Abstract
Brucea javanica is a traditional herbal medicine in China, and its antitumor activities are of research interest. Brucea javanica oil, extracted with ether and refined with 10% ethyl alcohol from Brucea javanica seed, was used to treat hepatoma H22-bearing mice in this study. The antitumor effect and probable mechanisms of the extracted Brucea javanica oil were studied in H22-bearing mice by WBC count, GOT, GPT levels, and western blotting. The H22 tumor inhibition ratio of 0.5, 1, and 1.5 g/kg bw Brucea javanica oil were 15.64%, 23.87%, and 38.27%. Brucea javanica oil could inhibit the involution of thymus induced by H22 tumor-bearing, but it could not inhibit the augmentation of spleen and liver. Brucea javanica oil could decrease the levels of WBC count and GOT and GPT in H22-bearing mice. The protein levels of GAPDH, Akt, TGF-β1, and α-SMA in tumor tissues decreased after being treated with Brucea javanica oil. Disturbing energy metabolism and neoplastic hyperplasia controlled by Akt and immunoregulation activity were its probable antitumor mechanisms in hepatoma H22-bearing mice.
1. Introduction
Cancer is a generic term for a large group of diseases that can affect any part of the body. One defining feature of cancer is the rapid creation of abnormal cells that grow beyond their usual boundaries and which can then invade adjoining parts of the body and spread to other organs. This process is referred to as metastasis. Metastases are the major cause of death from cancer. Cancer is a leading cause of death worldwide, accounting for 8.2 million deaths in 2012. The most common causes of cancer death are cancers of lung and liver [1]. Chinese medicine, one of the most popular complementary and alternative medicines, is an available option in many cancer centres in Asia, North America, and Europe [2].
Brucea javanica (B. javanica (L.) Merr.) is a shrub mostly originated in India, Southeast Asia, and Northern Australia [3]. Brucea javanica seed is used for oncotherapy in Chinese medicine. A series of chemical compounds have been isolated from this plant, such as alkaloids, lignans, terpenoids, alkaloid glycosides, quassinoid glycosides, and quassinoids [4–8]. In addition, a complex mixture of fatty acids and fatty acid derivatives (Brucea javanica oil), whose main activity components are oleic acid and linoleic acid, has been extracted from the seed of Brucea javanica. Oleic acid, linoleic acid, and quassinoids are known to be the major antitumor activity compounds [9]. It has been reported that Brucea javanica oil inhibited tumor cell growth via inhibition of DNA polymerase, overcoming tumor multidrug resistance, and the damage of tumor cell membrane system [10].
Brucea javanica oil is a natural plant product that possesses antitumor properties, but more molecular mechanisms of the antitumor effects of it are still unrevealed. Here we investigate the antitumor efficacy of Brucea javanica oil extracted with ether and refined with 10% ethyl alcohol in hepatoma H22-bearing mice. Furthermore, the protein levels of β-catenin, PI3K, Akt, TGF-β1, α-SMA, GAPDH, and β-actin (as internal control) in H22 tumor tissues treated in vivo with Brucea javanica oil were detected.
2. Materials and Methods
2.1. Mice and Cell Lines
Female Chinese Kunming mice (weight 18~20 g) were purchased from Fujian medical university laboratory animal center (Fuzhou, China). The mice were housed under normal condition and with free access to food and water. Animal experiments and animal care were carried out according to protocols approved by the institutional committee for animal care and also in accordance with the policy of the National Ministry of Health. Murine hepatoma cell line H22 was purchased from China Centre for Type Culture Collection (CCTCC, Wuhan, China), subcultured, and maintained in our laboratory according to the guidelines given.
2.2. Extraction and Refining of Brucea javanica Oil
Brucea javanica fruits (Chinese medicinal materials, place of origin: Xiamen, China) were purchased from Suzhou Hengfeng Ginseng & Deer Antler Commercial Firm, Jiangsu, China. Brucea javanica fruits were dried to constant weight at 80°C and shelled to get the seeds. Brucea javanica seeds were milled and soaked with ether to extract the seed oil. The crude seed oil was refined with 10% ethyl alcohol according to the patented method [11].
2.3. Acute Toxicity in Mice
Kunming mice were randomly divided into 8 groups according to the dose (n = 12 each group). The refined Brucea javanica oil was injected subcutaneously in the back with doses of 1.25, 2.5, 5, 6.25, 7.5, 8.75, 10, and 11.25 g/kg bw. After 24 h of injection, the number of mice surviving was recorded and the value of LD50 of Brucea javanica oil was calculated using the Bliss method with BL-420E software (Chengdu TME Technology Co., Ltd., China) [12].
2.4. Antitumor Efficacy on Mice Hepatoma
H22-Bearing Mice models were generated by subcutaneous injection of 2 × 106 H22 cells (mice hepatoma) in the armpit of left forelimb of each mouse. After injection, the mice models were randomly divided into 5 groups (n = 10 each group): soybean oil for injection (negative control), 0.5, 1, and 1.5 g/kg bw Brucea javanica oil (groups A, B, and C), and 25 mg/kg bw 5-Fu (positive control). And 10 other normal mice were set as normal control with injection of equal volume of soybean oil. The Brucea javanica oil was diluted to corresponding concentration with soybean oil for injection. The mice were injected subcutaneously in the back with corresponding medicines and the body weights before and after the experiment were measured. Seven days of continuous infusion later, all the mice were sacrificed, the tumor weights were recorded, and the whole blood, serum, thymus, spleen, heart, liver, kidney, and lung were collected. The blood white blood cells (WBC) were counted manually and the levels of GOT and GPT in serum were measured with automated biochemical analyzer (Hitachi, Japan). The organ coefficients (mg/g) of thymus, spleen, liver, kidney, and lung were calculated using the following formula: organ coefficient = organ weight/(body weight – tumor weight). Antitumor effects are expressed with inhibition ratio (%). The inhibition ratio (%) was calculated by the following formula: inhibition ratio (%) = [(A − B)/A] × 100%, where A is the average tumor weight of the negative control and B is the tumor weight of the treated group or positive control.
2.5. Antibodies for Western Blotting
Antibodies against β-catenin, Akt, and PI3K were from Cell Signaling Technology (Beverly, MA, USA). Antibodies against TGF-β1 and α-SMA were from Abcam (UK). The antibody against GAPDH was from Hangzhou Xianzhi Biological Technology (Zhejiang, CN). Anti-β-actin antibody was from Sigma. Anti-mouse IgG peroxidase-linked whole antibody and anti-rabbit IgG peroxidase-linked species-specific whole antibody were from Beyotime Institute of Biotechnology (Jiangsu, CN).
2.6. Tumor Harvesting and Western Blotting
The H22 tumors were excised from the mice of negative control group and 1.5 g/kg Brucea javanica oil groups and snap frozen in liquid nitrogen. Lysates were prepared in RIPA lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, CN) using a dounce homogenizer. Protein concentrations were quantified using BCA Protein Assay Reagent (Beyotime Institute of Biotechnology, Jiangsu, CN). Lysates were run at 40 μg per lane on 8% to 10% Bis-Tris gels and transferred to PVDF membranes (Invitrogen). Western blot band intensity quantification was done using Gel-Pro Analyzer software v4.0 (Media Cybernetics, Inc., USA). To account for differences in protein loading, all band intensities were corrected for β-actin.
2.7. Statistical Analysis
Student's t-test and ANOVA were used to analyze mean differences between groups of mice. P values of <0.05 were considered significant.
3. Results
3.1. Acute Toxicity in Mice
The LD50 was used to determine the acute toxicity, the changes in behavior, breathing, cutaneous effect, and sensory nervous system responses, and gastrointestinal effects were observed. The subcutaneous injection of Brucea javanica oil in doses ranging from 1.25 g/kg bw to 5 g/kg bw did not produce significant toxicity symptoms. Accompanying the increase of dose, towering hair, reduction in locomotor activity, and dull reactions were produced and the mortality was 100% in the dose of 11.25 g/kg bw. The LD50 of Brucea javanica oil and its 95% confidence limits were 8.36 and 7.07–10.05 g/kg bw.
3.2. Effect of Brucea javanica Oil on Body Weight of H22-Bearing Mice
The changes in body weights of mice before and after the experiment were as shown in Table 1; body weights increased markedly in all of the experimental groups. After experiment, the average body weights of mice did not have significant differences between six groups, although the average body weight of mice in positive control group was a little lower compared with normal control or negative control group. The subcutaneous injection of Brucea javanica oil for 7 days in dose no more than 1.5 g/kg bw did not affect the body weight growth.
Table 1.
Effect of Brucea Javanica oil on body weight of H22-bearing mice before and after experiment (, n = 10).
| Group | Dose (g/kg bw) | Number of animals | Average body weight of mice before the experiment (g) | Average body weight of mice after the experiment (g) |
|---|---|---|---|---|
| Normal control | 10 | 19.32 ± 1.86 | 23.67 ± 2.08 | |
| Negative control | 10 | 19.31 ± 1.63 | 22.35 ± 2.29 | |
| 5-Fu | 0.25 | 10 | 19.15 ± 1.49 | 21.14 ± 1.92 |
| A | 0.5 | 10 | 19.30 ± 1.73 | 23.49 ± 1.94 |
| B | 1 | 10 | 19.06 ± 1.67 | 22.35 ± 2.34 |
| C | 1.5 | 10 | 19.34 ± 1.79 | 22.39 ± 1.68 |
The average body weight of mice after the experiment excluding the tumor weight.
3.3. Effect of Brucea javanica Oil on Tumor Weight and Tumor Inhibition Ratio
The results for the effect of Brucea javanica oil on tumor weight and tumor inhibition ratio were as shown in Figure 1 and Table 2. There was a mouse in the negative control group that died in the sixth day, and only the body weight and tumor weight were recorded. The tumor excised from this mouse was not photographed.
Figure 1.
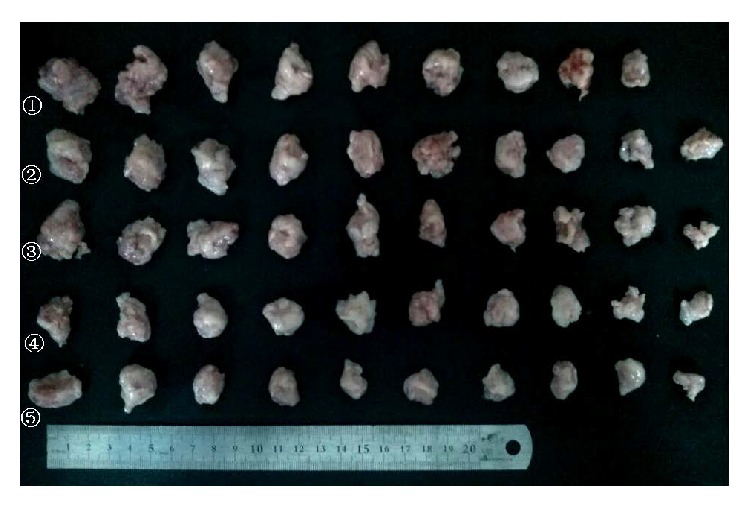
The tumors excised from the mice in the different groups. ① Negative group, ② 0.5 g/kg bw Brucea javanica oil, ③ 1 g/kg bw Brucea javanica oil, ④ 1.5 g/kg bw Brucea javanica oil, and ⑤ positive group (25 mg/kg bw 5-Fu).
Table 2.
Effect of Brucea Javanica oil on tumor weight and tumor inhibition ratio (, n = 10).
| Group | Dose (g/kg bw) | Number of animals | Average tumor weight (g) | Inhibition ratio (%) |
|---|---|---|---|---|
| Negative control | 10 | 2.43 ± 1.08 | — | |
| 5-Fu | 0.025 | 10 | 1.40 ± 0.62∗∗ | 42.39 |
| A | 0.5 | 10 | 2.05 ± 0.78 | 15.64 |
| B | 1 | 10 | 1.85 ± 0.73 | 23.87 |
| C | 1.5 | 10 | 1.50 ± 0.39∗∗ | 38.27 |
Comparison with the negative control group, ∗∗ P < 0.01.
3.4. Changes in Main Organ Coefficients of Mice
The main organ coefficients of mice, including thymus, spleen, liver, kidney, heart, and lung index, were as shown in Tables 3 and 4. Compared with the normal control group, H22 tumor-bearing decreased the thymus index and increased the spleen and liver indices significantly. Treated with 25 mg/kg bw 5-Fu, the thymus index of tumor-bearing mice decreased significantly. Compared with the negative control group, low dose Brucea javanica oil can increase the thymus index, but the high dose Brucea javanica oil decreased the thymus index instead (P = 0.08). The spleen index of tumor-bearing mice can be more or less increased by 0.5, 1, and 1.5 g/kg bw Brucea javanica oil. There were no significant differences in the indices of kidney, heart, and lung between all groups.
Table 3.
Changes in thymus index, spleen index, and liver index of mice ().
| Group | Dose (g/kg bw) | Number of animals | Thymus index (mg/g) | Spleen index (mg/g) | Liver index (mg/g) |
|---|---|---|---|---|---|
| Normal control | 10 | 5.01 ± 0.79 | 5.11 ± 1.35 | 46.79 ± 5.27 | |
| Negative control | 9 | 2.72 ± 1.06∗∗ | 8.76 ± 2.11∗∗ | 66.01 ± 6.29∗∗ | |
| 5-Fu | 0.025 | 10 | 1.22 ± 0.32∗∗,△△ | 7.64 ± 1.77∗ | 62.33 ± 6.89∗∗ |
| A | 0.5 | 10 | 3.72 ± 1.28∗∗,△ | 10.83 ± 1.09∗∗,△ | 68.56 ± 6.76∗∗ |
| B | 1 | 10 | 3.17 ± 0.95∗∗ | 11.80 ± 3.09∗∗,△△ | 64.80 ± 6.72∗∗ |
| C | 1.5 | 10 | 2.00 ± 0.75∗∗ | 10.37 ± 3.08∗∗ | 64.28 ± 8.36∗∗ |
Comparison with the normal control group, ∗ P < 0.05, ∗∗ P < 0.01. Comparison with the negative control group, △ P < 0.05, △△ P < 0.01.
Table 4.
Changes in kidney index, heart index, and lung index of mice ().
| Group | Dose (g/kg bw) | Number of animals | Kidney index (mg/g) | Heart index (mg/g) | Lung index (mg/g) |
|---|---|---|---|---|---|
| Normal control | 10 | 12.17 ± 0.84 | 5.29 ± 0.82 | 7.74 ± 2.46 | |
| Negative control | 9 | 12.07 ± 0.49 | 5.26 ± 0.47 | 6.01 ± 0.48 | |
| 5-Fu | 0.025 | 10 | 12.88 ± 1.57 | 5.48 ± 0.69 | 6.41 ± 0.69 |
| A | 0.5 | 10 | 13.49 ± 2.52 | 5.87 ± 0.87 | 7.16 ± 1.26 |
| B | 1 | 10 | 13.08 ± 1.58 | 5.44 ± 0.87 | 7.20 ± 1.47 |
| C | 1.5 | 10 | 12.21 ± 1.99 | 4.83 ± 0.70 | 7.25 ± 2.34 |
3.5. Effect of Brucea javanica Oil on WBC Count of Mice
The changes of average WBC count of mice were as shown in Table 5. It can be seen from the result H22 tumor-bearing increased WBC count of mice obviously and the WBC counts in 5-Fu treated or in Brucea javanica oil treated tumor-bearing mice decreased to the normal level.
Table 5.
Changes of WBC count of mice ().
| Group | Dose (g/kg bw) | Number of animals | WBC count (109 cells/L) |
|---|---|---|---|
| Normal control | 10 | 4.79 ± 1.36 | |
| Negative control | 9 | 13.88 ± 7.43∗∗ | |
| 5-Fu | 0.025 | 10 | 3.97 ± 1.22△△ |
| A | 0.5 | 10 | 5.25 ± 1.53△△ |
| B | 1 | 10 | 5.98 ± 2.36△△ |
| C | 1.5 | 10 | 5.58 ± 2.33△△ |
Comparison with the normal control group, ∗∗ P < 0.01; comparison with the negative control group, △△ P < 0.01.
3.6. Changes in GOT and GPT of Mice
The changes in the levels of GOT and GPT in serum of mice were as shown in Table 6. The results showed that Hepatoma H22-bearing increased the levels of GOT and GPT significantly and the levels of GOT and GPT decreased after being treated with 5-Fu or Brucea javanica oil compared with the negative control group.
Table 6.
Changes in the levels of GOT and GPT in serum of mice ().
| Group | Dose (g/kg bw) | Number of animals | GOT (U/L) | GPT (U/L) |
|---|---|---|---|---|
| Normal control | 10 | 146.00 ± 33.93 | 70.04 ± 14.47 | |
| Negative control | 9 | 780.16 ± 225.02∗∗ | 384.16 ± 70.41∗∗ | |
| 5-Fu | 0.025 | 10 | 395.00 ± 116.43∗∗,△△ | 172.67 ± 41.59∗∗,△△ |
| A | 0.5 | 10 | 589.60 ± 147.56∗∗,△ | 286.17 ± 71.84∗∗,△△ |
| B | 1 | 10 | 568.40 ± 248.91∗∗,△ | 276.60 ± 90.91∗∗,△△ |
| C | 1.5 | 10 | 665.66 ± 183.02∗∗ | 331.67 ± 91.01∗∗ |
Comparison with the normal control group, ∗∗ P < 0.01; comparison with the negative control group, △ P < 0.05, △△ P < 0.01.
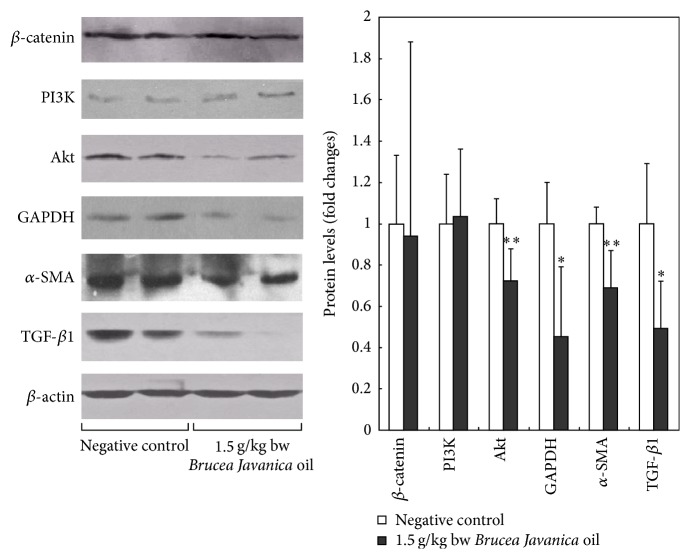
3.7. Changes in Protein Levels of β-catenin, PI3K, Akt, GAPDH, α-SMA, and TGF-β1
The protein levels of β-catenin, PI3K, Akt, GAPDH, α-SMA, and TGF-β1 in negative control group and 1.5 g/kg bw Brucea javanica oil group were as shown in Figure 2.
Figure 2.
The protein levels of β-catenin, PI3K, Akt, GAPDH, α-SMA, and TGF-β1 in negative control group and 1.5 g/kg bw Brucea javanica oil group. The β-actin was as internal control. There were 8 samples detected randomly per group and the error bars correspond to mean ± standard deviations. Comparison with the negative control group, ∗ P < 0.05, ∗∗ P < 0.01.
4. Discussion
Brucea javanica fruit is a kind of Chinese herb with toxicity. In China, the emulsion formulation of Brucea javanica oil has been used clinically widely in combination with conventional therapy to treat carcinoma, demonstrating efficacy enhancing, toxicity reducing effects, and immunoregulation activity [13–16], but the exact antitumor active components and corresponding molecular mechanisms have not been fully clarified [17].
Brucea javanica oil has been found to exhibit lethal toxicity to human or experimental animals, which has brought many difficulties to clinical application. The water-soluble quassinoid compounds were considered as the major material basis of its toxicity such as brucenol, bruceoside, brusatol and bruceine [18, 19]. In our past study, the LD50 of Brucea javanica oil extracted with ether directly was 2.26 g/kg bw in mice. We used the 10% ethyl alcohol to extract and refine the Brucea javanica oil. After the removal of residual water-soluble toxic components, the LD50 of refined Brucea javanica oil increased to 8.36 g/kg bw in mice. Oleic acid and linoleic acid were considered as the major antitumor activity compounds in Brucea javanica oil [9]. In addition, three terpene alcohols, lupeol, and taraxerol also had antitumor activity [20–22]. Our results clearly indicated that the refined Brucea javanica oil can inhibit the growth of implanted hepatoma H22 in mice in a dose-dependent manner. The antitumor efficacy of high dose Brucea javanica oil (1.5 g/kg bw) was slightly lower than 25 mg/kg bw 5-Fu.
Then we investigate probable mechanisms of Brucea javanica oil in hepatoma H22-bearing mice. The multiple pathways involved in the action of Brucea javanica oil were further identified [17, 23–26]. It could be proposed Brucea javanica oil induces apoptotic death of cancer cells via both the death receptors and the mitochondrial-related pathways. Brucea javanica oil also could inhibit the invasion and migration of tumor cells targeting at MRP-1/CD9 and integrin alpha-5. In addition, the autophagic process contributed to an increasing rate of cell death induced by Brucea javanica oil. In this study, we concluded immunoregulation activity and neoplastic hyperplasia controlled by Akt were probable antitumor mechanisms of Brucea javanica oil in H22-bearing mice.
The thymus is the major site of T cell differentiation and a key organ of the immune system. Many studies proved that tumor-bearing in mice could induce thymic atrophy due to the abnormal T cell development and apoptosis. In tumor-bearing mice, T cell recruitment from the thymus to the spleen and splenic excess augmentation could be observed. The splenic excess augmentation in H22-bearing mice was associated with the export of cells which was restrained after the cells entered the spleen, especially the CD8+T cell being detected. CD8+T cell was bound up with immunosuppression of spleen in H22-bearing mice [27–30]. Our results showed that low and medium dose of Brucea javanica oil could prevent the thymic atrophy of mice induced with H22 tumor-bearing in different extent, but the high dose of Brucea javanica oil could not. Treated with high dose of Brucea javanica oil, the splenic excess augmentation in H22 tumor-bearing mice became serious. Whether the more serious splenic excess augmentation could cause more serious immunosuppression of spleen in H22-bearing mice or not needs further study.
According to the result of effect of Brucea javanica oil on WBC count of mice, it was showed that H22 tumor-bearing induced the increase of WBC count and 5-Fu as a myelosuppressive agent could decrease the WBC count of tumor-bearing mice. Brucea javanica oil could also decrease the WBC count of tumor-bearing mice to the normal level. The effect of Brucea javanica oil on inhibiting the increase of WBC count is not relevant to myelosuppressive effect perhaps due to its anti-inflammatory property [31].
Hepatocellular carcinoma cell line H22 can express hepatocyte growth factor (HGF), which is a potent stimulator of DNA synthesis in a variety of epithelial cells, including hepatocytes, and has been implicated in liver regeneration [32, 33]. This study showed that the liver index of mice increased significantly after hepatoma H22 was implanted in mice. Neither 5-Fu nor Brucea javanica oil could inhibit the liver augmentation induced with H22 tumor-bearing. It was reported that the levels of GOT and GPT in serum would increase significantly when H22 cells were implanted into mice, and cytoxan (CTX) could decrease the levels in H22 tumor-bearing mice [34]. Our results indicated that both 5-Fu and Brucea javanica oil could decrease the levels of GOT and GPT in H22 tumor-bearing mice in different extent and Brucea javanica oil in dose no more than 1.5 g/kg bw didnot have obvious toxicity to the liver.
Disturbing energy metabolism and neoplastic hyperplasia controlled by Akt could be another probable antitumor mechanisms in hepatoma H22-bearing mice. Akt is a serine/threonine protein kinase that plays an important role in cell growth, proliferation, and survival. Numerous studies have revealed the blockage of Akt signaling to result in apoptosis and growth inhibition of tumor cells [35]. Akt counteracts apoptosis through a block of caspase-9, phosphorylation of proapoptotic members of the Bcl2-family of mitochondria-targeting proteins such as BAD, and stimulating signaling pathway of NF-kB and is also involved in the regulation of autophagy [36], DNA damage response and repair induced by commonly used genotoxic agents [37], and normal vascularization and pathological angiogenesis. Therefore, this signaling pathway of downstream of Akt has been considered to be a new target for effective cancer therapeutic strategies. In this study, treated with Brucea javanica oil the level of Akt in tumor tissue decreased, suggesting the potential of triggering apoptosis of Brucea javanica oil in hepatoma H22 cells.
In contrast to normal differentiated cells, which rely primarily on mitochondrial oxidative phosphorylation to generate the energy needed for cellular processes, most cancer cells instead rely on aerobic glycolysis, a phenomenon termed “the Warburg effect.” GAPDH is an important enzyme for energy metabolism and the production of ATP and pyruvate through anaerobic glycolysis in the cytoplasm. Additionally, it participates in apoptosis, membrane trafficking, iron metabolism, nuclear activities, and receptor mediated cell signaling. It was reported that overexpressed GAPDH in tumor could bind to active Akt and limit its dephosphorylation. This would lead to Bcl-xL overexpression and escape from caspase-independent cell death [38]. Recent reports indicate that GAPDH has the ability to interact with Akt in many settings [39, 40]. Since most cancers have evolved multiple strategies such as hypoxia to evade programmed cell death, it is suggested that GAPDH-dependent Akt expression is protecting cancer cells from hypoxia. In this study, treated with Brucea javanica oil the level of GAPDH and Akt in tumor tissue decreased. It is indicated that Brucea javanica oil could inhibit the growth of implanted hepatoma H22 in mice by disturbing energy metabolism and neoplastic hyperplasia controlled by Akt.
The TGF-β and PI3K/Akt signaling pathways are used in cells to control numerous responses, including proliferation, apoptosis, and metastasis. TGF-β is known for its cytostatic effects in premalignant states and its prooncogenic activity in advanced cancers. But in other ways, TGF-β1 can stimulate tumor cells to produce growth factors and promote tumor growth and development. It can also induce epithelial-mesenchymal transition (EMT) and promote the invasion and metastasis of tumor. In addition, TGF-β1 can inhibit tumor immunity to promote tumor progression [41]. Although early studies suggested that these two pathways might counteract each other in balancing cell survival, emerging evidence has uncovered multiple modes of intricate signal integration and obligate collaboration in driving cancer progression [42]. In this study, it was showed that the protein level of TGF-β1 and Akt decreased after treating with Brucea javanica oil in H22 tumor tissues, suggesting the collaboration of the two signaling pathways.
Alpha-smooth muscle actin (α-SMA) is commonly used as a marker of myofibroblast formation and tumor microvascular density associates with α-SMA expression [43, 44]. Our results showed that Brucea javanica oil could inhibit the growth of H22 tumors with downregulation of α-SMA in tumor tissues. It implied that Brucea javanica oil could inhibit tumor angiogenesis probably to block the growth of H22 tumors.
As mentioned above, immunoregulation activity and neoplastic hyperplasia controlled by Akt were probable antitumor mechanisms of Brucea javanica oil in H22-bearing mice. The work on Brucea javanica oil's antitumor components and compatibility with other herbs to reduce acute toxicity and enhance its antitumor activity is underway now in our laboratory and will be communicated in due course.
Acknowledgment
The authors gratefully acknowledge the financial support from the third round of foundation for colleges and universities in the construction of key subjects of Fujian Province, funds for integrated traditional Chinese and western medicine (no. 3003-905011010).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Wen-Rong Shi and Yan Liu supervised and directed the overall project. Yan Liu conceived and designed the experiments. Wen-Rong Shi, Xiao-Ting Wang, Xue-Rong Cai, Qiong-Ying Huang, and Shao-Rong Wu contributed to the antitumor experiments and data interpretation. Wen-Rong Shi and Yan Liu contributed to the western blot experiments. Wen-Rong Shi and Yan Liu discussed the results and analyzed the data and wrote the paper. All the authors were involved in the discussions.
References
- 1.World Health Organization. Cancer. Geneva, Switzerland: World Health Organization; 2014. http://www.who.int/mediacentre/factsheets/fs297/en/ [Google Scholar]
- 2.Lam Y.-C., Cheng C.-W., Peng H., Law C.-K., Huang X. Z., Bian Z. X. Cancer patients' attitudes towards Chinese medicine: a Hong Kong survey. Chinese Medicine. 2009;4, article 25:8. doi: 10.1186/1749-8546-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamperdick C., Van Sung T., Thuy T. T., Van Tri M., Adam G. (20R)-O-(3)-α-l-arabinopyranosyl-pregn-5-en-3β,20-diol from Brucea javanica . Phytochemistry. 1995;38(3):699–701. doi: 10.1016/0031-9422(94)00737-e. [DOI] [Google Scholar]
- 4.Karin C. S. L., Yang S. L., Roberts M. F., Phillipson J. D. Canthin-6-one alkaloids from cell suspension cultures of Brucea javanica . Phytochemistry. 1990;29(1):141–143. doi: 10.1016/0031-9422(90)89027-7. [DOI] [PubMed] [Google Scholar]
- 5.Luyengi L., Suh N., Fong H. H. S., Pezzuto J. M., Kinghorn A. D. A lignan and four terpenoids from Brucea javanica that induce differentiation with cultured HL-60 promyelocytic leukemia cells. Phytochemistry. 1996;43(2):409–412. doi: 10.1016/0031-9422(96)00258-0. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa I., Mahmud T., Simanjuntak P., Hori K., Uji T., Shibuya H. Indonesian medicinal plants. VIII. Chemical structures of three new triterpenoids, bruceajavanin A, dihydrobruceajavanin A, and bruceajavanin B, and a new alkaloidal glycoside, bruceacanthinoside, from the stems of Brucea javanica (Simaroubaceae) Chemical and Pharmaceutical Bulletin. 1994;42(7):1416–1421. doi: 10.1248/cpb.42.1416. [DOI] [PubMed] [Google Scholar]
- 7.Sakaki T., Yoshimura S., Tsuyuki T. Two new quassinoid glycosides, yadanziosides N and O isolated from seeds of Brucea javanica (L.) MERR. Tetrahedron Letters. 1986;27(5):593–596. doi: 10.1016/s0040-4039(00)84049-6. [DOI] [Google Scholar]
- 8.Kim I. H., Suzuki R., Hitotsuyanagi Y., Takeya K. Three novel quassinoids, javanicolides A and B, and javanicoside A, from seeds of Brucea javanica . Tetrahedron. 2003;59(50):9985–9989. doi: 10.1016/j.tet.2003.10.048. [DOI] [Google Scholar]
- 9.Bi Y. L. Quality analysis of Brucea javanica oil. Journal of Zhengzhou Institute of Technology. 2001;22(4):72–74. [Google Scholar]
- 10.Cui Y., Wu Z., Liu X., et al. Preparation, safety, pharmacokinetics, and pharmacodynamics of liposomes containing Brucea javanica oil. AAPS PharmSciTech. 2010;11(2):878–884. doi: 10.1208/s12249-010-9454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H. Java brucea oil-emulsion injecta with low-toxicity and preparing method thereof. CN, 03156023.7, 2014.
- 12.Li Q. X., Wang H., Xiao Q. Q., Kong R. The evaluation and calculation of median lethal dose (LD50) using Bliss method. Journal of Mathematical Medicine. 1995;4:318–320. [Google Scholar]
- 13.Lu Y.-Y., Huang X.-E., Cao J., et al. Phase II study on Javanica oil emulsion injection (Yadanzi) combined with chemotherapy in treating patients with advanced lung adenocarcinoma. Asian Pacific Journal of Cancer Prevention. 2013;14(8):4791–4794. doi: 10.7314/apjcp.2013.14.8.4791. [DOI] [PubMed] [Google Scholar]
- 14.Liu J., Huang X.-E., Tian G.-Y., et al. Phase II study on safety and efficacy of Yadanzi (Javanica oil emulsion injection) combined with chemotherapy for patients with gastric cancer. Asian Pacific Journal of Cancer Prevention. 2013;14(3):2009–2012. doi: 10.7314/apjcp.2013.14.3.2009. [DOI] [PubMed] [Google Scholar]
- 15.Nie Y.-L., Liu K.-X., Mao X.-Y., Li Y.-L., Li J., Zhang M.-M. Effect of injection of Brucea javanica oil emulsion plus chemoradiotherapy for lung cancer: a review of clinical evidence. Journal of Evidence-Based Medicine. 2012;5(4):216–225. doi: 10.1111/jebm.12001. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q., Wang M., He X., et al. Meta-analysis on treatment of non-small cell lung cancer with brucea javanica oil emulsion in combination with platinum-contained first-line chemotherapy. Zhongguo Zhongyao Zazhi. 2012;37(13):2022–2029. doi: 10.4268/cjcmm20121333. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H., Yang J. Y., Zhou F., et al. Seed oil of Brucea javanica induces apoptotic death of acute myeloid leukemia cells via both the death receptors and the mitochondrial-related pathways. Evidence-Based Complementary and Alternative Medicine. 2011;2011:14. doi: 10.1155/2011/965016.965016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuyama E., Gao L.-H., Yamazaki M. Studies on pharmacologically active principles from Indonesian crude drugs. III. Toxic components from Brucea javanica (L.) MERR. Yakugaku Zasshi. 1990;110(11):834–838. doi: 10.1248/yakushi1947.110.11_834. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L. L., Sun R. The effect of extracting methods on ‘quality toxicity’ comprehensive evaluation model of Fructus Bruce . Chinese Journal of Pharmacovigilance. 2012;19(5):269–271. [Google Scholar]
- 20.Li H., Tan L. Isolation and identification of triterpene alcohols in seed oil of Brucea javanica . Journal of Beijing Normal University (Natural Science) 1995;31(2):230–233. [Google Scholar]
- 21.Han Z., Li K., Zhang X., Li Q., Wang P., Wang J. Antitumor and apoptosis induction effects of lupeol on U14 cervical carcinoma bearing mice. Latin American Journal of Pharmacy. 2012;31(1):10–17. [Google Scholar]
- 22.Takasaki M., Konoshima T., Tokuda H., et al. Anti-carcinogenic activity of Taraxacum plant. II. Biological and Pharmaceutical Bulletin. 1999;22(6):606–610. doi: 10.1248/bpb.22.606. [DOI] [PubMed] [Google Scholar]
- 23.Nan Z., Li Y. H., Wu X. K., Wang G. Y., Cai D. Y., Han F. J. Effect of Brucea javanica fruit oil emulsion combined cisplatin on the growth inhibition of transplanted tumor in human ovarian cancer SKOV3 nude mice: an experimental study. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(1):57–62. [PubMed] [Google Scholar]
- 24.Yan Z., Zhang B., Huang Y., Qiu H., Chen P., Guo G. F. Involvement of autophagy inhibition in Brucea javanica oil emulsion-induced colon cancer cell death. Oncology Letters. 2015;9(3):1425–1431. doi: 10.3892/ol.2015.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lou G.-G., Yao H.-P., Xie L.-P. Brucea javanica oil induces apoptosis in t24 bladder cancer cells via upregulation of caspase-3, caspase-9, and inhibition of nf-κb and cox-2 expressions. American Journal of Chinese Medicine. 2010;38(3):613–624. doi: 10.1142/s0192415x10008093. [DOI] [PubMed] [Google Scholar]
- 26.Xu X., Xu D.-H., Jiang B., Lv Q.-H. Effects of brucea javanica oil on expression of vascular endothelial growth factor in A549 cell line. Zhongguo Zhong Yao Za Zhi. 2008;33(21):2517–2520. [PubMed] [Google Scholar]
- 27.Tanaka K., Koga Y., Taniguchi K., Kamikaseda K., Nomoto K. T cell recruitment from the thymus to the spleen in tumor-bearing mice. I. Analysis of recruited cells by surface markers. Cancer Immunology Immunotherapy. 1986;22(1):37–42. doi: 10.1007/bf00205714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrio R., Lopez D. M. Insights into thymic involution in tumor-bearing mice. Immunologic Research. 2013;57(1–3):106–114. doi: 10.1007/s12026-013-8446-3. [DOI] [PubMed] [Google Scholar]
- 29.Carrio R., Torroella-Kouri M., Iragavarapu-Charyulu V., Lopez D. M. Tumor-induced thymic atrophy: alteration in interferons and Jak/Stats signaling pathways. International Journal of Oncology. 2011;38(2):547–553. doi: 10.3892/ijo.2010.870. [DOI] [PubMed] [Google Scholar]
- 30.Dong H., Zheng G., Teng A., et al. Mechanism of splenic excess augmentation in tumor-bearing mice. Cancer Research on Prevention and Treatment. 2012;39(8):940–943. [Google Scholar]
- 31.Chen M., Chen R., Wang S., et al. Chemical components, pharmacological properties, and nanoparticulate delivery systems of Brucea javanica . International Journal of Nanomedicine. 2012;8:85–92. doi: 10.2147/ijn.s31636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian S., Zhou Y. Study on anti-cancer’s function of acupoint application of Cure-cancer Plaster associated with acupuncture in H22 liver cancer model mice. Journal of Liaoning University of TCM. 2007;9(5):173–175. [Google Scholar]
- 33.Roos F., Ryan A. M., Chamow S. M., Bennett G. L., Schwall R. H. Induction of liver growth in normal mice by infusion of hepatocyte growth factor/scatter factor. The American Journal of Physiology—Gastrointestinal and Liver Physiology. 1995;268(2):G380–G386. doi: 10.1152/ajpgi.1995.268.2.G380. [DOI] [PubMed] [Google Scholar]
- 34.Rao Z., Zheng J., Huang W., et al. Immune function indicators and effect of cyclophosphamide on H22 solid tumor-bearing mice. Laboratory Animal Science. 2013;30(3):10–15. [Google Scholar]
- 35.Tokunaga E., Oki E., Egashira A., et al. Deregulation of the akt pathway in human cancer. Current Cancer Drug Targets. 2008;8(1):27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- 36.Noguchi M., Hirata N., Suizu F. The links between AKT and two intracellular proteolytic cascades: ubiquitination and autophagy. Biochimica et Biophysica Acta. 2014;1846(2):342–352. doi: 10.1016/j.bbcan.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q., Turner K. M., Alfred Yung W. K., Chen K., Zhang W. Role of AKT signaling in DNA repair and clinical response to cancer therapy. Neuro-Oncology. 2014;16(10):1313–1323. doi: 10.1093/neuonc/nou058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacquin M. A., Chiche J., Zunino B., et al. GAPDH binds to active Akt, leading to Bcl-xL increase and escape from caspase-independent cell death. Cell Death and Differentiation. 2013;20(8):1043–1054. doi: 10.1038/cdd.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba T., Kobayashi H., Kawasaki H., Mineki R., Naito H., Ohmori D. Glyceraldehyde-3-phosphate dehydrogenase interacts with phosphorylated Akt resulting from increased blood glucose in rat cardiac muscle. FEBS Letters. 2010;584(13):2796–2800. doi: 10.1016/j.febslet.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Huang Q., Lan F., Zheng Z., et al. Akt2 kinase suppresses glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-mediated apoptosis in ovarian cancer cells via phosphorylating gapdh at threonine 237 and decreasing its nuclear translocation. Journal of Biological Chemistry. 2011;286(49):42211–42220. doi: 10.1074/jbc.m111.296905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng L., Xiang G.-Y., Chen D. Role of TGF-β1 and its receptors in malignant progression of hepatocellular carcinoma. World Chinese Journal of Digestology. 2012;20(33):3231–3236. [Google Scholar]
- 42.Zhang L., Zhou F., ten Dijke P. Signaling interplay between transforming growth factor-β receptor and PI3K/AKT pathways in cancer. Trends in Biochemical Sciences. 2013;38(12):612–620. doi: 10.1016/j.tibs.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Nagamoto T., Eguchi G., Beebe D. C. Alpha-smooth muscle actin expression in cultured lens epithelial cells. Investigative Ophthalmology and Visual Science. 2000;41(5):1122–1129. [PubMed] [Google Scholar]
- 44.Tonino P., Abreu C. Microvessel density is associated with VEGF and α-SMA expression in different regions of human gastrointestinal carcinomas. Cancers. 2011;3(3):3405–3418. doi: 10.3390/cancers3033405. [DOI] [PMC free article] [PubMed] [Google Scholar]