Abstract
Atrial fibrillation (AF) remains the most frequent sustained cardiac arrhythmia worldwide and its incidence increases with ageing, cardiovascular risk factors and comorbidities. Prevalence of diabetes mellitus (DM) is growing fast and is assuming pandemic proportions mostly due to overnutrition and sedentary habits. Experimental and clinical evidences suggest that DM and AF are strongly interconnected. The present review addresses in detail new molecular pathways implicated in the etiology of AF and their relevance for mechanism-based therapeutic strategies in this setting. Advances in risk stratification, drug therapy (i.e., novel anticoagulants) and catheter ablation are also described.
Keywords: Atrial fibrillation (AF), diabetes mellitus (DM), anticoagulants, catheter ablation
The mutual relationship between atrial fibrillation (AF) and diabetes mellitus (DM)
AF management represents a daily challenge for physicians in terms of controlling disabling symptoms and protecting from thromboembolism. AF is associated with multiple complications including heart failure and stroke. It is also related with a significant increase in mortality (1). The prevalence of AF is markedly related with advanced age. Due to a significant increase in overall survival AF prevalence is becoming dramatically high and it is nowadays the most prevalent cardiac sustained arrhythmia (2). Consensually the number of people with DM is alarmingly increasing worldwide due to the growing prevalence of lifestyle changes leading to obesity, or genetic susceptibility (3). Moreover, the number of affected people is expected to rise further if we consider ageing. AF is one of the main cardiovascular complications associated with diabetic disease. Data from the Framingham Heart Study showed that in addition to intrinsic cardiac causes such as valve disease and congestive heart failure, risk factors for cardiovascular disease also predispose to AF. Between these, DM conferred an odds ratio of 1.4 for men and 1.6 for women, after multivariable adjustment, for developing AF (4). The incidence of AF in patients with DM is reported to be 14.9%, and in the same study atrial flutter occurred in 4% of DM patients vs. 2.5% of the control group (P<0.0001) (5). Furthermore DM is cohesive to metabolic syndrome, including obesity which is an established risk factor for AF (6).
However, the link between AF and DM is much more mutual and reciprocal. Indeed, AF predicts worse prognosis in DM patients and accounts for high mortality rates. The main problem is that in many cases DM remains undiagnosed and people are aware of the disease only at an advanced stage. That implies that DM-related complications progress without any treatment for several years. For example, subtle diabetic neuropathy may mask cardiac symptoms of a first-detected episode of AF, thus increasing the chances to develop complications such heart failure and stroke. This may have major clinical implications since longer AF duration renders the arrhythmia more resistant to any cardioversion attempt. In 289 patients with persistent AF, logistic regression analysis showed that duration of AF (P<0.0001) and presence of DM (P=0.019) were independent predictors for AF recurrence (7).
Several efforts have been done in understanding how altered molecular pathways in DM patients may affect the initiation and/or progression of AF. These molecular insights have been translated into mechanism-based therapeutic approaches. Recent clinical trials have shown that development of novel anticoagulants as well as advances in technology yielded to consistent benefits in morbidity and mortality for AF patients. The present review provides a translational perspective, reporting seminal basic findings underpinning electrical and anatomical remodeling as well as their application in clinical practice.
New mechanistic insights into ionic currents flows and therapeutic implications
The current unifying most reliable electrophysiological hypothesis which may explain how AF initiates and perpetuates is that focal triggers inside pulmonary veins (PVs) are critically involved in the initiation of reentry circuits. Eventually, consequent atrial remodeling leads to additional focal triggers and perpetuation of such microwave reentry (8). Different alterations in cardiac action potential duration (APD) and its related ionic currents lead to arrhythmogenesis. Through the five phases of cardiac action potential (AP) ionic currents flow inward and outward the myocyte. Atrial peak INa blockers such as amiodarone, vernakalant have known therapeutic effects on suppression of AF. Their action is largely due to rate-dependent reduction of excitability, prolongation of APD and thus effective refractory period (ERP). The role of agents blocking Late INa current is less defined. However seems that Late INa inhibition may suppress the trigger that initiate AF (i.e., through a reduction of intracellular calcium loading), especially in the setting of prolonged APD and bradycardia. This condition could be present in long QT syndrome, congestive heart failure, atrial dilation and hypertension (9). Ranolazine is a Late INa current blockers whose anti-angina effects have been well characterized. More recently ranolazine showed to reduce reactive oxygen species (ROS) hydrogen peroxide-induced arrhythmogenic activity in pigs and rabbits inhibiting the INa mediated prolongation of activation potential due to reactive species (10). Several animal studies have confirmed antiarrhythmic effects of INa block as well as rapidly activating delayed rectifier potassium current (Ikr) also blocked by ranolazine (11,12). Clinical evidence is however limited and derives from small, uncontrolled studies. A post hoc analysis of MERLIN TIMI 36 trial showed a trend towards a few episodes of new-onset AF with a lower overall AF burden in patients with an acute coronary syndrome when allocated in the ranolazine group (13). In the same population Ranolazine significantly reduced levels of HbA(1c) in diabetic patients and the incidence of increased HbA(1c) in those without evidence of previous hyperglycemia (14). Ranolazine seems to be effective in facilitating restoration of sinus rhythm when added to amiodarone, especially in patients with dilated atria (15). This synergistic effect has been confirmed also with dronedarone in preliminary data from HARMONY trial (16). In conclusion, due to its metabolic, antiarrhythmic, anti-angina effects, ranolazine shows great promise for more effective pharmacologic management of AF in patients with DM.
Emerging role of transcription factors in AF
Over the past decade results of genetic studies have suggested that AF can be also a heritable disease (17). Monogenic mutations in AF families have been characterized, but common genetic variants have been discovered also in general population (18,19). In these settings mutations across transcription factors play a crucial role in AF susceptibility. They regulate the expression of a range of genes involved in electrical stability in the atrium, influencing atrial conduction velocity by atrial remodeling. Indeed, they shorten cellular refractory periods, thus increasing automaticity of PVs foci (20). For example the transcription factor nuclear factor-kappa B (NF-kB), influencing redox signaling pathway or angiotensin cascade, enhances conduction heterogeneity promoting reentry (21). NF-κB-mediated vascular inflammation in DM is well defined. In the vessels hyperglycemia determines overproduction of ROS and decreases nitric oxide (NO) availability, leading to NF-κB up-regulation which mediates transcription of pro-inflammatory genes (e.g., encoding for adhesion molecules) thus perpetuating inflammatory state (22). The pivotal role of NF-κB in oxidative stress, vascular and myocardial dysfunction and inflammation in diabetes as well as in the genesis of AF, renders this transcription factors a very reliable target to be modulated for diabetic patients with AF. This need to be confirmed by experimental and clinical studies yet. In the same way, PPAR-gamma has shown to increase atrial arrhythmias in cardiac myocytes. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) has proven anti-inflammatory effects. Using a case-control study design Chen et al. showed PPAR-gamma mRNA was markedly reduced in hypertensive patients with AF as compared with group without the arrhythmia. Moreover, it was significantly lower in persistent AF than paroxysmal AF (23). Thiazolidinediones (TZDs), that are peroxisome proliferator-activated receptor-gamma activator, have been already proven to have anti-inflammatory and anti-oxidant effects in addition to their anti-diabetic activity (Figure 1). A pilot study by Lin et al. showed in elderly patients with AF a strong correlation between lower levels of PPAR-gamma receptor protein and higher serum levels of inflammation markers as hs-CRP, IL-6 and TNF-alfa (24). Among 12,000 diabetic patients Chao and colleagues found a 1.6% of new onset AF. After adjustment for other variables, the TZDs independently protected patients from development of new AF (25). Indeed pioglitazone, a TZD, may retard the progression to permanent AF in patients with DM with firstly identified persistent AF, via reducing circulating levels of pro-collagen type I carboxy-terminal peptide (PICP) and advanced glycosylation end products (AGEs), thus influencing atrial remodeling (26). Further randomized multicenter trials are aimed to confirm a strong indication of these medications in patients with diabetes and AF.
Figure 1.
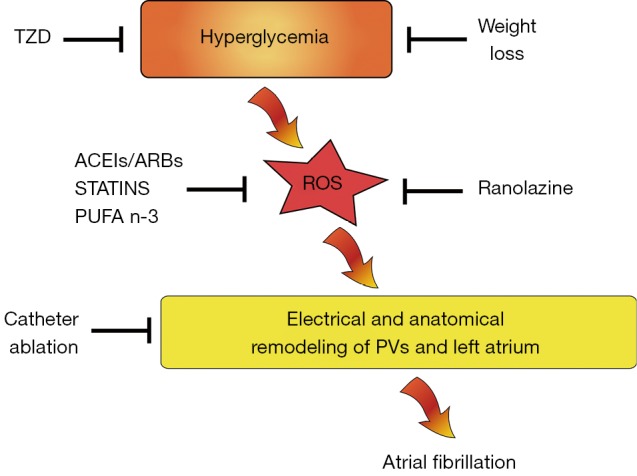
Mechanism linking hyperglycemia to atrial fibrillation (AF) and potential therapeutic interventions. TZD, thiazolidinediones; ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ROS, reactive oxygen species; PVs, pulmonary veins.
Pharmacological remodeling of atrial substrate: is upstream therapy satisfactory?
Detrimental effects of hyperglycemia in diabetes go beyond alterations in vascular homeostasis and target cardiac myocytes too. Increased inflammation and oxidative stress provokes formation of AGEs, cellular apoptosis, mitochondrial dysfunction, contraction-relaxation dysfunction, disorders of myocardial metabolism. AGEs infiltrate myocardium leading to interstitial fibrosis and hypertrophy (27). All these events, collected into the term DM cardiomyopathy, determine the substrate for anatomic and electrical atrial remodeling. In fact the presence of interstitial fibrosis lead to anisotropic impulse propagation underlying the initiation and perpetuating of microwave reentry that begets AF. Although a positive linear association has been demonstrated between HbA1c and risk of AF in patients with or without diabetes (28), contrary is not so obvious. In fact intensive glycemic control did not affect the rate of new-onset AF in a total of 10,082 patients with DM from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) cohort (29). Patients with DM and incident AF had an increased risk for morbidity and mortality compared with those without AF. However a strict control of all risk factors related to diabetes could contribute to reduce described mechanisms thus decreasing AF incidence. A very recent study by Pathak et al. showed how long-term sustained weight-loss, included avoidance of weight-fluctuation, is associated with significant reduction of AF burden and maintenance of sinus rhythm in patients with BMI >27 kg/m2 (30). Weight-loss >10% resulted in a six-fold (95% CI: 3.4-10.3, P<0.001) greater probability of arrhythmia-free survival compared to other less strict weight-loss managements. This highlights the pivotal role of lifestyle modifications in the treatment, as well as in the prevention of AF. An upstream pharmacological therapy has been proposed to prevent or delay myocardial remodeling associated with hypertension or heart failure deterring development of new AF or reducing its rate of recurrence. Angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), aldosterone antagonists, HMG-CoA reductase inhibitors (statins), and omega-3 polyunsaturated fatty acids (PUFAs) have been individualized as potential drugs that could act as anti-remodeling agents. Activation of the renin angiotensin aldosterone system (RAAS) plays a central role in DM-related myocardial fibrosis and inflammation (31). Results from meta-analysis and clinical studies are disappointing. In the LIFE study angiotensin II receptor blockade reduced new-onset AF (primary prevention) and subsequent stroke compared to atenolol in hypertensive patients (32). On the other hands valsartan, when added on top of optimal medical therapy in patients with cardiovascular risk factors and paroxysmal or recently cardioverted persistent AF, had no effect on the primary end-point of time to first AF recurrence in the GISSI-AF study (secondary prevention) (33). Roles of statins, especially atorvastatin, in the reduction of AF after coronary surgery are well defined but their added value in other AF patients’ cohorts is still to be validated. The reasons of this difference has been postulated to be that postoperative coronary surgery AF is characterized by severe inflammation and oxidative stress resulting from extracorporeal circulation and surgical manipulation of the epicardial coronary vessels. Thus the well-known anti-inflammatory and antioxidant effects of atorvastatin may account for its beneficial effects in these patients. On the contrary non-postoperative AF, especially recurrent AF, is often caused by atrial remodeling and changes in electrical, contractile, and structural properties of the atria and may be less responsive to statins compared with postoperative AF (34). In the same way, although some prior experimental results, in patients with paroxysmal or persistent AF, treatment with n-3 PUFAs neither reduced the recurrence of AF, nor was it able to affect markers of inflammation and oxidative stress (35). In conclusion, evidence in support of the use of up-stream therapy of AF is insufficient to make any recommendations in patients with DM. International guidelines only suggest ACEIs and ARBs should be considered for prevention of new-onset AF in patients with other compelling indications (e.g., heart failure and reduced ejection fraction, hypertension with left ventricular hypertrophy) (36). Further studies are ongoing in this field of research.
Interventional approaches against atrial electrical remodeling: the role of AF Ablation
During the last two decades catheter ablation has emerged as a useful tool to treat AF. The contemporary cornerstone of AF ablation is electrical isolation of the PVs by creation of circumferential lesions around the right and left PV ostia, potentially impacting both the trigger (PV ectopy) and substrate of AF by eliminating part of the atrial tissue located near the PV-atrium junction (37). The same basic treatment strategy of PV isolation, complemented with several additional ablation strategies, has been implemented for treatment of persistent as well as paroxysmal AF. However, optimal strategies have not yet been defined for the treatment of the various AF subforms, particularly in the case of extensive atrial remodeling and/or longstanding persistent AF (38). DM and AF may share pathophysiological common pathways, as evidenced by the clear impact DM has on the risk of developing AF (39). It is likely that catheter ablation is often performed in patients with DM, but little data exists studying this subgroup as a controlled, pre-specified population. The recent MANTRA-PAF trial compared radiofrequency ablation with antiarrhythmic drug therapy as first-line treatment in patients with paroxysmal AF, and found no significant differences between the treatment groups in the cumulative burden of AF over a period of 2 years. Patients enrolled in the ablation group, as well as in the drug therapy arm were relatively young (mean age 56 years), with hypertension (29%) and with a low thromboembolic risk (92% had a CHADS2 score of 0). DM was present only in 4% of patients (6/146) in the ablation arm and in 7% (10/148 pts) in the drug therapy group (40).
Similarly low numbers are present in the A4 trial, demonstrating the superiority of catheter ablation over antiarrhythmic drugs in patients with paroxysmal AF with regard to maintenance of sinus rhythm and improvement in symptoms, exercise capacity, and quality of life. In their analysis the authors report a DM prevalence of only 1/53 pts (1.9%) in the ablation group and 2/59 pts (3.4%) in the antiarrhythmics group (41). A long term follow-up report on middle aged patients 5 years after ablation for paroxysmal AF showed stable sinus rhythm in the majority of patients, and a low incidence of chronic AF. DM was present in 8/151 (5%) of patients (42). A specific paper randomizing a DM population to catheter ablation versus medical treatment consisted of 35 patients that received ablation and reports superiority of ablation over drug treatment in terms of achieving rhythm control (43). The low prevalence of DM in these and similar trials makes subgroup analysis for diabetic patients difficult in terms of assessing its value as a predictor of AF recurrence. A possible explanation for this low number of DM patients in these studies could be offered by the enrollment of subjects with paroxysmal AF, which typically occurs in relatively young subjects, without CV risk factors. One might expect in trials enrolling persistent AF or AF in the setting of heart failure, these rates should be higher because of the older age and presence of comorbidities. In 2012 Narayan and colleagues described rotor modulation as an additive approach to the ablation of AF. Among 107 procedures (mainly for persistent AF) 34 (31%) were done in patients with DM (44). Indeed, when selecting subjects with either persistent or high-burden paroxysmal AF for complex atrial fractionated electrograms (CAFE) ablation, Verma and colleagues found rates of diabetic patients between 10% and 16% (45).
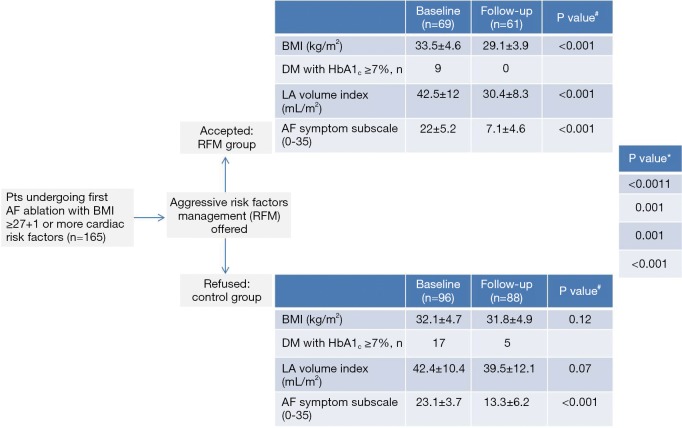
In the current pathogenetic model of AF, the changes in atrial cellular structure that are linked to DM contribute to the development and maintenance of AF in its various subtypes. Crucially, the question whether intervention for AF in an early disease stage may prevent later development of AF if these DM-related factors remain present, is still unanswered. The ongoing EAST, CABANA and other early intervention trials may provide important insights. The recent ARREST-AF trial provides spectacular new insights in this field, demonstrating that using a strategy of aggressive risk factor modification including weight loss and improved glycemic control, the odds of arrhythmia-free survival after ablation increased almost 5-fold (46) (Figure 2). A tailored approach targeting weight loss should be offered as first line and unlimited therapy in all obese patients referred for AF treatment, cohesively with other kind of pharmacological or more invasive treatments as AF ablation.
Figure 2.
ARREST-AF cohort study flow chart showing impact of risk factors management on cardiac and metabolic variables and AF severity scale. Values are expressed as mean ± SD or n. P value# is for within-group differences (baseline to follow-up). P value* is for between-group differences over time (group-time interaction). The median follow-up of the study was 42.8 months for the RFM group and 42.4 months for the control group. DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; LA, left atrium. Adapted from Pathak et al. (46).
Thromboembolic risk in patients with DM and role of non-vitamin K antagonist oral anticoagulants
Ischemic stroke is a leading cause of mortality and long-term disability worldwide. Patients with DM have an increased risk of stroke and worse outcome (47,48). This is why they have both increased susceptibility to atherosclerosis and increased prevalence of stroke risk factors such as arterial hypertension and dyslipidemia (49). The prothrombotic risk of DM in AF and heart failure is only partially understood. In a substudy to the SPAF-III trial (3rd stroke prevention in AF study), Varughese et al. showed that DM independently contributed to endothelial damage/dysfunction in patients with AF, and that this effect was exaggerated further in individuals with CHF, and increasing BMI but DM had no effect on platelet activation (50). Prevention of stroke in DM patients should take into account a multifactorial treatment including anti-hypertensive agents, statins, and glucose lowering drugs (51). The occurrence of AF is one of the main triggers underlying ischemic stroke in DM patients (52). Available evidence from community residents (Northern Manhattan Study; 21% white, 24% black, and 53% Hispanic) indicates that patients with DM and increased fasting glucose levels are at increased risk of stroke or vascular events compared to those with target glucose levels (53). At present, we dispose of several tools to stratify stroke risk in DM patients and this clinical information is invaluable to implement primary prevention strategies. Guidelines propose CHA2DS2-VASc [cardiac failure, hypertension, age ≥75 years (doubled), DM, stroke (doubled)-vascular disease, age 65-74 and sex category (female)] as a useful risk stratification tool to be used before prescribing oral anticoagulation in patients with AF (36). This index has shown to be rather specific since patients with a CHA2DS2-VASc score of 0 have a truly low risk of ischemic stroke, with an annual stroke rate of approximately 1% or even lower; in a nationwide Danish cohort in untreated patients 0.49 per 100 person-years (p-y) at 1 year of note, CHA2DS2-VASc includes DM and this might significantly contribute to improve the accuracy of such score in predicting stroke risk (54). On the other hand, DM is not included among well-established risk factors employed to assess bleeding risk in AF recipients on oral anticoagulation therapy (HASBLED, ATRIA, HEMORR2HAGES) (55-57). Evidence accumulated so far strongly suggests that oral anticoagulation is mandatory when diabetes coexists in AF patients. Vitamin K antagonists, namely warfarin, have been for decades the cornerstone in patients at high risk for cardioembolic stroke and low risk of hemorrhagic complications (58). However, their use in clinical practice has always been challenging because of their multiple interactions with food and drugs, requiring strict monitoring of drug effects. Moreover, DM has emerged as one of the clinical factors affecting quality of anticoagulation control during warfarin treatment, as assessed by SAMe-TT2R2 score (59). These data imply that warfarin may not be the best option in diabetic patients with AF. Over the last few years, several randomized trials were launched to investigate the efficacy and safety of new non-vitamin K-antagonist anticoagulant such as dabigatran, rivaroxaban, apixaban, and edoxaban as compared to warfarin in patients with non-valvular AF (Table 1). These studies demonstrated that these novel drugs were associated with rates of stroke and systemic embolism similar or inferior to those associated with warfarin, as well as with equal or lower rates of major haemorrhage (60-63). Patients with DM are well represented in these trials, and their outcome was comparable to non-diabetic patients. A recent meta-analysis showed the consistency of the safety and efficacy of the new oral anticoagulants dabigatran, rivaroxaban, and apixaban across subgroups of patients with AF. They noted a trend towards interaction with heart failure with respect to reduction of stroke and systemic embolism (greater in patients not presenting heart failure) but not for DM. In contrast, reduction of major bleeding showed a non-significant trend (P=0.06) towards a difference between patients with (RR =0.97) and without (RR =0.76) DM (64). The proportion of patients with DM (5,695; 39.9%) in ROCKET AF was greater than in RE-LY (23.3%), in AVERROES (apixaban versus acetylsalicylic acid to prevent strokes) (19.2%), in ARISTOTLE (25%) and in ENGAGE-AF (effective anticoagulation with factor Xa next generation in AF—thrombolysis in myocardial infarction with edoxaban) (36%). In more detail, of the 18,113 patients in RE-LY trial (randomized evaluation of long-term anticoagulation therapy), 4,221 (23.3%) had DM. In this substudy, AF patients with DM had a higher prevalence of cardiovascular diseases, poorer INR control, and increased risk of adverse outcomes including bleeding. Relative benefits of dabigatran over warfarin were comparable among diabetics and non-diabetics. However, the diabetic patients displayed a greater absolute reduction in embolic events (65). A substudy of the ROCKET-AF trial (rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in AF) explored the efficacy and safety of rivaroxaban and warfarin in patients with and without diabetes. The rates of primary efficacy endpoints (stroke and systemic embolism) were similar between diabetic and non-diabetic patients (P=0.60 for interaction). Rates of major and non-major clinically relevant bleeding were similar between rivaroxaban and warfarin in diabetic patients (HR: 0.98; 95% CI: 0.79-1.21; P=0.84 and HR: 0.97; 95% CI: 0.86-1.08; P=0.57, respectively) (66). The ARISTOTLE trial (apixaban for reduction in stroke and other thromboembolic events in AF) showed that in patients with AF, apixaban was superior to warfarin in preventing stroke or systemic embolism (0.66 to 0.95; P<0.001 for non-inferiority; P=0.01 for superiority), caused less bleeding, and resulted in lower mortality. Apixaban-related benefits were also observed in the subgroup of 4,547 DM patients with 1.4 events as compared to 1.9% observed with warfarin. However, significance was reached only among non-DM subjects (n=13,654) (62). In the ENGAGE-AF trial, there was no difference in the primary outcome parameter of stroke or systemic embolism between edoxaban at high dose (P for interaction =0.54) or low dose (P=0.35) and warfarin. The same applies to major bleeding (P for interaction =0.70 and 0.52, respectively) (63). Remarkably, compared with warfarin, the relative efficacy and safety of novel oral anticoagulants are similar in patients with and without DM.
Table 1. Main clinical features and prevalence of diabetes mellitus (DM) in recent randomized AF trials with novel non-vitamin K antagonist oral anticoagulants.
| Study | RE-LY | ROCKET-AF | ARISTOTLE | ENGAGE-AF |
|---|---|---|---|---|
| Drug | Dabigatran | Rivaroxaban | Apixaban | Edoxaban |
| Age >75 years | 40% | 43.7% | 31.2% | 40.5% |
| CHADS2 mean | 2.2 | 3.48 | 2.1 | 2.8 |
| Previous TIA/stroke | 20.3% | 54.9% | 19.2% | 28.1% |
| Hypertension | 78.9% | 90.3% | 87.3% | 93.7% |
| Diabetes | 23.3% | 39.9% | 25% | 36.4% |
| CHF | 31.8% | 62.6% | 35.5% | 58.2% |
AF, atrial fibrillation; CHF, chronic heart failure; TIA, transient ischaemic attack; CHADS2, cardiac failure, hypertension, age, diabetes, stroke (doubled).
Conclusions
The occurrence of AF is one of the main triggers underlying ischemic stroke and cardiovascular morbidity in DM patients. Available evidence indicates that the association between AF and DM may significantly amplify morbidity and mortality. In this review, we have provided mechanistic insights and their potential translation to the clinical setting. Advances in understanding the factors predisposing to AF in DM has led to a better definition of therapeutic strategies, including the development of effective anticoagulants and refinement of electrophysiological procedures, namely catheter ablation. However, AF occurrence is driven by a cluster of different factors and we do still lack a detailed comprehension of the molecular cues linking hyperglycemia, insulin resistance with an arrhythmogenic substrate. Efforts in this direction will be invaluable for the design of novel mechanism-based approaches to reduce AF burden in patients with diabetes.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Francesco De Sensi, Tom De Potter and Alberto Cresti declare they have no associations (either commercial or industrial) that may pose a conflict of interest. Silva Severi received support from Menarini, Boehringer Ingelheim, Bayer, Bristol-Myers Squibb. Günter Breithardt is consultant to Bayer Health Care, Johnson & Johnson, Boehringer Ingelheim, Sanofi-Aventis, Bristol-Myers Squibb, Pfizer, MSD, Portola. Günter Breithardt received grants and support for research to AFNET from German Ministry of Education and Research, Sanofi-Aventis, St Jude, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, MEDA Pharma, and Biosense.
References
- 1.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920-5. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008;92:17-40, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paneni F, Costantino S, Cosentino F. Molecular mechanisms of vascular dysfunction and cardiovascular biomarkers in type 2 diabetes. Cardiovasc Diagn Ther 2014;4:324-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840-4. [PubMed] [Google Scholar]
- 5.Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol 2005;105:315-8. [DOI] [PubMed] [Google Scholar]
- 6.Pathak RK, Mahajan R, Lau DH, et al. The implications of obesity for cardiac arrhythmia mechanisms and management. Can J Cardiol 2015;31:203-10. [DOI] [PubMed] [Google Scholar]
- 7.Soran H, Younis N, Currie P, et al. Influence of diabetes on the maintenance of sinus rhythm after a successful direct current cardioversion in patients with atrial fibrillation. QJM 2008;101:181-7. [DOI] [PubMed] [Google Scholar]
- 8.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace 2007;9:335-79. [DOI] [PubMed] [Google Scholar]
- 9.Burashnikov A, Antzelevitch C. Role of late sodium channel current block in the management of atrial fibrillation. Cardiovasc Drugs Ther 2013;27:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Y, Shryock JC, Wagner S, et al. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 2006;318:214-22. [DOI] [PubMed] [Google Scholar]
- 11.Sicouri S, Burashnikov A, Belardinelli L, et al. Synergistic electrophysiologic and antiarrhythmic effects of the combination of ranolazine and chronic amiodarone in canine atria. Circ Arrhythm Electrophysiol 2010;3:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frommeyer G, Milberg P, Uphaus T, et al. Antiarrhythmic effect of ranolazine in combination with class III drugs in an experimental whole-heart model of atrial fibrillation. Cardiovasc Ther 2013;31:e63-71. [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM, Belardinelli L, Chaitman BR, et al. Effect of ranolazine on atrial fibrillation in patients with non-ST elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 trial. Europace 2015;17:32-7. [DOI] [PubMed] [Google Scholar]
- 14.Morrow DA, Scirica BM, Chaitman BR, et al. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation 2009;119:2032-9. [DOI] [PubMed] [Google Scholar]
- 15.Fragakis N, Koskinas KC, Vassilikos V. Ranolazine as a promising treatment option for atrial fibrillation: electrophysiologic mechanisms, experimental evidence, and clinical implications. Pacing Clin Electrophysiol 2014;37:1412-20. [DOI] [PubMed] [Google Scholar]
- 16.Kowey PR, Reiffel JA, Camm J. The effect of the combination of ranolazine and low dose dronedarone on atrial fibrillation burden in patients with paroxysmal atrial fibrillation (HARMONY trial). Presented paper at 35th Annual Scientific Sessions for the Late Breaking Clinical Trials III. San Francisco, USA, 2014. [Google Scholar]
- 17.Kirchhof P, Lip GY, Van Gelder IC, et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options--a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012;14:8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnar DO, Thorvaldsson S, Manolio TA, et al. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J 2006;27:708-12. [DOI] [PubMed] [Google Scholar]
- 19.Ellinor PT, Lunetta KL, Glazer NL, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet 2010;42:240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahida S. Transcription factors and atrial fibrillation. Cardiovasc Res 2014;101:194-202. [DOI] [PubMed] [Google Scholar]
- 21.Kim YH, Lim DS, Lee JH, et al. Gene expression profiling of oxidative stress on atrial fibrillation in humans. Exp Mol Med 2003;35:336-49. [DOI] [PubMed] [Google Scholar]
- 22.Paneni F, Beckman JA, Creager MA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J 2013;34:2436-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Bing Z, He J, et al. Downregulation of peroxisome proliferator-activated receptor-gamma expression in hypertensive atrial fibrillation. Clin Cardiol 2009;32:337-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Q, Jia L, Sun Y. A pilot study of circulating PPAR-γ receptor protein in elderly patients with atrial fibrillation. Arch Med Sci 2012;8:471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao TF, Leu HB, Huang CC, et al. Thiazolidinediones can prevent new onset atrial fibrillation in patients with non-insulin dependent diabetes. Int J Cardiol 2012;156:199-202. [DOI] [PubMed] [Google Scholar]
- 26.Liu B, Wang J, Wang G. Beneficial effects of pioglitazone on retardation of persistent atrial fibrillation progression in diabetes mellitus patients. Int Heart J 2014;55:499-505. [DOI] [PubMed] [Google Scholar]
- 27.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 2010;11:31-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dublin S, Glazer NL, Smith NL, et al. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med 2010;25:853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fatemi O, Yuriditsky E, Tsioufis C, et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol 2014;114:1217-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathak RK, Middeldorp ME, Meredith M, et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol 2015;65:2159-69. [DOI] [PubMed] [Google Scholar]
- 31.Volpe M, Cosentino F, Tocci G, et al. Antihypertensive therapy in diabetes: the legacy effect and RAAS blockade. Curr Hypertens Rep 2011;13:318-24. [DOI] [PubMed] [Google Scholar]
- 32.Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol 2005;45:712-9. [DOI] [PubMed] [Google Scholar]
- 33.GISSI-AF Investigators , Disertori M, Latini R, et al. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med 2009;360:1606-17. [DOI] [PubMed] [Google Scholar]
- 34.Yang Q, Qi X, Li Y. The preventive effect of atorvastatin on atrial fibrillation: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2014;14:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darghosian L, Free M, Li J, et al. Effect of omega-three polyunsaturated fatty acids on inflammation, oxidative stress, and recurrence of atrial fibrillation. Am J Cardiol 2015;115:196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European Heart Rhythm Association , European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. [DOI] [PubMed] [Google Scholar]
- 37.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [DOI] [PubMed] [Google Scholar]
- 38.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812-22. [DOI] [PubMed] [Google Scholar]
- 39.Huxley RR, Filion KB, Konety S, et al. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol 2011;108:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587-95. [DOI] [PubMed] [Google Scholar]
- 41.Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498-505. [DOI] [PubMed] [Google Scholar]
- 42.Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010;122:2368-77. [DOI] [PubMed] [Google Scholar]
- 43.Forleo GB, Mantica M, De Luca L, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. J Cardiovasc Electrophysiol 2009;20:22-8. [DOI] [PubMed] [Google Scholar]
- 44.Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma A, Sanders P, Champagne J, et al. Selective complex fractionated atrial electrograms targeting for atrial fibrillation study (SELECT AF): a multicenter, randomized trial. Circ Arrhythm Electrophysiol 2014;7:55-62. [DOI] [PubMed] [Google Scholar]
- 46.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222-31. [DOI] [PubMed] [Google Scholar]
- 47.Arboix A. Stroke prognosis in diabetes mellitus: new insights but questions remain. Expert Rev Cardiovasc Ther 2009;7:1181-5. [DOI] [PubMed] [Google Scholar]
- 48.Mankovsky BN, Ziegler D. Stroke in patients with diabetes mellitus. Diabetes Metab Res Rev 2004;20:268-87. [DOI] [PubMed] [Google Scholar]
- 49.Benson RT, Sacco RL. Stroke prevention: hypertension, diabetes, tobacco, and lipids. Neurol Clin 2000;18:309-19. [DOI] [PubMed] [Google Scholar]
- 50.Varughese GI, Patel JV, Tomson J, et al. The prothrombotic risk of diabetes mellitus in atrial fibrillation and heart failure. J Thromb Haemost 2005;3:2811-3. [DOI] [PubMed] [Google Scholar]
- 51.Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580-91. [DOI] [PubMed] [Google Scholar]
- 52.McFarlane SI, Sica DA, Sowers JR. Stroke in patients with diabetes and hypertension. J Clin Hypertens (Greenwich) 2005;7:286-92; quiz 293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boden-Albala B, Cammack S, Chong J, et al. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS). Diabetes Care 2008;31:1132-7. [DOI] [PubMed] [Google Scholar]
- 54.Lip GY, Skjøth F, Rasmussen LH, et al. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol 2015;65:1385-94. [DOI] [PubMed] [Google Scholar]
- 55.Olesen JB, Lip GY, Hansen PR, et al. Bleeding risk in 'real world' patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost 2011;9:1460-7. [DOI] [PubMed] [Google Scholar]
- 56.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011;58:395-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713-9. [DOI] [PubMed] [Google Scholar]
- 58.Blackshear JL, Kusumoto F. Stroke prevention in atrial fibrillation: warfarin faces its challengers. Curr Cardiol Rep 2005;7:16-22. [DOI] [PubMed] [Google Scholar]
- 59.Apostolakis S, Sullivan RM, Olshansky B, et al. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT2R2 score. Chest 2013;144:1555-63. [DOI] [PubMed] [Google Scholar]
- 60.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [DOI] [PubMed] [Google Scholar]
- 61.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [DOI] [PubMed] [Google Scholar]
- 62.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. [DOI] [PubMed] [Google Scholar]
- 63.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104. [DOI] [PubMed] [Google Scholar]
- 64.Lega JC, Bertoletti L, Gremillet C, et al. Consistency of safety and efficacy of new oral anticoagulants across subgroups of patients with atrial fibrillation. PLoS One 2014;9:e91398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Darius H, Clemens A, Healey JS, et al. Abstract 15937: comparison of dabigatran versus warfarin in diabetic patients with atrial fibrillation: results from the RE-LY trial. Circulation 2012;126:A15937. [DOI] [PubMed] [Google Scholar]
- 66.Halperin JL, Bloomgarden Z, Hellkamp A, et al. Abstract 15544: rivaroxaban compared with warfarin in patients with atrial fibrillation and diabetes: a subgroup analysis of the ROCKET AF trial. Circulation 2012;126:A15544. [Google Scholar]