Abstract
Although central PYY delivery potently increases food intake, the sites of action and mechanisms mediating these hyperphagic effects are not fully understood. The present studies investigate the contribution of lateral parabrachial nucleus (lPBN) PYY-Y receptor signaling to food intake control, as lPBN neurons express Y receptors and receive PYY fibers and are known to integrate circulating and visceral sensory signals to regulate energy balance. Immunohistochemical results identified a subpopulation of gigantocellular reticular nucleus PYY-producing neurons that project monosynaptically to the lPBN, providing an endogenous source of PYY to the lPBN. lPBN microinjection of PYY-(1–36) or PYY-(3–36) markedly increased food intake by increasing meal size. To determine which receptors mediate these behavioral results, we first performed quantitative real-time PCR to examine the relative levels of Y receptor expression in lPBN tissue. Gene expression analyses revealed that, while Y1, Y2, and Y5 receptors are each expressed in lPBN tissue, Y1 receptor mRNA is expressed at fivefold higher levels than the others. Furthermore, behavioral/pharmacological results demonstrated that the hyperphagic effects of PYY-(3–36) were eliminated by lPBN pretreatment with a selective Y1 receptor antagonist. Together, these results highlight the lPBN as a novel site of action for the intake-stimulatory effects of central PYY-Y1 receptor signaling.
Keywords: peptide YY, parabrachial nucleus, gigantocellular reticular nucleus, Y1 receptor, food intake, obesity
peptide yy (PYY), a member of a peptide family that also includes neuropeptide Y (NPY) and pancreatic polypeptide (PP), is produced primarily in cells of the colon and small intestine and released in response to local nutrient stimulation (31). The two endogenous circulating isoforms of PYY [PYY-(1–36) and PYY-(3–36)] bind to Y1, Y2, and Y5 receptors (6, 34) with different affinities; whereas PYY-(1–36) binds to all of these receptors, PYY-(3–36) has the highest affinity for the Y2 receptor (22). The discovery that systemic PYY-(3–36) reduces food intake through action on Y2 receptors in the periphery and in the arcuate hypothalamic nucleus (ARC) (3) dramatically increased research on the role of peripheral PYY signaling in the control of energy balance (25).
In contrast to effects of peripheral PYY signaling, the role of central PYY signaling in the control of feeding has received considerably less attention despite the characterization of PYY-producing neurons in the hindbrain gigantocellular reticular nucleus (Gi) by several groups (14, 15, 24). Also, in contrast to peripheral PYY signaling, central [lateral, 3rd, and 4th intracerebroventricular (icv)], administration of either PYY-(1–36) or PYY-(3–36) potently stimulates food intake (9–11, 26, 32). PYY microinjected into the paraventricular hypothalamic nucleus (36) or the hippocampus (18) increases food intake. However, the hindbrain sites of action mediating the hyperphagic effects of 4th icv PYY administration, as well as the receptors mediating these effects, have not been investigated.
Here, we study the role of PYY-Y receptor signaling in the lateral parabrachial nucleus (lPBN), whose neurons integrate visceral information including that involved in the control of food intake (e.g., taste and gastrointestinal signals) (4, 21). lPBN neurons receive input from and project to key brain regions involved in energy balance control [e.g., nucleus tractus solitarius (NTS), Gi, as well as several nuclei of the hypothalamus and amygdala] (28, 29, 38). Additionally, the lPBN processes a host of energy balance-relevant signals, such as GLP-1 (1, 33, 37), leptin (2, 13), cannabinoids (12), opiates (40, 42), and glutamate (43, 44). Yet, despite available evidence showing that 1) Gi neurons project to the lPBN (38), 2) PYY fibers are found in the lPBN (15), and 3) Y receptors are expressed in the lPBN (23, 35), the effects of lPBN PYY-Y receptor signaling in the regulation of food intake are unexplored.
Studies described here use neuroanatomical tracing, immunohistochemical, qPCR, and behavioral/pharmacological techniques to study Gi PYY projections to the lPBN, the relative expression of Y receptors in the lPBN, and the effects of lPBN PYY-Y receptor signaling on the control of food intake. Results indicate that a subpopulation of Gi PYY neurons project monosynaptically to the lPBN and that lPBN PYY signals through the Y1 receptor to increase food intake.
METHODS
Subjects and Drugs
Adult male Sprague-Dawley rats (250–265 g upon arrival; Charles River Laboratories, Wilmington, MA) were individually housed in hanging wire bottom metal cages and had ad libitum access to pelleted chow (Purina Rodent Chow, 5001) and water, unless otherwise noted, on a 12:12-h light-dark cycle. All procedures conformed to and received approval from the institutional standards of the University of Pennsylvania Animal Care and Use Committee.
PYY-(1–36) and PYY-(3–36) (Bachem Americas, Torrence, CA), as well as the selective Y1 receptor antagonist LY-1229U91 (20) (Sigma-Aldrich, St. Louis, MO), were dissolved in sterile saline. The monosynaptic retrograde tracer Fluorogold (Fluorochrome, Denver, CO) was dissolved (2%) in distilled water.
Surgery
For all surgeries, rats received an intramuscular anesthetic [ketamine (90 mg/kg; Butler Schein Animal Health Supply, Dublin, OH), xylazine (2.7 mg/kg; Anased, Shenandoah, IA), and acepromazine (0.64 mg/kg; Butler Animal Health Supply) and subcutaneous analgesia (2.0 mg/kg Meloxicam and 0.1 mg/kg buprinorphine; Midwest Veterinary Supply, Norristown, PA)].
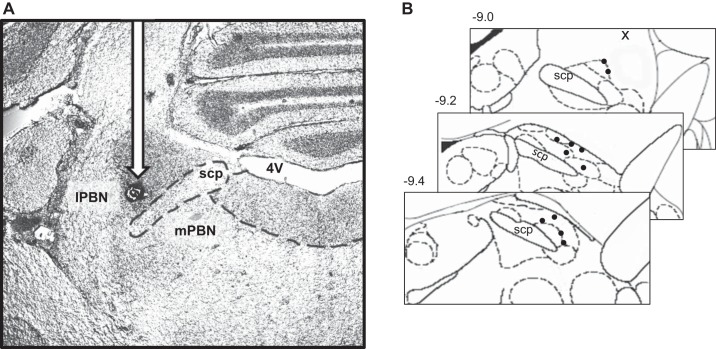
Unilateral 26-gauge guide cannulae (Plastics One, Roanoke, VA) were stereotaxically implanted into the lPBN according to the following coordinates: ± 2.0 mm from midline, 0.6 mm anterior from lambda, and 5.7 mm ventral from skull surface using a 20° angle (negative slope in anterior-to-posterior direction) with the injection aimed 2.0 mm below the end of the guide cannula. Cannulae were secured to the skull with screws and dental cement. Cannula placements were histologically confirmed post mortem with injection of Chicago Sky Blue ink (2% wt/vol). Figure 1A is a representative image of a lPBN injection site, and Fig. 1B provides a schematic diagram of injection sites for a cohort of rats included in the current study. Animals whose injection sites were not located within the lPBN were excluded from data analyses.
Fig. 1.
A: representative image of lateral parabrachial nucleus (lPBN) injection site (black arrow). B: diagram of injection sites in a cohort of rats in this study; numbers represent position (mm) relative to bregma; • represents hits; x represents misses.
Experimental Procedures
Experiment 1: lPBN Fluorogold tracing and Gi PYY immunohistochemistry.
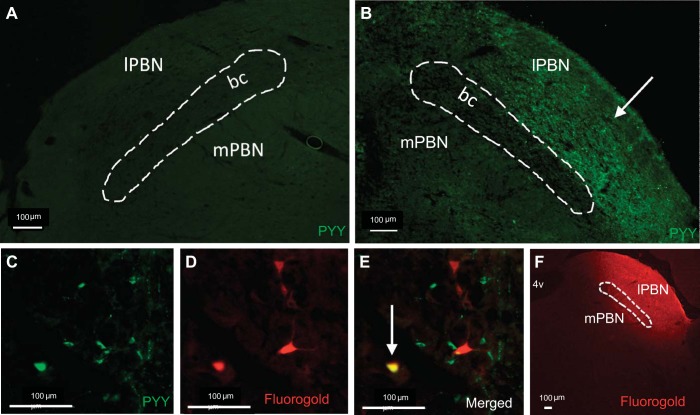
Rats (n = 5) were anesthetized and placed in a stereotaxic instrument where a unilateral cannula was positioned above the lPBN. Rats received a 300-nl injection of 2% (wt/vol) Fluorogold (FG) via an automated syringe pump (PHD Ultra; Harvard Apparatus, Holliston, MA). Other rats (n = 2) did not receive FG injection and were used to confirm the presence of PYY fibers in the lPBN. Four days later, rats were anesthetized and transcardially perfused with 0.1 M pH 7.4 phosphate-buffered saline (PBS; Boston Bioproducts, Ashland, MA), followed by 4% paraformaldehyde (PFA; Boston Bioproducts). Brains were removed and postfixed in PFA for 4 h and stored in 30% sucrose until brains no longer floated. Coronal sections (35 μm) were cut from the hindbrain at the level of the Gi as well as the lPBN, collected in glass jars, and stored at 4°C until the start of immunohistochemistry (IHC). A representative image of an lPBN FG injection is provided in Fig. 2F.
Fig. 2.
A: omission of peptide YY (PYY) primary antibody (negative control) resulted in no PYY fiber immunofluorescence in the lPBN. B: PYY immunohistochemistry confirmed the presence of PYY fibers (white arrow) in the lPBN. C–E: representative ×20 magnification image of a Gi section: (C) green immunofluorescence represents PYY, (D) red immunofluorescence represents lPBN-injected Fluorogold(FG), (E) merged image shows colocalization of FG and PYY (white arrow). F: representative image of lPBN FG injection.
IHC was performed with a protocol similar to what we have previously reported (1). Briefly, brain sections were treated with 1% H2O2 in methanol, 0.5% sodium borohydride, and blocking solution (5% normal donkey serum in 0.1 M PBS with 2.5% Triton X) and then incubated with the PYY primary antibody (Rabbit anti-PYY; Bachem Americas, Torrance, CA) at a 1:10,000 concentration in blocking solution for 16 h at room temperature. This antibody has demonstrated selectivity for PYY and does not exhibit cross-reactivity with NPY in the rat (15). Brain sections were washed in 0.1 M PBS and then incubated with a secondary antibody (DyLight 488; Jackson ImmunoResearch Laboratories, West Grove, PA) at a 1:500 concentration in blocking solution for 2 h. FG immunofluorescence was detected under a special filter (C-FL UV-2A; Nikon Instruments, Melville, NY).
Brain sections were mounted on glass slides and coverslipped with FG (Electron Microscopy Sciences; Hatfield, PA). Fluorescence microscopy (Nikon 80i; NIS Elements AR 3.0) was used at ×10 and ×20 magnification to quantify neurons (≥6 coronal sections containing the Gi per brain) expressing FG and PYY in the Gi from bregma −10.8 mm to −11.5 mm (15, 30).
Experiment 2: effects of lPBN PYY-(1–36) and PYY-(3–36) on chow intake.
Rats (n = 14) that were previously habituated to experimental procedures received a 100-nl unilateral injection of sterile saline or PYY-(1–36) (0.01, 0.1, or 1 μg) in the lPBN in a within-subjects, counterbalanced experimental design immediately before onset of the dark cycle. Chow intake was measured at 1, 3, 6, and 24 h postinjection, accounting for spillage. Body weight and water intake were also measured 24 h postinjection. At least 48 h elapsed between drug injection conditions. A second cohort of rats (n = 12) underwent identical experimental conditions but was injected with the isoform PYY-(3–36) (0.01, 0.1, or 1 μg) in the lPBN.
Experiment 3: effects of lPBN PYY-(1–36) and PYY-(3–36) on meal patterns.
Rats (n = 12) were housed in a custom automated feedometer consisting of hanging wire bottom cages with an access hole to a food cup that rested on an electronic scale. The feedometer was connected to software (LabView) that records the weight of the food cup every 10 s. Following a 5-day habituation to the feedometer caging and to powdered chow, rats received a 100-nl unilateral injection of sterile saline or PYY-(1–36) (1 μg) in a within-subjects counterbalanced experimental design immediately before onset of the dark cycle. Food cup measurements were automatically recorded every 10 s; food spillage and body weight were manually measured 24 h postinjection. Meal size and number were subsequently analyzed, with a meal defined as any intake ≥0.25 g; ≥10 min must elapse for feeding bouts to be considered two separate meals. At least 48 h elapsed between drug injection conditions. A second cohort of rats (n = 12) underwent identical experimental conditions but was injected with the isoform PYY-(3–36) (1 μg) in the lPBN.
Experiment 4: relative gene expression of Y receptors in lPBN.
Rats (n = 6) were euthanized by decapitatation, and brains were rapidly removed, flash-frozen in isopentane, and stored at −80°C. Brains were mounted on a cryostat, and 1 × 1 × 1-mm micropunches were taken bilaterally from the lPBN. Total RNA was extracted from the lPBN micropunches using TRIzol (Invitrogen) and the RNeasy kit (Qiagen). cDNA was synthesized from the RNA, and qPCR was done in duplicate using TaqMan Universal qPCR Master Mix (Applied Biosystems). The primer/probe sets for the Y1R, Y2R, and Y5R were from Applied Biosystems (Rn02769337_s1, Rn04219516_g1, Rn0208986_s1, respectively). Relative mRNA expression was calculated using the comparative CT method as previously described (19).
Experiment 5: effects of lPBN Y1 receptor antagonist pretreatment on PYY-(3–36) hyperphagia.
Rats (n = 11) that were previously habituated to experimental procedures received a 200-nl unilateral injection of the selective Y1 receptor (Y1R) antagonist (LY-1229U91, 1 μg) in the lPBN 20 min prior to a 100-nl lPBN injection of PYY-(3–36) (1 μg) in a within-subjects counterbalanced design immediately before the onset of the dark cycle. Food intake measurements were taken at 30 min, 1 h, 2 h, 3 h, 4 h, 6 h, and 24 h, accounting for spillage. Body weight was also measured at 24 h postinjection. At least 48 h elapsed between drug injection conditions.
Statistical Analyses
Data from the experiments were analyzed using Statistica (version 7; StatSoft, Tulsa, OK) and expressed as means ± SE. For behavioral experiments, repeated-measures or two-way ANOVA and post hoc Neuman-Keuls comparisons were made. Alpha levels were set to α = 0.05 for all analyses.
RESULTS
Experiment 1: a Subpopulation of PYY Neurons in the Gi Project Directly to the lPBN
Analysis of lPBN brain sections confirmed the presence of PYY fibers specifically in the lPBN (Fig. 2B). Omission of the PYY primary antibody resulted in no PYY fiber immunofluorescence (Fig. 2A). Analysis of Gi brain sections showed an average of 32 ± 2.1 PYY-expressing neurons and 31.6 ± 4.9 FG-expressing neurons per 35-μm coronal section. Quantification of the colocalization of PYY and FG immunofluorescence showed that 8.3 ± 0.8% of Gi PYY-producing neurons project monosynaptically to the lPBN. Representative images of single-labeled neurons are shown in Fig. 2, C and D, and a representative image of double-labeled neurons is shown in Fig. 2E.
Experiment 2: lPBN-Injected PYY-(1–36) or PYY-(3–36) Increases Cumulative Food Intake
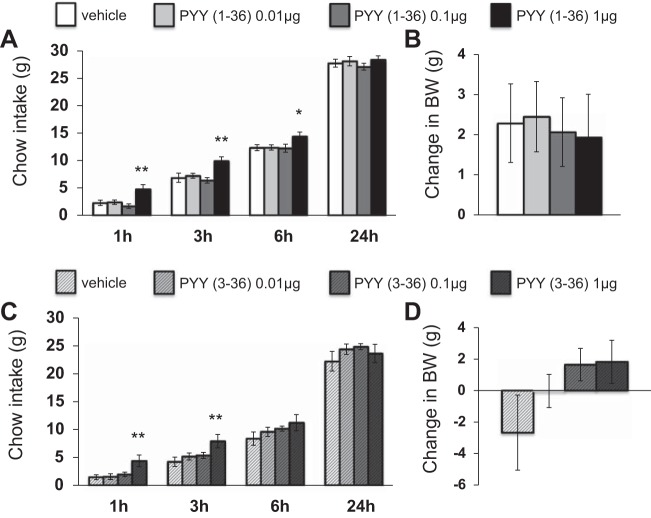
lPBN delivery of PYY-(1–36) increased cumulative chow intake at 1 h [F(3,39)=7.88, P < 0.01], 3 h [F(3,39)=7.75, P < 0.01], and 6 h [F(3,39)=4.42, P < 0.01] compared with vehicle treatment (Fig. 3A). lPBN delivery of PYY-(3–36) also increased cumulative chow intake at 1 h [F(3,33)=5.57, P < 0.01] and 3 h [F(3,33)=4.70, P < 0.01] (Fig. 3C). Neither PYY-(1–36) (Fig. 3B) nor PYY-(3–36) (Fig. 3D) affected 24-h change in body weight [F(3,39)=0.076 and F(3,33)=2.0, respectively]. Neither PYY-(1–36) nor PYY-(3–36) affected 24-h water intake [F(3,39)=0.18 and F(3,33)=0.64, respectively; data not shown].
Fig. 3.
Intra-lPBN PYY-(1–36) microinjection (A) significantly increased cumulative chow intake but (B) had no effect on 24-h change in body weight. Likewise, intra-lPBN PYY-(3–36) microinjection (C) significantly increased cumulative chow intake but (D) had no effect on 24-h change in body weight (means ± SE; *P < 0.05, **P < 0.01).
Experiment 3: lPBN-Injected PYY-(1–36) or PYY-(3–36) Increases Meal Size
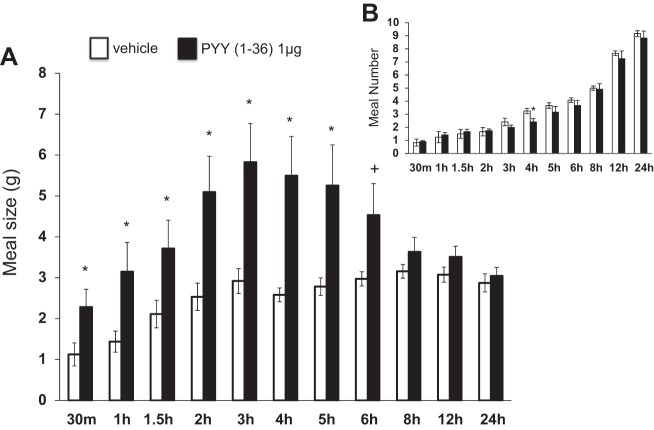
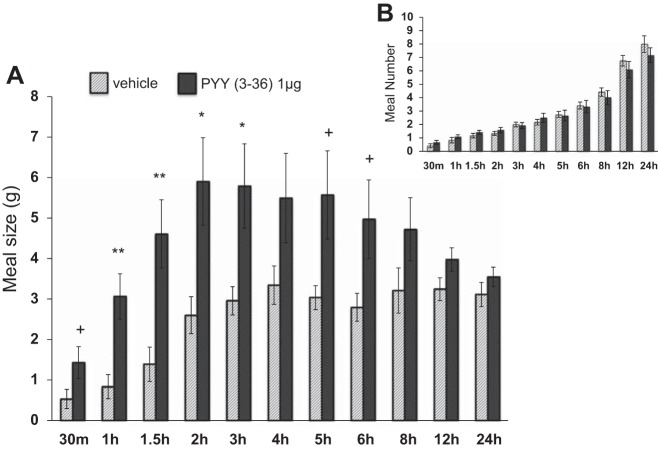
lPBN delivery of PYY-(1–36) increased average meal size from 30 min to 5 h postinjection [5.20≤F(1,11)≤8.49, P < 0.05; Fig. 4A]. lPBN delivery of PYY-(3–36) increased average meal size from 1–4 h postinjection [5.07≤F(1,11)≤11.5, P < 0.05; Fig. 5A]. There was very little effect of PYY-(1–36) or PYY-(3–36) on average meal number (Figs. 4B and 5B, respectively), although meal number was reduced following lPBN PYY-(1–36) at 4 h postinjection [F(1,11)=6.70, P < 0.05].
Fig. 4.
Intra-lPBN PYY-(1–36) microinjection (A) significantly increased meal size and (B) had little effect on meal number (means ± SE; *+P < 0.10, *P < 0.05).
Fig. 5.
Intra-lPBN PYY-(3–36) microinjection (A) significantly increased meal size and (B) had no effect on meal number (means ± SE; +P < 0.10, *P < 0.05, **P < 0.01).
Experiment 4: Y1R Is Expressed About Five Times Higher Than Y2R and Y5R in lPBN Tissue
Quantitative PCR in lPBN tissue revealed that Y1R mRNA levels were approximately fivefold higher than Y2 or Y5 receptor mRNA levels (Fig. 6A).
Fig. 6.
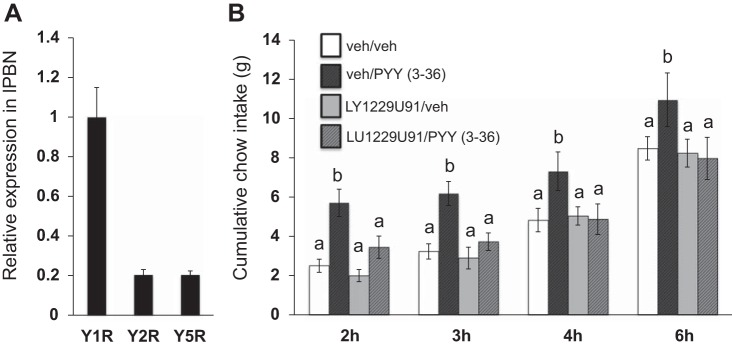
A: qPCR in lPBN tissue revealed that Y1 receptor (Y1R) mRNA was expressed ∼5 times higher than Y2R and Y5R mRNA. B: lPBN pretreatment with the selective Y1R antagonist LY-1229U91 attenuated the orexigenic effects of lPBN PYY-(3–36). Within a time bin, bars with different letters are significantly different from each other (means ± SE; P < 0.05).
Experiment 5: Pretreatment with a Selective Y1R Antagonist Attenuates the Intake-Stimulatory Effects of PYY-(3–36)
Pretreatment with the selective Y1R antagonist LY-1229U91 significantly attenuated the intake-stimulatory effects of PYY-(3–36) and produced a drug × drug interaction at 2 h [F(1,10)=8.51, P < 0.05; vehicle/PYY-(3–36) vs. LY-1229U91/PYY-(3–36) P < 0.01], 3 h [F(1,10)=12.34, P < 0.05; vehicle/PYY-(3–36) vs. LY-1229U91/PYY-(3–36) P < 0.001] and 4 h [F(1,10)=60.39, P < 0.0001; vehicle/PYY-(3–36) vs. LY-1229U91/PYY-(3–36) P < 0.001], with a strong trend for drug × drug interaction at 6 h postinjection [F(1,10)=4.60, P = 0.058, vehicle/PYY-(3–36) vs. LY-1229U91/PYY-(3–36) P < 0.05] (Fig. 6B). At each aforementioned time point, post hoc comparisons revealed a significant increase in food intake with lPBN PYY-(3–36) compared with the vehicle treatment and a reversal of lPBN PYY-(3–36) hyperphagia with LY-122U91 pretreatment. There was no effect of the Y1R antagonist alone on food intake at any time point examined.
DISCUSSION
Although central (i.e., icv) PYY potently stimulates feeding (17), the specific brain sites mediating the hyperphagic effects of PYY are unresolved. Given that lPBN neurons express Y receptors (23, 35) and integrate a multitude of circulating and visceral sensory signals involved in the regulation of feeding behavior (1, 2, 21), studies here examined the potential role of lPBN PYY-Y receptor signaling in the control of food intake. Immunohistochemical results identified a population of Gi PYY-producing neurons that project directly to the lPBN. Microinjection of either PYY-(1–36) or PYY-(3–36) in the lPBN increased food intake specifically via an increase in meal size. Quantitative gene expression analyses showed that the Y1, Y2, and Y5 receptors are expressed in lPBN tissue, with Y1 as the most highly expressed receptor (5× higher than others). On the basis of these data, we examined the role of Y1R signaling in mediating the effects of lPBN administration of PYY-(3–36). Indeed, the intake-stimulatory effects of PYY-(3–36) were eliminated by lPBN pretreatment with a selective Y1R antagonist. Together, results highlight the lPBN as a novel site of action for the hyperphagic effects of central PYY-Y1R signaling.
Previous studies show direct neuronal projections from the Gi to the lPBN (38), yet the neurochemical phenotype of these neurons has not been previously identified. Since the Gi represents the only known central source of PYY (15), and PYY fibers are present throughout the hindbrain including the lPBN (15), we employed a strategy similar to that of Alhadeff et al. (1) by injecting the monosynaptic retrograde tracer Fluorogold into the lPBN and examining colocalization of Fluorogold and PYY immunofluorescence in the Gi. Results identified a population of Gi PYY-producing neurons that projects directly to the lPBN, providing a potential endogenous pathway by which central PYY may act in the lPBN. Our results also identified a population of Gi PYY-producing neurons that do not project to the lPBN, as well as Gi lPBN-projecting neurons that do not express PYY. Further characterization of these neuron populations is warranted. As there are no PYY fibers present throughout the forebrain (15), it is likely that the PYY-producing neurons within the Gi project only locally within the hindbrain. Given that a high density of PYY fibers has been localized in the hindbrain NTS (15), it is possible that Gi PYY-producing neurons project to the NTS in addition to the lPBN. Furthermore, the conditions under which Gi PYY neurons are stimulated are unknown. In the Gi, fibers containing the orexigenic hypothalamic peptides orexin or melanin-concentrating hormone (MCH) make close appositions onto PYY neurons. Thus, the role of orexin and MCH signaling on PYY neuron excitability should be investigated in future studies.
To determine the role of PYY-Y receptor signaling in the lPBN in the control of energy balance, PYY-(1–36) or PYY-(3–36) was administered directly into the lPBN. Microinjection of either PYY isoform in the lPBN significantly increased food intake. This finding was intriguing, since PYY-(3–36) in the ARC reduces food intake (3); however, it is consistent with other data showing that ventricular (lateral and hindbrain) administration of either PYY isoform markedly increases food intake (10, 11) and highlights the lPBN as a potential site of action mediating these effects. It is also interesting to note that both isoforms potently increased meal size but had little effect on meal number. Given that lPBN neurons are well known to process gastrointestinal signals relayed through the visceral afferent signaling pathway (21), the effects of PYY on meal size indicate a potential interaction with the processing of within-meal gastrointestinally derived satiation signals. Direct examination of such interactions is a compelling topic for future research.
While we have identified a potential endogenous source of PYY to the lPBN that can putatively bind to lPBN Y receptors to regulate food intake, the current studies do not rule out the contribution of peripheral PYY or other Y receptor ligands in mediating these effects. Previous studies have suggested that peripheral PYY crosses the blood-brain barrier (27) and acts in the brain, particularly the ARC (3), to affect food intake. Although it is conceivable that peripheral PYY could reach the lPBN, it is unlikely that this mechanism explains our results, given that peripheral administration of PYY reduces food intake but ventricular or intra-lPBN PYY administration increases food intake. Additionally, there are two other endogenous ligands of the Y family of receptors, NPY and PP, and it is possible that these ligands (especially NPY, which is highly expressed throughout the brain) may act on lPBN Y receptors to exert effects on energy balance. In fact, an established projection of ARC NPY/agouti-related peptide (AgRP)/γ-aminobutyric acid (GABA) neurons to the lPBN is involved in food intake control (43). The current experiments conclusively demonstrate a role for exogenous lPBN PYY signaling in the control of food intake, but it will be important to determine the role of each of these endogenously relevant projections and peptides that may contribute to lPBN Y receptor feeding effects.
As mentioned previously, it was somewhat surprising that PYY-(3–36) was as effective as PYY-(1–36) in increasing food intake and meal size when injected into the lPBN, given that that peripheral or ARC PYY-(3–36) injections reduce food intake via action on the Y2 receptor (3). Since PYY-(3–36) can also bind to Y1 and Y5 receptors (16, 41), we explored the possibility that PYY-(3–36) acts via these receptors in the lPBN. First, we performed real-time qPCR in lPBN tissue and determined that the Y1 receptor is expressed fivefold higher than the Y2 and Y5 receptors. On the basis of these data, we hypothesized that the Y1 receptor might mediate the hyperphagic effects of PYY-(3–36). Indeed, pretreatment with a selective Y1R antagonist in the lPBN completely blocked the increase in food intake following lPBN PYY-(3–36) injection. Thus, on the basis of the qPCR and behavioral evidence, we conclude that exogenous lPBN PYY signaling through the Y1R is involved in the control of food intake, providing a site of action for the hyperphagic effects of hindbrain ventricle-delivered PYY.
An important feature of the present experimental design was the selection of a dose of Y1R antagonist that had no effect on food intake when injected in the lPBN alone. Indeed, the antagonist was used specifically to attenuate the hyperphagic effects of lPBN PYY. Our preliminary studies (data not shown) indicated that the current dose (1 μg) and one higher dose (2 μg) of Y1R antagonist were both subthreshold for effect on food intake. Future studies could use higher doses of the Y1R antagonist in the lPBN to examine whether endogenous lPBN Y1R activity is physiologically relevant to the control of food intake.
Although Y1 receptors are highly expressed in lPBN neurons (23), the neurochemical phenotypes and neuroanatomical targets of these neurons are unknown. Y1R stimulation, at least in some hindbrain and forebrain regions, results in postsynaptic inhibitory currents (7, 39). Thus, it is possible that Y1 receptors are expressed on and inhibit neurons in the lPBN that reduce food intake when activated. For example, a population of lPBN calcitonin gene-related peptide (CGRP)/glutamate neurons that project to the central nucleus of the amygdala (CeA) has recently received attention for a role in mediating anorexia and malaise (8). The possibility that Y1 receptors inhibit these neuronal projections to the CeA to increase food intake warrants further investigation. In addition to CGRP and glutamate, a variety of other neurochemical signals, including cholecystokinin, neurotensin, substance P, enkephalin, and somatostatin, among others, are expressed in the lPBN (5, 8) and therefore may be involved in mediating lPBN Y1R signaling.
Collectively, these data demonstrate a role for exogenous lPBN PYY-Y receptor signaling in the control of food intake. Both lPBN PYY-(1–36) and PYY-(3–36) microinjection increased cumulative food intake by increasing meal size, and identification of direct PYY projections from the Gi provides a potential endogenous source for these ligands in the lPBN to modulate feeding behavior. Y1R is highly expressed in lPBN tissue, and selective lPBN Y1R antagonist injection abolishes the hyperphagic effects of PYY-(3–36). Future studies should examine the functional relevance of Gi PYY projections to the lPBN as well as the neural signals and circuits mediating lPBN Y1R stimulation-induced increases in feeding. Overall, these data provide a novel site of action for the hyperphagic effects of central PYY-Y receptor signaling, and, more broadly, they add to the growing body of literature highlighting the role of lPBN signaling in the control of food intake and energy balance.
GRANTS
This work was funded by National Institutes of Health Grant DK-21397 (H. J. Grill), DK-096139 (M. R. Hayes), and F31-NS-084633 (A. L. Alhadeff).
DISCLOSURES
H. J. Grill has received funding from Novo Nordisk, where he is also on the Obesity Advisory Board, and M. R. Hayes has received funding from the Dairy Research Institute; however, the support from these companies has no relevance to the current paper. Thus, no conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.L.A., M.R.H., and H.J.G. conception and design of research; A.L.A. and D.G. performed experiments; A.L.A. and D.G. analyzed data; A.L.A., M.R.H., and H.J.G. interpreted results of experiments; A.L.A. prepared figures; A.L.A. drafted manuscript; A.L.A., M.R.H., and H.J.G. edited and revised manuscript; A.L.A., D.G., M.R.H., and H.J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Rachel Batterham for helpful advice, as well as Derek Zimmer, Dr. Zhi Yi Ong, Elizabeth Schleissinger, and Hallie Wald for technical assistance.
REFERENCES
- 1.Alhadeff AL, Baird JP, Swick JC, Hayes MR, Grill HJ. Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology 39: 2233–2243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhadeff AL, Hayes MR, Grill HJ. Leptin receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake. Am J Physiol Regul Integr Comp Physiol 307: R1338–R1344, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Blessing WW. The Lower Brainstem and Bodily Homeostasis. New York: Oxford Univ. Press, 1997. [Google Scholar]
- 5.Block CH, Hoffman GE. Neuropeptide and monoamine components of the parabrachial pontine complex. Peptides 8: 267–283, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Blomqvist AG, Herzog H. Y-receptor subtypes—how many more? Trends Neurosci 20: 294–298, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil 21: 1309–e1126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature 503: 111–114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark JT, Sahu A, Kalra PS, Balasubramaniam A, Kalra SP. Neuropeptide Y (NPY)-induced feeding behavior in female rats: comparison with human NPY ([Met17]NPY), NPY analog ([norLeu4]NPY) and peptide YY. Regul Pept 17: 31–39, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Corp ES, McQuade J, Krasnicki S, Conze DB. Feeding after fourth ventricular administration of neuropeptide Y receptor agonists in rats. Peptides 22: 493–499, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Corp ES, Melville LD, Greenberg D, Gibbs J, Smith GP. Effect of fourth ventricular neuropeptide Y and peptide YY on ingestive and other behaviors. Am J Physiol Regul Integr Comp Physiol 259: R317–R323, 1990. [DOI] [PubMed] [Google Scholar]
- 12.DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci 28: 9702–9709, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flak JN, Patterson CM, Garfield AS, D'Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JM, Germani M, Jones JC, Rajala M, Satin L, Rhodes CJ, Olson DP, Kennedy RT, Heisler LK, Myers MG Jr. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci 17: 1744–1750, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelegen C, Chandarana K, Choudhury AI, Al-Qassab H, Evans IM, Irvine EE, Hyde CB, Claret M, Andreelli F, Sloan SE, Leiter AB, Withers DJ, Batterham RL. Regulation of hindbrain Pyy expression by acute food deprivation, prolonged caloric restriction, and weight loss surgery in mice. Am J Physiol Endocrinol Metab 303: E659–E668, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glavas MM, Grayson BE, Allen SE, Copp DR, Smith MS, Cowley MA, Grove KL. Characterization of brainstem peptide YY (PYY) neurons. J Comp Neurol 506: 194–210, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Gobbi M, Mennini T, Vezzani A. Autoradiographic reevaluation of the binding properties of 125I-[Leu31,Pro34]peptide YY and 125I-peptide YY3-36 to neuropeptide Y receptor subtypes in rat forebrain. J Neurochem 72: 1663–1670, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Hagan MM. Peptide YY: a key mediator of orexigenic behavior. Peptides 23: 377–382, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Hagan MM, Castaneda E, Sumaya IC, Fleming SM, Galloway J, Moss DE. The effect of hypothalamic peptide YY on hippocampal acetylcholine release in vivo: implications for limbic function in binge-eating behavior. Brain Res 805: 20–28, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hegde SS, Bonhaus DW, Stanley W, Eglen RM, Moy TM, Loeb M, Shetty SG, DeSouza A, Krstenansky J. Pharmacological evaluation of 1229U91, a novel high-affinity and selective neuropeptide Y-Y1 receptor antagonist. J Pharmacol Exp Ther 275: 1261–1266, 1995. [PubMed] [Google Scholar]
- 21.Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res 957: 193–206, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Keire DA, Bowers CW, Solomon TE, Reeve JR Jr. Structure and receptor binding of PYY analogs. Peptides 23: 305–321, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hokfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience 111: 443–532, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg JM, Terenius L, Hokfelt T, Tatemoto K. Comparative immunohistochemical and biochemical analysis of pancreatic polypeptide-like peptides with special reference to presence of neuropeptide Y in central and peripheral neurons. J Neurosci 4: 2376–2386, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning S, Batterham RL. The role of gut hormone peptide YY in energy and glucose homeostasis: twelve years on. Annu Rev Physiol 76: 585–608, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Morley JE, Levine AS, Grace M, Kneip J. Peptide YY (PYY), a potent orexigenic agent. Brain Res 341: 200–203, 1985. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3-36 in the mouse. J Pharmacol Exp Ther 306: 948–953, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience 3: 207–218, 1978. [DOI] [PubMed] [Google Scholar]
- 29.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol 166: 17–30, 1976. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic, 2005. [Google Scholar]
- 31.Pedersen-Bjergaard U, Host U, Kelbaek H, Schifter S, Rehfeld JF, Faber J, Christensen NJ. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand J Clin Lab Invest 56: 497–503, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Raposinho PD, Pierroz DD, Broqua P, White RB, Pedrazzini T, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol Cell Endocrinol 185: 195–204, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Richard JE, Farkas I, Anesten F, Anderberg RH, Dickson SL, Gribble FM, Reimann F, Jansson JO, Liposits Z, Skibicka KP. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological and behavioral evidence. Endocrinology en20141248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva AP, Cavadas C, Grouzmann E. Neuropeptide Y and its receptors as potential therapeutic drug targets. Clin Chim Acta 326: 3–25, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Stanic D, Brumovsky P, Fetissov S, Shuster S, Herzog H, Hokfelt T. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J Comp Neurol 499: 357–390, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides 6: 1205–1211, 1985. [DOI] [PubMed] [Google Scholar]
- 37.Swick JC, Alhadeff AL, Grill HJ, Urrea P, Lee SM, Roh H, Baird JP. Parabrachial nucleus contributions to glucagon-like peptide-1 receptor agonist-induced hypophagia. Neuropsychopharmacology 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokita K, Inoue T, Boughter JD Jr. Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience 161: 475–488, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Heuvel JK, Furman K, Gumbs MC, Eggels L, Opland DM, Land BB, Kolk SM, Narayanan N, Fliers E, Kalsbeek A, DiLeone RJ, la Fleur SE. Neuropeptide Y activity in the nucleus accumbens modulates feeding behavior and neuronal activity. Biol Psychiatry 77: 633–641, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward HG, Simansky KJ. Chronic prevention of mu-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology (Berl) 187: 435–446, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Widdowson PS, Buckingham R, Williams G. Distribution of [Leu31,Pro34]NPY-sensitive, BIBP3226-insensitive [125I]PYY(3–36) binding sites in rat brain: possible relationship to Y5 NPY receptors. Brain Res 778: 242–250, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 285: R1055–R1065, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature 483: 594–597, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q, Zheng R, Srisai D, McKnight GS, Palmiter RD. NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus. Proc Natl Acad Sci USA 110: 14765–14770, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]