Abstract
Proinflammatory cytokines impact islet β-cell mass and function by altering the transcriptional activity within pancreatic β-cells, producing increases in intracellular nitric oxide abundance and the synthesis and secretion of immunomodulatory proteins such as chemokines. Herein, we report that IL-1β, a major mediator of inflammatory responses associated with diabetes development, coordinately and reciprocally regulates chemokine and insulin secretion. We discovered that NF-κB controls the increase in chemokine transcription and secretion as well as the decrease in both insulin secretion and proliferation in response to IL-1β. Nitric oxide production, which is markedly elevated in pancreatic β-cells exposed to IL-1β, is a negative regulator of both glucose-stimulated insulin secretion and glucose-induced increases in intracellular calcium levels. By contrast, the IL-1β-mediated production of the chemokines CCL2 and CCL20 was not influenced by either nitric oxide levels or glucose concentration. Instead, the synthesis and secretion of CCL2 and CCL20 in response to IL-1β were dependent on NF-κB transcriptional activity. We conclude that IL-1β-induced transcriptional reprogramming via NF-κB reciprocally regulates chemokine and insulin secretion while also negatively regulating β-cell proliferation. These findings are consistent with NF-κB as a major regulatory node controlling inflammation-associated alterations in islet β-cell function and mass.
Keywords: chemokine, inflammation, insulin secretion, interleukin-1, nitric oxide
the progression to both type 1 (T1DM) and type 2 diabetes mellitus (T2DM) proceeds via immune cell-associated alterations in islet β-cell mass and function. Alterations in islet β-cell mass and function are two major determinants controlling the total amount of insulin produced and secreted in response to physiological stimuli (e.g., glucose). Proinflammatory cytokines such as IL-1β and IFNγ contribute significantly to losses in islet β-cell viability and insulin secretion. Islet β-cells exposed to IL-1β and IFNγ undergo extensive genetic reprogramming, which includes transcriptional increases in the inducible nitric oxide synthase (iNOS) gene (13, 26) and various genes encoding chemokines (8–10, 12, 44).
IL-1β induces the expression of the iNOS gene, promoting marked accumulation of iNOS protein, a phenotype potentiated by the addition of IFNγ (13, 16, 17, 26). The active iNOS enzyme produces nitric oxide, a free radical signaling molecule that impacts numerous cellular functions (5, 28, 46). In pancreatic β-cells, nitric oxide influences insulin secretion, DNA damage and repair, and overall cellular viability. In addition to controlling the abundance of iNOS, IL-1β also promotes increased production of a variety of chemokines (8, 44), which are soluble secreted factors that regulate immune cell recruitment and activation (25).
For example, CCL2 (a.k.a. MCP-1) is elevated in islets isolated from diabetic mice (8) and from human islets exposed to cytokines (21, 44). Transgenic mice with CCL2 production driven within pancreatic β-cells display enhanced recruitment of immune cells into the pancreatic islets, although disease outcome differs depending on genetic background (35, 36). The chemokine CCL20 (a.k.a. LARC/MIP-3α) is also elevated within islets from mouse, rat, and humans during inflammation (11, 14, 44). CCL20 and CCL2 recruit distinct populations of leukocytes via the use of specific receptors. CCL2 signals through CCR2 (present on monocytes and macrophages), whereas CCL20 is a ligand for the CCR6 receptor (42).
Interestingly, CCL2, CCL20, and iNOS are all bona fide target genes controlled by the NF-κB family of transcription factors (8, 11, 13). NF-κB subunits include p65 (RelA), RelB, c-Rel, p50 (NF-κB1), and p52 (NF-κB2). The NF-κB pathway is one of the major intracellular systems regulating inflammatory responses (2). Therefore, understanding the mechanisms underlying the IL-1β-mediated, NF-κB-regulated production of chemokines and other signaling molecules, such as nitric oxide, within pancreatic β-cells is essential for developing novel therapeutic strategies to prevent or reverse β-cell death and dysfunction. However, the precise mechanisms underlying the phenotypic changes in pancreatic β-cells in response to IL-1β and nitric oxide are not completely understood.
Toward this end, we have undertaken a systematic analysis of the timing of insulin secretion, nitric oxide accumulation, and chemokine production and release from pancreatic β-cells. We report herein that chemokine secretion increases, whereas insulin secretion decreases, in response to IL-1β. The NF-κB pathway is the central mediator of these outcomes. We further found that elevations in nitric oxide negatively regulate insulin secretion but have no effect on chemokine release. Moreover, the secretion of chemokines is not influenced by changes in glucose concentration but rather is controlled directly by NF-κB activity. We conclude that NF-κB is the central regulator of the reciprocal and coordinated changes in insulin and chemokine secretion in pancreatic β-cells during receipt of proinflammatory signals.
MATERIALS AND METHODS
Cell culture, islet isolation, adenoviral vectors, and reagents.
Culture of 832/13 and 833/15 rat insulinoma cells and isolation of islets from Wistar and Zucker diabetic fatty (ZDF) rats were carried out as described previously (17, 18). All islet isolation protocols were approved by the Duke University and the University of Tennessee institutional animal care and use committees. All cell lines were confirmed to be free of mycoplasma contamination. The generation and use of adenoviruses encoding β-galactosidase (27) and IκBαSR (32) have been reported. IL-1β was purchased from Thermo Fisher Scientific (Waltham, MA), IFNγ was purchased from Shenandoah Biotechnology (Warwick, PA), NG-monomethyl-l-arginine (l-NMMA) was from Cayman Chemical (Ann Arbor, MI), and dimethyl malate was from Sigma Aldrich (St. Louis, MO). The spin trap N-methyl-d-glucamine dithiocarbamate (MGD) was from ENZO Life Sciences (Farmingdale, NY).
Total RNA isolation, cDNA synthesis, and RT-PCR.
RNA isolation from cell lines and rat islets, cDNA synthesis, and analysis of real-time RT-PCR data has been described in detail previously (12). For all transcripts studied, the relative mRNA abundance was normalized to that of the housekeeping gene ribosomal S9. Primers used to detect transcript levels of ribosomal S9, iNOS, IL-1β, and Nkx6.1 were designed using Primer3Plus software and are available upon request.
Cellular fractionation and immunoblot analysis.
Cell lysis and extraction of cytoplasmic and nuclear protein fractions was performed using the NE-PER kit (Thermo Fisher Scientific) according to the manufacturer's directions. The protein concentration of the lysate was determined using the bicinchoninic acid assay (Thermo Fisher Scientific), using BSA as the standard. SDS-PAGE, transfer to polyvinylidene difluoride membranes, membrane blocking before antibody incubation, and downstream subsequent detection using a ChemiDoc imaging system (Bio-Rad) have been described previously (16). Antibodies detecting β-actin or tubulin were used as loading controls. Antibodies used were from the following sources: iNOS (Santa Cruz Biotechnology), β-actin (Cell Signaling Technology), IκBα (Santa Cruz Biotechnology), tubulin (Cell Signaling Technology), and Pdx-1 (Cell Signaling Technology). The antibody recognizing Nkx6.1 has been described (34). Immunoblot images were quantified using Image Lab software (Bio-Rad).
ELISA.
832/13 cells were grown in 12-well plates and treated as indicated. RPMI was supplemented with 0.3% BSA, and media were collected at the indicated time points. In addition, the cells were lysed using M-PER lysis reagent (Thermo Fisher Scientific) to quantify total intracellular protein content. CCL2 and CCL20 secretion into the media was measured by CCL2 Quantikine ELISA kit (R & D Systems, Minneapolis, MN) and CCL20 DuoSet ELISA kit (R & D Systems) according to the manufacturer's protocol. ELISA data were normalized to total protein to account for any differences in cell number between treatment groups.
Nitrite and MTS assay.
Nitrite in the cell culture media was measured as an index of NO production using the Griess assay kit (Promega, Madison, WI), whereas cellular reduction of the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) dye was determined using the MTS 1 solution kit (Promega) according to the manufacturer's protocol.
Electron paramagnetic resonance spectroscopy.
The spin trap N-methyl-d-glucamine dithiocarbamate-iron complex [(MGD)2-Fe2+] was prepared fresh in each experiment by making stock solutions of 500 mM MGD and 100 mM Fe2+ (from FeSO4.7 H2O) in ultrapure water under anaerobic conditions. A final concentration of 25 mM MGD and 5 mM Fe2+ was introduced onto cells in culture using serum-free media with a 30-min incubation period. Supernatants were then collected for the measurements of the (MGD)2-iron-NO complex. All electron paramagnetic resonance (EPR) measurements were carried out in a quartz flat cell at room temperature. Samples were transferred directly into the flat cell, which was then placed into the cavity of a Bruker EMX Plus spectroscope. Typical instrumental conditions were as follows: 20 mW of microwave power, 5.0-G modulation amplitude, 1 × 105 gain, 0.163-s time constant, and 80-G scan range. Quantitation was carried out by measuring and comparing the first peak heights on the spectra.
Insulin secretion and tritiated thymidine incorporation assays.
832/13 cells were grown in 12-well plates and treated as indicated. Glucose-stimulated insulin secretion (GSIS) assays were performed as described previously (29). Insulin secretion by cell lines into the media was measured using either the High Range Rat Insulin ELISA kit (Mercodia, Uppsala, Sweden) or by rat islets using the insulin radioimmunoassay (Siemens, Malvern, PA) according to the company directions. In addition, cells were lysed using M-PER lysis reagent (Thermo Fisher Scientific) to quantify total intracellular protein content via BCA assay (Thermo Fisher Scientific). Insulin secretion data were normalized to total protein to account for any differences in cell number between treatment groups. Incorporation of tritiated thymidine into DNA as a cellular index of proliferation was carried out as described (45).
Live cell calcium imaging.
832/13 cells were plated into six-well plates containing poly-d-lysine-coated coverslips (Neuvitro, Vancouver, WA). At 50% confluence, cells were treated for 18 h with one of four experimental groups: 1 ng/ml IL-1β alone, 1 mM l-NMMA alone, IL-1β + l-NMMA, or normal growth media. Immediately prior to imaging, cells were preloaded with Calcium Green 1 AM (CG, C3012; Life Technologies) by transferring coverslips to a solution of 10 μM CG in 20% Pluronic F127 in DMSO (Life Technologies) made up in carbogenated (95% O2-5%CO2) normal Krebs. The recipe for normal Krebs solution was (in mM) 124 NaCl, 25 NaHCO3, 2 glucose, 3 KCl, 2 CaCl2, 1.5 NaH2PO4, and 1 MgSO4-7H2O, which corrected to pH 7.4 with HCl. After incubation in the CG solution for 30 min, coverslips were transferred to carbogenated normal Krebs at 29°C.
Coverslips were transferred to a recording chamber on a fixed stage Zeiss Axioskop2 microscope equipped with a Perkin Elmer Ultraview/Yokogawa C10 spinning disk laser confocal illuminator. Cells were illuminated with the 488 nm laser; CG-calcium fluorescence signals were collected at 509 nm. Cells were visualized with a ×40 water immersion objective. Images were collected with a Hammamatsu Ocra-ER charge-coupled device camera. The confocal system, including image collection, was controlled by Perkin-Elmer Improvision software.
Cells on coverslips were constantly perfused with normal Krebs solution at 29°C at a rate of 3 ml/min. After ∼10 min, the perfusion solution was switched to one containing 12 mM glucose and proportionally less sodium chloride to maintain osmolality. The 12 mM glucose challenge was applied for 5 min, followed by a 5 min washout period. Images of CG-labeled cells were collected continuously during the challenge period at a rate of 20/min, with each frame taking 1 s. Images were collected as TIFF stacks and processed using Nikon Elements AR software. Individual cells in the field were designated as regions of interest (ROI), and their fluorescence signal over time was captured. Background fluorescence was subtracted from the fluorescence signal of individual ROIs. The relative changes in cytoplasmic calcium in the cells were expressed as changes in fluorescence [(ΔF/F)%], where F is the intensity of the baseline fluorescence signal before stimulation and ΔF is the difference between the peak fluorescence intensity and the baseline signal. Therefore, magnitude of response was normalized for each individual ROI.
Oxygen consumption assays.
Oxygen consumption rates (OCR) were measured using the XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Chicopee, MA). 832/13 cells were grown on cell culture plates for 2 days prior to OCR assays being run. Cells were treated with vehicle, 1 ng/ml IL-β, 1 mM l-NMMA, or both IL-1β (1 ng/ml) plus l-NMMA (1 mM) for 18 h prior to OCR being measured. The oxygen consumption rate was measured over a 2-h period. Data represent the calculated average area under the curve from six wells per treatment group over multiple independent experiments.
Mitochondrial/nuclear DNA ratio analysis.
Genomic DNA was isolated using a DNeasy kit from Qiagen (Valencia, CA) per the manufacturer's protocol and quantified via quantitative PCR using iTaq Universal SYBR Green Supermix (Bio-Rad). Mitochondrial number was determined as the expression of genes encoded by mitochondrial DNA (CO1 and ATP6) relative to nuclear genes (NDUFA and SDHA) using theΔΔCT method. This methodology has been described previously (24). Primer sequences are available upon request.
Electrophysiology.
Cell culture dishes were mounted on the stage of an Olympus IX70 inverted microscope. A reference Ag/AgCl pellet served to ground the bath. Patch electrodes were pulled from thick-walled borosilicate glass with a filament (outer diameter: 1.5 mm; inner diameter: 0.86 mm; Sutter Instruments, Novato, CA) using a Flaming-Brown Micropipette Puller (Sutter Instruments). Electrode resistance values ranged from 4 to 7 MΩ. Ruptured patch, whole cell voltage clamp recordings were made using an Axopatch 1D-patch clamp amplifier (Molecular Devices, Sunnyvale, CA). Data were recorded using pClamp 10.0 software (Molecular Devices). Unless otherwise indicated, all reagents were purchased from Sigma-Aldrich (St. Louis, MO). External solutions consisted of the following (in mM): 116.7 NaCl, 20.0 TEACl, 3.0 CaCl2, 0.4 MgCl2, 4.0 glucose, and 10.0 HEPES. Voltage clamp experiments were performed in the ruptured patch configuration using the following internal solution (in mM): 100.00 CsAc, 10.0 CsCl, 2.0 MgCl2, 0.1 CaCl2, and 10.0 HEPES. Also included was an ATP regeneration system to preserve Ca2+ currents [3.0 ATP (dipotassium), 1.0 ATP (disodium), 20.0 phosphocreatine, 2.0 GTP, and 50 U/ml creatine phosphokinase]. Cesium was used as a substitute for K+ to minimize currents through voltage-gated K+ channels. Tetrodotoxin (300 nM; Alomone Laboratories, Jerusalem, Israel) was included in the external solution to block voltage-gated Na+ currents. The liquid junction potential error for these solutions was estimated to be −13 mV (pClamp). Solutions were adjusted to pH 7.4 with NaOH for external solutions and with CsOH for internal solutions. A pressurized gravity flow perfusion system (1.5–2 ml/min) was used to deliver the external solutions (AutoMate Scientific, Berkeley, CA).
Statistical analysis.
All statistical analyses were performed using GraphPad (La Jolla, CA) Prism 6.0 using one-way ANOVA, followed by Tukey's post hoc correction. For the live cell calcium imaging, mean magnitude of responses for each experimental condition were determined and analyzed using one-way ANOVA with Dunnett's multiple comparison pos-test analysis. Significance values are given in the figure legends.
RESULTS
IL-1β reciprocally regulates chemokine and insulin secretion.
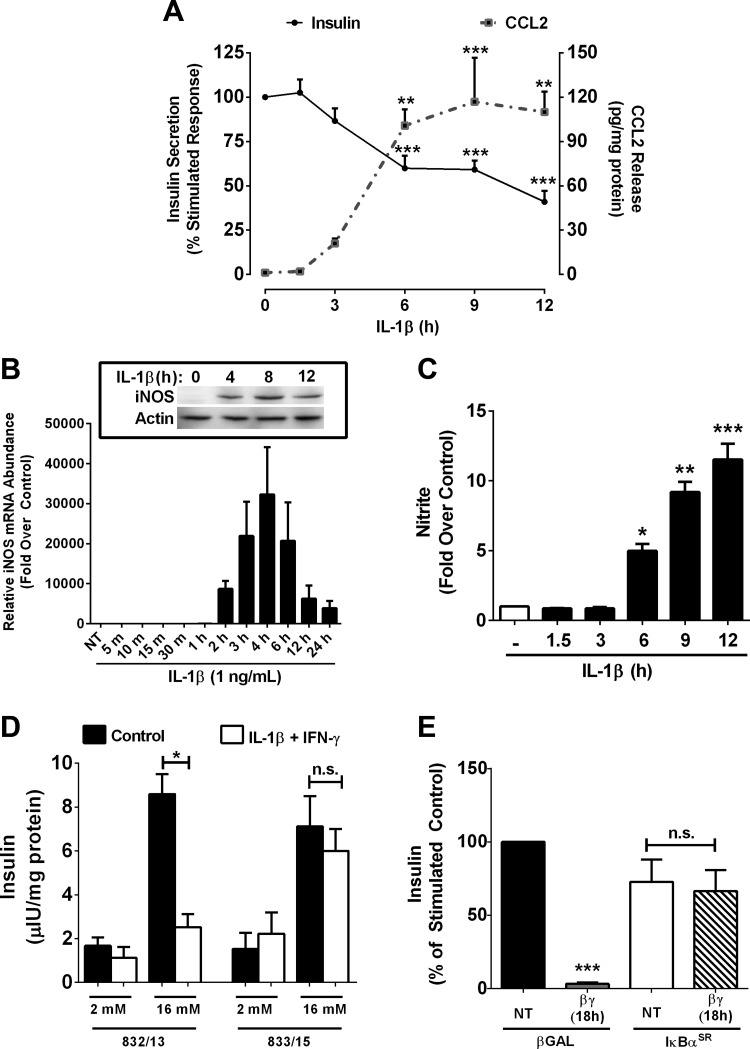
For decades, IL-1β has been associated with inhibition of GSIS (43). Indeed, we observed a steady decrease in GSIS over a time course of exposure to 1 ng/ml IL-1β, with a 40 and 59% decrease in GSIS by 6 and 12 h, respectively (Fig. 1A). In contrast to GSIS, the expression and secretion of the chemokine CCL2 are markedly elevated in response to IL-1β (Fig. 1A and data not shown). These data demonstrate clearly the reciprocal regulation of chemokine and insulin secretion by IL-1β (Fig. 1A).
Fig. 1.
IL-1β reciprocally regulates chemokine and insulin secretion. A: 832/13 cells were stimulated with 1 ng/ml IL-1β for the indicated times. Secretion of insulin (solid line) or CCL2 (dashed line) into the culture media was quantified by ELISA. B: 832/13 cells were stimulated with 1 ng/ml IL-1β for the indicated times. Relative mRNA abundance of inducible nitric oxide synthase (iNOS) was quantified by RT-PCR, with protein abundance determined by immunoblotting (inset). C: 832/13 were treated with 1 ng/ml IL-1β for the indicated times, and nitrite accumulation in the culture media was assessed using the Griess assay. D: 832/13 and 833/15 insulinoma cells were either untreated (control) or stimulated with both 1 ng/ml IL-1β and 100 U/ml IFNγ for 18 h. Insulin secretion was measured in response to basal (2 mM) or stimulatory (16 mM) glucose concentrations. E: isolated rat islets were treated with AdCMV-β-galactosidase (β-Gal) or AdCMV-IκBα super-repressor (IκBαSR) in the presence or absence of 1 ng/ml IL-1β and 100 U/ml IFNγ. Insulin secretion during static incubation was measured and represented as percent of the stimulated control. ***P < 0.001 vs. untreated (NT; black bars). Data are presented as means ± SE of 3 independent experiments. **P < 0.01; *P < 0.05. NS, not significant.
Because elevations in nitric oxide have been linked with decreases in GSIS (19, 20), we investigated the timing of iNOS mRNA accumulation in response to IL-1β. iNOS expression is undetectable at both the transcript and protein level in the absence of IL-1β but is rapidly induced upon cellular exposure to IL-1β (Fig. 1B). The marked elevation in transcript abundance (Fig. 1B) is congruent with the increase in iNOS protein (Fig. 1B, inset) and nitrite accumulation (Fig. 1C), an index of nitric oxide production. Thus, the accumulation of nitrite correlates with the decrease in GSIS (compare Fig. 1, A and C). We conclude that IL-1β reciprocally regulates insulin and chemokine secretion in pancreatic β-cells.
NF-κB is required for cytokine-mediated decreases in insulin secretion and cellular production of nitric oxide.
The NF-κB pathway is strongly activated by IL-1β in pancreatic β-cells (6, 16), and the proinflammatory cytokines IL-1β and IFNγ impair β-cell function and viability through activation of specific signaling pathways such as NF-κB and STAT1 (16, 17, 26). The clonal β-cell line 833/15 is resistant to losses in viability upon exposure to IL-1β and IFNγ (17, 47). Using clonal β-cell lines, which are either sensitive (832/13) or resistant (833/15) to killing by IL-1β + IFNγ, we tested whether or not insulin secretion was preserved in the cytokine-resistant 833/15 cells. Under normal conditions (i.e., no cytokines present), GSIS was similar between 832/13 and 833/15 cells (Fig. 1D, black bars). However, when cultured overnight in the presence of IL-1β and IFNγ, only 833/15 cells retained the ability to secrete insulin in response to a glucose stimulus (Fig. 1D, open bars).
An inherent defect in NF-κB-mediated gene activation in the 833/15 cell line was reported (15), which we interpreted as the reason for their protection against cytokine-mediated impairments in GSIS. To directly test this possibility, we used adenoviral delivery of a mutated form of IκBα that contains S32A/S36A substitutions, termed the IκBα super-repressor (IκBαSR). The IκBαSR retains the p65 subunit of NF-κB in the cytosol and thus decreases NF-κB transcriptional activity (11, 16). Culturing rat islets in IL-1β and IFNγ overnight completely suppressed GSIS; this loss in function was reversed in islets transduced with the IκBαSR, but not in islets receiving a control virus expressing β-galactosidase (Fig. 1E).
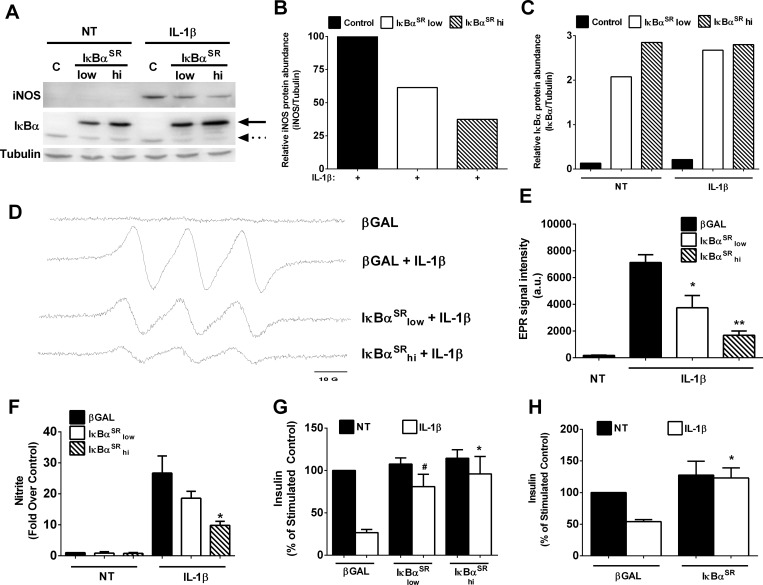
To investigate the contribution of NF-κB to nitric oxide production after cellular exposure to IL-1β, we first analyzed iNOS protein abundance in the presence and absence of the IκBαSR. Again, iNOS protein is undetectable in the absence of a cytokine stimulus but accumulates upon cellular exposure to IL-1β (Fig. 2A). Inhibiting NF-κB transcriptional activity, using adenoviral transduction of 832/13 cells with IκBαSR, decreases iNOS protein abundance by 73% at the highest viral dose (Fig. 2A; quantified in Fig. 2, B and C).
Fig. 2.
NF-κB is required for cytokine-mediated decreases in insulin secretion and cellular production of nitric oxide. A–F: 832/13 cells were transduced with adenoviruses expressing either AdCMV-β-Gal [control (C)] or increasing doses of AdCMV-IκBαSR (low and high). Eight hours after adenoviral transduction, cells were then NT or stimulated for an additional 18 h with 1 ng/ml IL-1β. A: immunoblot analysis of whole cell lysates probed with antibodies against iNOS, IκBα, and tubulin. Solid arrow, adenovirally overexpressed IκBα; dashed arrow, endogenous IκBα. B and C: densitometric analysis of iNOS (B) and IκBα (C) protein abundance from the immunoblot shown in A. D: nitric oxide determination by electron paramagnetic resonance (EPR) spectroscopy combined with N-methyl-d-glucamine dithiocarbamate (MGD)2Fe2+ spin trap. E: quantification of the EPR spectroscopy data shown in D. F: nitrite accumulation in the media was measured by Griess assay. G and H: 832/13 cells were cultured with the indicated adenoviruses for 8 h, followed by exposure to 1 ng/ml IL-1β for either 6 (G) or 18 h (H). Insulin secretion was measured in response to basal (2 mM) and stimulatory (16 mM) glucose concentrations, with data shown as %stimulated control. The high dose of AdCMV-IκBαSR was used in H. *P < 0.05 vs. β-Gal-IL-1β; **P < 0.01 vs. β-Gal-IL-1β; #P < 0.1 vs. β-Gal-IL-1β. Data represent means ± SE of 3 independent experiments.
Using EPR spectroscopy in conjunction with the spin trap (MGD)2Fe2+, we observed robust production of nitric oxide in response to IL-1β (spectra shown in Fig. 2D). This technique specifically measures nitric oxide radicals. Reducing iNOS expression with the IκBαSR diminished nitric oxide production in a manner consistent with the decrease in iNOS protein (Fig. 2, A and D). Quantification of the signal intensity from multiple EPR experiments is shown in Fig. 2E. The Griess assay, which measures total nitrite accrual in the media from both organic and inorganic sources, also revealed decreased nitrite production when iNOS protein levels were reduced by blocking NF-κB activity (Fig. 2F). This diminution in nitric oxide levels correlated directly with the complete recovery of IL-1β-mediated impairments in GSIS in 832/13 clonal β-cells cultured for 6 (Fig. 2G) and 18 h (Fig. 2H) with IL-1β. We interpreted these data to indicate that transcriptional activation of the iNOS gene by NF-κB controls iNOS enzyme accumulation and subsequent nitric oxide production, which serves as a negative regulator of insulin secretion.
NF-κB is required for IL-1β-mediated decreases in cellular proliferation.
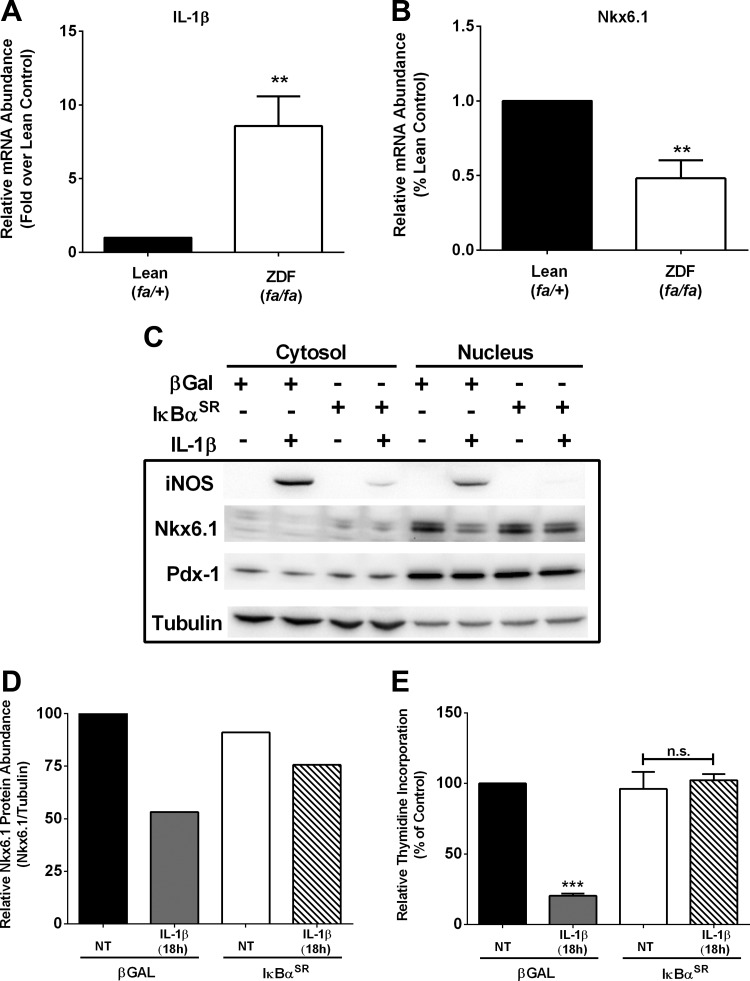
A reduction in islet β-cell mass and function is a hallmark of both T1DM and T2DM. The Zucker diabetic fatty (ZDF) rat is a model of obesity and diabetes resulting from reduced functional β-cell mass (4). Using isolated islets from 8-wk-old ZDF rats, we found that IL-1β expression was elevated 8.6-fold (Fig. 3A). Nkx6.1 expression was reduced by 52% in islets isolated from ZDF rats relative to their lean littermate controls (Fig. 3B). Because the transcription factor Nkx6.1 regulates β-cell proliferation (45), we next examined its abundance under conditions of IL-1β exposure in the presence or absence of the IκBαSR. 832/13 cells exposed to IL-1β for 4 h displayed robust iNOS abundance, which was blocked by the IκBαSR (Fig. 3C). Nkx6.1 was largely nuclear, and its abundance was decreased by 47% after IL-1β exposure (Fig. 3, C and D). Moreover, the ability to incorporate thymidine into DNA, an index of proliferation, was reduced markedly in 832/13 β-cells exposed to IL-1β for 18 h but was restored in cells expressing the IκBαSR (Fig. 3D). Thus, blocking NF-κB activity with the IκBαSR restored Nkx6.1 abundance (Fig. 3C), which was consistent with the recovery in cellular thymidine incorporation (Fig. 3E). The amount of Pdx-1 protein was unchanged under these conditions. These observations are consistent with IL-1β-mediated decreases in proliferation being controlled by NF-κB activation.
Fig. 3.
NF-κB is required for IL-1β-mediated decreases in cellular proliferation. A and B: islets were isolated from 8-wk-old lean (fa/+) and obese (fa/fa) Zucker diabetic fatty (ZDF) rats (n = 8 rats/group). RT-PCR measurement of IL-1β (A) and Nkx6.1 (B) is shown. C: 832/13 cells were transduced with AdCMV-β-Gal or the high dose of AdCMV-IκBαSR. Following an overnight incubation with the indicated adenoviruses, cells were either left untreated or exposed to 1 ng/ml IL-1β for 4 h. Cytosolic and nuclear fractions were immunoblotted using antibodies directed against iNOS, Nkx6.1, Pdx-1, and tubulin. D: densitometric analysis of Nkx6.1 protein abundance normalized to the abundance of tubulin. E: 832/13 cells were transduced with AdCMV-β-Gal or the high dose of AdCMV-IκBαSR for 8 h, followed by 18 h of exposure to 1 ng/ml IL-1β. Tritiated thymidine incorporation into DNA was measured at the end of the 18-h cytokine exposure. Data represent means ± SE of a minimum of 3 independent experiments, whereas the immunoblot represents 2 independent experiments. **P < 0.01 vs. lean (fa/+) control; ***P < 0.001 vs. β-Gal-NT.
IL-1β generates elevations in nitric oxide production and decreases in oxygen consumption.
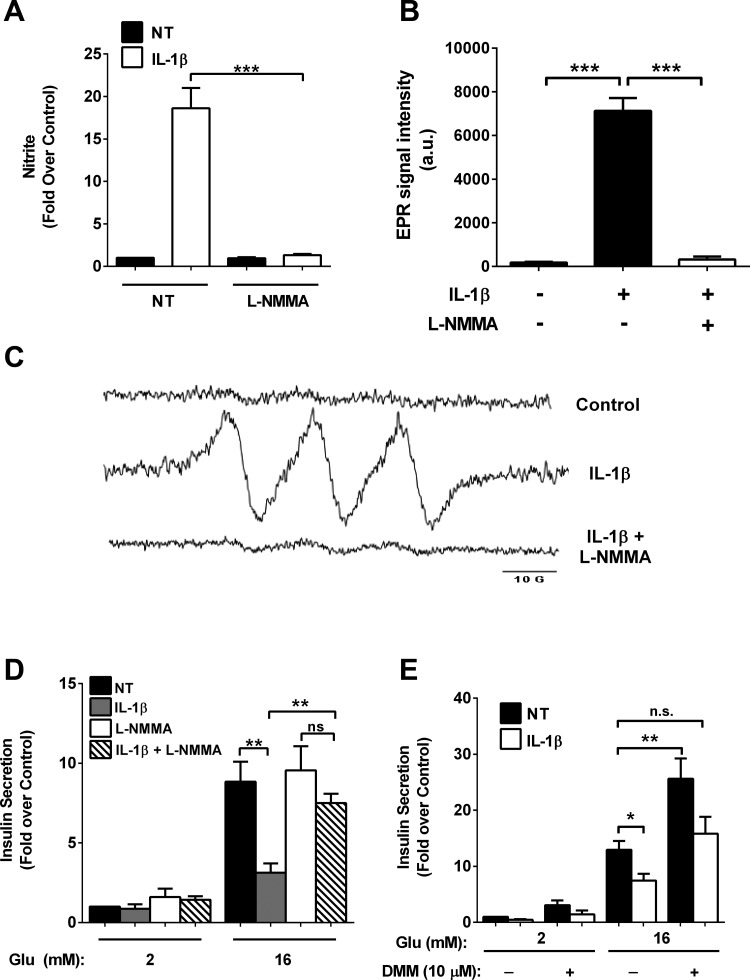
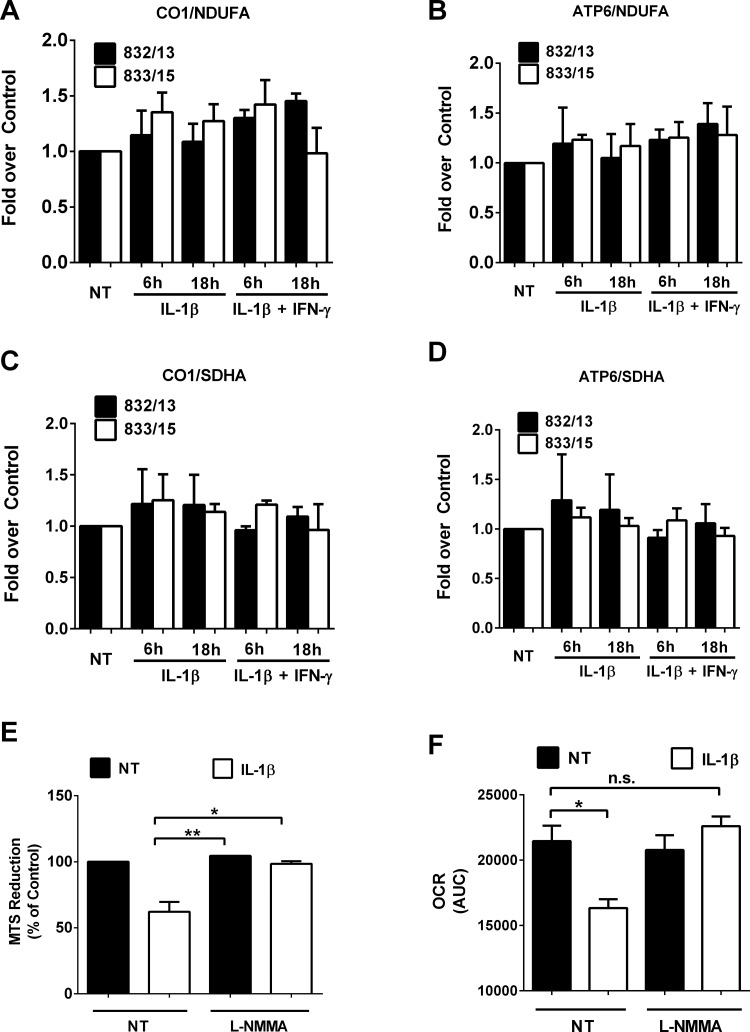
IL-1β promotes robust accumulation of the iNOS protein (Fig. 1 and Ref. 26) and nitrite accumulation, which impairs insulin secretion; this effect can be prevented by blocking NF-κB transcriptional activity (Figs. 1 and 2). Therefore, we used l-NMMA, an arginine analog that inhibits all known nitric oxide synthase isoforms (39), and found that the increase in total nitrite production by cellular exposure to IL-1β was completely inhibited (Fig. 4A). The EPR (MGD)2 Fe2+ spin trap methodology also revealed a lack of IL-1β-mediated elevations in nitric oxide formation when l-NMMA was present (Fig. 4B; representative spectra shown in Fig. 4C). Therefore, we next addressed whether l-NMMA prevented the suppression of insulin secretion upon exposure to IL-1β. We observed an almost complete recovery of insulin secretion in the presence of IL-1β + l-NMMA (Fig. 4D). In addition, insulin secretion could be recovered by dimethyl malate (Fig. 4E), a malate analog, indicating that mitochondrial metabolism was still active in the presence of IL-1β.
Fig. 4.
IL-1β generates elevations in nitric oxide production and decreases in oxygen consumption. A–D: 832/13 cells were cotreated with 1 ng/ml IL-1β and 1 mM NG-monomethyl-l-arginine (l-NMMA) for 18 h. A: nitrite was measured in the culture media using the Griess method. B and C: nitric oxide was measured by EPR spectroscopy combined with (MGD)2Fe2+ spin trapping. D: insulin secretion was measured in response to basal (2 mM) or stimulatory (16 mM) glucose concentrations. E: 832/13 cells were NT or exposed to 1 ng/ml IL-1β for 18 h. Glucose-stimulated insulin secretion was measured in response to basal (2 mM) or stimulatory (16 mM) glucose concentrations in the presence or absence of 10 μM dimethylmalate (DMM), with DMM added only during the final 2 h of the secretion experiment. Data are presented as means ± SE of 3 independent experiments. ***P < 0.001; **P < 0.01; *P < 0.05.
To address whether mitochondrial number or mitochondrial function is altered by IL-1β, we first measured the mitochondrial/nuclear DNA ratios, which have been used to track changes in mitochondrial biogenesis (24). We did not detect any alterations in mitochondrial/nuclear DNA ratios (Fig. 5, A–D), which we interpreted as evidence that mitochondrial number was not being altered in response to either IL-1β or the combination of IL-1β + IFNγ.
Fig. 5.
Nitric oxide decreases oxygen consumption rate without affecting mitochondrial number. A–D: 832/13 and 833/15 cells were NT or treated with 1 ng/ml IL-1β alone or IL-1β + 100 U/mL IFNγ for either 6 or 18 h. Mitochondrial number was determined using the ratio of mitochondrial DNA (CO1 or ATP6) relative to nuclear DNA (NDUFA or SDHA). E and F: 832/13 cells were exposed to 1 ng/ml IL-1β in the presence or absence of 1 mM l-NMMA for 18 h. Cellular ability to reduce the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] dye (E) and area under the curve (AUC) analysis of oxygen consumption rate (OCR; F) are shown. Values represent means ± SE of 3 independent experiments. **P < 0.01; *P < 0.05.
Therefore, we next investigated whether mitochondrial function was reduced. We found a 38% decrease in reduction of the MTS dye after IL-1β exposure (Fig. 5E). This decrease was fully recovered in the presence of the NOS inhibitor l-NMMA (Fig. 5E). In addition, a 25% decrease in cellular oxygen consumption rate was identified, which was also prevented by the addition of 1 mM l-NMMA (Fig. 5F). We conclude that enhanced cellular abundance of nitric oxide diminished mitochondrial activity but did not alter total mitochondrial number.
Nitric oxide mediates the IL-1β-induced impairment in glucose-stimulated calcium elevations.
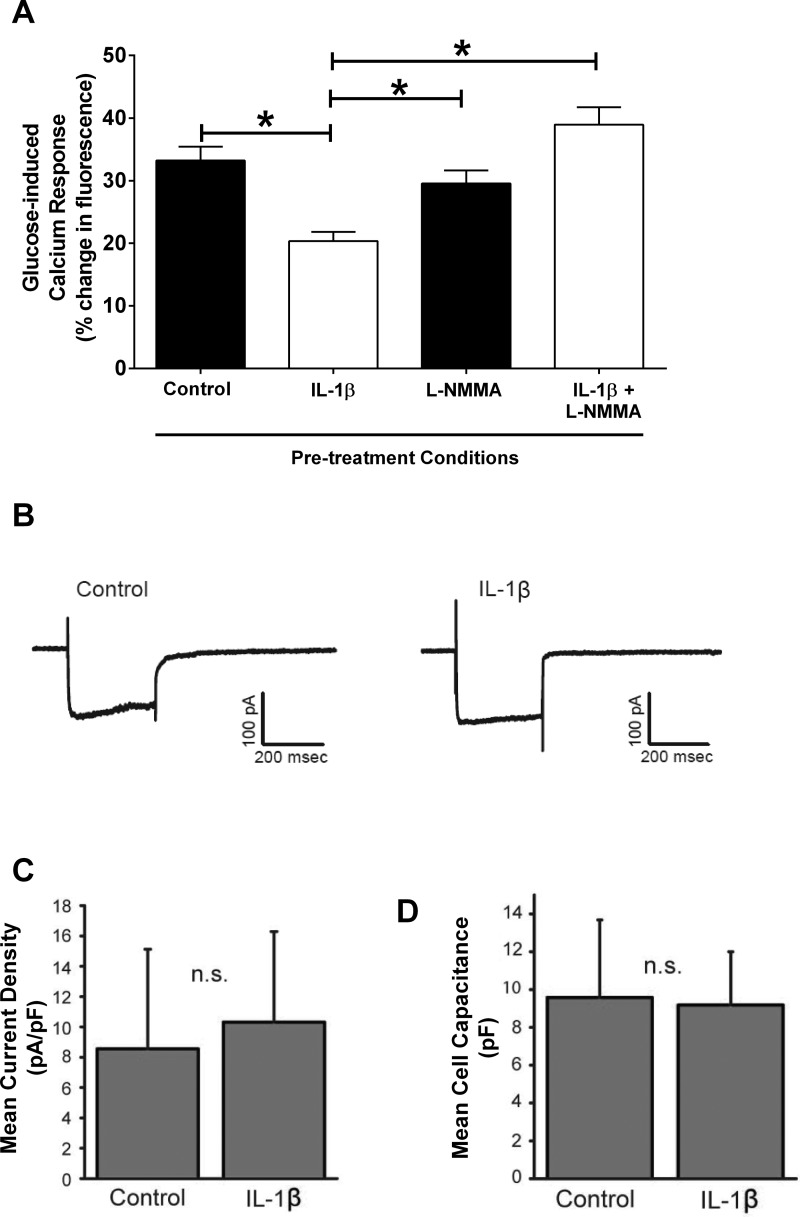
Nitric oxide suppresses insulin secretion in mouse, rat, and human islets (5). Using 832/13 rat clonal β-cells, which display similar impairments in insulin secretion, as observed in isolated islets, we examined changes in cytoplasmic calcium using the reporter dye calcium green in conjunction with confocal microscopy. In control pretreated cells, ∼97% of the cells responded to the glucose challenge with an averaged response magnitude of 33% change in fluorescence levels. In cells pretreated with IL-1β alone, only 61% of the cells responded to the glucose challenge at an average response magnitude of 20%. However, the pretreatment of cells with l-NMMA plus IL-1β rescued both the number of responsive cells (89%) and the magnitude of the response (30%) of these cells to the glucose challenge. Cells pretreated with l-NMMA alone differed neither in the number of responders nor in the magnitude of the responses to the glucose challenge relative to control pretreatment. Thus, the responses of cells pretreated with IL-1β alone were reduced by 39% relative to the responses of the control group (Fig. 6A). This reduced change in intracellular calcium observed with IL-1β was completely prevented by 1 mM l-NMMA (Fig. 6A), indicating a requirement for nitric oxide production to alter the glucose-induced increase in cytoplasmic calcium levels.
Fig. 6.
Nitric oxide mediates the IL-1β-mediated impairment in glucose-stimulated calcium responses. A: 832/13 cells were cotreated for 18 h with 1 ng/ml IL-1β and 1 mM l-NMMA, followed by a bath-applied glucose challenge to evoke elevations in intracellular calcium levels as measured by changes in fluorescence. *P < 0.05. B: whole cell voltage clamp recordings from a control and an IL-1β-treated cell. Cells were held at −70 mV and then stepped for 300 ms to a series of voltages ranging from −103 mV to +17 mV. Shown are the currents generated by the step to −3 mV. Voltage step values are corrected for the liquid junction potential (−13 mV). C: mean current densities (voltage step to −3 mV) were plotted for control and IL-1β-treated cells. No significant difference in current amplitude was observed (n = 25 for both). D: capacitance values for the 2 groups of cells indicate that cell size for IL-1β-treated cells was also not significantly different from control (n = 25 for both).
To test for a decrease in the activity of voltage-gated Ca2+ channels, whole cell voltage clamp recordings were made from individual 832/13 clonal β-cells (Fig. 6B). Voltage-gated Ca2+ current density was not significantly different in control or IL-1β-treated cells (Fig. 6C), and IL-1β pretreatment also had no effect on cell size as indicated by whole cell capacitance measurements (Fig. 6D). We conclude that nitric oxide accumulation most likely decreases calcium release from internal stores and not by reducing the activity of voltage-gated calcium channels.
NF-κB, but not glucose or nitric oxide concentration, controls production of the chemokines CCL2 and CCL20.
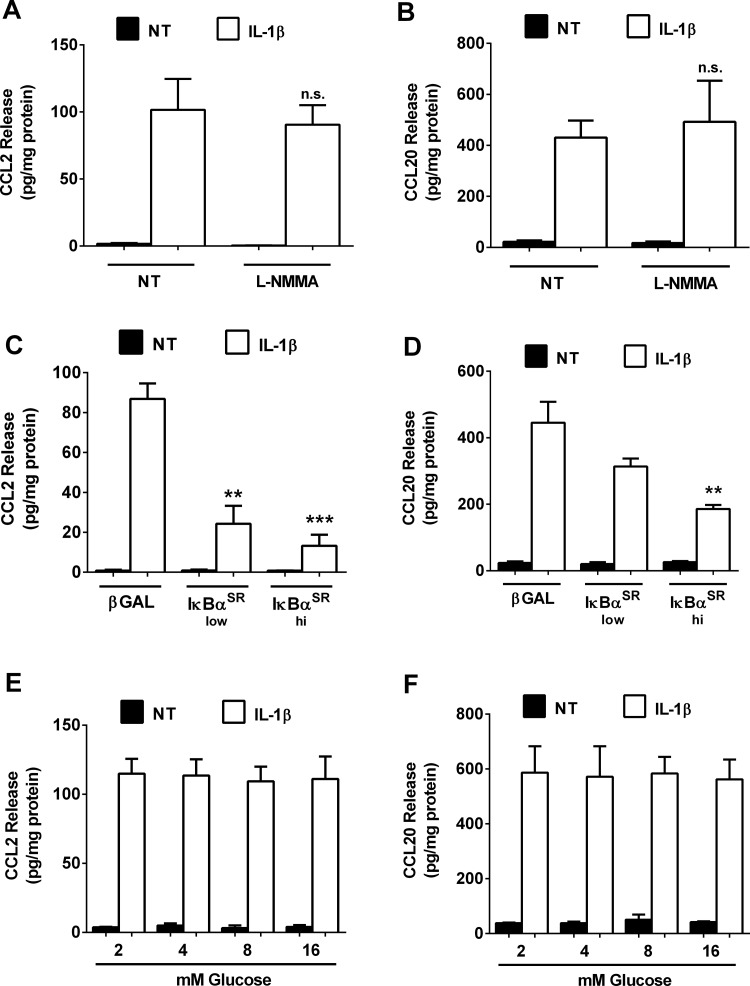
Because nitric oxide is a signaling molecule whose elevated abundance reduces insulin secretion, we next investigated whether chemokine secretion, a process also driven by IL-1β, was altered by changes in either glucose or nitric oxide concentration. IL-1β strongly induced the synthesis and secretion of CCL2 in pancreatic β-cells, which are not altered by the addition of 1 mM l-NMMA (Fig. 7A). Similarly, CCL20 secretion is not impacted by suppressing nitric oxide production using l-NMMA (Fig. 7B). By contrast, inhibiting NF-κB transcriptional activity via adenoviral delivery of the IκBαSR dose dependently decreases the expression of the CCL2 gene (Ref. 10 and data not shown) and release of CCL2 protein (Fig. 7C). Similar results were observed for CCL20 release (Fig. 7D). Finally, we also investigated whether chemokine secretion was dependent on the glucose concentration. Although glucose concentration is the major determinant controlling insulin secretion (37), the IL-1β-induced secretion of both CCL2 and CCL20 is not different over a range of glucose concentrations (Fig. 7, E and F).
Fig. 7.
NF-κB, but not glucose or nitric oxide concentration, controls production of the chemokines CCL2 and CCL20 in response to IL-1β. A and B: 832/13 cells were cotreated for 6 h with 1 ng/ml IL-1β and 1 mM l-NMMA. C and D: 832/13 cells were transduced with adenoviruses overexpressing either AdCMV-β-Gal or increasing doses of AdCMV-IκBαSR (low and high). Twelve hours after adenoviral transduction, cells were then NT or exposed to 1 ng/ml IL-1β for an additional 6 h. E and F: 832/13 cells were NT or exposed to 1 ng/ml IL-1β for 6 h in the presence of increasing concentrations of glucose (2, 4, 8, or 16 mM). Release of CCL2 (A, C, and E) and CCL20 (B, D, and F) into the culture media was measured by ELISA assays. Data represent means ± SE of a minimum of 3 independent experiments. ***P < 0.001 vs. β-Gal-IL-1β; **P < 0.01 vs. β-Gal-IL-1β.
DISCUSSION
A diminution in the function and mass of pancreatic islet β-cells is a hallmark of both major forms of diabetes (1, 7). Although extended exposure of islet β-cells to proinflammatory cytokines decreases viability, less is known about the early changes that occur in response to the initial transcriptional reprogramming events. Therefore, we undertook the present study to investigate the acute functional responses of β-cells to the proinflammatory cytokine IL-1β. Several novel observations emerged. 1) Chemokine secretion occured rapidly (within 3–6 h) after cellular exposure to IL-1β; 2) insulin secretion began to decrease by 6 h, concomitant with elevations in nitric oxide production and reduction in the abundance of Nkx6.1. 3) the decline in proliferation after exposure to IL-1β was consistent with decreases in Nkx6.1 abundance; 4) all of these processes were reversed by blocking NF-κB transcriptional activity; and 5) chemokine release was not dependent on glucose concentration or nitric oxide accumulation, whereas elevations in cellular nitric oxide levels negatively regulated insulin secretion.
The 5.5-fold increase in nitrite accrual (Fig. 1B) at 6 h is connected with a 36% decrease in GSIS, whereas a 13-fold increase in nitrite is associated with a 54% diminution in GSIS (Fig. 1). Because measurements of total nitrite production by the Griess method account for both organic and inorganic nitrite-containing compounds, we used the MGD spin trap coupled with EPR spectroscopy to unequivocally measure nitric oxide production. Accrual of nitric oxide was inversely correlated with insulin secretion, implicating this free radical signaling molecule as a negative regulator of insulin release in β-cells. However, the mitochondria are still functional in β-cells exposed to IL-1β, as evidenced by enhanced insulin secretion in the presence of dimethyl malate (DMM; a malate analog). Because mitochondrial substrate metabolism is tightly linked with insulin secretion (37), the ability of DMM to rescue insulin secretion is consistent with nitric oxide functioning as an intracellular signaling intermediary to decrease insulin secretion after exposure to IL-1β.
DMM also improves insulin secretion in islets isolated from ZDF rats and clonal β-cells cultured in lipids (4). We have shown herein that ZDF islets display elevated levels of IL-1β mRNA relative to lean control islets (Fig. 3). This finding is consistent with IL-1β serving as a common link to alterations in islet function and mass in both T1DM and T2DM. Furthermore, if nitric oxide is removed from cells within the first 24 h of IL-1β exposure, mitochondrial metabolism and insulin secretion are restored (5). In this study, we found that voltage-gated calcium channel activity is not different between control and IL-1β-exposed cells, but the ability to increase intracellular calcium in response to a glucose challenge is diminished (Fig. 6). Thus, nitric oxide accumulation most likely prevents the release of calcium from internal storage depots, which is consistent with reduced insulin release observed in our studies and complementary to findings from previous studies (38). Collectively, we interpret these data to indicate that the mitochondria are responsive to both nitric oxide and metabolic signals and that an initial increase in intracellular nitric oxide production acts as a rheostat to rapidly and reversibly diminish insulin secretion in response to a specific inflammatory signal (e.g., IL-1β) via multiple mechanisms.
In contrast to insulin secretion, the synthesis and release of chemokines in response to IL-1β is completely independent of either the glucose concentration or nitric oxide accumulation. We discovered that the major control point for both IL-1β-mediated reduction in insulin secretion and enhancements in chemokine production is NF-κB activation. Indeed, several chemokines are primary response genes in pancreatic β-cells exposed to IL-1β (Refs. 8 and 12 and data not shown), which may be explained at least in part by the exquisite sensitivity of the pancreatic β-cell to IL-1 receptor (IL-1R) activation. The marked sensitivity of β-cells to IL-1β is due largely to the high level of IL-1R expression (3). Thus, at least one of the major consequences of islet β-cell signaling via IL-1R ligands (e.g., IL-1β) is the rapid and reciprocal regulation of chemokine and insulin secretion. Consequently, the islet β-cell may play a much more important role in regulating immune system function than has been recognized previously. Along these lines, insulin has documented anti-inflammatory properties (30, 31). Therefore, β-cell insulin release may have a greater impact on innate and adaptive immunity than realized previously, which would fit with a need to coordinately downregulate insulin secretion during periods of inflammation. Because chemokines control both immune cell recruitment as well as immune cell activity, changes in the islet β-cell ratio of insulin to chemokine secretion could have important consequences for both local and systemic inflammatory responses as well as for metabolic homeostasis.
The progression to both T1DM and T2DM requires immune cell-associated alterations in islet β-cell mass and function. With many discrete chemokines made in response to IL-1β and IFNγ in pancreatic β-cells (8, 44), the β-cell influence on tissue leukocytosis and overall pancreatic inflammatory responses may be underappreciated. It also appears that NF-κB is the major regulatory factor controlling chemokine production in response to IL-1β (9, 10, 12), whereas STAT1 is the predominant transcription factor mediating the increased chemokine production by IFNγ (9, 12). Furthermore, NF-κB is the key determinant of the chemokine/insulin secretion ratio, suggesting that modulation of NF-κB would be beneficial for improving islet function by suppressing inflammatory responses. The results of a variety of in vitro and in vivo studies support this rationale (21–23, 33, 40, 41), and a recent computational model predicted elevated IL-1β signaling as necessary and sufficient to promote progression to T2DM (48).
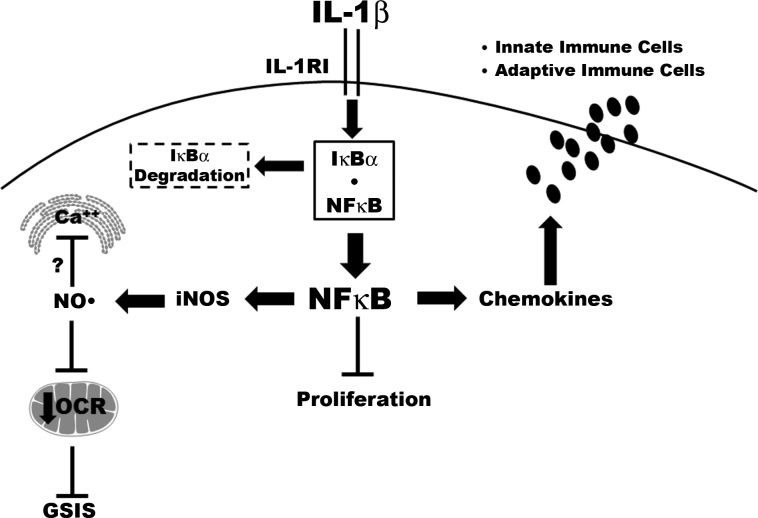
In summary, we have shown herein that IL-1β signaling via NF-κB controls both insulin and chemokine secretion and also mediates decreases in β-cell proliferation (Fig. 8). Thus, the transcriptional reprogramming of β-cells, such as that which occurs after exposure to IL-1β, is a major biochemical and molecular process controlling both islet β-cell insulin secretion and pancreatic leukocyte infiltration via chemokine release. Understanding the molecular mechanisms underlying these signal-induced genetic events should provide novel insights into therapeutic possibilities to prevent loss in β-cell function and mass.
Fig. 8.
NF-κB coordinately regulates chemokine and insulin secretion, with concomitant alterations in proliferation during exposure to IL-1β. IL-1β signals through the IL-1 receptor I (IL-1RI), which promotes the phosphorylation-induced degradation of the IκBα regulatory protein. Degradation of IκBα allows the p65 subunit of NF-κB to move into the nucleus. NF-κB controls the expression of multiple target genes, including iNOS and numerous chemokines. The coordinated changes in gene transcription include increased expression of the iNOS gene, which leads to enhanced production of nitric oxide (NO·). NO· serves as a negative regulator of glucose-stimulated insulin secretion (GSIS). Intracellular calcium is stored in both endoplasmic reticulum (ER) and mitochondria, and the signal-specific release of calcium (e.g., in response to glucose) is potentially inhibited by elevations in intracellular nitric oxide, contributing to diminished insulin secretion. The decrease in cellular OCR is also consistent with the depletion of ATP observed in β-cells exposed to cytokines (17). In addition, IL-1β decreases proliferation in an NF-κB-dependent manner, with concomitant upregulation of chemokine synthesis and secretion. Increasing the amount of chemokines released from β-cells influences both innate and adaptive immune responses. Unlike insulin secretion, β-cell chemokine secretion is independent of cellular nitric oxide levels.
GRANTS
This study used the Pennington Biomedical Research Center Genomics core facilities, which are supported in part by Center of Biomedical Research Excellence (P20-GM-103528) and Nutrition Obesity Research Center (P30-DK-072476) grants from the National Institutes of Health (NIH). This study was also partially supported by NIH Grants DK-52142 and NS-60664 (to R. C. Rogers and G. E. Hermann) and National Institute of General Medical Sciences Grant P20-GM-103528 (to J. J. Collier). Research from Dr. Stadler's laboratory was supported by a DiaComp Pilot and Feasibility Grant (14GHSU1393; Georgia Regents University/NIH) and through the Pennington Foundation. Studies from Dr. Gleason's laboratory were supported by a grant from the National Science Foundation (1256782).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.B. and J.J.C. conception and design of research; S.J.B., K.S., D.L., E.L.G., A.H., D.R.D., R.R., G.E.H., M.D.K., and J.J.C. performed experiments; S.J.B., K.S., D.L., E.L.G., A.H., D.R.D., R.R., G.E.H., M.D.K., and J.J.C. analyzed data; S.J.B., K.S., D.L., E.L.G., D.R.D., R.R., G.E.H., M.D.K., and J.J.C. interpreted results of experiments; S.J.B., K.S., D.L., E.L.G., A.H., D.R.D., R.R., G.E.H., M.D.K., and J.J.C. prepared figures; S.J.B. and J.J.C. drafted manuscript; S.J.B., K.S., D.L., E.L.G., A.H., D.R.D., R.R., G.E.H., M.D.K., and J.J.C. edited and revised manuscript; S.J.B., K.S., D.L., E.L.G., A.H., D.R.D., R.R., G.E.H., M.D.K., and J.J.C. approved final version of manuscript.
REFERENCES
- 1.Ashcroft FM, Rorsman P. Diabetes mellitus and the beta cell: the last ten years. Cell 148: 1160–1171, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab 13: 11–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC, Donath MY. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150: 5218–5229, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Boucher A, Lu D, Burgess SC, Telemaque-Potts S, Jensen MV, Mulder H, Wang MY, Unger RH, Sherry AD, Newgard CB. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem 279: 27263–27271, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Broniowska KA, Oleson BJ, Corbett JA. β-Cell responses to nitric oxide. Vitam Horm 95: 299–322, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Burke SJ, Collier JJ. The gene encoding cyclooxygenase-2 is regulated by IL-1beta and prostaglandins in 832/13 rat insulinoma cells. Cell Immunol 271: 379–384, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Burke SJ, Collier JJ. Insulitis and diabetes: a perspective on islet inflammation. Immun Res 10: e002, 2014. [Google Scholar]
- 8.Burke SJ, Collier JJ. Transcriptional regulation of chemokine genes: a link to pancreatic islet inflammation? Biomolecules 5: 1020–1034, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke SJ, Goff MR, Lu D, Proud D, Karlstad MD, Collier JJ. Synergistic expression of the CXCL10 gene in response to IL-1β and IFN-γ involves NF-κB, phosphorylation of STAT1 at Tyr701, and acetylation of histones H3 and H4. J Immunol 191: 323–336, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Burke SJ, Goff MR, Updegraff BL, Lu D, Brown PL, Minkin SC Jr, Biggerstaff JP, Zhao L, Karlstad MD, Collier JJ. Regulation of the CCL2 gene in pancreatic β-cells by IL-1β and glucocorticoids: role of MKP-1. PLoS One 7: e46986, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke SJ, Karlstad MD, Regal KM, Sparer TE, Lu D, Elks CM, Grant RW, Stephens JM, Burk DH, Collier JJ. CCL20 is elevated during obesity and differentially regulated by NF-kappaB subunits in pancreatic beta-cells. Biochim Biophys Acta 1849: 637–652, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke SJ, Lu D, Sparer TE, Masi T, Goff MR, Karlstad MD, Collier JJ. NF-κB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metab 306: E131–E149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke SJ, Updegraff BL, Bellich RM, Goff MR, Lu D, Minkin SC Jr, Karlstad MD, Collier JJ. Regulation of iNOS gene transcription by IL-1β and IFN-γ requires a coactivator exchange mechanism. Mol Endocrinol 27: 1724–1742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia 46: 255–266, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Hohmeier HE, Gasa R, Tran VV, Newgard CB. Selection of insulinoma cell lines with resistance to interleukin-1beta- and gamma-interferon-induced cytotoxicity. Diabetes 49: 562–570, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Collier JJ, Burke SJ, Eisenhauer ME, Lu D, Sapp RC, Frydman CJ, Campagna SR. Pancreatic β-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. PLoS One 6: e22485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier JJ, Fueger PT, Hohmeier HE, Newgard CB. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes 55: 1398–1406, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Collier JJ, White SM, Dick GM, Scott DK. Phosphatidylinositol 3-kinase inhibitors reveal a unique mechanism of enhancing insulin secretion in 832/13 rat insulinoma cells. Biochem Biophys Res Commun 324: 1018–1023, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Corbett JA, Sweetland MA, Wang JL, Lancaster JR Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci USA 90: 1731–1735, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbett JA, Wang JL, Sweetland MA, Lancaster JR Jr, McDaniel ML. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest 90: 2384–2391, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowley MJ, Weinberg A, Zammit NW, Walters SN, Hawthorne WJ, Loudovaris T, Thomas H, Kay T, Gunton JE, Alexander SI, Kaplan W, Chapman J, O'Connell PJ, Grey ST. Human islets express a marked proinflammatory molecular signature prior to transplantation. Cell Transplant 21: 2063–2078, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Ding X, Wang X, Xue W, Tian X, Li Y, Jiao F, Feng X, Zheng J. Blockade of the nuclear factor kappa B pathway prolonged islet allograft survival. Artif Organs 36: E21–E27, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, Christofori G, Peled A, Carel JC, Boitard C, Klein T, Serup P, Eizirik DL, Melloul D. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA 103: 5072–5077, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis JM, Mentock SM, Depetrillo MA, Koves TR, Sen S, Watkins SM, Muoio DM, Cline GW, Taegtmeyer H, Shulman GI, Willis MS, Coleman RA. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs Fatty Acid oxidation and induces cardiac hypertrophy. Mol Cell Biol 31: 1252–1262, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol 2: 108–115, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Heitmeier MR, Scarim AL, Corbett JA. Interferon-gamma increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem 272: 13697–13704, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Herz J, Gerard RD. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci USA 90: 2812–2816, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill BG, Dranka BP, Bailey SM, Lancaster JR Jr, Darley-Usmar VM. What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. J Biol Chem 285: 19699–19704, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49: 424–430, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Jeschke MG, Klein D, Bolder U, Einspanier R. Insulin attenuates the systemic inflammatory response in endotoxemic rats. Endocrinology 145: 4084–4093, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg 239: 553–560, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, Sartor RB. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J Immunol 160: 410–418, 1998. [PubMed] [Google Scholar]
- 33.Kanak MA, Takita M, Itoh T, SoRelle JA, Murali S, Kunnathodi F, Shahbazov R, Lawrence MC, Levy MF, Naziruddin B. Alleviation of instant blood-mediated inflammatory reaction in autologous conditions through treatment of human islets with NF-κB inhibitors. Transplantation 98: 578–584, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klinck R, Serup P, Madsen OD, Jorgensen MC. Specificity of four monoclonal anti-NKx6-1 antibodies. J Histochem Cytochem 56: 415–424, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriegel MA, Rathinam C, Flavell RA. Pancreatic islet expression of chemokine CCL2 suppresses autoimmune diabetes via tolerogenic CD11c+ CD11b+ dendritic cells. Proc Natl Acad Sci USA 109: 3457–3462, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes 57: 3025–3033, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem 64: 689–719, 1995. [DOI] [PubMed] [Google Scholar]
- 38.O'Neill CM, Lu C, Corbin KL, Sharma PR, Dula SB, Carter JD, Ramadan JW, Xin W, Lee JK, Nunemaker CS. Circulating levels of IL-1B+IL-6 cause ER stress and dysfunction in islets from prediabetic male mice. Endocrinology 154: 3077–3088, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol 101: 746–752, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rink JS, Chen X, Zhang X, Kaufman DB. Conditional and specific inhibition of NF-kappaB in mouse pancreatic beta cells prevents cytokine-induced deleterious effects and improves islet survival posttransplant. Surgery 151: 330–339, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rink JS, McMahon KM, Zhang X, Chen X, Mirkin CA, Thaxton CS, Kaufman DB. Knockdown of intraislet IKKbeta by spherical nucleic acid conjugates prevents cytokine-induced injury and enhances graft survival. Transplantation 96: 877–884, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy I, Evans DB, Dwinell MB. Chemokines and chemokine receptors: update on utility and challenges for the clinician. Surgery 155: 961–973, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandler S, Andersson A, Hellerstrom C. Inhibitory effects of interleukin 1 on insulin secretion, insulin biosynthesis, and oxidative metabolism of isolated rat pancreatic islets. Endocrinology 121: 1424–1431, 1987. [DOI] [PubMed] [Google Scholar]
- 44.Sarkar SA, Lee CE, Victorino F, Nguyen TT, Walters JA, Burrack A, Eberlein J, Hildemann SK, Homann D. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes 61: 436–446, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, Becker TC, Naziruddin B, Levy M, Mirmira RG, Newgard CB. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol 28: 3465–3476, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tapia-Limonchi R, Díaz I, Cahuana GM, Bautista M, Martín F, Soria B, Tejedo JR, Bedoya FJ. Impact of exposure to low concentrations of nitric oxide on protein profile in murine and human pancreatic islet cells. Islets 6: e995997, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran VV, Chen G, Newgard CB, Hohmeier HE. Discrete and complementary mechanisms of protection of beta-cells against cytokine-induced and oxidative damage achieved by bcl-2 overexpression and a cytokine selection strategy. Diabetes 52: 1423–1432, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Zhao G, Dharmadhikari G, Maedler K, Meyer-Hermann M. Possible role of interleukin-1β in type 2 diabetes onset and implications for anti-inflammatory therapy strategies. PLoS Comput Biol 10: e1003798, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]