Abstract
Objective
This study examined HIV superinfection (HIV SI) in HIV-infected women postpartum, and its association with mother-to-child transmission (MTCT).
Design
Plasma samples were obtained from HIV-infected women who transmitted HIV to their infants after 6 weeks of age (transmitters, n=91) and HIV-infected women who did not transmit HIV to their infants (non-transmitters, n=91). These women were originally enrolled in a randomized trial for prevention of MTCT of HIV in Malawi (PEPI-Malawi).
Methods
Two HIV genomic regions (p24 and gp41) were analyzed by next generation sequencing (NGS) for HIV SI. HIV SI was established if the follow-up sample contained a new, phylogenetically distinct viral population. HIV SI and transmission risk were examined by multiple logistic regression, adjusted for PEPI study arm, baseline viral load, baseline CD4 cell count, time to resumption of sex, and breastfeeding duration.
Results
Transmitters had lower baseline CD4 cell counts (p=0.001) and higher viral loads (p<0.0001) compared to non-transmitters. There were five cases of SI among transmitters [rate of SI=4.7/100pys person year (pys)] compared to five cases among the non-transmitters (rate of SI=4.4/100pys; p=0.78). HIV SI was not associated with increased risk of post-natal MTCT of HIV after controlling for maternal age, baseline viral load and CD4 cell count (adjusted odds ratio=2.32, p=0.30). Longer breastfeeding duration was independently associated with a lower risk of HIV SI after controlling for study arm and baseline viral load (p=0.05).
Conclusions
There was a significant level of HIV SI in women postpartum, but this was not associated with an increased risk of MTCT via breastfeeding.
Introduction
HIV superinfection (SI) occurs when an HIV-infected individual is infected with a new, phylogenetically distinct HIV strain [1]. HIV SI has been demonstrated in multiple cohorts around the world, and occurs at varying rates that can be similar to the corresponding primary HIV incidence rate in the population, although this has not been observed in all studies [2-7]. Rates of HIV SI appear to be higher in populations at increased risk of primary HIV infection, such as female sex-workers [8]. HIV SI can cause temporary and sustained increases in viral load, which could increase the risk of HIV transmission to other individuals [4, 9, 10]. Examining the role of SI and sexual transmission in HIV discordant sexual partnerships is difficult since it is often impossible to establish if the SI event occurred prior to the subsequent transmission, or vice versa [7]. In contrast, studying SI in the context of MTCT of HIV through breastfeeding is simpler, because there is no question about the directionality of SI and subsequent transmission, and because there are fewer confounding behavioral factors.
The Post-Exposure Prophylaxis of Infants trial in Malawi (PEPI-Malawi, 2004-2009) demonstrated that the risk of MTCT of HIV via breastfeeding was significantly reduced by providing infants an extended regimen of nevirapine or nevirapine plus zidovudine prophylaxis up to age 14 weeks, compared to infants who received a short regimen for prophylaxis (single dose nevirapine with one week of zidovudine) [11]. Several studies in sub-Saharan Africa, including Malawi, have demonstrated that postpartum women are at increased risk of HIV infection, particularly at the time when sexual activity is resumed [12-14]. Genital tract infections and risk behaviors were the main factors associated with HIV acquisition postpartum. It is not known if there is an increased risk of HIV SI in HIV-infected women postpartum or if maternal HIV SI increases the risk of MTCT of HIV during breastfeeding.
Methods
Ethical Considerations
Women provided written informed consent for participation in the PEPI-Malawi trial (NCT00115648). The study was approved by the institutional review boards in Malawi and the United States. [11].
Study Population
A detailed description of the PEPI-Malawi trial has been described previously [11]. Briefly, eligible HIV-infected pregnant women presenting for either antenatal or delivery services in Blantyre, Malawi were offered participation in the PEPI-Malawi trial. Infants who were HIV-uninfected at birth were then randomized to either a control regimen (single-dose nevirapine and one week of zidovudine, the standard of care at the time) or the control regimen plus one of two extended antiretroviral (ARV) prophylaxis regimens (daily nevirapine, or daily nevirapine plus zidovudine) for up to 14 weeks of age [11]. Women whose infants tested HIV-negative at six weeks of age and HIV-positive by 24 months of age were identified as transmitters. Transmitters were included in the current study if they had plasma samples available at both baseline and at the time of their infant's HIV diagnosis, or at a later time point up to age 24 months (n=120). Transmitters were included in the analysis if their samples were successfully amplified and analyzed by next generation sequencing (NGS), for at least one genomic region of both samples screened (n=91). Transmitters were initially matched as a group on study arm and duration of follow-up to women who did not transmit HIV to their infants (non-transmitters, n=454). Appropriate baseline and follow-up samples according to length of follow-up were available for 164 non-transmitters. Amplification and NGS of at least one genomic region of both samples was successful for 91 non-transmitters who were included in the current study. HIV viral load and CD4 cell count testing were performed on baseline samples in the PEPI-Malawi trial [15].
HIV superinfection analysis
Maternal plasma samples were analyzed using NGS to identify HIV SI, as described previously [1, 8]. Briefly, HIV RNA was extracted from 140μL plasma, reverse-transcribed, and amplified using a nested-polymerase chain reaction (PCR) to produce amplicons corresponding to portions of the viral p24 (~390 basepairs) and gp41 (~324 basepairs) genomic regions. Samples that were successfully amplified for both study visits (baseline and follow-up) in at least one region were sequenced using the 454 DNA Sequencing platform as previously described, with adjustments to use a 2-region format (Roche, Branford, CT) [1, 2, 8]. Pools of samples were processed using emPCR Amplification Manual-Lib-L-LV – June 2013 (Roche Branford, CT) using 25% of the recommended amplification primer amount and a 0.2 copy-per-bead ratio [1].
The resulting sequencing reads were analyzed and similar sequences were combined into a single consensus sequence. Consensus sequences that encompassed a cluster of at least ten individual, near-identical sequence reads were determined [1, 2]. In order to remove any residual contaminating sequences a representative sequence from all distinct viral populations for each sample run in a given NGS sequencing plate were combined in a neighbor-joining tree, and any micro-contamination or spillover sequences that localize with another unrelated sample are removed. The clean sequences from the women's two sample time points were analyzed in a neighbor-joining tree with a variety of HIV reference sequences containing representative sequences of the major African subtypes (n=23 for p24 and n=25 for gp41) as well as a collection of sequences from Malawi (n=13 for p24, and n=30 for gp41).
HIV SI was defined when the follow-up sample yielded two or more distinct consensus sequences forming a phylogenetic cluster that was distinct from the consensus sequences in the baseline sample, and were of adequate genetic distance from the baseline sequences to rule out evolutionary drift [1]. Genetic distance cut-off for HIV SI was a rate ≥0.59% per year for the p24 region, or ≥0.98% per year for the gp41 region, which is equal to the mean plus twice the standard deviation of the intra-person viral divergence from a previous analysis[1]. Any SI events were re-amplified and sequenced in a separate reaction to confirm SI [2]. The consensus sequences for gp41 and p24 are available upon request (aredd2@jhmi.edu).
Statistical analysis
Differences between women in the transmitter and non-transmitter groups were compared using Fisher's exact test for categorical variables. For continuous variables, medians and interquartile ranges (IQR) are reported and non-parametric tests were used for comparisons since data were not normally distributed (Wilcoxon Rank Sum/Kruskal-Wallis). Similar methods were used to compare characteristics across three groups of non-transmitters to assess for possible bias: a) all non-transmitters, b) non-transmitters selected for sequencing, c) non-transmitters with samples and NGS results available for analysis. The association between HIV SI and transmission risk was examined by multiple logistic regression adjusted for maternal age, baseline log10 viral load and baseline CD4 cell count. Rates of SI were calculated as confirmed SI events divided by person years of follow-up. Incidence rate ratios (IRR) were estimated using Stata 11. Risk factors for HIV SI were examined by logistic regression; PEPI study arm, baseline CD4 cell count and breastfeeding duration were included in the multivariate model.
Results
Characteristics of the 91 non-transmitter women included in this analysis were not significantly different from those of all other non-transmitting women enrolled in the PEPI-Malawi trial (Table 1). Non-transmitters analyzed for HIV SI had significantly higher baseline CD4 cell counts (p=0.001), lower baseline HIV viral loads (p<0.0001), and slightly shorter breastfeeding duration than transmitters (n=91, p=0.048, Table 1). Transmitters and non-transmitters included in the analysis did not differ significantly in age or time to postpartum resumption of sexual activity (Table 1).
Table 1.
Demographic and clinical characteristics of study participants.
| Non-transmitters (Non-TM) |
||||||
|---|---|---|---|---|---|---|
| Characteristics | Transmitters (TM) N=91 | Non-TM included N=91 | Non-TM eligible for sequencing N=363 | All other Non-TM N=2297 | p-value* TM vs Non-TM included | p-value® (3 Non-TM groups) |
| Median maternal age (IQR), yrs | 25 (24-28) | 25 (23-29) | 25 (22-30) | 26 (23-29) | 0.76 | 0.89 |
| Median parity (IQR) | 3 (2-4) | 3 (2-4) | 3 (2-4) | 3 (2-4) | 0.10 | 0.49 |
| PEPI study arm, n (%) | 1.00 | 1.00 | ||||
| Control | 28 (30.8) | 28 (30.8) | 112 (30.9) | 717 (31.2) | ||
| Intervention | 63 (69.2) | 63 (69.2) | 251 (69.1) | 1580 (68.8) | ||
| Median baseline CD4 (IQR), cells/μL | 301.0 (208.0-451.0) | 427.0 (274.0-583.0) | 391.0 (268.0-595.0) | 401.5 (267.0-588.0) | 0.001 | 0.91 |
| Median baseline log10 VL (IQR), copies | 4.6 (4.1-5.1) | 4.0 (3.6-4.4) | 4.0 (3.4-4.6) | 4.0 (3.4-4.6) | <0.0001 | 0.70 |
| Median time to resumption of sexual activity (IQR), mos | 6.1 (5.9-9.0) | 6.0 (5.9-9.0) | 6.0 (5.9-9.0) | 6.0 (5.9-9.0) | 0.77 | 0.82 |
| Median BF duration (IQR), mos | 9.0 (8.9-17.9) | 9.0 (8.8-9.4) | 9.0 (8.9-10.0) | 9.0 (8.9-11.9) | 0.048 | 0.61 |
| Superinfection, n (%) | 5 (5.5) | 5 (5.5) | 1.00 | |||
Abbreviations: N: number of individuals; TM: transmitter; non-TM: non-transmitter; IQR: interquartile range; PEPI: Post-exposure Prophylaxis of Infants in Malawi trial; yrs: years; mos: months; BF: breastfeeding.
p-values were calculated using Fisher's exact test for categorical variables, Wilcoxon Rank Sum test for continuous variables.
p-values were calculated using Fisher's exact test for categorical variables, Kruskal-Wallis test for continuous variables.
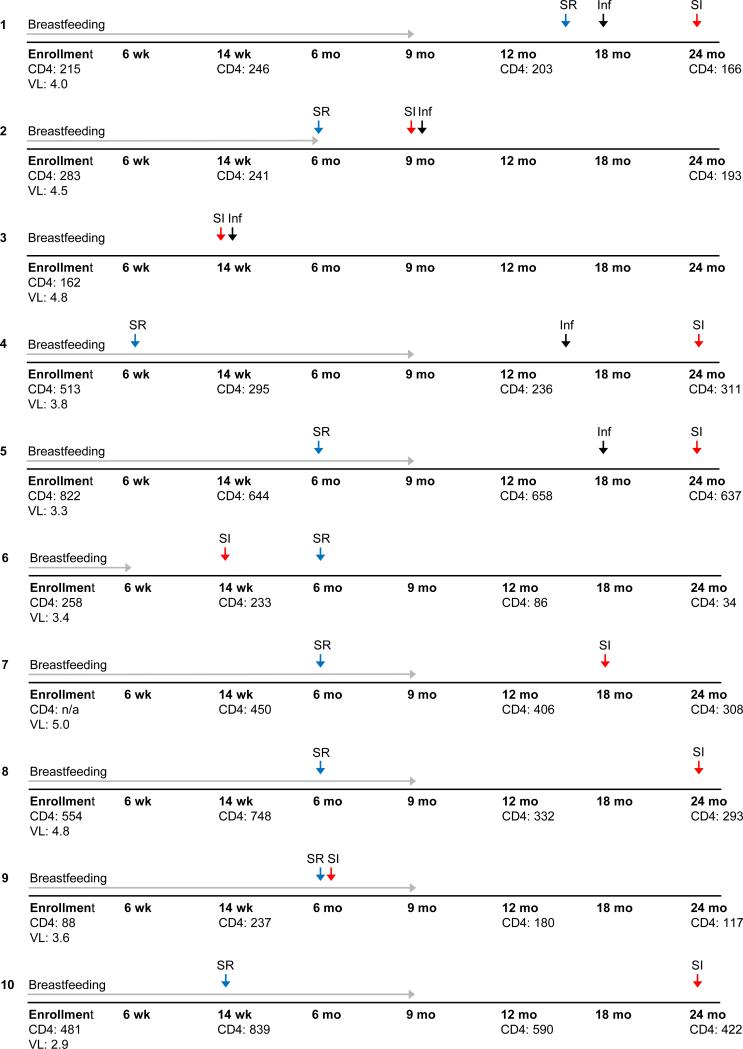
Five cases of SI were identified in women from the transmitter group compared to five in the non-transmitter group (p=1.0, Table 1, Figure 1, and supplementary figure 1). Three of the ten SI events involved complete replacement of the original strain by the new superinfecting strain [2]. The rates of SI in the transmitters and non-transmitters were 4.7/100 person years (pys) [95% confidence interval (CI)=1.5-11.0] and 4.4/100 pys (95% CI=1.4-10.3), respectively (IRR=1.1, 95% CI=0.3-4.7; p=0.92). As part of the original trial women were asked if they had a sexual partner (excluding husband) since their last visit, and if they were using condoms with that partner. Of the women examined here, 180 reported no extra sexual partners, with two women having missing data; therefore no association was found between extra-partners and risk of SI.
Figure 1.
Clinical and trial data by woman for the ten cases of HIV superinfection were identified. Five cases from the transmitting women (Cases #1-5) and five cases from the non-transmitters (Cases #6-10) are shown (corresponding phylogenetic trees are shown in supplemental figure 1). Grey arrow: indicates duration of breastfeeding based on self-report; Black arrow: indicates the visit where infant was diagnosed with HIV; Red arrow: indicates the visit HIV superinfection was detected; Blue arrow: indicates the visit the woman reported resumption of sexual activity. Abbreviations: Inf: Infant; SI: superinfection; SR: sex resumed; wk: weeks; mo: months; CD4: CD4 cell count (expressed as cells/mm3); n/a: not applicable; VL: HIV viral load (expressed as log10copies/mL).
Maternal HIV SI did not increase the odds of MTCT of HIV via breast-feeding when controlling for maternal age, baseline CD4 cell count, and baseline viral load (adjusted OR=2.32, 95% CI=0.47-11.47; p=0.30, Table 2). In univariate and adjusted models, longer breastfeeding duration was borderline significantly associated with a lower risk of HIV SI after controlling for baseline viral load and PEPI study arm (p=0.05, Table 3).
Table 2.
Association between maternal superinfection (SI) and mother-to-child transmission of HIV via breastfeeding.
| Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|
| Factors | n/N | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Superinfection | 1.00 | 0.30 | |||
| No (ref) | 172/182 | 1 | 1 | ||
| Yes | 10/182 | 1.66 (0.52-5.27) | 2.32 (0.47-11.47) | ||
| Maternal age | 0.44 | 0.53 | |||
| 17-24 yrs (ref) | 68/182 | 1 | 1 | ||
| ≥25 yrs | 114/182 | 1.32 (0.73-2.42) | 0.79 (0.37-1.66) | ||
| PEPI study arm | 1.00 | ||||
| Control (ref) | 56/182 | 1 | |||
| Intervention | 126/182 | 1.00 (0.51-1.97) | |||
| Baseline CD4 cell count | 0.002 | 0.11 | |||
| <250 cells/μl (ref) | 49/172 | 1 | 1 | ||
| ≥250 cells/μl | 123/172 | 0.33 (0.15-0.69) | 0.50 (0.22-1.16) | ||
| Baseline log10 VL | 0.001 | 0.004 | |||
| <4 log10 copies (ref) | 55/157 | 1 | 1 | ||
| ≥4 log10 copies | 102/157 | 3.44 (1.64-7.43) | 3.06 (1.44-6.54) | ||
Abbreviations: N: total number of individuals; n: number of individuals with given trait; OR: odds ratio; CI: confidence intervals; PEPI: Post-exposure Prophylaxis of Infants in Malawi trial; VL: viral load.
Table 3.
Association between selected risk factors and superinfection
| Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|
| Factors | n/N | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Maternal age | 0.86 | - | - | ||
| 17-24 yrs | 68/182 | 1 | |||
| ≥25 yrs | 114/182 | 0.89 (0.24-3.27) | |||
| Parity | 0.84 | - | - | ||
| 1-2 | 60/182 | 1 | |||
| >2 | 122/182 | 1.16 (0.29-4.64) | |||
| PEPI study arm | 0.19 | 0.45 | |||
| Control | 56/182 | 1 | 1 | ||
| Intervention | 126/182 | 0.42 (0.12-1.52) | 0.54 (0.11-2.71) | ||
| Baseline CD4 cell count, 100 cells/μL | 0.97 (0.69-1.34) | 0.84 | |||
| Baseline log10 VL | 0.46 (0.17-1.24) | 0.12 | 0.40 (0.13-1.19) | 0.10 | |
| Time to resumption of sexual activity, mos | 0.90 (0.73-1.11) | 0.32 | |||
| BF duration, mos | 0.78 (0.62-0.99) | 0.04 | 0.75 (0.56-1.00) | 0.05 | |
Abbreviations: N: total number of individuals; n: number of individuals with given trait; OR: odds ratio; CI: confidence intervals; PEPI: Post-exposure Prophylaxis of Infants in Malawi trial; VL: viral load; yrs; years; mos: months; BF: breastfeeding.
Discussion
HIV SI has been observed in multiple populations and settings, and is associated with underlying HIV risk in the same populations [4]. Postpartum women in Malawi have been shown to be at increased risk for primary HIV infection [12]. The rate of HIV SI observed among HIV-infected women in the PEPI-Malawi trial corroborates this risk of exposure and vulnerability to HIV primary infection in the postpartum period. The study also demonstrated a significant association of maternal viral load with MTCT of HIV, which is a well-established risk factor. Longer breastfeeding duration was associated with decreased odds of SI, although the mechanism for this relationship is not clear. A previous analysis of this population found that women who breastfed had a 48% lower likelihood of resuming sexual activity early, which may be associated with the lower risk of SI [16]. Additionally, in at least four of the transmission events the mother's reported cessation of breastfeeding prior to the period when their child was infected. It should be noted that this study examined viral populations from the mothers only, and does not address what viral strain was transmitted to their infants.
One limitation of this study was that the transmitter group was selected for analysis based on the assumption that MTCT would be associated with SI. While this association was not seen in this population, inclusion of a significant proportion of transmitters in this study (50% of the women assessed for SI) may have biased the overall rate of SI. Another limitation of this study is that for five of the women who appeared to be superinfected a sample available post-SI was not possible because their SI event was detected in samples collected at their last study visit. Additionally, this study was a secondary analysis of a well-studied cohort of patients and therefore in the other five cases later samples were not readily available. These facts limited the ability to verify the SI events in a second sample post-SI, and means that in the cases of full replacements a sample mix-up cannot be ruled out. However, all SI events were re-amplified to confirm SI, which should help address this concern. The limited sample volume for these samples also did not allow to perform HIV viral load analysis on the follow-up samples, although the enrollment viral load was previously measured as part of the original trial.
Case studies, as well as a cohort study of Kenyan female bar workers, have demonstrated that HIV SI is associated with both transient and persistent increases in HIV viral load, one of the main drivers of HIV transmission [4, 9, 10, 17, 18]. It is unknown what role HIV SI has on MTCT or sexual transmission, but given the effect it has on viral load, it is possible that SI could temporarily increase the infectiousness of the individual. However, it is difficult to establish the directionality and relationship of SI and transmission events in the setting of sexual transmission [4, 7]. This issue is not relevant in MTCT of HIV, since transmission only occurs vertically. In this study, although the rate of SI was significant in women after delivery, SI was not associated with an increased risk of MTCT of HIV through breastfeeding. It should be pointed out that this study might have been underpowered to detect this association.
The current best clinical practice in Africa for prevention of MTCT of HIV is to provide all HIV-infected mothers with highly active ARV therapy (ART) during pregnancy and to continue ART for the rest of their lives (i.e., Option B+)[19]. Continued provision of ART after delivery should reduce the risk of HIV SI. The high rates of SI observed in this study support the use of Option B+ moving forward, since it is likely to reduce the risk of MTCT and potential adverse effects of HIV SI, such as higher viral loads.
Supplementary Material
Acknowledgements
The authors would like to thank the study participants and staff in Malawi. The authors have no conflicts to report. This study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by grant 1R01-AI087139 (Eshleman, principal investigator). The PEPI trial was supported by a cooperative agreement (5-U50-PS022061-05; award U50-CC0222061) from the Centers for Disease Control and Prevention and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
ClinicalTrials.gov number, NCT00115648.
References
- 1.Redd AD, Collinson-Streng A, Martens C, Ricklefs S, Mullis CE, Manucci J, et al. Identification of HIV superinfection in seroconcordant couples in Rakai, Uganda using next generation deep sequencing. J Clin Microbiol. 2011;49:2859–2867. doi: 10.1128/JCM.00804-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redd AD, Mullis CE, Serwadda D, Kong X, Martens C, Ricklefs SM, et al. The Rates of HIV Superinfection and Primary HIV Incidence in a General Population in Rakai, Uganda. J Infect Dis. 2012;206:267–274. doi: 10.1093/infdis/jis325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redd AD, Mullis CE, Wendel SK, Sheward D, Martens C, Bruno D, et al. Limited HIV-1 superinfection in seroconverters from the CAPRISA 004 Microbicide Trial. J Clin Microbiol. 2014;52:844–848. doi: 10.1128/JCM.03143-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redd AD, Quinn TC, Tobian AA. Frequency and implications of HIV superinfection. Lancet Infect Dis. 2013;13:622–628. doi: 10.1016/S1473-3099(13)70066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronen K, McCoy CO, Matsen FA, Boyd DF, Emery S, Odem-Davis K, et al. HIV-1 Superinfection Occurs Less Frequently Than Initial Infection in a Cohort of High-Risk Kenyan Women. PLoS Pathog. 2013;9:e1003593. doi: 10.1371/journal.ppat.1003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner GA, Pacold ME, Kosakovsky Pond SL, Caballero G, Chaillon A, Rudolph AE, et al. Incidence and Prevalence of Intrasubtype HIV-1 Dual Infection in At-Risk Men in the United States. J Infect Dis. 2014;209:1032–1038. doi: 10.1093/infdis/jit633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraft CS, Basu D, Hawkins PA, Hraber PT, Chomba E, Mulenga J, et al. Timing and source of subtype-C HIV-1 superinfection in the newly infected partner of Zambian couples with disparate viruses. Retrovirology. 2012;9:22. doi: 10.1186/1742-4690-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redd AD, Ssemwanga D, Vandepitte J, Wendel SK, Ndembi N, Bukenya J, et al. Rates of HIV-1 superinfection and primary HIV-1 infection are similar in female sex workers in Uganda. AIDS. 2014;28:2147–2152. doi: 10.1097/QAD.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronen K, Richardson BA, Graham SM, Jaoko W, Mandaliya K, McClelland RS, et al. HIV-1 superinfection is associated with an accelerated viral load increase but has a limited impact on disease progression. AIDS. 2014;28:2281–2286. doi: 10.1097/QAD.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 11.Kumwenda NI, Hoover DR, Mofenson LM, Thigpen MC, Kafulafula G, Li Q, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 12.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Taha TE, Dallabetta GA, Hoover DR, Chiphangwi JD, Mtimavalye LA, Liomba GN, et al. Trends of HIV-1 and sexually transmitted diseases among pregnant and postpartum women in urban Malawi. AIDS. 1998;12:197–203. doi: 10.1097/00002030-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 15.Taha TE, Li Q, Hoover DR, Mipando L, Nkanaunena K, Thigpen MC, et al. Postexposure prophylaxis of breastfeeding HIV-exposed infants with antiretroviral drugs to age 14 weeks: updated efficacy results of the PEPI-Malawi trial. J Acquir Immune Defic Syndr. 2011;57:319–325. doi: 10.1097/QAI.0b013e318217877a. [DOI] [PubMed] [Google Scholar]
- 16.Makanani B, Kumwenda J, Kumwenda N, Chen S, Tsui A, Taha TE. Resumption of sexual activity and regular menses after childbirth among women infected with HIV in Malawi. Int J Gynaecol Obstet. 2010;108:26–30. doi: 10.1016/j.ijgo.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, et al. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature. 2002;420:434–439. doi: 10.1038/nature01200. [DOI] [PubMed] [Google Scholar]
- 18.Jost S, Bernard MC, Kaiser L, Yerly S, Hirschel B, Samri A, et al. A patient with HIV-1 superinfection. N Engl J Med. 2002;347:731–736. doi: 10.1056/NEJMoa020263. [DOI] [PubMed] [Google Scholar]
- 19.WHO Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013:272. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.