Butler AJ, Kadah YM, Epstein CM, Hu XP. Functional magnetic resonance imaging and transcranial magnetic stimulation: effects of motor imagery, movement and coil orientation. Clin Neurophysiol 116: 1601–1610, 2005. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Oliveira MA, Hsu J, Park J, Clark JE, Shim JK. Age-related changes in multi-finger interactions in adults during maximum voluntary finger force production tasks. Hum Mov Sci 27: 714–727, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin SA, d'Avella A, Carmena JM, Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron 76: 1071–1077, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin SA, d'Avella A, Roh J, Bizzi E. Modulation of muscle synergy recruitment in primate grasping. J Neurosci 28: 880–892, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso GO, Cunic DI, Gunraj CA, Chen R. Representation of facial muscles in human motor cortex. J Physiol 567: 323–336, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saif A, Cheney PD. Properties of primary motor cortex output to forelimb muscles in rhesus macaques. J Neurophysiol 92: 2968–2984, 2004. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res 71: 491–507, 1988. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. [DOI] [PubMed] [Google Scholar]
- Santello M, Baud-Bovy G, Jörntell H. Neural bases of hand synergies. Front Comput Neurosci 7: 23, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. Individuated finger movements of rhesus monkeys-a means of quantifying the independence of the digits. J Neurophysiol 65: 1381–1391, 1991. [DOI] [PubMed] [Google Scholar]
- Schomers MR, Kirilina E, Weigand A, Bajbouj M, Pulvermüller F. Causal influence of articulatory motor cortex on comprehending single spoken words: TMS evidence. Cereb Cortex. First published December 1, 2014; doi: 10.1093/cercor/bhu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senna I, Bolognini N, Maravita A. Grasping with the foot: goal and motor expertise in action observation. Hum Brain Mapp 35: 1750–1760, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senot P, D'Ausilio A, Franca M, Caselli L, Craighero L, Fadiga L. Effect of weight-related labels on corticospinal excitability during observation of grasping: a TMS study. Exp Brain Res 211: 161–167, 2011. [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci 25: 9339–9346, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Kiyosawa M, Mochizuki M, Ishiwata K, Ishii K. The pre-supplementary and primary motor areas generate rhythm for voluntary eye opening and closing movements. Tohoku J Exp Med 222: 97–104, 2010. [DOI] [PubMed] [Google Scholar]
- Waldert S, Vigneswaran G, Philipp R, Lemon RN, Kraskov A. Modulation of the intracortical LFP during action execution and observation. J Neurosci 35: 8451–8461, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WS, van Duinen H, Gandevia SC. Limits to the control of the human thumb and fingers in flexion and extension. J Neurophysiol 103: 278–289, 2010. [DOI] [PubMed] [Google Scholar]