Abstract
Defects in primary cilia lead to a variety of human diseases. One of these, polycystic kidney disease, can be caused by defects in a Ca2+-gated ion channel (TRPP2) found on the cilium. Other ciliary functions also contribute to cystogenesis, and defects in apical Ca2+ homeostasis have been implicated. By recording directly from the native cilia of mIMCD-3 cells, a murine cell line of renal epithelial origin, we have identified a second Ca2+-gated channel in the ciliary membrane: the transient receptor potential cation channel, subfamily M, member 4 (TRPM4). In excised primary cilia, TRPM4 was found to have a low sensitivity to Ca2+, with an EC50 of 646 μM at +100 mV. It was inhibited by MgATP and by 9-phenanthrol. The channel was not permeable to Ca2+ or Cl− and had a permeability ratio PK/PNa of 1.42. Reducing the expression of Trpm4 mRNA with short hairpin (sh) RNA reduced the TRPM4 current by 87% and shortened primary cilia by 43%. When phospholipase C was inhibited, the sensitivity to cytoplasmic Ca2+ greatly increased (EC50 = 26 μM at +100 mV), which is consistent with previous reports that phosphatidylinositol 4,5-bisphosphate (PIP2) modulates the channel. MgATP did not restore the channel to a preinactivation state, suggesting that the enzyme or substrate necessary for making PIP2 is not abundant in primary cilia of mIMCD-3 cells. The function of TRPM4 in renal primary cilia is not yet known, but it is likely to influence the apical Ca2+ dynamics of the cell, perhaps in tandem with TRPP2.
Keywords: primary cilium, TRPM4, polycystic kidney disease, TRPP2
many cells in the body, when they are not dividing, possess a single primary cilium (72, 81). The primary cilium is a thin projection that extends from the surface of the cell. Defects in primary cilia have wide-ranging deleterious impacts on human health (7). Often, though, the specific functions of primary cilia in health and disease are not well understood. Primary cilia are believed to be cellular antennae that detect chemical, mechanical, osmotic, and gravitational stimuli (7, 28, 51, 61, 73). The membranes of various primary cilia have ion-conducting channels (4, 16, 18, 28, 37, 46, 60, 68, 85; see Refs. 36 and 62 for review). It is apparent that activity of channels in a cilium may have substantial consequences for the entire cell. During flow sensing, for example, renal epithelial cells show a large cellular Ca2+ increase that requires the cilium, external Ca2+, and the channel subunits TRPP2 and TRPV4 (37, 52, 63, 64). TRPP2 (also known as polycystin-2 or PC2) and TRPV4 are members of the TRP family of channel proteins and conduct Ca2+ (27, 30). In general, opening of ciliary channels should have two consequences. First, some channels allow entry of Ca2+, which modulates many cellular processes. Second, opening or closing of ciliary channels can change the membrane potential, which in turn may influence voltage-dependent processes such as electrogenic ion transport. Whether these effects are confined to the cilium itself is an area of active investigation (48).
Several renal cystic diseases are caused by mutations in ciliary proteins (9, 23, 77). Nearly all cases of autosomal dominant polycystic kidney disease result from mutations in TRPP2, a channel protein found in the cilium (4, 37, 46, 60, 85), or PC1 (polycystin-1), a ciliary membrane protein that interacts with TRPP2 (27, 60, 65, 70, 78, 85). In the absence of PC1 or TRPP2, the presence of the primary cilium exacerbates the cystic phenotype (45). It is believed that aberrant Ca2+ homeostasis contributes to polycystic kidney disease (1, 38, 52, 75, 83; for a review see Ref. 12). In particular, control of apical Ca2+ entry is proposed to affect cyst formation (75). Channels in the primary cilium provide one possible route for apical Ca2+ entry. Because of the primary cilium's small size, though, it has been difficult to learn exactly how it contributes to the initiation, amplification, and termination of Ca2+ signals in renal epithelial cells.
In a preliminary study, we described two channels in the cilia of mIMCD-3 renal epithelial cells that are activated by cytoplasmic Ca2+ and by depolarization (33). Here we report that one of these is a TRPM4-dependent channel. TRPM4 is a voltage-dependent, Ca2+-activated channel that conducts monovalent cations and is expressed in many tissues, including the kidney (50). TRPM4 currents have been identified in many cells, including HEK-293 cells (2, 41), Chinese hamster ovary cells (84), pancreatic β cells (13, 47), and vascular smooth muscle cells (19, 69). However, TRPM4 has not been previously identified in a primary cilium, nor has a role for TRPM4 in renal physiology been described. We discuss possible roles for TRPM4 in Ca2+ signaling in the renal primary cilium.
MATERIALS AND METHODS
Electrical recording.
Electrical recordings were made from primary cilia of mIMCD-3 cells as described previously (33). In short, mIMCD-3 cells (murine epithelial cells from the renal inner medullary collecting duct, CRL-2123, American Type Culture Collection, Manassas, VA) (67) were cultured on beads that were free to move in the recording chamber. Suction was applied to a recording pipette so that a single primary cilium entered the pipette. After a resistance of at least 1 GΩ formed between the membrane and the pipette, the cilium was excised from the cell. This left the cilium inside the recording pipette in the inside-out configuration. The pipette containing the cilium could then be transferred among different solutions that bathed the cytoplasmic face of the membrane.
During recording, the beads coated with cells were stored in a standard external solution (Table 1). The recording pipettes also contained this solution except as noted. For every cilium, a control current was measured with the cytoplasmic face of the cilium bathed in a solution with free Ca2+ buffered to 0.1 μM (Table 1). Currents measured in this solution were taken to represent leak and capacitive currents. In all figures and analyses shown except for Fig. 1A, this current has been subtracted. To activate TRPM4 currents, cytoplasmic solutions contained from 0.3 μM to 10 mM free Ca2+ as noted. A minority of the cilia showed a large-conductance channel that is also activated by voltage and by cytoplasmic Ca2+ but reverses at a negative potential (33); those cilia were excluded from the present study. For some recordings as noted, inside-out excised patches of apical membrane were used instead of cilia. All recordings were done under voltage-clamp at room temperature (24°C). The recording pipette and chamber were coupled by Ag/AgCl electrodes to an Axopatch 200B patch-clamp amplifier with a CV203BU headstage and Digidata 1200A BNC data-acquisition system, controlled by pCLAMP 5.7.1 software (all from Axon Instruments/Molecular Devices, Sunnyvale, CA). The pipette was positioned with a Narishige MHW-3 hydraulic micromanipulator (Narishige, Tokyo, Japan). During acquisition, currents were low-pass filtered at 2 kHz and digitized at 5 kHz. As described elsewhere (34), corrections were applied for each of two liquid junction potentials. The first, which was <5 mV, existed between each cytoplasmic bath and its salt bridge. The second potential existed during the patch procedure whenever the pipette solution was different from the solution bathing the cells. The largest such correction was 7 mV between high-Ca2+ (pipette) and standard (bath) external solutions. All potentials are given as membrane potential (i.e., cytoplasmic relative to external). Except as noted, the holding potential between recordings was 0 mV. Software for analysis included Origin 7.0 (OriginLab, Northampton, MA) and QuB 1.5 (www.qub.buffalo.edu, State University of New York, Buffalo, NY).
Table 1.
Compositions of solutions (mM)
| KCl | NaCl | NaMeSO3 | CaCl2 | CaGluc | MgCl2 | MgGluc | HEPES | BAPTA | Dibromo-BAPTA | d-Glucose | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasmic | |||||||||||
| 0.1–0.3 μM Free Ca2+ | 140 | 5 | 0.7–1.2 | 2 | 5 | 2 | 5 | ||||
| 3 μM Free Ca2+ | 140 | 5 | 1.4 | 2 | 5 | 2 | 5 | ||||
| Unbuffered Ca2+ | 140 | 5 | 0.03–10 | 2 | 5 | 5 | |||||
| High-Na+ (Ca2+unbuffered) | 145 | 1 | 2 | 5 | 5 | ||||||
| High-K+ (Ca2+unbuffered) | 145 | 0.1–1 | 2 | 5 | 5 | ||||||
| Cl−-free (Ca2+unbuffered) | 145 | 1 | 2 | 5 | 5 |
| NaCl | KCl | CaCl2 | MgCl2 | HEPES | NaPyr | d-Glucose | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| External | |||||||||||
| Standard | 140 | 5 | 2 | 2 | 5 | 2 | 9.4 | ||||
| High-K+ | 145 | 2 | 2 | 5 | 2 | 9.4 | |||||
| High-Ca2+ | 100 | 2 | 5 | 9.4 |
For each reagent, total concentrations are shown. NaMeSO3, sodium methanesulfonate; CaGluc, calcium gluconate; MgGluc, magnesium gluconate; NaPyr, sodium pyruvate. Total and free Ca2+ were the same in the unbuffered high-Ca2+ solutions. BAPTA and dibromo-BAPTA were added as the tetrapotassium salts. Solutions were adjusted to pH 7.4 with NaOH (high-Na+ solutions), KOH (high-K+ solutions), or Tris base (high-Ca2+ external solution).
Fig. 1.
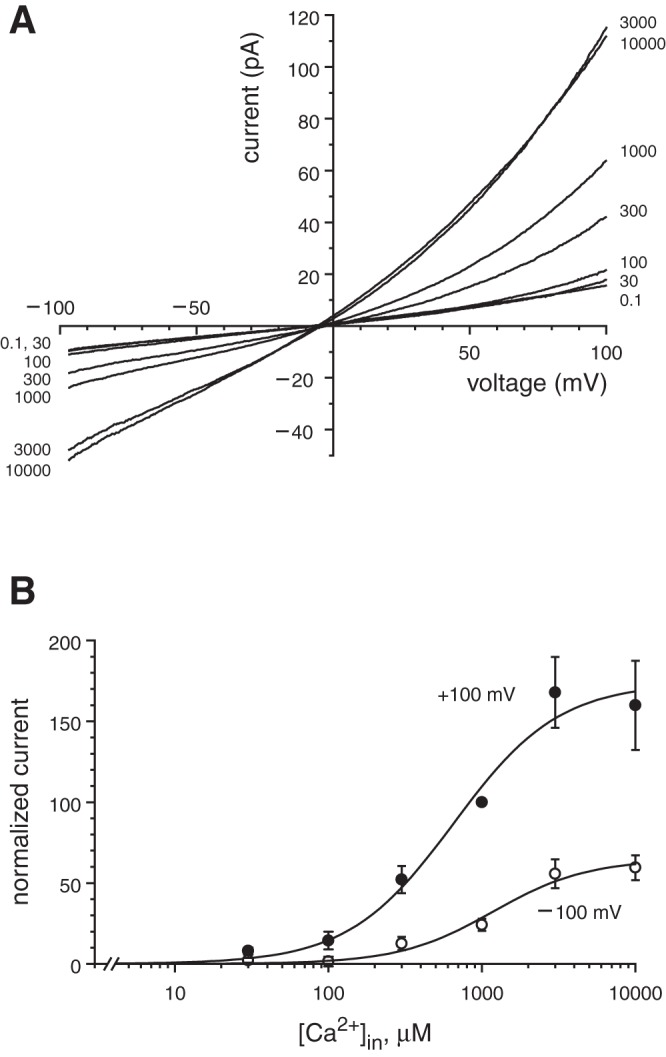
Dose-response properties of the macroscopic ciliary transient receptor potential cation channel, subfamily M, member 4 (TRPM4) current. A: mean macroscopic current-voltage relationships as a function of free cytoplasmic Ca2+ concentration ([Ca2+]in). The numbers at the right and left of each curve represent [Ca2+]in in μM. Each curve is the average of the relationship measured in 24 cilia (for 1,000 μM Ca2+) or 12 cilia (all other concentrations). Cilia were bathed in the standard external solution and cytoplasmic solutions with the concentration of free Ca2+ indicated (Table 1). B: normalized TRPM4 current as a function of [Ca2+]in at +100 and −100 mV. This represents the same set of recordings shown in A, but for each cilium every current measured was normalized to the current in 1,000 μM cytoplasmic Ca2+ at +100 mV, which was assigned a value of 100. Leak current was subtracted (part B only) as described in materials and methods. Each line shown is the best fit to a Hill equation of form I = (Imax × cn)/(cn + EC50n), where I is the normalized macroscopic current, Imax is the maximum current, c is [Ca2+]in, EC50 is the concentration for half-maximal effect, and n is the Hill coefficient.
BAPTA or dibromoBAPTA was used to buffer the concentration of free Ca2+ in the range 0.1 to 3 μM (Table 1). In buffered solutions, concentrations of free Ca2+ were estimated by the method of Bers (8) as described previously (35). No Ca2+ buffer was added to solutions with free Ca2+ in the range 30 μM to 10 mM. U-73122 hydrate, 9-phenanthrol, ATP, and BAPTA were purchased from Sigma-Aldrich (St. Louis, MO). U-73122 and 9-phenanthrol were initially prepared as stock solutions in dimethyl sulfoxide (5 mM U-73122, with warming; 100 mM 9-phenanthrol). The final concentration of dimethyl sulfoxide present at the cilium was 0.1 or 0.2% (vol/vol). DibromoBAPTA was purchased from Molecular Probes/Invitrogen (Carlsbad, CA).
Knockdown of Trpm4 mRNA.
To confirm that we were recording from TRPM4, we generated a cell line in which Trpm4 mRNA was stably knocked down by lentiviral transduction of short hairpin RNA (shRNA) plasmids into the mIMCD-3 cell line. As a control, we generated a cell line transduced with a non-target control shRNA plasmid (i.e., one not expected to knock down any mammalian mRNA). The sequence of the murine Trpm4 knockdown shRNA (TRCN0000068683, Sigma-Aldrich) was CCGGCCTGGGTAATGTGGTCAGTTACTCGAGTAACTGACCACATTACCCAGGTTTTTG. The sequence for the non-target control shRNA (SHC002, Sigma-Aldrich) was CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT. Both were in the pLKO.1 vector. The plasmid was packaged in lentivirus by the Cincinnati Children's Hospital Lenti-shRNA Library Core. The mIMCD-3 cells (passage 19) were incubated overnight with the lentivirus and 8 μg/ml hexadimethrine bromide. After transduction, selection was enforced by 2 μg/ml puromycin dihydrochloride. To develop clonal lines, cells were diluted to 750 cells/ml and passaged into 96-well plates (1 μl/well). After confirmation that a given well contained only one cell, medium was added and the cell lines were expanded. We prepared cell-coated beads with the Trpm4 shRNA knockdown (passages 24 and 25) and non-target control (passages 27 and 29) lines as described previously (33) except that puromycin was included. The electrophysiologist was not told whether he was recording from knockdown or nontarget control cells until analysis of the data was completed.
We used RT-quantitative (q) PCR to monitor the expression of Trpm4 mRNA in the wild-type, Trpm4 knockdown, and non-target control lines. Total RNA was extracted from cultured cells (RNeasy 74104, Qiagen, Valencia, CA), treated with DNase (EN0525, Thermo Fisher Scientific, Waltham, MA), and reverse-transcribed to cDNA with random hexamers (Maxima reverse transcriptase, EP0742, Thermo Fisher Scientific). For reverse transcription, DNase-treated RNA was added to a final concentration of 0.03 μg/μl. The RT reaction product was diluted 25-fold in water. For qPCR, each 15-μl reaction contained 0.66 μl of diluted RT reaction product. PCR was done with HotStart-IT SYBR Green qPCR Master Mix (75762, Affymetrix, Santa Clara, CA) and was followed on a OneStepPlus thermal cycler (Thermo Fisher Scientific) with an annealing temperature of 64°C. Primer pairs were selected using Primer-BLAST (National Center for Biotechnology Information, Database: Mus musculus refseq_rna). Primers were designed to cross at least one intron and to be specific for the gene of interest (Table 2). Using LinRegPCR software (71), we determined the starting amount of mRNA transcript (N0, in arbitrary fluorescence units) for each qPCR reaction/well in a 96-well plate. The efficiency of the PCR amplification was calculated for each reaction, and an average efficiency for a given primer pair was calculated. (Rare efficiencies that varied from the median by >2.5% were excluded from the calculation of the mean efficiency.) This mean efficiency was taken into account when calculating the starting amount of transcript (N0). Relative expression of mRNA (the vertical axis in Fig. 5A) was calculated by dividing N0 for the target transcript (Trpm4) by N0 for the reference transcript (B2m or Sdha). As negative controls, we used samples processed without RT or reactions with water in place of cDNA. The data were examined with a one-way analysis of variance followed by the all pairwise multiple comparison procedure: Tukey test (SigmaPlot, Systat Software, San Jose, CA). Three cell-culture passages (passages 26–28) were analyzed for each of the three cell lines: wild-type, Trpm4 knockdown, and non-target control. Two to three wells per condition were used as technical replicates. Melt curves were used to confirm that only the targeted product was made. Sequencing confirmed the identities of the PCR products.
Table 2.
PCR primer sequences
| Gene | Product Size, bp | Primer Sequence (5′-3′) | NCBI Accession No. |
|---|---|---|---|
| B2m | 211 | F: CACAGTTCCACCCGCCTCACA | NM_009735.3 |
| R: TCTCGATCCCAGTAGACGGTCTTGG | |||
| Sdha | 75 | F: AAGAAGGCATCAGCTAAAGTTTCA | NM_023281.1 |
| R: CACAGCATCAAATTCATGATCCAC | |||
| Trpm4 | 146 | F: ACGGCTCCGAGGAGTTTGAGACTA | NM_175130.4 |
| R: CCCACGGAAAAGTTCACTTTGGGC |
F, forward; R, reverse; NCBI, National Center for Biotechnology Information.
Fig. 5.
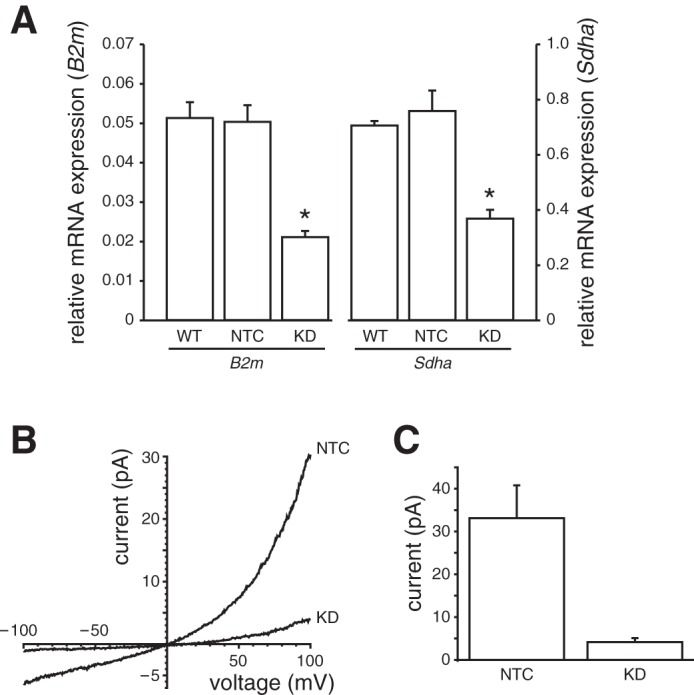
Effect of short hairpin (sh) RNA knockdown of TRPM4 on Ca2+-activated current in apical membrane patches from mIMCD-3 cells. A: RT-quantitative (q) PCR for Trpm4 mRNA expression. RT-qPCR was performed to determine Trpm4 mRNA expression relative to the reference genes B2m and Sdha. Stable transduction of mIMCD-3 cells with Trpm4 shRNA (knockdown; KD) reduced Trpm4 mRNA expression compared with wild-type (WT) cells or cells treated with a non-target control (NTC) shRNA. The percent reductions compared with WT were 59 and 48% using the B2m and Sdha reference genes, respectively (n = 3 passages). *Significantly different from WT and NTC for a given reference gene (P < 0.007, Tukey test). B: the current-voltage relationship was determined in cytoplasmic solution with 1 mM Ca2+ (Table 1) in apical membrane patches from cells in which TRPM4 was knocked down (KD) and in NTC. Pipettes contained the standard external solution. Leak current was subtracted as described in materials and methods. C: mean Ca2+-activated current at +100 mV in patches from each of the two cell types. Current-voltage relationships and currents are averages from 12 (KD) and 14 (NTC) patches.
Determination of ciliary length and percentage of cells with cilia.
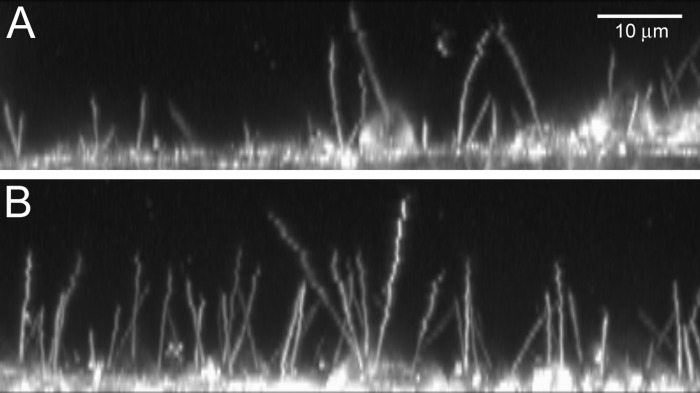
The dye di-8-ANEPPS (D-3167, Life Technologies/Thermo Fisher Scientific, Waltham, MA) was chosen because of its ability to label the membrane of live cells; we did not use its voltage-sensing properties. mIMCD-3 cells were plated on 25-mm glass coverslips at 200,000 cells/ml, had been confluent for 6–9 days, and were last fed 1 day before use. We mixed di-8-ANEPPS (1 μl of a 1.7 mM stock in dry dimethyl sulfoxide) with 2.4 μl of 10% Pluronic F-127 (P-6866, Life Technologies) and then added 497 μl of standard external solution (Table 1). Final concentrations of di-8-ANEPPS and Pluronic F-127 were 3 μM and 0.05%, respectively. The cells were rinsed with standard external solution and then labeled with the di-8-ANEPPS for 20 min before being rinsed again with standard external solution. The coverslips were viewed in an Attofluor cell chamber (A-7816, Life Technologies) at room temperature. To document the ciliary length, images were acquired every 0.7 μm from the cell body to just beyond the distal tip of the cilium. These image stacks were acquired using a high-speed confocal microscope (LSM 7 LIVE, Carl Zeiss, Oberkochen, Germany) with a 63×/1.20 NA, C-Apochromat, water-immersion objective with voxel dimensions of 0.2 × 0.2 × 0.7 μm and eight-bit depth. The 488-nm laser was used for excitation; the emission filter was a 505-nm long-pass filter. For both Trpm4 knockdown and non-target control cells, ciliary lengths were measured using passages 27 (6–7 days after confluence, 11 days in culture) and 28 (7–9 days after confluence, 15 days in culture; 30 cilia for each strain and passage, total 120 cilia). The acquisition parameters were the same for all images except for a difference in digital offset for the two passages (0.008 for passage 28 and 0.015 for passage 27). Contrast was enhanced by the same amount for all images, and γ was kept at 1 using Zen 2012 (Zeiss). Ciliary length was measured in Imaris (Bitplane, Zürich, Switzerland) using the Slice“ view and ”polygon" 3-dimensional (3D) measuring tool. With Imaris, we measured the length by noting the position of a cilium in each slice of a stack of 2D, fluorescent images. That is, we used raw data, rather than maximum-intensity projections or other 3D visualization methods, to measure ciliary length. The person measuring was blinded to the cell type. Despite the fast acquisition, there was small movement near the tips of some of the cilia (see Fig. 6), but the measurements were not affected enough to explain the difference in ciliary length between the two groups. Figure 6 shows maximum projections made with LSM4.2 (Zeiss) and Zen 2012 software and processed in Photoshop 6.0 (Adobe Systems, San Jose, CA). To determine the percentage of cells with cilia, 749 cells from 12 image stacks were analyzed (3 stacks for each strain and passage). To be counted, the entire apical surface of the cell had to be present in the image stack.
Fig. 6.
Ciliation in Trpm4 shRNA KD vs. control. mIMCD-3 cells that had been stably transduced with Trpm4 shRNA (A) had shorter cilia and were less likely to be ciliated than cells treated with the NTC shRNA (B). Cells from both lines were labeled with a fluorescent membrane dye while live, and stacks of images at various depths were acquired on a confocal microscope. Maximum-intensity projections of representative stacks are shown. The scale bar is the same for both the vertical (z, depth) and horizontal (x) axes.
Statistical analyses.
Results of repeated experiments are reported as means ± SE. Student's t-tests reported are two-tailed (SigmaPlot).
RESULTS
When the primary cilium is excised from an mIMCD-3 cell, one can record the ciliary transmembrane current. The recording configuration is equivalent to an inside-out patch, but in this case the patch consists of one whole cilium. In this configuration, high levels of cytoplasmic Ca2+ activated a transmembrane current in most of the primary cilia tested. Fig. 1A shows the ciliary current-voltage relationship as the concentration of cytoplasmic Ca2+ was increased from 0.1 μM to 10 mM. The activated current showed mild outward rectification. The current was observed in 114 of 124 cilia tested; the mean current at +100 mV in 1 mM Ca2+ was 49.6 ± 4.5 pA (n = 88). The dose-response relationships for cytoplasmic Ca2+ at −100 and +100 mV are well-fit by Hill equations (Fig. 1B), with parameters EC50 = 646 ± 150 μM and n = 1.27 ± 0.28 at +100 mV and EC50 = 1,166 ± 280 μM and n = 1.42 ± 0.37 at −100 mV. The parameter EC50 represents the Ca2+ concentration for half-maximal activation of the ciliary current. The Ca2+-activated current was not exclusive to the cilium. In excised patches of apical cell membrane, each of 35 patches tested showed a Ca2+-activated current; the mean current at +100 mV in 1 mM Ca2+ was 40.6 ± 5.1 pA.
We determined the selectivity for monovalent ions of the ciliary Ca2+-activated macroscopic current. Excised cilia were tested in three cytoplasmic baths, each containing a different monovalent ion. In all cases, the external (pipette) solution was primarily NaCl. Voltage ramps from −100 mV to +100 mV were applied in each solution (Fig. 2A). In nearly symmetrical NaCl solutions, the reversal potential of the macroscopic Ca2+-activated current was, as expected, not significantly different from 0 mV (−2.2 ± 2.2 mV, n = 12; Fig. 2A, black). When cytoplasmic NaCl was replaced with sodium methanesulfonate, the reversal potential was not changed (−1.0 ± 2.0 mV, n = 12; Fig. 2A, red). Thus the channels do not select between Cl− and methanesulfonate−; they are likely impermeable to anions. When cytoplasmic NaCl was replaced by KCl, though, the reversal potential shifted to −7.8 ± 2.1 mV (n = 12; Fig. 2A, blue). This equates to a PK/PNa permeability ratio for monovalent cations of 1.42. We also recorded from excised cilia using a pipette (external) solution containing isotonic (100 mM) CaCl2 and no K+ or Na+. The average current-voltage relationship was not seen to reverse even at −100 mV (Fig. 2B). This indicates that Ca2+ is not a carrier of the Ca2+-activated current.
Fig. 2.
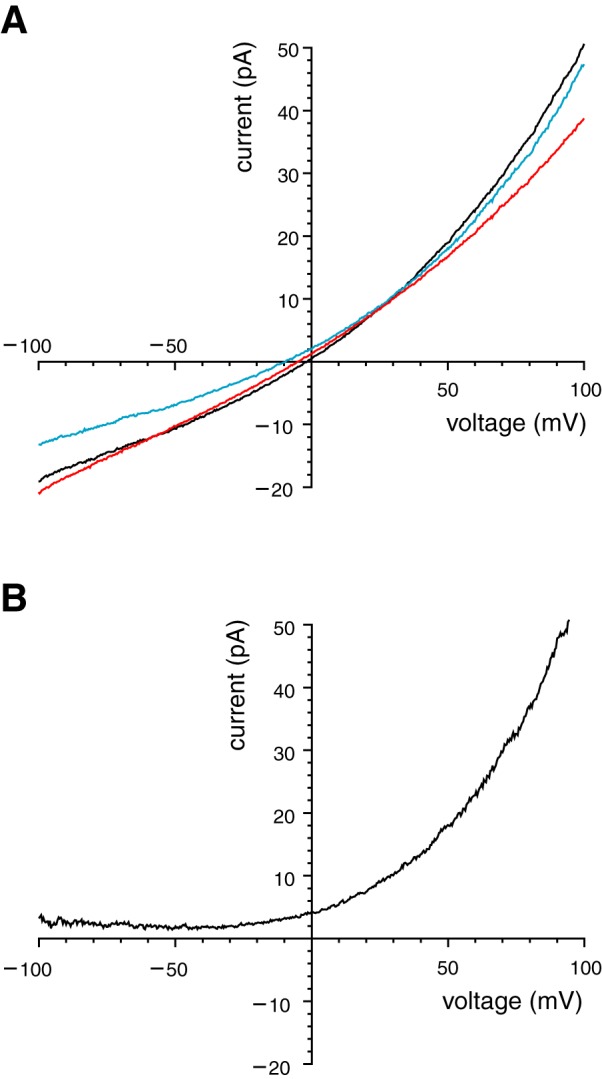
Ionic selectivity of the ciliary TRPM4 current. A: selectivity for monovalent ions. Mean macroscopic current-voltage relationships were determined in each of 3 modified cytoplasmic solutions (Table 1): high-Na+ (black), high-K+ (blue), or Cl− free (red). Each of these contained 1 mM Ca2+. In all cases, the pipette contained the standard external solution, which is primarily NaCl. Each curve is the average of the relationship measured in 12 cilia. B: nonpermeability to Ca2+. Pipette solution contained 100 mM CaCl2 (Table 1, high-Ca2+ external solution). The cytoplasmic solution is shown in Table 1 as unbuffered Ca2+ (with 1 mM CaCl2). The principal cytoplasmic cation was K+ (140 mM). The relationship shown is the average of the relationship measured in 4 cilia. For both A and B, leak current was subtracted as described in materials and methods.
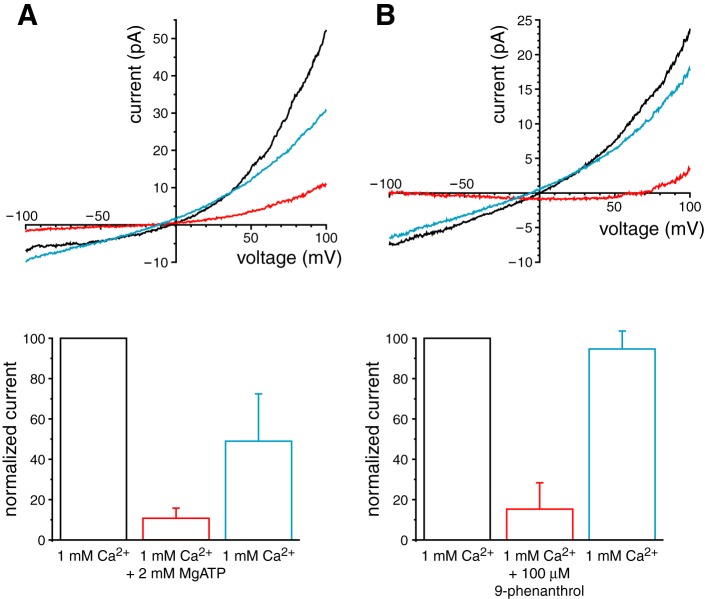
The properties of the ciliary current, activation by cytoplasmic Ca2+ and permeability to monovalent but not divalent cations, are characteristic of TRPM4 and TRPM5 channels (43, 50). Cytoplasmic ATP blocks TRPM4 channels exogenously expressed in HEK-293 cells (56–58, 79) but does not block TRPM5 (79). Figure 3A shows that MgATP reversibly blocked the current activated by 1 mM cytoplasmic Ca2+ in excised cilia at +100 mV. Mean current was reduced from 53.1 ± 9.3 pA in 1 mM Ca2+ to 8.6 ± 2.0 pA in 1 mM Ca2++2 mM MgATP (n = 23); the reduction was significant (P < 0.0001 by t-test for repeated measures). Only 82 ± 4% of the current was blocked, which is close to the value reported elsewhere for TRPM4 (∼80%) (58). We also tested the inhibitor 9-phenanthrol, which blocks TRPM4 (2, 24, 26, 29) but not TRPM5 (29). At +100 mV, 100 μM 9-phenanthrol reversibly inhibited 85 ± 13% of the total Ca2+-activated current (Fig. 3B). In 10 cilia tested, mean current was reduced from 22.2 ± 8.1 pA in 1 mM Ca2+ to 3.4 ± 2.1 pA in 1 mM Ca2++100 µM 9-phenanthrol; the reduction was significant (P < 0.05 by t-test for repeated measures).
Fig. 3.
Reversible inhibitors of the macroscopic ciliary TRPM4 current. A: block by 2 mM cytoplasmic MgATP. Top: the current-voltage relationship was determined in cytoplasmic solution with 1 mM Ca2+ (black, Table 1), then in the same solution plus 2 mM MgATP (red), and finally in the initial solution that lacked MgATP (blue). In all cases, the pipette contained the standard external solution. Bottom: mean Ca2+-activated current at +100 mV in each of the 3 solutions, normalized to the value in the first solution. Current-voltage relationships and currents are averages from 5 cilia. B: block by 100 μM cytoplasmic 9-phenanthrol. Procedures were the same as for A except that 100 μM 9-phenanthrol replaced 2 mM MgATP. Current-voltage relationships and currents are averages from 10 cilia. For both A and B, leak current was subtracted as described in materials and methods.
Current in apical cell patches was also inhibited by MgATP and 9-phenanthrol. On average, 2 mM MgATP inhibited 87 ± 8% of the total Ca2+-activated current at +100 mV. In nine patches tested, mean current was significantly reduced from 41.7 ± 11.8 pA in 1 mM Ca2+ to 3.1 ± 1.4 pA in 1 mM Ca2++2 mM ATP (P < 0.02 by t-test for repeated measures). On average, 100 μM 9-phenanthrol inhibited 90 ± 4% of the total Ca2+-activated current at +100 mV. In nine patches tested, mean current was significantly reduced from 52.5 ± 7.5 pA in 1 mM Ca2+ to 5.5 ± 2.4 pA in 1 mM Ca2++100 μM 9-phenanthrol (P < 0.0001 by t-test for repeated measures).
With cytoplasmic Ca2+ at or below 300 μM, it was often possible to observe single channels that likely underlie the macroscopic current (Fig. 4A). Channel openings were more frequent at depolarized potentials (Fig. 4A). In symmetrical solutions, the average single-channel conductance between −100 and +100 mV was 23 pS. However, the conductance was higher at positive potentials than at negative potentials (Fig. 4B). Like the macroscopic current, the single channels activated by cytoplasmic Ca2+ were blocked by 2 mM cytoplasmic MgATP (Fig. 4C).
Fig. 4.
Properties of single TRPM4 channels observed in excised primary cilia. A: at left are current fluctuations from opening and closing of single channels at +80 and −120 mV. Numbers at the right of each recording indicate the number of channels open at each current level; the levels are indicated by dashed lines. At right are amplitude histograms from the full 20 s of recorded current at each potential. At least 9 channels were present in this cilium. The average single-channel current is 2.2 pA in the top histogram (+80 mV) and −2.6 pA in the bottom histogram (−120 mV). All data shown are from the same cilium. The high-K+ cytoplasmic and external solutions (Table 1) were used; the cytoplasmic solution contained 300 μM Ca2+. The recordings were low-pass filtered at 1 kHz following acquisition. Leak currents of 10 pA (+80 mV) and −28 pA (−120 mV) were subtracted. B: single-channel current-voltage relationship. For each voltage, single-channel current was measured as shown in A. Solutions were as in A, except that both 100 and 300 μM cytoplasmic Ca2+ were used. The slope (single-channel conductance) is 17 pS at negative potentials and 31 pS at positive potentials. Numbers along the relation show the number of cilia studied at each voltage. C: block of single channels by 2 mM cytoplasmic MgATP at a membrane potential of −120 mV. The top recording was made in the standard cytoplasmic solution (Table 1), the middle recording in cytoplasmic solution with 100 μM Ca2+, and the bottom recording in the latter solution plus 2 mM MgATP. The dashed lines indicate the current levels when all TRPM4 channels were closed. The recordings were low-pass filtered at 700 Hz.
As additional evidence that the Ca2+-activated current in mIMCD-3 cells is TRPM4 dependent, we knocked down Trpm4 expression in mIMCD-3 cells using shRNA. The percentage reductions of Trpm4 mRNA expression compared with wild-type were 59 and 48% using the B2m and Sdha reference genes, respectively, and were significant (Fig. 5A, P < 0.007, Tukey test). We compared the total Ca2+-activated current at +100 mV in Trpm4 knockdown cells and in cells transfected with a non-target control shRNA. In both cell types, 1 mM cytoplasmic Ca2+ activated some current that showed mild outward rectification (Fig. 5B). At +100 mV, the mean Ca2+-activated current in apical membrane patches was 33.1 ± 7.3 pA (n = 12) in non-target control cells and 4.2 ± 0.9 pA (n = 14) in Trpm4 knockdown cells (Fig. 5C). This reduction was significant (P < 0.001 by t-test for independent measures). Each of the 12 patches from non-target control cells had a detectable Ca2+-activated current; in patches from knockdown cells, 12 of 14 cells had a detectable current. The mean current in apical membrane patches from the non-target control cells was not significantly different from that in apical patches from non-transfected cells (t-test for independent measures). For these studies, it was necessary to use apical membrane patches rather than cilia for both lines because cilia that were long enough for recording were rare in the Trpm4 knockdown line. We measured the lengths of live cilia from the two lines using the fluorescent membrane dye di-8-ANEPPS and a fast-scanning confocal microscope (Fig. 6). The mean ciliary length for the non-target control cells was 11.6 ± 0.7 μm, while the mean ciliary length for Trpm4 knockdown cells was 6.5 ± 0.6 μm. This difference was significant (60 cilia/cell line; P < 0.001 by 2-tailed t-test of independent measures). Moreover, although the cells were plated at the same density and grown for the same length of time, the percentage of cells with cilia was significantly smaller for the Trpm4 knockdown cells (52 ± 7%) than for the non-target control cells (75 ± 4%; 6 fields/cell line, 749 cells, P < 0.03 by t-test for independent measures).
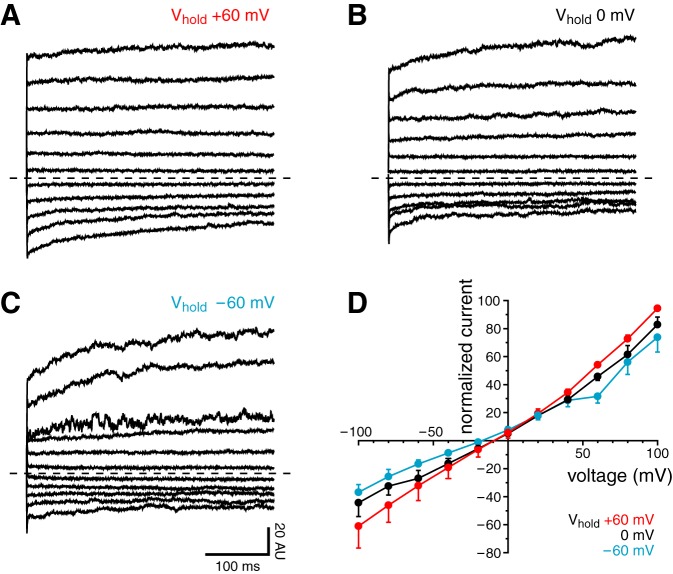
According to previous reports, the TRPM4 current depends on the holding potential before the measurement; there is more inward current when the holding potential is more positive (54, 76). We applied voltage jumps to potentials ranging from −100 to +100 mV from a holding potential of −60, 0, or +60 mV (Fig. 7, A–C). We did not observe a significant dependence of the resulting currents on holding potential. More positive holding potentials yielded slightly more current (Fig. 7D), but this difference was not significant.
Fig. 7.
Effect of holding potential (Vhold) on the macroscopic ciliary TRPM4 current. A–C: cilia were subjected to a series of voltage jumps as the Vhold between jumps was varied. The voltage protocol consisted of 400-ms test pulses to potentials ranging from −100 to +100 mV (increment +20 mV). Vhold between jumps was +60 (A), 0 (B), or −60 mV (C). Leak current was subtracted as described in materials and methods. For each cilium, all TRPM4 currents were normalized to the mean current at +100 mV and Vhold at +60 mV; this current was assigned a value of 100 arbitrary units (AU). The dashed lines indicate 0 current. All of the records shown are averages of determinations in 8 cilia. D: initial normalized currents from the jumps, the averages of which are shown in A–C.
It is generally found that TRPM4 currents inactivate or desensitize under some recording conditions and that the desensitization results from exposure to cytoplasmic Ca2+ (25, 31, 53, 54, 56, 57, 76, 79, 86; see also Ref. 41). In excised cilia from mIMCD-3 cells, exposure to cytoplasmic Ca2+ did cause an inactivation of the TRPM4 current. Exposure to 1 mM Ca2+ for 5 min resulted in a reduction to 48 ± 8% of the maximum current at +100 mV (Fig. 8A, ●). In TRPM4-transfected HEK-293 cells, addition of a phospholipase C (PLC) inhibitor prevents inactivation of TRPM4 (53). Since phosphatidylinositol 4,5-bisphosphate (PIP2) increases the sensitivity of TRPM4 to cytoplasmic Ca2+ (53, 86), it is believed that Ca2+ desensitizes (inactivates) TRPM4 by activating PLC, an enzyme that cleaves PIP2. Consistent with this, we found that addition of the PLC inhibitor U-73122 greatly reduced inactivation (Fig. 8A, ○). The TRPM4 current was 78 ± 5% of the maximum current after the cytoplasmic membrane of the cilium was exposed to 1 mM Ca2++5 μM U-73122 for 5 min. As reported elsewhere (53), sensitivity to cytoplasmic Ca2+ was much greater when PLC was inhibited (Fig. 8B). We found that inhibition of PLC greatly reduced the EC50 for cytoplasmic Ca2+, to 26 ± 12 μM at +100 mV and 17 ± 7 μM at −100 mV, with Hill coefficients n = 0.74 ± 0.20 (+100 mV) and 0.64 ± 0.12 (−100 mV). It has also been reported that ATP restores the current to its preinactivation amplitude when applied between Ca2+ applications (53, 86), probably by enhancing the production of PIP2. However, we found that in excised cilia, addition of 4 mM MgATP to the cytoplasmic solution did not restore TRPM4 currents to preinactivation levels (Fig. 8A, ● at 480 ms). After 5 min in 1 mM Ca2+ followed by 90 s in 4 mM MgATP, the TRPM4 current was 25 ± 7% of the maximum current.
Fig. 8.
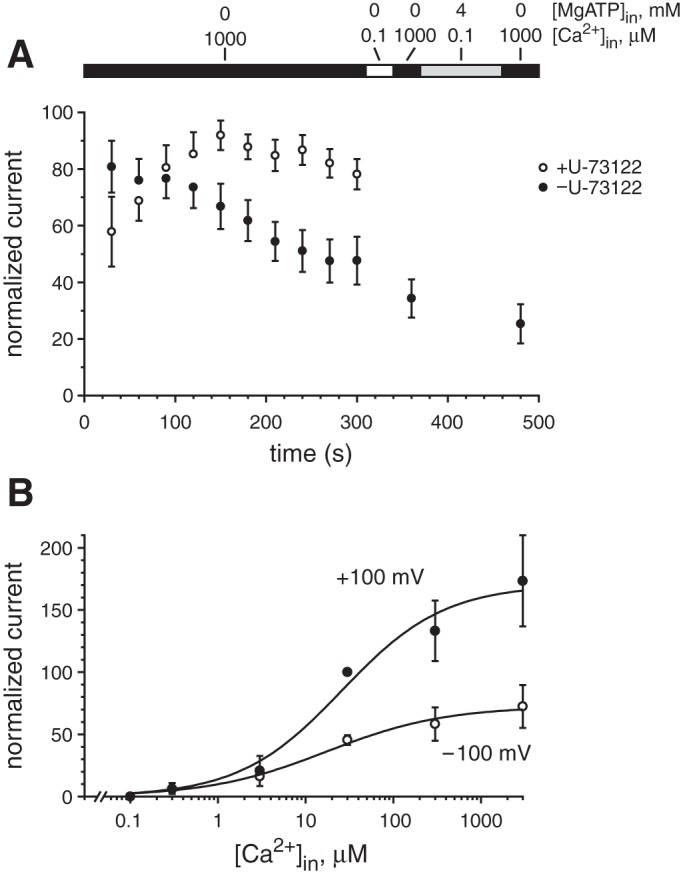
Desensitization of the macroscopic ciliary TRPM4 current. A: current at +100 mV was measured repeatedly with the cilium in a cytoplasmic solution containing 1 mM Ca2+ (Table 1, unbuffered Ca2+). For each cilium, a leak current was subtracted; this was measured in a cytoplasmic solution with 0.1 μM Ca2+ (Table 1) at 330 ms. Currents for each cilium were also normalized to the largest current recorded for that cilium; this current was assigned a value of 100. The bar at the top indicates the changes in cytoplasmic solution as follows: 1 mM Ca2+ (black); 0.1 μM Ca2+ (white); 0.1 μM Ca2++4 mM MgATP (gray). For the cilia represented by ○, the cytoplasmic solutions also contained 5 μM U-73122. The data shown as ● are averages from 10 cilia; ○ represent 6 cilia. B: normalized TRPM4 current as a function of free [Ca2+]in at +100 and −100 mV in the presence of 5 μM U-73122. For each cilium, every current measured was normalized to the current in 30 μM cytoplasmic Ca2+ at +100 mV, which was assigned a value of 100. Leak current was subtracted as described in materials and methods. Each line shown is the best fit to a Hill equation as described in the legend to Fig. 1.
DISCUSSION
We report that the primary cilia and apical membrane of mIMCD-3 cells have TRPM4-dependent channels. The channels and macroscopic current closely match those associated with TRPM4 in previous studies (50). The current is activated by cytoplasmic Ca2+ and by depolarization. It conducts Na+ and K+ but not Ca2+ or Cl−. The underlying single channels have the expected unitary conductance. The macroscopic current desensitizes to Ca2+ in a Ca2+- and PLC-dependent manner, and it decreases when Trpm4 expression is reduced by shRNA knockdown. mIMCD-3 primary cilia are already known to have TRP channel subunits TRPP2 (44), TRPC1 (4), and PKD2L1 (18). A functional channel observed in mIMCD-3 cilia is very similar to a channel in another cell line that depends on both PKD2L1 and PKD1L1 (16). To the best of our knowledge, TRPM4 has not been previously identified in any cilium.
Like TRPM4 currents, the ciliary currents described here are inhibited by cytoplasmic MgATP and by 9-phenanthrol. Neither blocker is highly selective for TRPM4. Among channels that conduct both Na+ and K+ (such as the one reported here), 9-phenanthrol blocks TRPM4 (2, 24, 26, 29) but not TRPM5 (29), TRPM7 (32), TRPC3, or TRPC6 (26). In smooth muscle, 9-phenanthrol also fails to block BKCa, KIR, KV, or voltage-dependent Ca2+ channels (26). On the other hand, 9-phenanthrol has been found to block K+ and L-type Ca2+ channels in cardiac myocytes (74) as well as a Ca2+-activated Cl− channel (11) in smooth muscle. It activates a Ca2+-activated K+ channel in endothelial cells (22). Cytoplasmic MgATP blocks TRPM4 but also TRPM6 and (as a source of Mg2+) TRPM7 (14, 20). Unlike the current reported here, TRPM6 and TRPM7 are permeable to Ca2+ and have larger single-channel conductances (39–186 pS) (6, 20).
In HEK-293 cells, both the native and exogenously expressed TRPM4 currents have been extensively examined. In studies of inside-out excised membrane patches of HEK-293 cells, EC50 for cytoplasmic Ca2+has ranged from 370 nM (58) to ∼200 μM (19). Some native currents that appear to be TRPM4 dependent have still higher EC50 values (e.g., 500 μM) (42). Our value for the native ciliary current is high (646 μM at +100 mV) but decreases to 26 μM at +100 mV when PLC is inhibited. In other reports, the sensitivity of TRPM4 to Ca2+ has been found to depend on many factors, including the phosphorylation status of the channel (57), the availability of PIP2 (53, 86), whether the current is endogenous or exogenously expressed (2, 41), and associated channel subunits. We have not determined whether the ciliary TRPM4-dependent channels include other subunits. In other systems, TRPM4 associates with TRPC3 (59) or with SUR1, which increases the channel's sensitivity to Ca2+ (82).
In the renal primary cilium, the current-voltage relationship of the TRPM4-dependent current showed mild outward rectification (Figs. 1A, 2A, and 3). When TRPM4 has been exogenously expressed, the degree of rectification has been variable. Strong outward rectification has often (29, 54, 55, 58, 76) but not always (41) been observed. After holding at a depolarized potential, voltage jumps yield instantaneous currents with little rectification (54, 76). Treatment with PIP2 or inhibition of PLC produces a nearly linear current-voltage relationship (53, 86), as does deletion of a C-terminal calmodulin-binding site in TRPM4 (57). Less is known about the current-voltage properties of native TRPM4 currents. In vomeronasal neurons, a native current that appears to be TRPM4 dependent shows mild outward rectification like that reported here (42).
In most previous studies, TRPM4 current has inactivated quickly, particularly at negative potentials (25, 31, 53, 54, 56, 57, 76, 79, 86; see also Ref. 41). The ciliary TRPM4-dependent current also inactivates in the presence of high cytoplasmic Ca2+, but relatively slowly (Fig. 8A, ●). In ChoK1 and HEK-293 cells, inactivation of exogenously expressed TRPM4 channels was greatly reduced by supplying cytoplasmic PIP2, which increased the sensitivity of the channels to cytoplasmic Ca2+ (53, 86). Inhibiting PLC, an enzyme that hydrolyzes PIP2, also strongly reduced inactivation (53), and we have observed a similar effect in the ciliary current (Fig. 8A, ○). In contrast to other reports (53, 86), though, we were unable to restore the ciliary current by incubating the cilium with MgATP (Fig. 8A, ● at 480 ms). This incubation is believed to facilitate the synthesis of PIP2 (53, 86). In the cilium, three properties of the TRPM4-dependent current suggest that it may be somewhat desensitized from the moment the cilium is excised: the sensitivity to cytoplasmic Ca2+ is low (Fig. 1B); the rate of inactivation is slow (Fig. 8A); and the inactivation is only weakly voltage-dependent (Fig. 7). The ineffectiveness of MgATP in restoring the TRPM4 current may indicate that an enzyme or substrate required for the production of PIP2 is not abundant in the primary cilia of mIMCD-3 cells. Recent evidence indicates that absence of Ca2+ buffering also contributes to inactivation of TRPM4 (25), but we have not examined that.
At lower concentrations of cytoplasmic Ca2+, we observed current fluctuations consistent with the presence of TRPM4 channels (Fig. 4). The average single-channel conductance of the ciliary channels was 23 pS, which matches values reported elsewhere for TRPM4 (22–25 pS) (2, 19, 29, 41, 54). Like other TRPM4 channels (2, 19, 41, 55), these channels conduct Na+ and K+ but not Cl− or Ca2+. The relative cationic permeability PK/PNa for native TRPM4-dependent channels in primary cilia is 1.42, which is somewhat higher than the values reported in HEK-293 cells (0.84 and 0.87) (2, 55). In primary cilia, the single-channel conductance was voltage dependent, being 17 pS at negative potentials and 31 pS at positive potentials (Fig. 4B). This rectification closely matches that seen in the macroscopic currents (Fig. 1A). In most other reports, the unitary conductance has been linear over a similar range of potentials (2, 19, 29), although an S-shaped rectification has also been reported (41). Most often, the outward rectification of the macroscopic current is attributed not to a voltage-dependent unitary conductance but to voltage dependence of channel gating (41, 54). In one case, though, TRPM4 gating was found to be voltage independent (19). Our initial results suggest that gating of the ciliary channel is also voltage dependent (Fig. 4A). However, we were unable to directly measure the relationship between voltage and open probability of the single channels. A given cilium or membrane patch usually has numerous channels (e.g., Fig. 4A), and the exact number of channels is not certain.
When we knocked down Trpm4 mRNA expression, cilia were 43% shorter and a smaller percentage of cells were ciliated (52 vs. 75%) compared with cells treated with the control shRNA. Many cells disassemble the cilium when dividing (66), so seeing shorter cilia and fewer ciliated cells could result from a TRPM4-related increase in mitotic rate. Alternatively, TRPM4 may play a more direct role in the control of ciliary length, as several molecules (reviewed in Ref. 10) have been found to do.
Although TRPM4 itself does not conduct Ca2+, it influences Ca2+ uptake in many cells. Endogenous TRPM4 currents have been identified in nonexcitable cells such as HEK-293 cells, where they appear to modulate Ca2+ currents (21, 41, 59), and in T cells, where they regulate Ca2+ oscillations (40). While Launay et al. (41) demonstrated that TRPM4 current in HEK-293 cells reduces Ca2+ entry by depolarizing the membrane, Fliegert et al. (21) showed that outward K+ currents through TRPM4 can facilitate Ca2+ entry by repolarizing the plasma membrane. In excitable cells such as pancreatic β cells (13, 47), vascular smooth muscle cells (19, 69), and pre-Bötzinger complex neurons (15), TRPM4-mediated depolarization promotes opening of voltage-dependent Ca2+ channels. Studies in TRPM4 knockout animals (reviewed in Refs. 39 and 50) suggest that TRPM4 activity modulates blood pressure, the immune response, control of inflammation, formation of secondary hemorrhages, and the duration of the cardiac action potential. Many of these effects may be accounted for by the ability of TRPM4 to modulate the driving force for Ca2+ entry. In humans, dominantly inherited mutations in TRPM4 underlie several diseases of cardiac conduction (39).
In mIMCD-3 cells derived from renal epithelium, we have found TRPM4 in both the primary cilium and the cytoplasmic membrane. A given protein may serve different functions in the cell membrane and in the cilium (3, 49, 62), and we do not yet know the function of TRPM4 in either compartment. mIMCD-3 cells are considered nonexcitable, but TRPM4 in the primary cilium may function as it does in excitable cells. The cilia of mIMCD-3 cells have TRPP2-dependent channels (44), which, like TRPM4, are activated by cytoplasmic Ca2+ (38, 80). Like the voltage-gated Ca2+ channels of excitable cells, TRPP2 is activated by depolarization (5) and conducts Ca2+ (27, 30). Evidence suggests that TRPP2 is gated when the cilia are deflected by flow in the renal filtrate (52). TRPP2 should then allow Ca2+ to enter the cilium. This should in turn activate TRPM4, which should further depolarize the cilium. This depolarization should have two competing effects. It should increase the open probability of TRPP2, which would promote further Ca2+ influx. At the same time, depolarization should decrease the driving force for Ca2+ entry, which would tend to reduce the influx of Ca2+ through TRPP2 channels. This model assumes that intraciliary Ca2+ can reach levels sufficient to gate TRPM4 channels. The sensitivity of TRPM4 to cytoplasmic Ca2+ varies greatly depending on the level of PIP2 (53, 86). When hydrolysis of PIP2 is inhibited, the ciliary TRPM4 channel is activated by Ca2+ concentrations of a few micromolar (Fig. 8B). Because of the cilium's high surface-to-volume ratio, a modest influx of Ca2+ can easily bring intraciliary Ca2+ to very high levels. In olfactory cilia, internal Ca2+ reaches at least 100 μM during the odor response (17). This would be more than sufficient to activate the ciliary TRPM4 channels in their more sensitive state.
Ca2+ signaling within renal epithelial cells is influential in the development of renal cysts (12, 62). As noted elsewhere (12), molecules that influence this signaling are attractive targets for pharmacological intervention in polycystic kidney disease. It now seems likely that the TRPM4 channel is among the molecules of interest.
GRANTS
S. J. Kleene received support from National Institutes of Health (NIH) Grant R21 DK091917.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.J.F., N.K.K., and S.J.K. provided conception and design of research; R.J.F., N.K.K., and S.J.K. performed experiments; R.J.F., N.K.K., and S.J.K. analyzed data; R.J.F., N.K.K., and S.J.K. interpreted results of experiments; R.J.F., N.K.K., and S.J.K. prepared figures; R.J.F., N.K.K., and S.J.K. drafted manuscript; R.J.F., N.K.K., and S.J.K. edited and revised manuscript; R.J.F., N.K.K., and S.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Priscilla Amofa for excellent technical assistance and the Cincinnati Children's Hospital Medical Center Confocal Imaging Core and Dr. Matt Kofron for access to and training with the Imaris software.
REFERENCES
- 1.Aguiari G, Trimi V, Bogo M, Mangolini A, Szabadkai G, Pinton P, Witzgall R, Harris PC, Borea PA, Rizzuto R, del Senno L. Novel role for polycystin-1 in modulating cell proliferation through calcium oscillations in kidney cells. Cell Prolif 41: 554–573, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarouch MY, Syam N, Abriel H. Biochemical, single-channel, whole-cell patch clamp, and pharmacological analyses of endogenous TRPM4 channels in HEK293 cells. Neurosci Lett 541: 105–110, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson KF, Kathem SH, Jin X, Muntean BS, Abou-Alaiwi WA, Nauli AM, Nauli SM. Dopaminergic signaling within the primary cilia in the renovascular system. Front Physiol 6: 103, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9: 472–479, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai CX, Kim S, Li WP, Streets AJ, Ong ACM, Tsiokas L. Activation of TRPP2 through mDia1-dependent voltage gating. EMBO J 27: 1345–1356, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates-Withers C, Sah R, Clapham DE. TRPM7, the Mg2+ inhibited channel and kinase. Adv Exp Med Biol 704: 173–183, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol 19: R526–R535, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bers DM. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol Cell Physiol 242: C404–C408, 1982. [DOI] [PubMed] [Google Scholar]
- 9.Bissler JJ, Dixon BP. A mechanistic approach to inherited polycystic kidney disease. Pediatr Nephrol 20: 558–566, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Broekhuis JR, Leong WY, Jansen G. Regulation of cilium length and intraflagellar transport. Int Rev Cell Mol Biol 303: 101–138, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Burris SK, Wang Q, Bulley S, Neeb ZP, Jaggar JH. 9-Phenanthrol inhibits recombinant and arterial myocyte TMEM16A channels. Br J Pharmacol 172: 2459–2468, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chebib FT, Sussman CR, Wang X, Harris PC, Torres VE. Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat Rev Nephrol 11: 451–464, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R. TRPM4 controls insulin secretion in pancreatic β-cells. Cell Calcium 41: 51–61, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chubanov V, Gudermann T. Trpm6. Handb Exp Pharmacol 222: 503–520, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex. J Physiol 582: 1047–1058, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504: 315–318, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado R, Bacigalupo J. Cilium-attached and excised patch-clamp recordings of odourant-activated Ca-dependent K channels from chemosensory cilia of olfactory receptor neurons. Eur J Neurosci 20: 2975–2980, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature 504: 311–314, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Fleig A, Chubanov V. Trpm7. Handb Exp Pharmacol 222: 521–546, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fliegert R, Glassmeier G, Schmid F, Cornils K, Genisyuerek S, Harneit A, Schwarz JR, Guse AH. Modulation of Ca2+ entry and plasma membrane potential by human TRPM4b. FEBS J 274: 704–713, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Garland CJ, Smirnov SV, Bagher P, Lim CS, Huang CY, Mitchell R, Stanley C, Pinkney A, Dora KA. TRPM4 inhibitor 9-phenanthrol activates endothelial cell intermediate conductance calcium-activated potassium channels in rat isolated mesenteric artery. Br J Pharmacol 172: 1114–1123, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gascue C, Katsanis N, Badano JL. Cystic diseases of the kidney: ciliary dysfunction and cystogenic mechanisms. Pediatr Nephrol 26: 1181–1195, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 299: C279–C288, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzales AL, Earley S. Endogenous cytosolic Ca2+ buffering is necessary for TRPM4 activity in cerebral artery smooth muscle cells. Cell Calcium 51: 82–93, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzales AL, Garcia ZI, Amberg GC, Earley S. Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol 299: C1195–C1202, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, LaRusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA 104: 19138–19143, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grand T, Demion M, Norez C, Mettey Y, Launay P, Becq F, Bois P, Guinamard R. 9-phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br J Pharmacol 153: 1697–1705, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol 13: 1153–1158, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Kim BJ, Kim SY, Lee S, Jeon JH, Matsui H, Kwon YK, Kim SJ, So I. The role of transient receptor potential channel blockers in human gastric cancer cell viability. Can J Physiol Pharmacol 90: 175–186, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Kleene NK, Kleene SJ. A method for measuring electrical signals in a primary cilium. Cilia 1: 17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleene SJ. A simple intrapipette salt bridge. J Neurosci Methods 46: 11–16, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. J Neurosci 11: 3624–3629, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleene SJ, Van Houten JL. Electrical signaling in motile and primary cilia. Bioscience 64: 1092–1102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Kruse M, Pongs O. TRPM4 channels in the cardiovascular system. Curr Opin Pharmacol 15: 68–73, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science 306: 1374–1377, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109: 397–407, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Liman ER. Regulation by voltage and adenine nucleotides of a Ca2+-activated cation channel from hamster vomeronasal sensory neurons. J Physiol 548: 777–787, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liman ER. Trpm5. Handb Exp Pharmacol 222: 489–502, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol 23: 2600–2607, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marigo V, Courville K, Hsu WH, Feng JM, Cheng H. TRPM4 impacts on Ca2+ signals during agonist-induced insulin secretion in pancreatic β-cells. Mol Cell Endocrinol 299: 194–203, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Masyuk AI, Gradilone SA, LaRusso NF. Calcium signaling in cilia and ciliary-mediated intracellular calcium signaling: are they independent or coordinated molecular events? Hepatology 60: 1783–1785, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masyuk AI, Huang BQ, Radtke BN, Gajdos GB, Splinter PL, Masyuk TV, Gradilone SA, Larusso NF. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol 304: G1013–G1024, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathar I, Jacobs G, Kecskes M, Menigoz A, Philippaert K, Vennekens R. TRPM4. Handb Exp Pharmacol 222: 461–487, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Moorman SJ, Shorr AZ. The primary cilium as a gravitational force transducer and a regulator of transcriptional noise. Dev Dyn 237: 1955–1959, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 25: 467–478, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem 278: 30813–30820, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Nilius B, Prenen J, Janssens A, Owsianik G, Wang C, Zhu MX, Voets T. The selectivity filter of the cation channel TRPM4. J Biol Chem 280: 22899–22906, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Nilius B, Prenen J, Janssens A, Voets T, Droogmans G. Decavanadate modulates gating of TRPM4 cation channels. J Physiol 560: 753–765, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem 280: 6423–6433, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Nilius B, Prenen J, Voets T, Droogmans G. Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflügers Arch 448: 70–75, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Park JY, Hwang EM, Yarishkin O, Seo JH, Kim E, Yoo J, Yi GS, Kim DG, Park N, Ha CM, La JH, Kang D, Han J, Oh U, Hong SG. TRPM4b channel suppresses store-operated Ca2+ entry by a novel protein-protein interaction with the TRPC3 channel. Biochem Biophys Res Commun 368: 677–683, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol 15: 105–110, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Phua SC, Lin YC, Inoue T. An intelligent nano-antenna: primary cilium harnesses TRP channels to decode polymodal stimuli. Cell Calcium 58: 415–422, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179–183, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Quarmby LM, Parker JDK. Cilia and the cell cycle? J Cell Biol 169: 707–710, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol Renal Fluid Electrolyte Physiol 265: F416–F424, 1993. [DOI] [PubMed] [Google Scholar]
- 68.Raychowdhury MK, McLaughlin M, Ramos AJ, Montalbetti N, Bouley R, Ausiello DA, Cantiello HF. Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem 280: 34718–34722, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Reading SA, Brayden JE. Central role of TRPM4 channels in cerebral blood flow regulation. Stroke 38: 2322–2328, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, Zhang Q, Thompson PA, Miller JP, Harris PC; CRISP Consortium. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 18: 2143–2160, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman AFM. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37: e45, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol 69: 377–400, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci 123: 499–503, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simard C, Salle L, Rouet R, Guinamard R. Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br J Pharmacol 165: 2354–2364, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol 290: F1320–F1328, 2006. [DOI] [PubMed] [Google Scholar]
- 76.Takezawa R, Cheng H, Beck A, Ishikawa J, Launay P, Kubota H, Kinet JP, Fleig A, Yamada T, Penner R. A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol Pharmacol 69: 1413–1420, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 76: 149–168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 94: 6965–6970, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium 37: 267–278, 2005. [DOI] [PubMed] [Google Scholar]
- 80.Vassilev PM, Guo L, Chen XZ, Segal Y, Peng JB, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye CP, Brown EM, Hediger MA, Zhou J. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun 282: 341–350, 2001. [DOI] [PubMed] [Google Scholar]
- 81.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int 20: 73–81, 1996. [DOI] [PubMed] [Google Scholar]
- 82.Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem 288: 3655–3667, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006. [DOI] [PubMed] [Google Scholar]
- 84.Yarishkin OV, Hwang EM, Park JY, Kang D, Han J, Hong SG. Endogenous TRPM4-like channel in Chinese hamster ovary (CHO) cells. Biochem Biophys Res Commun 369: 712–717, 2008. [DOI] [PubMed] [Google Scholar]
- 85.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem 280: 39185–39192, 2005. [DOI] [PubMed] [Google Scholar]