Abstract
It is well known that eye movement patterns are influenced by both goal- and salience-driven factors. Recent studies, however, have demonstrated that objects that are nonsalient and task irrelevant can still capture our eyes if moving our eyes to those objects has previously produced reward. Here we demonstrate that training such an association between eye movements to an object and delivery of reward is not needed. Instead, an object that merely signals the availability of reward captures the eyes even when it is physically nonsalient and never relevant for the task. Furthermore, we show that oculomotor capture by reward is more reliably observed in saccades with short latencies. We conclude that a stimulus signaling high reward has the ability to capture the eyes independently of bottom-up physical salience or top-down task relevance and that the effect of reward affects early selection processes.
Keywords: reward, visual attention, oculomotor capture, eye movements
picture yourself at a crowded market, looking at the various fruits and vegetables of a market stand. Even though you want to purchase a particular kind of fruit, distinctively colored or oddly shaped fruits may capture your eyes before you finally fixate the fruit you were looking for. Likewise, fruits that you tend to buy frequently may also stand out, as they have repeatedly given you a rewarding experience when you have eaten them.
In situations like these, the visual system is overwhelmed by an abundance of stimuli that compete for selection. Covert or overt selection has traditionally been described to be controlled by voluntary goal-directed processes (top down) or driven by the features of the stimulus or object at hand (bottom up; for reviews see, e.g., Theeuwes 2010; Wolfe 2007). It has, however, recently been argued that categorization in terms of bottom-up and top-down processes alone cannot explain all effects on visual selection (Awh et al. 2012). More specifically it has been suggested that reward-based selection history prioritizes stimuli in such a way as to change their pertinence to the visual system, quite possibly by interacting with ongoing bottom-up or top-down processes (Awh et al. 2012; Hickey et al. 2010; Hickey and van Zoest 2012).
Classic studies investigating the neural basis of reward processing have long pointed to the parallels in the neural processes of reward and attention. For instance, activity in the lateral intraparietal sulcus, which is a brain area that is usually associated with attentional processing (e.g., Bisley and Goldberg 2010; Gottlieb et al. 1998), has been shown to be affected by reward contingencies (Dorris and Glimcher 2004; Louie et al. 2011; Sugrue et al. 2004). Based on such evidence, some theories have aimed to describe how reward affects selection and decision-making (Berridge and Robinson 1998; Mackintosh 1975). For example, the incentive salience hypothesis of Berridge and Robinson (1998) postulates that stimuli associated with reward come to trigger the release of mesencephalic dopamine. This release of dopamine then elicits motivated behavior prioritizing these reward-associated stimuli by modulating the degree to which they become “liked” or “wanted.” The degree to which stimuli are liked or wanted eventually determines a range of different behaviors toward these stimuli. Similar theories have now been applied to provide a theoretical framework describing how reward may also affect visual selection (e.g., Awh et al. 2012; Hickey et al. 2010; Raymond and O'Brien 2009).
There is now substantial empirical evidence for an effect of reward on attention in the context of visual selection. For example, in Anderson et al. (2011a, 2011b) participants engaged in a visual search task. During a training phase, they were asked to search for certain colors of circles and report the orientation of a line inside these circles. Depending on the color of the target circle, participants either received relatively high reward (e.g., 10 cents) or relatively low reward (e.g., 1 cent). In a subsequent test phase, participants no longer received any reward. In contrast to the training, they were now asked to search for a unique shape (i.e., shape singleton) and to ignore any color information. Even though participants were told to ignore color information, a colored nontarget caused more interference in search time when it was of the color that was previously associated with high reward compared with when none of these colors was present. The authors argued that the increase in search time was due to attentional capture by the stimulus associated with high reward. Crucially, this occurred even though that stimulus was physically nonsalient, task irrelevant, not part of the task set, and no longer predictive for any reward during the test phase. Similar conclusions were drawn in several other studies (for reviews see Anderson 2013; Chelazzi et al. 2013).
Distraction in visual selection due to reward is not solely restricted to covert search. Similar findings have been reported in overt search tasks. For instance, Theeuwes and Belopolsky (2012; see also Anderson and Yantis 2012) used an oculomotor task to demonstrate that saccades toward a target object were also affected by previously learned stimulus-reward relationships. During a training phase, participants had to make a saccade to a target object (horizontal or vertical bar) presented among other objects. Accurate and fast saccades to one target object received a relatively high reward, and those to another object received low reward. In a subsequent test phase, participants engaged in a color singleton search task in which they could no longer earn any reward. In this task, they had to make a saccade to the color singleton and ignore all other stimuli. However, on two-thirds of the trials one of the previously rewarded objects was presented as a distractor. The results showed that participants made more erroneous saccades to the distractor that was previously associated with high reward compared with the distractor that was previously associated with low reward. It was concluded that stimuli associated with reward continue to capture the eyes even when they are task irrelevant and nonpredictive for reward.
Taken together, these studies demonstrate that rewarding a response (i.e., an oculomotor or covert attentional shift) to a stimulus may train that response, such that it persists into a subsequent test phase, even if that response to the stimulus is now inappropriate (and unrewarded). Since the stimulus that previously predicted reward continues to elicit an orienting response during the test phase, one will observe performance benefits when the stimulus happens to appear at the location of the target (see, e.g., Failing and Theeuwes 2014; Kiss et al. 2009; Raymond and O'Brien 2009) and costs when it appears somewhere other than the target (e.g., Anderson et al. 2011a, 2011b; Failing and Theeuwes 2014; Theeuwes and Belopolsky 2012). As such, one may argue that these studies represent some form of instrumental conditioning in which a response to a stimulus feature or object that was predictive for reward is reenacted as soon as that conditioned stimulus reappears. This process of conditioning a response to a stimulus is known as shaping (Dayan and Balleine 2002; Schultz 2006; Sutton and Barto 1998).
A recent study by Le Pelley, Pearson, Griffiths, and Beesley (2015), however, demonstrates that the influence of reward on visual selection is not only through instrumental response shaping. In one of their experiments, on each trial participants were required to make a rapid saccade to a shape singleton (a diamond among circles). On most trials, one of the nontargets was colored either red or blue; all other shapes were gray (additional singleton task; e.g., Theeuwes 1992). The color of the nontarget color singleton signaled the amount of reward that was available on that particular trial. For example, a red distractor might signal that a rapid saccade to the shape singleton target would receive high reward, while a blue distractor signaled that a rapid saccade to the target would receive low reward. Importantly, however, if participants looked at or near the distractor prior to looking at the target on a particular trial, the reward on that trial was omitted. The critical finding was that, nevertheless, participants were more likely to look at the distractor when it appeared in the color signaling high reward than the color signaling low reward. Moreover, participants were slower in moving their eyes to the shape singleton target when the display contained a distractor rendered in the color that predicted high reward compared with the low-reward color.
These results are striking because they suggest that reward influenced the extent to which distractors elicited oculomotor capture, even though selecting the distractor was never necessary in this task. In fact, it was detrimental for obtaining reward, since looking at the distractor always caused reward omission. The authors argued that there was therefore less scope for instrumental response shaping under these conditions, since participants were never rewarded for selecting the distractor. Instead, these results suggest that merely signaling reward is sufficient to change the extent to which stimuli elicit oculomotor capture. In other studies, Le Pelley et al. (2015) demonstrated a similar influence of reward signaling on covert selection.
However, the stimuli signaling reward availability in Le Pelley et al.'s (2015) studies were always physically salient, since they were the only colored stimuli in the display on each trial (i.e., they were color singletons). It is well documented that physically salient stimuli “pop out” because of bottom-up processes, capturing attention both covertly and overtly in a reflexive manner (e.g., Theeuwes 1992, 1994; Theeuwes et al. 1999; for a review see Theeuwes 2010). Thus it is likely that the salient, reward-signaling stimulus in Le Pelley et al.'s (2015) procedure would pop out. It is therefore feasible that capture was initially driven by the physical salience of the stimulus and reward merely modulated the degree of capture. On this account, if the stimulus that signaled reward availability had not been physically salient, no capture would have occurred and so no modulation of capture by reward prediction would have been observed. As such, one could argue that the reward effect observed by Le Pelley et al. (2015) may not be that different from the previously discussed studies that used top-down task relevance during reward learning to shape the selection of the stimulus that signals reward (see Anderson 2013; Chelazzi et al. 2013; Theeuwes and Belopolsky 2012).
The present study was designed to investigate whether task-irrelevant stimuli that signal reward capture the eyes even when these stimuli are not physically salient. Crucially, selecting these task-irrelevant stimuli is and was never necessary to obtain reward. Participants performed an oculomotor search task. On each trial they were required to fixate a shape singleton (e.g., a diamond) that was displayed among five nontarget shapes (e.g., circles). The color of one of the nontarget shapes in each display indicated the magnitude of reward (high or low) that could be earned for a quick and correct saccade to the target. Crucially, and unlike in the study by Le Pelley et al. (2015), this task-irrelevant, reward-signaling distractor was not physically salient, since it was not a color singleton: all stimuli in the display were uniquely colored. If a stimulus captures the eyes merely because it signals reward, irrespective of task relevance or physical salience, we would expect a stimulus signaling relatively high reward to capture the eyes more often than a stimulus signaling low reward. If, however, reward availability only modulates the capture that is initially produced by physical salience, then we would not expect to observe capture by the nonsalient, reward-signaling stimuli. Instead, we would expect observers to be able to ignore these stimuli, regardless of the level of reward availability that they signal, and simply make a saccade directly to the target.
EXPERIMENT 1
Methods
Participants.
Twenty-two naive students of the VU University Amsterdam (11 women, 11 men; mean age ±22 yr) with reported normal or corrected-to-normal vision participated in the experiment after giving written informed consent. The experiment was approved by the Ethics Committee of the VU University Amsterdam. Participants received monetary compensation of between €11.42 and €12.91 (mean payout ±€12.20) based on their performance. Data from two participants were excluded because of relatively low overall performance (averaged proportion of correct responses >2.5 SD below the group mean) and data from two other participants because of apparatus malfunction that caused data loss. This left data sets from 18 participants (10 women, 8 men; mean age ±22 yr).
Apparatus.
Stimuli were created in OpenSesame (Mathôt et al. 2012) and presented on a Lacie electron22blueIV monitor (1,024 × 768 pixels, 100 Hz). Eye movements were registered with the Eyelink 1000 Tower mount system (1,000-Hz temporal resolution, <0.01° gaze resolution, and a gaze position accuracy of <0.5°). An automatic algorithm detected saccades using minimum velocity and acceleration criteria of 35°/s and 9,500°/s2, respectively. Participants' heads were positioned on a chin rest at a distance of 50 cm from the screen.
Stimuli.
The experimental task was based on the additional singleton paradigm (Theeuwes 1992). Accordingly, the search display consisted of six filled shapes: either one diamond and five circles or one circle and five diamonds. Each shape was uniquely colored. The full set of colors used in this study was as follows: blue, CIE: x = 0.146, y = 0.072, 2.1 cd/m2; red, CIE: x = 0.615, y = 0.346, 5.6 cd/m2; green, CIE: x = 0.292, y = 0.614, 16.4 cd/m2; yellow, CIE: x = 0.412, y = 0.515, 21.9 cd/m2; pink, CIE: x = 0.313, y = 0.226, 11.7 cd/m2; brown, CIE: x = 0.538, y = 0.411, 8.5 cd/m2; cyan, CIE: x = 0.220, y = 0.331, 18.5 cd/m2. Each display contained exactly one nontarget shape that was colored either red or blue; this was the reward-signaling stimulus in experimental blocks (henceforth called the distractor). The colors of other shapes were randomly sampled from the remaining set of colors without replacement. All shapes were presented at equal distances on an imaginary circle (10.1° radius) around a white fixation dot (0.23°) on a black background. The location of the target was randomly determined on each trial. The location of the distractor was random, with the constraint that the distractor was never placed next to the target but was either two or three positions away from the target (i.e., the smallest distance from target to distractor was 17.4° visual degrees or 120° polar angle).
Procedure and design.
Each trial consisted of a fixation display, a search display, and a feedback display (see Fig. 1). Once drift correction was successful (i.e., participant fixating within 2° of the center of the screen), the actual trial started with the presentation of the fixation display for 300–500 ms. After the fixation display, the search display consisting of the six uniquely colored shapes was displayed for 1,000 ms or until response. Participants were asked to fixate the shape singleton (target) as quickly and accurately as possible and to continue fixating it until the search display disappeared. A region of interest (ROI) with a radius of 3.5° was defined around the target. Once participants had fixated within this target ROI for >100 ms, a correct response was registered. Then the display blanked for 250 ms, followed by the feedback display (“correct” during the practice block) for 1,250 ms, subsequent to which the feedback display was presented for 1,250 ms. To foster quick responses during practice, we set a latency limit of 650 ms (based on a pilot experiment). When responses were correct but slower than this cutoff time, the feedback display informed participants that their response was “correct but too slow” during practice. If 1,000 ms elapsed before participants registered a correct response, the feedback display instead read “too slow.”
Fig. 1.
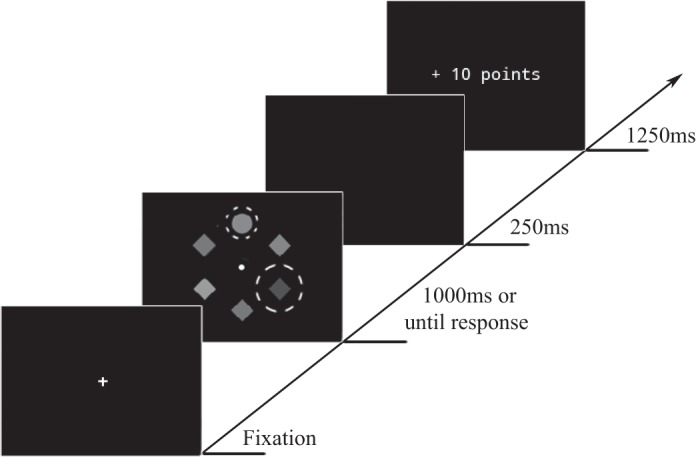
Trial sequence of experiment 1. Participants had to respond by fixating the unique shape (target singleton) for 100 ms. One of the nontarget shapes (distractor) signaled the magnitude of the reward that could be earned, if participants made a fast saccade to the target. Depending on the color of this distractor (red or blue), participants received either high or low reward. Dashed lines (not visible to the participants) indicate the region of interest (ROI) around the target and the reward-signaling distractor. If participants fixated in the distractor ROI at any point in time, the trial was marked as an omission trial and no reward was delivered.
With the start of the experimental blocks, two crucial design features changed: First, participants were informed prior to the start of the first experimental block that they could now earn points depending on their performance and that these points would correspond to money paid out to them at the end of the experiment (no information was given about how many points corresponded to how much money). In particular, participants were informed that the presence of either a red or a blue nontarget shape in the display would indicate how many points could be earned for that particular trial. Participants were also informed that they would receive reward only if they managed to fixate the target before a variable latency limit (as opposed to the fixed latency limit in the practice block). This variable latency limit was based on the third quartile of all response times (i.e., times taken to fixate the target for 100 ms on each trial) from the previous block, in order to promote quick responses throughout the experiment.
The colors red and blue were counterbalanced as the signals of high and low reward magnitudes across participants. That is, for half of the participants the presence of a red distractor indicated that a high reward (+10 points) would be earned for a quick and correct response, while the presence of a blue distractor indicated that a low reward (+1 point) would be earned for a quick and correct response; for the other half of the participants this was reversed. However, if any gaze was recorded inside an ROI with a radius of 5.1° around the distractor prior to a correct response, reward was omitted (i.e., participants earned 0 points on that trial). Note that on these trials on which gaze was recorded on the distractor and reward was omitted, the search display still remained on until participants registered a correct response (fixation in the target ROI for 100 ms) or the trial timed out after 1,000 ms. For trials on which response time was slower than the latency limit, or no response was registered within 1,000 ms (timeout), 0 points were earned. In the experimental blocks, the feedback screen following each search display indicated only how much reward was earned (+10, +1, or 0 points).
Each participant performed one block of practice and six experimental blocks of 72 trials each, yielding a total of 504 trials. Half of the trials in each block featured a red distractor, and the other half featured a blue distractor.
Discarded data.
Timeouts (3.2% of all trials) were excluded from analyses. We furthermore discarded trials in which participants engaged in anticipatory saccades, i.e., in which the first saccade latency was <80 ms (0.9% of the trials), and trials in which the time between the start of the drift correction and the presentation of the search display exceeded 7,000 ms (1.7% of the trials).
Results
Omission trials.
To investigate whether a physically nonsalient and task-irrelevant stimulus signaling relatively high reward interferes with target search, we analyzed the proportion of trials in which participants fixated the distractor before fixating the target for 100 ms (i.e., omission trials). The results with the factors reward (high vs. low) and block (1–6) were submitted to a within-subjects analysis of variance (ANOVA). There was a significant main effect of reward [F(1,17) = 20.824, P < 0.001, ηp2 = 0.551] and no main effect of block [F(5,85) = 0.486, P = 0.706] or interaction [F(5,85) = 0.214, P = 0.909]. Participants fixated the distractor on a larger proportion of the trials when it signaled a high reward [8.2 ± 5.6% (mean ± SD)] compared with when it signaled a low reward (2.9 ± 2.6%). This pattern remained relatively stable over the course of the experiment (Fig. 2).
Fig. 2.
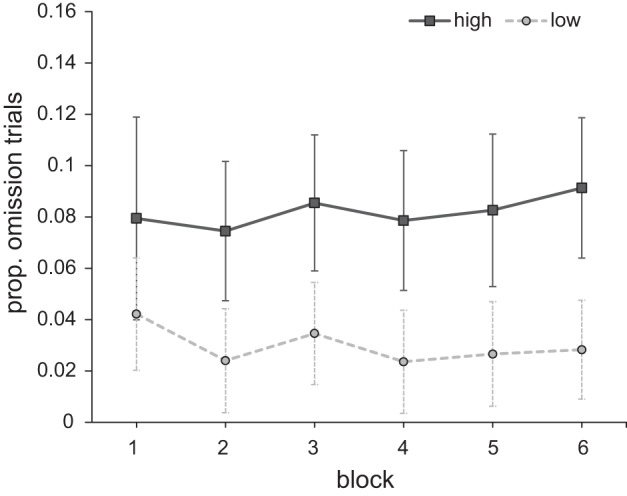
Experiment 1: proportion of omission trials for both reward conditions across blocks. Error bars here and in all subsequent figures represent within-subject 95% confidence intervals (Cousineau 2005; Morey 2008).
Time to fixate the target.
The preceding analysis shows that participants' eyes were more often captured by the distractor that signaled high reward than the distractor that signaled low reward. We examined whether this difference was also reflected in the time taken for participants' eyes to reach the target. A paired-samples t-test showed that the time until fixation on the target ROI was significantly longer on trials with a high-reward distractor (439 ± 46 ms) than on trials with a low-reward distractor (427 ± 52 ms) {t(17) = 2.719, P = 0.015, CI 95% [2.6 20.7]}.
First saccade latencies and destinations.
The preceding finding, relating to time to fixate the target, is consistent with the idea that the high-reward distractor was more likely to produce rapid oculomotor capture. Alternatively, the latency difference may have arisen because participants learned to delay their first saccade on high-reward trials, possibly to reduce conflict arising due to the distractor's presence. To test for this latter possibility, we examined the first saccade latencies for the two different reward conditions over blocks (for the full first saccade latency distributions see appendix, Fig. A1). An ANOVA with reward (high vs. low) and block (1–6) as factors showed no main effect of reward [F(1,17) = 0.343, P = 0.566] or block [F(5,85) = 1.699, P = 0.176] and no interaction of reward and block [F(5,85) = 1.220, P = 0.311]. This suggests that participants did not delay the first saccade when the high-reward compared with the low-reward distractor was present.
Fig. A1.
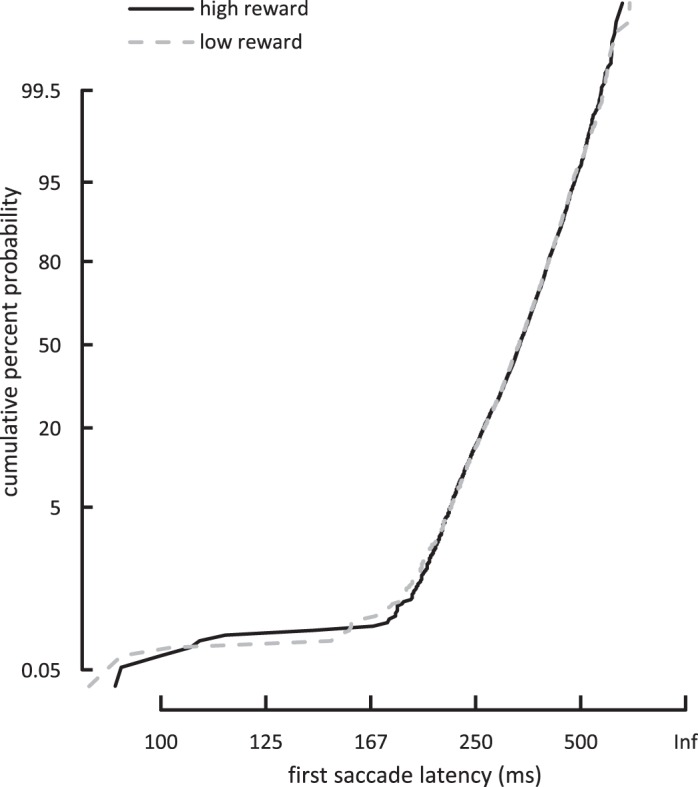
First saccade latency distribution across participants as a function of the reward condition for experiment 1. Here and in Fig. A2, latency data are plotted on a probability, or probit, scale to form a reciprobit plot (for more details on the method see, e.g., LATER model; Carpenter and Williams 1995).
To investigate in more detail whether reward availability affected early eye movements, we further analyzed latencies of first saccades as a function of their direction. A first saccade was defined as going in the direction of the target or in the direction of the distractor when the end point had an angular deviation of <30° to the left or the right (i.e., half the distance between objects) from the center of the target or distractor on the imaginary circle around the central fixation point (Theeuwes et al. 1998, 1999). Saccadic latencies for target and distractor end points collapsed over both reward conditions revealed that latencies were generally faster for first saccades to the distractor (293 ± 40 ms) than for those going to the target (334 ± 49 ms; Fig. 3A) {t(17) = 7.401, P < 0.001, 95% CI [29.3, 52.7]}.
Fig. 3.
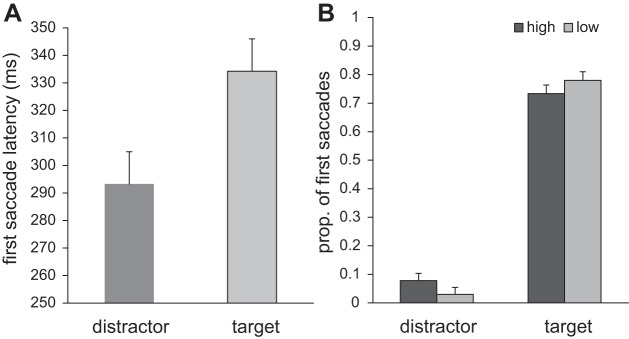
Experiment 1: results of the first saccade latency and its destination. A: latencies of first saccades going to either the distractor or the target. B: proportion of first saccades going either to the distractor or to the target for both reward conditions separately.
In terms of the direction of first saccades, this analysis revealed that a greater proportion of first saccades went toward the reward distractor signaling high reward compared with the distractor that signaled low reward (7.8 ± 5.2% vs. 3.1 ± 2.3%) {t(17) = 4.110, P = 0.005, 95% CI [1.6, 7.7]}. Moreover, fewer first saccades went toward the target on trials with a high-reward vs. a low-reward distractor (73.3 ± 10.2% vs. 78.0 ± 10.8%; Fig. 3B) {t(17) = 3.233, P = 0.001, 95% CI [2.4, 7.3]}.
Time course of oculomotor capture.
To further investigate the time course of the effect of reward on overt selection, we investigated the onset times of the first saccades with the Vincentizing procedure (Ratcliff 1979). We calculated the mean saccade latencies and the proportion of first saccades going toward the distractor separately for each reward condition and each decile of the individual saccade latency distribution.
An ANOVA of reward (high vs. low) and decile (1st–10th) on the proportion of first saccades toward the distractor showed a main effect of decile [F(9,153) = 15.107, P < 0.001, ηp2 = 0.471], with the proportion of first saccades toward the distractor decreasing as first saccade latency increased (see Fig. 4). A main effect of reward [F(1,17) = 16.953, P = 0.001, ηp2 = 0.499] reflected a greater proportion of first saccades toward the high-reward than the low-reward distractor. Importantly, a significant interaction [F(9,153) = 5.283, P = 0.008, ηp2 = 0.237] reflected the finding that the influence of reward was more reliably observed in short first saccades latencies than for longer latencies. To further investigate the development of this interaction effect, we compared the reward levels for each decile with paired-samples t-tests. Significantly more first saccades went to the distractor in the high- compared with the low-reward trials for deciles 1, 2, 3, 4, 7, and 10 {decile 1: t(17) = 3.084, P = 0.007, 95% CI [5.4, 29.2]; decile 2: t(17) = 3.472, P = 0.003, 95% CI [2.9, 12.1]; decile 3: t(17) = 2.949, P = 0.009, 95% CI [1.6, 10.0]; decile 4: t(17) = 2.752, P = 0.014, 95% CI [1.1, 8.8]; decile 5: t(17) = 1.275, P = 0.220, 95% CI [−1.2, 5.0]; decile 6: t(17) = 1.518, P = 0.147, 95% CI [−0.6, 3.8]; decile 7: t(17) = 2.168, P = 0.045, 95% CI [0.2, 7.9]; decile 8: t(17) = 0.536, P = 0.599, 95% CI [−1.9, 3.3]; decile 9: t(17) = 1.431, P = 0.171, 95% CI [−0.8, 3.9]; decile 10: t(17) = 2.171, P = 0.044, 95% CI [0.81, 5.7]}. This suggests that the high reward-signaling stimulus exerted its effect particularly on early first saccade onset times.
Fig. 4.
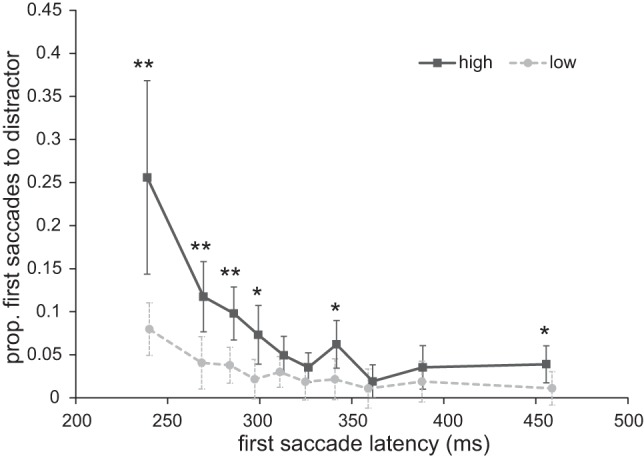
Experiment 1: proportion of first saccades going toward the distractor as a function of first saccade latency for both reward conditions. *P < 0.05, **P < 0.01.
Discussion
Experiment 1 shows that a physically nonsalient and task-irrelevant stimulus that signals relatively high reward captures the eyes more often than an otherwise equivalent stimulus that instead signals the availability of a low reward. Notably, this oculomotor capture by the distractor occurred even though such capture was detrimental for actual reward payout, since fixating the distractor caused the omission of reward that would otherwise have been earned. In other words, even though capture was counterproductive, participants failed to adopt a strategy that prevented capture from occurring.
With experiment 1 we extend the findings of Le Pelley et al. (2015). They showed that physically salient but task-irrelevant stimuli that signal reward are prioritized in search, causing significantly more capture when they signal relatively high reward. However, it was not clear from their experiments how far physical salience contributed to the effect. It is well documented that physically salient stimuli are capable of capturing attention of the eyes even if they are otherwise task irrelevant (e.g., Theeuwes 1992, 1994, 2010; Theeuwes et al. 1999). As such, Le Pelley at al.'s findings can be explained in terms of capture that is driven by physical salience, with reward merely modulating its degree. The present experiment, however, clearly shows that physical salience is not necessary to obtain this effect.
Notably, the effect of reward on oculomotor capture was more reliably found in early first saccades, suggesting that the influence of reward occurs early in the selection process. This has been suggested by several other studies (e.g., Anderson et al. 2011a, 2011b, 2012; Failing and Theeuwes 2014, 2015; Hickey et al. 2010; Theeuwes and Belopolsky 2012; Wang et al. 2013), but direct evidence for this claim is rather scarce. We return to this point in general discussion.
EXPERIMENT 2
The finding that the effect of reward was more reliably found in early first saccades in experiment 1 provides evidence for very early oculomotor capture by reward-signaling stimuli. In experiment 2 we wanted to elicit faster saccades to further “zoom in” on the effect of reward on these very fast saccades. For that purpose, we made use of the so-called gap effect (e.g., Kingstone and Klein 1993; Reuter-Lorenz et al. 1991; Saslow 1967). The gap effect describes the phenomenon whereby saccade latencies typically reduce when there is a gap between the offset of the fixation stimulus and the onset of the target. In other words, when the fixation cross is removed before the onset of the search display and the moment in time at which the target may appear is uncertain [e.g., by varying stimulus onset asynchronies (SOA)], it is more likely that participants will make fast saccades (see, e.g., Mulckhuyse et al. 2009).
In experiment 2 we varied the SOA between offset of the fixation stimulus and onset of the target. In light of the findings of experiment 1, we hypothesized that the reward effect would be further modulated by the fixation offset procedure. That is, we expected that the earlier the fixation offset relative to the onset of the search display, the larger the capture by the high- compared with the low-reward distractor.
To further increase the likelihood of saccades toward the reward distractor, we increased the competition between the target and the reward distractor by reducing the minimum possible distance between these stimuli in the search display (cf. Desimone 1998). In experiment 2, the distractor could now be positioned either next to the target or two positions away (rather than 2 or 3 as in experiment 1).
Methods
Participants.
Eighteen naive students of the VU University Amsterdam (11 women, 7 men; mean age ±25 yr) with reported normal or corrected-to-normal vision participated in experiment 2. All participants provided written informed consent, and the experiment was approved by the Ethics Committee of the VU University Amsterdam. Participants received monetary compensation of between €10.28 and €12.67 (mean payout ±€11.80) based on their performance.
Apparatus.
The apparatus was identical to experiment 1.
Stimuli.
The setup of the stimuli was identical to experiment 1 with a single exception. In experiment 2, the reward-signaling distractor could be either one or two positions away from the target instead of two or three positions as in experiment 1 (i.e., the smallest distance from target to distractor was 10.1° visual degrees or 60° polar angle).
Procedure and design.
The procedure and design were similar to experiment 1 with the following exceptions. In experiment 2, the fixation display remained on for 250–400 ms after successful drift correction. To manipulate the fixation offset SOA, subsequent presentation of the search display varied between being shown either immediately or 150 ms later. The fixation cross disappeared prior to (−150 ms), at the same time as (0 ms), or after (150 ms) the presentation of the search display (see Fig. 5, illustrating a trial with an SOA of −150 ms).
Fig. 5.
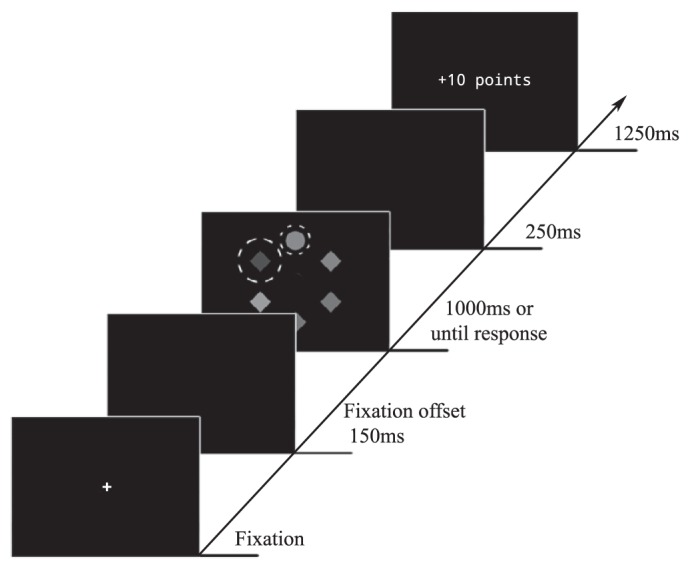
Trial sequence of experiment 2. In this experiment, the fixation offset relative to the search display onset was manipulated. In this example, the fixation offset was 150 ms prior to the onset of the search display.
Discarded data.
Criteria for discarding data were identical to those in experiment 1; 2.7% of all trials timed out before participants registered a correct response, 0.6% of the trials contained anticipatory saccades, and in 2.9% of the trials the time between the start of the drift correction and the presentation of the search display exceeded 7,000 ms.
Results
Omission trials.
As in experiment 1, we determined whether physically nonsalient and task-irrelevant stimuli signaling reward would interfere with target search. An ANOVA with the factors reward (high vs. low) and block (1–6) on the proportion of omission trials revealed a main effect of reward [F(1,17) = 10.663, P = 0.005, ηp2 = 0.385] but no main effect of block [F(5,85) = 1.955, P = 0.130] and no interaction [F(5,85) = 1.521, P = 0.211]. Replicating findings from experiment 1, participants fixated the distractor more often when it signaled high reward (20.7 ± 16.2%) compared with when it signaled low reward (9.1 ± 4.7%), an effect that remained relatively stable throughout experiment 2 (Fig. 6).
Fig. 6.
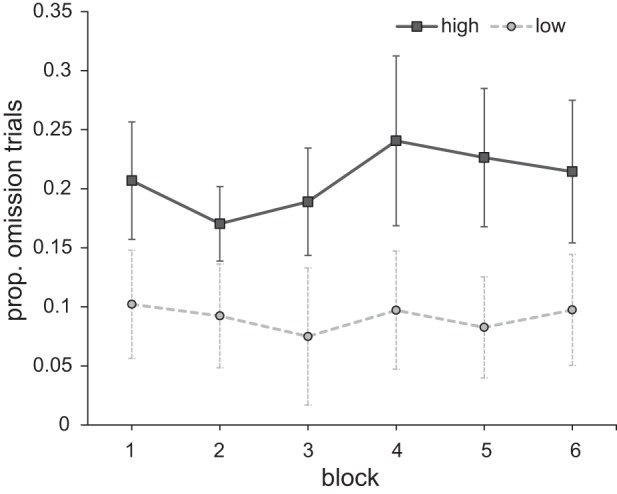
Experiment 2: proportion of omission trials for both reward conditions across blocks.
Time to fixate the target.
As in experiment 1, the time until fixation on the target ROI was significantly longer on trials with a high-reward distractor (423 ± 49 ms) than on trials with a low-reward distractor (403 ± 45 ms) {t(17) = 3.624, P = 0.002, 95% CI [8.4, 31.9]}.
First saccade latencies and destinations.
Saccade latencies were analyzed as in experiment 1. First, we examined whether first saccade latencies in general were influenced by the magnitude of reward signaled by the distractor (for the full first saccade latency distributions see appendix, Fig. A2). An ANOVA with reward (high vs. low) and block (1–6) as factors showed neither a main effect of reward [F(1,17) = 0.378, P = 0.547] or block [F(5,85) = 1.374, P = 0.266] nor an interaction [F(5,85) = 0.325, P = 0.820], suggesting that participants did not merely delay their first saccade on high-reward trials relative to low-reward trials.
Fig. A2.
First saccade latency distribution across participants as a function of the reward condition for experiment 2.
Saccades were classified as going to either the target or the distractor with the same criteria as in experiment 1. Again, comparing the saccadic latencies for target and distractor end points collapsed over both reward conditions showed generally lower latencies for first saccades to the distractor (277 ± 41 ms) than to the target (306 ± 42 ms; Fig. 7A) {t(17) = 6.240, P < 0.001, 95% CI [19.6, 40.0]}. In terms of first saccade directions, a greater proportion of first saccades went toward the high-reward distractor than toward the low-reward distractor (19.6 ± 15.2% vs. 7.4 ± 3.9%) {t(17) = 3.552, P = 0.002, 95% CI [4.9, 19.5]}. Moreover, fewer first saccades were directed toward the target on trials with a high-reward vs. a low-reward distractor (62.2 ± 16.1% vs. 72.9 ± 11.3%; Fig. 7B) {t(17) = 3.977, P = 0.001, 95% CI [5.1, 16.5]}.
Fig. 7.
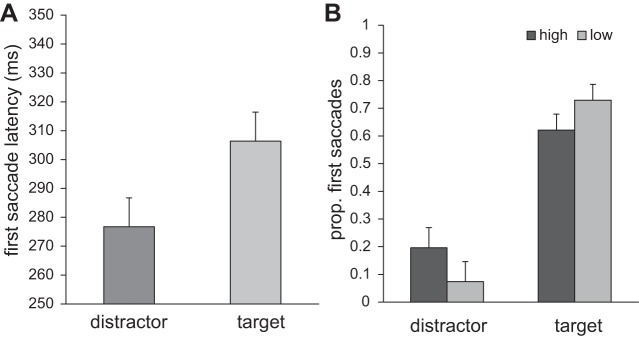
Experiment 2: results of the first saccade latency and its destination. A: latencies of first saccades going to either the distractor or the target. B: proportion of first saccades going either to the distractor or to the target for both reward conditions separately.
As noted above, the absolute distance of the distractor's position relative to the target was changed in experiment 2 compared with experiment 1, to increase competition between target and distractor so as to increase the likelihood of capture by the distractor. Specifically, in experiment 2 the distractor could be either one or two positions away from the target. To see whether this manipulation indeed influenced capture, we submitted the proportion of first saccades toward the distractor to an ANOVA with reward (high vs. low) and distance (1 vs. 2 positions) as factors. There was a main effect of reward [F(1,17) = 12.653, P = 0.002, ηp2 = 0.427] and distance [F(1,17) = 73.663, P < 0.001, ηp2 = 0.812] but no interaction [F(1,17) = 1.938, P = 0.182]. In other words, there were significantly more first saccades toward the distractor when it was adjacent to the target (17.7%) compared with when it was two positions away (9.3%). However, this pattern was not further modulated by the availability of reward.
Time course of oculomotor capture.
To assess whether the pattern of stronger oculomotor capture by the reward distractor was particularly evident in fast saccades as in experiment 1, we ran an ANOVA on proportion of first saccades toward the distractor using reward and decile as factors. There was a main effect of reward [F(1,17) = 12.667, P = 0.002, ηp2 = 0.427] and decile [F(9,153) = 9.737, P < 0.001, ηp2 = 0.364] and interaction [F(9,153) = 3.549, P = 0.015, ηp2 = 0.173]. As in the previous experiment, the interaction seems to reflect the pattern that the difference in proportion of first saccades toward the distractor between the high- and low-reward conditions decreased as a function of decile (see Fig. 8). To further examine the development of this interaction effect, we compared the reward levels for each decile with paired-samples t-tests. Significantly more first saccades went to the distractor in the high-reward compared with the low-reward trials for deciles 1, 2, 3, 4, 5, 6, and 8 {decile 1: t(17) = 2.966, P = 0.009, 95% CI [5.0, 30.0]; decile 2: t(17) = 3.389, P = 0.003, 95% CI [7.8, 33.6]; decile 3: t(17) = 3.306, P = 0.004, 95% CI [6.7, 30.3]; decile 4: t(17) = 2.487, P = 0.024, 95% CI [2.1, 25.3]; decile 5: t(17) = 2.813, P = 0.012, 95% CI [4.1, 28.4]; decile 6: t(17) = 3.746, P = 0.002, 95% CI [5.8, 20.8]; decile 7: t(17) = 1.676, P = 0.112, 95% CI [−0.16, 14.0]; decile 8: t(17) = 2.120, P = 0.049, 95% CI [0.0, 16.8]; decile 9: t(17) = 2.015, P = 0.060, 95% CI [−0.2, 7.6]; decile 10: t(17) = 1.814, P = 0.087, 95% CI [−0.6, 8.2]}. Replicating the findings from experiment 1, this suggests that the high reward-signaling distractor exerted its effect particularly in early first saccade onset times.
Fig. 8.
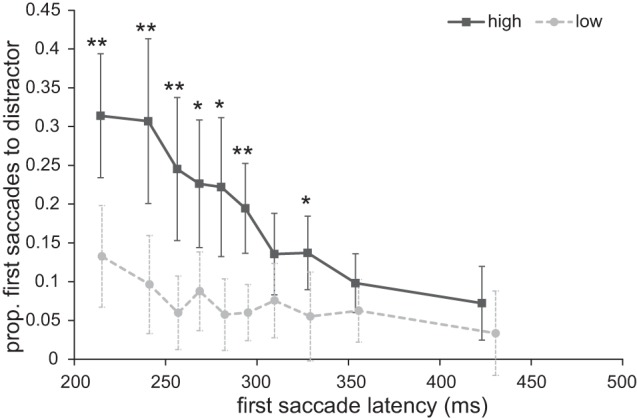
Experiment 2: proportion of first saccades going toward to the distractor as a function of first saccade latency for both reward conditions. *P < 0.05, **P < 0.01.
Fixation offset SOA.
In experiment 2 we manipulated the offset of the fixation with respect to the onset of the search display. To test whether this generally influenced saccade latencies of the first saccade, we ran an ANOVA with fixation offset SOA (−150 ms, 0 ms, 150 ms) as factor. This revealed a significant effect of fixation offset SOA [F(2,34) = 111.950, P < 0.001, ηp2 = 0.868]. Paired-samples t-tests revealed that first saccade latencies differed significantly between the conditions. Saccades were faster for trials with SOA of −150 ms (SOA−150 = 277 ± 43 ms) compared with trials with SOA of 0 ms (SOA0 = 296 ± 41 ms) {t(17) = 7.062, P < 0.001, 95% CI [13.6, 25.1]} and compared with trials with SOA of 150 ms (SOA150 = 319 ms ± 43; Fig. 9A) {t(17) = 13.536, P < 0.001, 95% CI [35.6, 48.8]; SOA0 vs. SOA150: t(17) = 8.838, P < 0.001, 95% CI [17.4, 28.2]}.
Fig. 9.
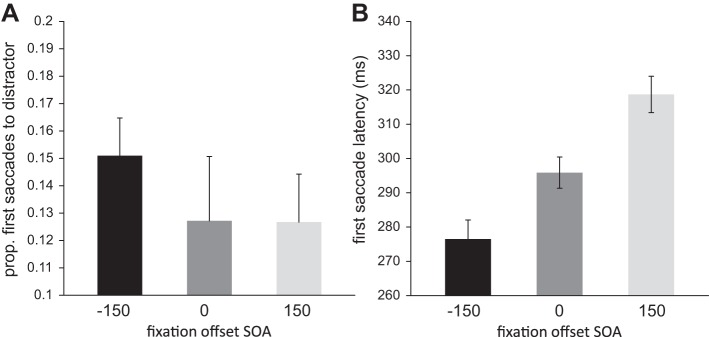
Experiment 2: results of first saccade latency and proportion of first saccade going either to the distractor or to the target. A: first saccade latency for all 3 levels of the fixation offset manipulation separately. B: proportion of first saccades to the distractor for all 3 levels of the fixation offset stimulus onset asynchrony (SOA) separately.
To determine whether there was more oculomotor capture by the distractor due to the fixation offset manipulation and, more importantly, whether the effect of fixation offset interacted with the effect of reward, we ran an ANOVA on first saccades toward the distractor using reward (high vs. low) and fixation offset SOA (−150 ms, 0 ms, 150 ms) as factors. There was a main effect of reward [F(1,17) = 12.120, P = 0.003, ηp2 = 0.416], a marginally significant main effect of fixation offset SOA [F(2,34) = 2.795, P = 0.092, ηp2 = 0.141], and no interaction [F(2,34) = 453, P = 0.600]. Planned comparisons revealed that there was a significant difference in the proportion of first saccades to the distractor for trials with SOA of −150 ms (SOA−150 = 15.1% ± 9.4) compared with either trials with SOA of 0 ms (SOA0 = 12.7 ± 7.5%) {t(17) = 2.198, P = 0.042, 95% CI [1.0, 4.7]} or trials with SOA of 150 ms (SOA150 = 12.7% ± 9.1; Fig. 9B) {t(17) = 3.647, P = 0.002, 95% CI [1.0, 3.8]} {SOA0 vs. SOA150: t(17) = 0.038, P = 0.970, 95% CI [−2.6, 2.7]}. In other words, the earlier the fixation offset, the higher the proportion of first saccades to the distractor. However, the results also suggest that capture by the reward distractor did not differ between the different SOAs.
Comparison to experiment 1.
In light of previous research on the gap effect (e.g., Kingstone and Klein 1993; Reuter-Lorenz et al. 1991; Saslow 1967), we expected that the introduction of the fixation offset procedure in experiment 2 would generally result in greater and faster capture by the distractor compared with experiment 1. For the following tests, only trials in which the distractor was placed two positions away from the target were used, as these were the only trials that were comparable over the two experiments. Planned comparisons showed that introducing the fixation offset procedure did generally reduce mean first saccade latency {298 ± 42 ms vs. 327 ± 47 ms, t(17) = 1.963, P (1-tailed) = 0.029, 95% CI [−1.0, 59.4]} and increased capture by the distractor {5.7 ± 3.5% vs. 9.3 ± 7.3% of the first saccades went to the distractor, t(24.551) = 1.894, P (1-tailed) = 0.045, 95% CI [0.3, 7.5]} when comparing experiment 1 with experiment 2.
To examine how the capture by the distractor was modulated because of reward availability when comparing both experiments, we ran a mixed ANOVA on proportion of first saccades toward the distractor with decile, reward, and experiment as factors. The results showed a main effect of reward [F(1,34) = 19.940, P < 0.001, ηp2 = 0.370] and decile [F(9,306) = 18.141, P < 0.001, ηp2 = 0.348] and a marginally significant main effect of experiment [F(1,34) = 3.645, P = 0.065, ηp2 = 0.097]. The analysis further revealed a significant interaction between reward and decile [F(9,306) = 4.777, P = 0.001, ηp2 = 0.123], no interaction between decile and experiment [F(9,306) = 1.588, P = 0.179], and no interaction between reward and experiment [F(1,34) = 2.709, P = 0.109]. This suggests that even though there was evidence for generally more capture when comparing both experiments, the difference in capture by reward was not further modulated by the introduction of the fixation offset manipulation (see Fig. 10).
Fig. 10.
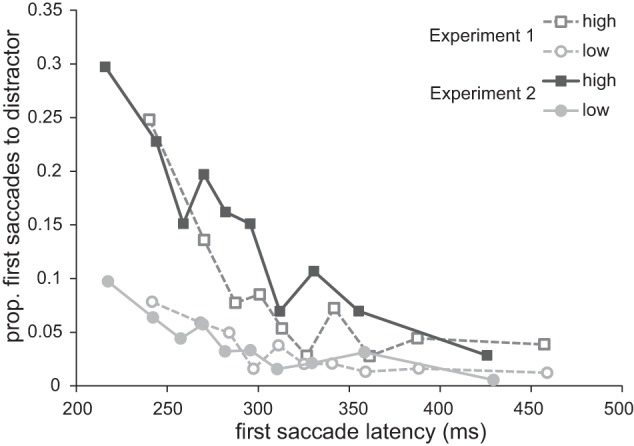
Proportion of first saccades going to the distractor as a function of first saccade latency for both reward conditions and each experiment separately. Note that this graph only depicts data from trials in which the distractor was 2 positions away from the target.
Discussion
Experiment 2 replicated the main findings of experiment 1. Again, task performance was hampered when a physically nonsalient and task-irrelevant stimulus signaling relatively high reward was present in the search display. Participants' gaze was more often drawn to that reward-signaling stimulus, particularly so for early first saccades.
By manipulating the fixation offset we were able to generate faster initial saccades than in experiment 1, and a larger proportion of these very fast saccades went to the high-reward distractor than the low-reward distractor. This finding is consistent with the competitive integration model by Godijn and Theeuwes (2002), who argued that fast saccades are basically exogenous in origin as top-down effects need time to develop. The implication is that the influence of reward on oculomotor capture observed in the present experiments is comparable to the exogenous effect that physically salient stimuli have on the oculomotor system.
GENERAL DISCUSSION
The present study shows that physically nonsalient and task-irrelevant stimuli signaling relatively high reward interfere with overt target search more often than otherwise comparable stimuli that signal low reward. Clearly, participants' eyes went more often in the direction of the stimulus signaling high reward compared with the stimulus signaling low reward, and this difference was particularly evident in very early saccades. Notably, oculomotor capture by the reward-signaling distractor occurred even though it was never necessary for obtaining reward. Instead, moving the eyes to the reward-signaling stimulus caused the omission of reward. We attribute this eye movement pattern to reward increasing the selection priority of stimuli signaling relatively high reward. By signaling reward, stimuli gain subjective salience, making them more likely to cause oculomotor shifts in the direction of those stimuli (cf. Berridge and Robinson 1998; Mackintosh 1975).
The present findings are consistent with a recent study by Le Pelley et al. (2015). In their study task-irrelevant distractors that signaled relatively high reward caused attentional and oculomotor capture in visual search for a target. However, the reward-signaling stimuli in their experiments were always physically salient. Physically salient stimuli are known to “pop out,” causing capture in bottom-up fashion irrespective of the current task set or their task relevance (e.g., Theeuwes 1992, 1994; Theeuwes et al. 1999). Therefore, it is unclear from Le Pelley et al.'s (2015) study whether capture by stimuli signaling reward is dependent on their physical salience. The present study clearly demonstrates that physical salience is not necessary for stimuli signaling reward to cause oculomotor capture. Merely by signaling reward, a stimulus that by its physical appearance does not stand out from the background grabs the eyes.
Together with Le Pelley et al. (2015), the present findings highlight a mechanism by which reward affects visual selection that is quite different from that explored in previous studies. In previous studies (e.g., Anderson et al. 2011a, 2011b; Chelazzi et al. 2013; Failing and Theeuwes 2014, 2015; Hickey et al. 2010; Kiss et al. 2009; Raymond and O'Brien 2009; Theeuwes and Belopolsky 2012), the stimulus signaling reward was either physically salient or task relevant at some point during the experiment (e.g., during a training phase prior to the test phase). The previous studies may therefore have merely demonstrated the process of training a (motor) response (i.e., an attentional or oculomotor shift of attention) toward the reward-signaling stimulus via known bottom-up or top-down processes. As a consequence of prolonged training, this response toward the reward-signaling stimulus may have persisted to influence subsequent behavior even when that stimulus was no longer physically salient or task relevant. In the present study, however, training or shaping a response to the reward-signaling distractor was never part of the task. Task instructions, as well as the fact that reward was omitted if the reward-signaling stimulus was selected, strongly discouraged shaping. Despite this, the association between the stimulus and the reward triggered oculomotor capture on a relatively large proportion of trials.
Many studies on the influence of reward-associated stimuli in visual selection (whether investigating covert or overt selection) have argued not only that the effect of reward is involuntary but also that it affects the selection process at a very early stage (e.g., Anderson et al. 2011a, 2011b, 2012; Failing and Theeuwes 2014, 2015; Hickey et al. 2010; Theeuwes and Belopolsky 2012; Wang et al. 2013). Indeed, attentional search studies using electroencephalography have provided evidence for this claim by showing modulations in the early lateral selection component N2pc (e.g., Eimer 1996). The N2pc in these studies was significantly earlier and larger for high- than low-reward stimuli whether they were targets (Kiss et al. 2009) or distractors (Hickey et al. 2010; Qi et al. 2013;). This finding was interpreted as evidence that reward affects visual search early in time by either mimicking or interacting with the effect of physical salience (cf. Hickey and van Zoest 2012; Wang et al. 2013). However, in all these studies either the stimuli currently or previously signaling reward were physically salient or task relevant or their selection had previously been necessary for the delivery of reward during a given stage of the experiment. Here we showed a pronounced influence of reward availability on the very earliest saccades toward stimuli that had been nonsalient and task irrelevant throughout the entire experiment. Modulations in proportion and latencies of first saccades due to reward occurred even though selecting these stimuli was never necessary for obtaining reward but actually produced omission of subsequent reward payout. Importantly, we also demonstrated that the modulations due to reward reduced as a function of time. In other words, the influence of reward on oculomotor capture was smaller the later the first saccade was initiated. This is consistent with the idea that reward acts early in the selection process, when selection is largely driven by involuntary selection processes (e.g., Theeuwes 2010). It is only later in time (i.e., late first saccades) that the priority signal of the reward-signaling stimulus can be suppressed by top-down processes. A similar pattern that is characterized by differential effects on short relative to long first saccade latencies has been reported previously (e.g., Mackay et al. 2012). Mackay et al. (2012) found that the effect of salience is particularly pronounced for saccades with a short latency. Assuming that reward affects visual search by mimicking or interacting with salience (Hickey and van Zoest 2012; Wang et al. 2013), this is consistent with the present results. Taken together, the present study provides evidence that physically nonsalient and task-irrelevant stimuli signaling reward cause oculomotor capture early in the selection process even if that oculomotor response is never explicitly trained or encouraged but detrimental for obtaining reward.
A potentially important difference between the procedure of the present study and that of Le Pelley et al. (2015) is that here participants were explicitly informed prior to the experiment that the presence of a certain color of a nontarget in the search display would indicate the reward that could be earned on that trial. No such instructions were provided in Le Pelley et al.'s (2015) study. It is feasible to assume that this instruction may have motivated participants to search for the reward-signaling colors in the display in order to establish which color predicted which level of reward. However, it is unlikely that such a process underlies the influence of reward on oculomotor capture that we observed. First, there was no reason for participants to engage in such a search. The task demands made it clear that they should be moving their eyes to the target, not the distractor, as quickly as possible in order to achieve reward. Even notwithstanding this, once participants had established which was the high-reward and which the low-reward color, there was no reason at all for them to continue to orient gaze selectively to the high-reward distractor more than to the low-reward distractor. Finally, the fact that the effect of reward was most reliably found at the very shortest saccade latencies argues against an interpretation of this effect in terms of a strategic search process driven by instructions.
One could argue that the distractor in the present study is not task irrelevant, as its location is informative about the location of the target. Indeed, target and distractor are never presented at the same location, which implies that the presence of a distractor reduces the number of possible target locations. It may be argued that participants moved their eyes toward the distractor location as a way to “know” the location where the target is not located. Even though feasible, such a search strategy seems unlikely, as there was only a relatively small proportion of omission trials overall and only a relatively small proportion of first saccades going toward the distractor compared with the target. Moreover, the target had a unique shape, while the distractor had a nonunique color and was always embedded among multiple colors of the nontarget items. Searching for a nonunique color is much more difficult than searching for a unique shape (Theeuwes 1992). It seems unlikely that the benefit of reducing potential target locations would outweigh the costs arising from the use of a much more demanding color search strategy. Note that this explanation also cannot account for the difference between the reward conditions we observed and why it was more reliably observed in saccades with short latencies.
The mechanism underlying the early effect of reward on visual search can be explained in terms of the competitive integration model proposed by Godijn and Theeuwes (2002). According to this model overt search patterns are best described by a hierarchical priority map (saccade map). Activity on the saccade map is assumed to spread to neighboring locations but inhibits activity of more distant locations through lateral inhibition. When the activity somewhere on the map passes a certain threshold, a saccade toward that location is executed. Modulations of activity are thought to occur through an integration of early bottom-up (e.g., physical salience) and late top-down (e.g., task-relevance) signals that evolve after the onset of the search display (e.g., Mulckhuyse et al. 2009; Trappenberg et al. 2001). Accordingly, when a physically salient distractor activates a certain location on the map early in time, activity from stimuli in other locations is inhibited because of lateral inhibition. Only later, top-down processes inhibit the location of the distractor and activate (i.e., boost the activity of) the target location. Observing capture by reward-signaling but otherwise nonsalient stimuli particularly on the earliest first saccades suggests that the reward signal is already integrated at an early stage of the selection process. At this stage, the location of the reward-signaling stimulus has a high activity on the saccade map that inhibits activity of other distant locations (e.g., the location of the target) through lateral inhibition. It is thus at this early stage that saccades toward the reward-signaling stimulus are particularly promoted. This claim is consistent with primate studies that demonstrate an early influence of reward in the visual system at the level of the superior colliculus (e.g., Ikeda and Hikosaka 2003; Weldon et al. 2008), a brain area believed to be the host of the saccade map.
We modulated the fixation offset in experiment 2 in order to speed up saccades, allowing us to investigate the limits of the reward effect on early first saccades. We demonstrated that the fixation offset procedure was indeed successful, as initial saccades were executed faster in experiment 2 than in experiment 1. However, we did not find evidence that the gap effect was further modulated by reward. In the context of the competitive integration model this suggests that both manipulations affected the selection process differently. On one hand, when fixation is released because of the offset of the fixation cross, fixation cells in the superior colliculus stop firing (cf. Munoz and Wurtz 1993a, 1993b), thereby removing lateral inhibition of other distant locations on the saccade map. This in turn causes a rise in baseline activity at these locations, reducing the activity necessary for eliciting a saccade. On the other hand, we suggest that the reward signal modulates the threshold that must be surpassed in order to elicit a saccade, with increasing expected value (i.e., integration of reward magnitude and probability) lowering the threshold. In other words, an increase in reward magnitude given the same probability of payout (as in our experiments) lowers the threshold, resulting in an increased probability of a saccade.
Taken together, the present experiments show that reward-signaling but otherwise nonsalient and task-irrelevant stimuli cause oculomotor capture even when selecting them is detrimental for obtaining reward. This form of oculomotor capture (cf. Le Pelley et al. 2015) is different from other reported forms of reward-related capture and occurs early in the selection process. The findings expand upon the growing evidence that suggests that visual selection cannot fully be explained in terms of known top-down or bottom-up processes (Awh et al. 2012). Reward either in the form of a trained response (see Anderson 2013; Chelazzi et al. 2013) or, as in the present experiments, in the form of a priority signal penetrates top-down control guiding visual selection.
APPENDIX: FIRST SACCADE LATENCY DISTRIBUTIONS
The full first saccade latency distributions for experiment 1 are shown in Fig. A1. The full first saccade latency distributions for experiment 2 are shown in Fig. A2.
GRANTS
This research was supported by an ERC advanced grant (ERC-2012-AdG-323413, J. Theeuwes) and an Australian Research Council Future Fellowship (FT100100260, M. Le Pelley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.F. and J.T. conception and design of research; M.F. and T.N. performed experiments; M.F. and T.N. analyzed data; M.F., T.N., M.L.P., and J.T. interpreted results of experiments; M.F. and T.N. prepared figures; M.F. drafted manuscript; M.F., T.N., D.P., M.L.P., and J.T. edited and revised manuscript; M.F., T.N., D.P., M.L.P., and J.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the anonymous reviewers for their valuable comments.
REFERENCES
- Anderson BA. A value-driven mechanism of attentional selection. J Vis 13: 7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proc Natl Acad Sci USA 108: 10367–10371, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Learned value magnifies salience-based attentional capture. PLoS One 6: e27926, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Yantis S. Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Atten Percept Psychophys 74: 1644–1653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn Sci 16: 437–443, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28: 309–369, 1998. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L, Perlato A, Santandrea E, Della Libera C. Rewards teach visual selective attention. Vision Res 85: 58–72, 2013. [DOI] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature 377: 59–62, 1995. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson's method. Tutor Quant Methods Psychol 1: 42–45, 2005. [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron 36: 285–298, 2002. [DOI] [PubMed] [Google Scholar]
- Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philos Trans R Soc Lond B Biol Sci 353: 1245–1255, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Glimcher PW. Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron 44: 365–378, 2004. [DOI] [PubMed] [Google Scholar]
- Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalogr Clin Neurophysiol 99: 225–234, 1996. [DOI] [PubMed] [Google Scholar]
- Failing MF, Theeuwes J. Exogenous visual orienting by reward. J Vis 14: 6, 2014. [DOI] [PubMed] [Google Scholar]
- Failing MF, Theeuwes J. Nonspatial attentional capture by previously rewarded scene semantics. Vis Cogn 23: 82–104, 2015. [Google Scholar]
- Godijn R, Theeuwes J. Programming of endogenous and exogenous saccades: evidence for a competitive integration model. J Exp Psychol Hum Percept Perform 28: 1039–1054, 2002. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature 391: 481–484, 1998. [DOI] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. J Neurosci 30: 11096–11103, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, van Zoest W. Reward creates oculomotor salience. Curr Biol 22: R219–R220, 2012. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron 39: 693–700, 2003. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Klein RM. Visual offsets facilitate saccadic latency: does predisengagement of visuospatial attention mediate this gap effect? J Exp Psychol Hum Percept Perform 19: 1251–1265, 1993. [DOI] [PubMed] [Google Scholar]
- Kiss M, Driver J, Eimer M. Reward priority of visual target singletons modulates event-related potential signatures of attentional selection. Psychol Sci 20: 245–251, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pelley ME, Pearson D, Griffiths O, Beesley T. When goals conflict with values: counterproductive attentional and oculomotor capture by reward-related stimuli. J Exp Psychol Gen 144: 158–171, 2015. [DOI] [PubMed] [Google Scholar]
- Louie K, Grattan LE, Glimcher PW. Reward value-based gain control: divisive normalization in parietal cortex. J Neurosci 31: 10627–10639, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay M, Cerf M, Koch C. Evidence for two distinct mechanisms directing gaze in natural scenes. J Vis 12: 9, 2012. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: variations in the associability of stimuli with reinforcement. Psychol Rev 82: 276, 1975. [Google Scholar]
- Mathôt S, Schreij D, Theeuwes J. OpenSesame: an open-source, graphical experiment builder for the social sciences. Behav Res Methods 44: 314–324, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RD. Confidence intervals from normalized data: a correction to Cousineau (2005). Tutor Quant Methods Psychol 4: 61–64, 2008. [Google Scholar]
- Mulckhuyse M, Van der Stigchel S, Theeuwes J. Early and late modulation of saccade deviations by target distractor similarity. J Neurophysiol 102: 1451–1458, 2009. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70: 559–575, 1993a. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol 70: 576–589, 1993b. [DOI] [PubMed] [Google Scholar]
- Qi S, Zeng Q, Ding C, Li H. Neural correlates of reward-driven attentional capture in visual search. Brain Res 1532: 32–43, 2013. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Group reaction time distributions and an analysis of distribution statistics. Psychol Bull 86: 446, 1979. [PubMed] [Google Scholar]
- Raymond JE, O'Brien JL. Selective visual attention and motivation: the consequences of value learning in an attentional blink task. Psychol Sci 20: 981–988, 2009. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Hughes HC, Fendrich R. The reduction of saccadic latency by prior offset of the fixation point: an analysis of the gap effect. Percept Psychophys 49: 167–175, 1991. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J Opt Soc Am 57: 1024–1029, 1967. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 57: 87–115, 2006. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science 304: 1782–1787, 2004. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Introduction to Reinforcement Learning. Cambridge, MA: MIT Press, 1998. [Google Scholar]
- Theeuwes J. Perceptual selectivity for color and form. Percept Psychophys 51: 599–606, 1992. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Stimulus-driven capture and attentional set: selective search for color and visual abrupt onsets. J Exp Psychol Hum Percept Perform 20: 799–806, 1994. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol 135: 77–99, 2010. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Belopolsky AV. Reward grabs the eye: oculomotor capture by rewarding stimuli. Vision Res 74: 80–85, 2012. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Kramer AF, Hahn S, Irwin DE. Our eyes do not always go where we want them to go: capture of the eyes by new objects. Psychol Sci 9: 379–385, 1998. [Google Scholar]
- Theeuwes J, Kramer AF, Hahn S, Irwin DE, Zelinsky GJ. Influence of attentional capture on oculomotor control. J Exp Psychol Hum Percept Perform 25: 1595–1608, 1999. [DOI] [PubMed] [Google Scholar]
- Trappenberg T, Dorris M, Munoz D, Klein R. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. J Cogn Neurosci 13: 256–271, 2001. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu H, Zhou X. Interaction between value and perceptual salience in value-driven attentional capture. J Vis 13: 5, 2013. [DOI] [PubMed] [Google Scholar]
- Weldon DA, Patterson CA, Colligan EA, Nemeth CL, Rizio AA. Single unit activity in the rat superior colliculus during reward magnitude task performance. Behav Neurosci 122: 183–190, 2008. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 4.0: current progress with a model of visual search. In: Integrated Models of Cognitive Systems, edited by Gray W. New York: Oxford Univ. Press, 2007, p. 99–119. [Google Scholar]


