Abstract
Vasopressin (VP) stimulates a signaling cascade that results in phosphorylation and apical membrane accumulation of aquaporin-2 (AQP2), leading to water reabsorption by kidney collecting ducts. However, the roles of most C-terminal phosphorylation events in stimulated and constitutive AQP2 recycling are incompletely understood. Here, we generated LLC-PK1 cells containing point mutations of all potential phosphorylation sites in the AQP2 C terminus: S226, S229, T244, S256, S261, S264, and S269, to determine their impact on AQP2 trafficking. We produced an All Null AQP2 construct in which these serine (S) or threonine (T) residues were mutated to alanine (A) or glycine (G), and we then reintroduced the phosphorylation mimic aspartic acid (D) individually to each site in the All Null mutant. As expected, the All Null mutant does not accumulate at the plasma membrane in response to VP but still undergoes constitutive recycling, as shown by its membrane accumulation when endocytosis is blocked by methyl-β-cyclodextrin (MβCD), and accumulation in a perinuclear patch at low temperature (20°C). Single phosphorylation mimics S226D, S229D, T244D, S261D, S264D, and S269D were insufficient to cause membrane accumulation of AQP2 alone or after VP treatment. However, AQP2 S256 reintroduced into the All Null mutant maintains its trafficking response to VP. We conclude that 1) constitutive recycling of AQP2 does not require phosphorylation at any C-terminal sites; 2) forced “phosphorylation” of sites in the AQP2 C terminus is insufficient to stimulate membrane accumulation in the absence of S256 phosphorylation; and 3) phosphorylation of S256 alone is necessary and sufficient to cause membrane accumulation of AQP2.
Keywords: aquaporin-2, phosphorylation, vasopressin, LLC-PK1, endocytosis
water transport in principal cells of the renal collecting duct is dependent on the vasopressin (VP)-induced translocation of aquaporin-2 water channels (AQP2) from intracellular vesicles to the apical membrane. Circulating vasopressin binds to the vasopressin receptor (V2R) located on the basolateral side of principal cells in the kidney, causes elevation of cAMP, which leads to PKA activation, and subsequent phosphorylation of serine-256 (S256) in the C terminus of AQP2. Phosphorylation of AQP2 at S256 results in membrane accumulation of AQP2 (5, 10, 17, 23), water reabsorption, and urinary concentration. Dysfunction of the VP-V2R and AQP2 axis results in either urinary concentrating defects, such as diabetes insipidus (DI), or urinary dilutional defects, such as hyponatremia, as seen in heart failure and cirrhosis (19).
The phosphorylation state of C-terminal residues is critical for AQP2 trafficking and its ultimate effect on membrane water permeability. In addition to phosphorylation at AQP2 S256, additional phosphorylation sites in the AQP2 C terminus have been identified, including S261, S264, and S269 (1, 2, 9, 23). The physiological role of these putative AQP2 phosphorylation sites has been examined by several groups (2, 4, 8, 13, 17, 22, 23). Substitution of alanine (A) at S256 prevents the plasma membrane accumulation of AQP2 in response to VP or forskolin (FK). Conversely, substitution of phosphorylation mimetic aspartic acid (D) at S256 promotes VP-independent plasma membrane accumulation of AQP2 in cells (7, 16, 20, 23). Increases in phosphorylated S264 and S269 and a decrease in phosphorylated S261 (8) are observed after VP-dependent phosphorylation at S256 in Madin-Darby canine kidney (MDCK) cells, but no phosphorylation at S264 or S269 occurred in S256L mutant mice (2, 4, 7). These observations suggest that AQP2 trafficking is modulated by a complex series of phosphorylation events.
Our group and others have shown that AQP2 is constitutively recycling between the plasma membrane and intracellular compartments without vasopressin stimulation (6, 11, 12). Inhibiting AQP2 endocytosis alone causes membrane accumulation of AQP2 (6, 17, 20). Importantly, this VP-independent AQP2 recycling does not depend on phosphorylation at AQP2 S256 (13, 14). All of this complexity has made understanding definitive mechanisms underlying constitutive and regulated AQP2 trafficking somewhat elusive, but dissecting AQP2 phosphorylation events is of clear importance to move the field forward. With this in mind, and knowing that there is very limit knowledge of kinases other than PKA that phosphorylate AQP2, we have applied a “discovery” approach of performing an unbiased analysis of all seven potential phosphorylation sites on C-terminal AQP2 S226, S229, T244, S256, S261, S264, and S269, regardless of whether they are known or unknown, predicted or not predicted phosphorylation sites. Therefore, we removed all potential phosphorylation sites through mutation (AQP2 all S/A) and assessed the impact on both regulated trafficking (using VP/FK as the stimulation) and constitutive recycling of AQP2. Then, we mutated back the phosphorylation of each potential residue, to test the effect of each individual phosphorylation on the regulated trafficking and constitutive recycling of AQP2, and examined whether they were interdependent on the phosphorylation of other residues in the AQP2 C terminus. We found that S256 phosphorylation alone is sufficient for VP- and FK-stimulated AQP2 trafficking, and constitutive recycling of AQP2 occurs independently of any known C-terminal phosphorylation events.
MATERIALS AND METHODS
Antibodies and reagents.
The following antibodies were used in the study: mouse monoclonal antibody against c-Myc (9E10 hybridoma cell line, ATCC, Manassas VA), goat anti-AQP2 antibody (sc9882, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-β-actin antibody (Chemicon), Cy3-conjugated donkey anti-mouse, and anti-goat secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove PA). Methyl-β-cyclodextrin (MβCD), FK, lysine VP, and cycloheximide were purchased from Sigma-Aldrich (St. Louis, MO).
Generating DNA constructs and establishing stable cell lines.
A list of the primers used in generating AQP2 DNA constructs is shown in Fig. 1A. Briefly, a cloning construct containing full-length rat wild-type AQP2 with a c-Myc tag on the C terminus was generated by PCR using a sense primer F1 and an anti-sense primer R1(Fig. 1A) using pcDNA1.AQP2 as a template (3). Through ligation, we generated a new cloning template, pcDNA3-wtAQP2. All serine or threonine residues (S226, S229, T244, S256, S261, S264, and S269) on the C terminus of AQP2 were mutated to mimic a dephosphorylation state, alanine (A) or glycine (G). The choice of a G vs. A mutation was dictated by the least number of nucleotide changes needed to produce the amino acid substitution. We first generated the first half-fragment of the construct by PCR amplification using pcDNA3-wtAQP2 as a template and primer pairs F1 and R2 that introduced point mutations S226A and S229G. This PCR product was inserted in the pcDNA3-wtAQP2. Then, we generated the second half-fragment by PCR using primer F2, which introduced a point mutation of T244A, and a reverse primer R3, which introduced point mutations S256A, S261A, S264G, and S269G (Fig. 1A). The PCR product was inserted in pcDNA3.m1AQP2.
Fig. 1.
Summary of primers used to generate aquaporin-2 (AQP2) C-terminal phosphorylation mutants and sequence of generated AQP2 mutants. A: list of primers used to generate wild-type AQP2 and AQP2 all A/G mutant. B: comparison of the amino acid sequence of AQP2 C terminus between human and rodents. C: list of primer pairs used for site-directed mutagenesis to generate phosphorylation mimics.
Unique AQP2 mutants mimicking phosphorylation (aspartic acid; D) at potential phosphorylated sites on the AQP2 C terminus were generated using the above pcDNA3.m2AQP2 as a template and a site-directed mutagenesis kit (Agilent Technologies) following the manufacturer's instructions. The primer pairs used for generating individual point mutation are listed in Fig. 1C.
All constructs were sequenced twice to confirm the correct mutations. Constructs expressing wild-type and various phosphorylated AQP2 mimics were transfected into the LLC-PK1 cells using Lipofectamine 2000 Reagent (Invitrogen). Stable cell lines were cloned using the limiting dilution method and selected by geneticin. Both immunoblots and immunofluorescence staining confirmed all positive clones. Stably transfected cells were cultured as previously described (12).
Immunofluorescence staining and immunoblotting.
Immunofluorescence staining and immunoblotting were performed as previously reported (12).
Cold block: LLC-PK1.
The cold-block experiment was performed as previously reported (20). Briefly, LLC-PK1 cells were retreated with 10 μM cycloheximide for 60 min, then the cold block was performed by incubating cells at 20°C for various times. Cells were fixed and immunostained. Quantification of the accumulation of fluorescence signal in the “perinuclear patches” was performed as a measure of the extent and rate of endocytosis of AQP2, as previously described (20).
RESULTS
Establishing LLC-PK1 stable cell lines expressing AQP2 phosphorylation mutants.
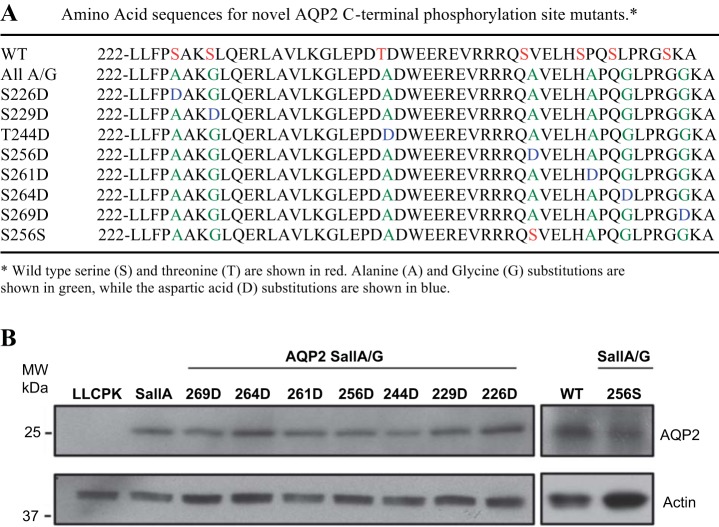
The alignment of the C terminus of human, mouse, and rat AQP2 is shown in Fig. 1B. With the exception of S231 and S226, rodent AQP2 shares six similar, predicted phosphorylation sites with human AQP2, and seven predicted phosphorylation sites are conserved in rodents. A total of nine mutant constructs were generated: AQP2 with seven putative C-terminal phosphorylation sites mutated to alanine or glycine (AQP2-SallA/G), AQP2-SallA/G with only intact S256 (SallA/G 256S), and AQP2-SallA/G with a single aspartic acid at any of the seven putative phosphorylation sites (SallA/G S226D, S229D, T244D, S256D, S261D, S264D, S269D, respectively). All constructs were sequenced twice (Fig. 2A). All AQP2 constructs contained a c-Myc tag fused to the C terminus as previously described (20). LLC-PK1 cells were transfected with the above nine constructs. At least two stable clones were selected for each construct. The expression of mutant AQP2 protein was similar in all transfected LLC-PK1 cell lines as revealed by immunoblotting (Fig. 2B).
Fig. 2.
Expression of AQP2 C-terminal phosphorylation mutants in LLC-PK1 cells. A: summary of the amino acid sequence of AQP2 C terminus of wild-type and phosphorylation mutants that were generated. B: immunoblotting of lysates from untransfected and AQP2-transfected LLC-PK1 cells to confirm AQP2 expression.
Phosphorylation of S256 alone is critical for regulated trafficking.
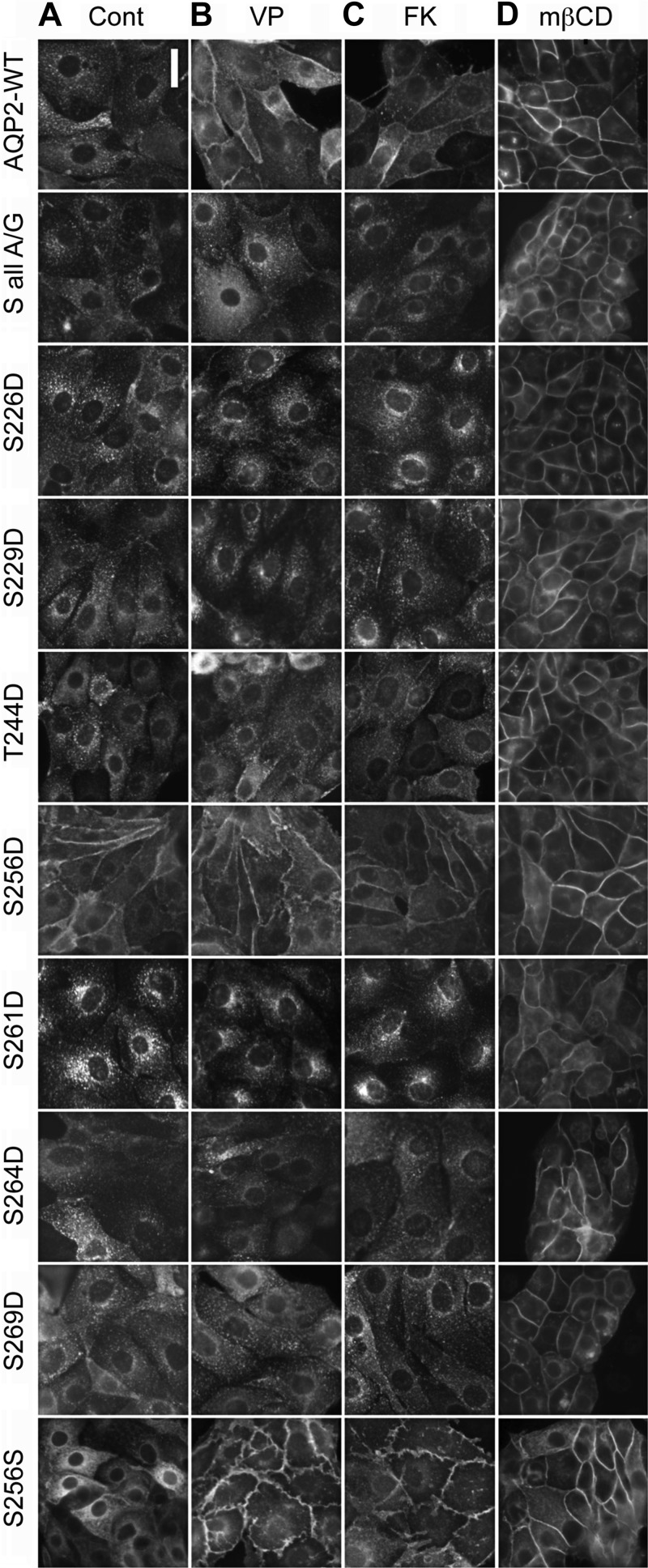
AQP2-WT, AQP2-S all A/G, AQP2-S all A/G S256S, and AQP2-S all A/G containing individual phosphorylation mimics (S226D, S229D, T244D, S256D, S261D, S264D, and S269D) were examined for regulated trafficking in LLC-PK1 cells. Under basal conditions, AQP2-WT and AQP2-SallA/G were localized similarly in intracellular vesicles (Fig. 3A). When treated with either VP or FK, AQP2-SallA/G mutants failed to accumulate on the plasma membrane while AQP2-WT responded robustly, leading to clear membrane accumulation (Fig. 3, B and C). AQP2-SallA/G S256D accumulated on the plasma membrane with or without treatment with VP or FK (Fig. 3, A–C) independently of the phosphorylation state of any other putative phosphorylation site in the C terminus of AQP2. This again confirms the critical role of phosphorylation at S256 on membrane accumulation of AQP2 and is consistent with previous reports using AQP2-S256D (18, 20, 23). In AQP2-SallA/G S256S cells, AQP2 was primarily observed in intracellular vesicles under baseline conditions and accumulated at the cell membrane in response to VP and FK treatment (Fig. 3, A–C). These results suggest that intact S256 is solely responsible for VP-induced membrane accumulation independently of the phosphorylation state of any other sites in the C terminus. AQP2-SallA/G S226D, S229D, T244D, S261D, S264D, and S269D were all located in intracellular vesicles under baseline conditions and did not exhibit any notable membrane accumulation even when stimulated by VP or FK, suggesting that individual phosphorylation at S226, S229, T244, S261, S264, and S269 alone is not sufficient for membrane accumulation of AQP2 (Fig. 3, A–C). Thus phosphorylation at S256 alone can induce AQP2 membrane accumulation and appears to be necessary and sufficient for VP-induced AQP2 trafficking.
Fig. 3.
Regulated trafficking and constitutive recycling of AQP2 in LLC-PK1 cells transfected with AQP2 phosphorylation mutants. Cells grown on coverslips were treated with vehicle (Cont; A), 10 nM vasopressin (VP; B), 10 μM forskolin (FK; C), or 10 mM methyl-β-cyclodextrin (MβCD; D) for 30 min. Immunofluorescence staining of AQP2 was performed using anti-c-myc antibody. Similar experiments were performed at least 3 times. Scale bar = 10 μm.
AQP2 C-terminal phosphorylation is not required for constitutive recycling.
Constitutive recycling of AQP2 was examined in wild-type and AQP2-SallA/G mutant-expressing cells. In the presence of MβCD, a cholesterol-chelating reagent known to block endocytosis (12, 21), both AQP2-WT and AQP2-SallA/G accumulated on the plasma membrane (Fig. 3D). This suggests that the AQP2-SallA/G mutant is constitutively recycling, and its recycling does not depend on any phosphorylation in the C terminus of AQP2. Treatment with MβCD also caused membrane accumulation of AQP2 SallA/G mutants with individual phosphorylation mimics at S226, S229, T244, S261, S264, and S269 (Fig. 3D), indicating that these AQP2 mutants are also undergoing constitutive recycling similar to the wild-type AQP2 and that this recycling mechanism is not adversely affected by the phosphorylation state at S226, S229, T244, S261, S264, and S269 of AQP2. Taken together, our results suggest that in contrast to VP/cAMP-regulated trafficking of AQP2, constitutive recycling of AQP2 is independent of any phosphorylation of AQP2 in its C terminus.
AQP2 endocytosis is inhibited by S256 phosphorylation.
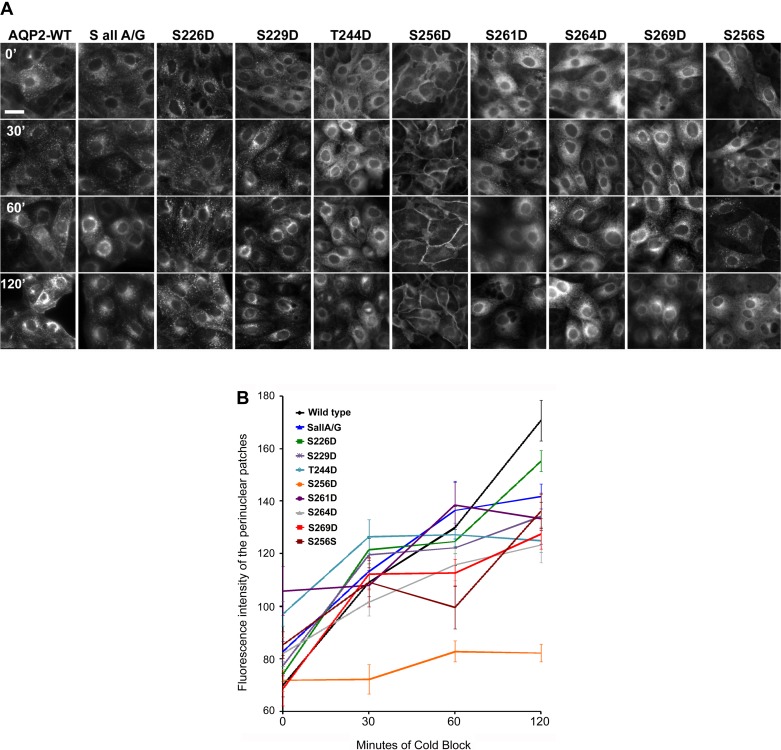
We and others previously reported that AQP2-S256D was highly resistant to endocytosis (17, 20) compared with AQP2-WT. AQP2-SallA/G S256D is also highly resistant to endocytosis and did not accumulate in the perinuclear region after 120 min of 20°C cold block, a temperature at which endocytosis can still occur, but the secretory pathway is inhibited by blocking the release of transport vesicles from the trans-Golgi network (6, 14) (Fig. 4, A and B). This is similar to the previously reported pattern of perinuclear patch formation by AQP2-S256D in the wild-type background (20). In contrast, AQP2-WT and all other AQP2-SallA/G mutants including S226D, S229D, T244D, S261D, S264D, S269D, and S256S clearly showed the presence of perinuclear patches (derived mainly from internalized AQP2) in cells after 60–120 min of cold block (Fig. 4A), although we did notice subtle differences in the rate and compactness of the perinuclear patches among various AQP2-SallA/G mutants. This might indicate differences in subcellular trafficking among various mutants that cannot be easily characterized by our current methodology. Overall, however, we conclude that constitutive phosphorylation at individual sites other than S256 does not appear to significantly impede or promote AQP2 endocytosis in this model system.
Fig. 4.
Assessment of endocytosis in LLC-PK1 cells expressing AQP2 C-terminal mutant by 20°C cold block. A: AQP2 distribution in cells after cold block by immunofluorescence staining. Cells were incubated at 20°C for 30, 60, and 120 min to block AQP2 release from the trans-Golgi network, forming a “perinuclear patch.” Scale bar = 10 μm. B: quantification of AQP2 perinuclear patch formation after 20°C cold block. The accumulation of intracellular AQP2 fluorescence signal in the perinuclear patches over time was measured by quantifying mean patch fluorescence intensity using Velocity software as was described previously (20). Values are means ± SE; n = ≥13 for each data point. Similar experiments were repeated at least 3 times.
DISCUSSION
Through this unbiased, discovery approach as mentioned above, we have shown that constitutive recycling of AQP2 does not require phosphorylation at any C-terminal site; forced “phosphorylation” of sites in the AQP2 C terminus is insufficient to stimulate membrane accumulation in the absence of S256 phosphorylation; phosphorylation of S256 alone is necessary and sufficient to cause membrane accumulation of AQP2 (10, 20). Our findings, therefore, support a number of previous reports on the role of AQP2 phosphorylation (mostly studies related to S256, 261, 264, and 269), but extend current understanding by examining the potential involvement of other C-terminal sites in the stimulated and constitutive AQP2 recycling process.
Our demonstration that AQP2-SallA/G S256D and AQP2 SallA/G localize at the plasma membrane or in intracellular vesicles, respectively, both with or without VP/FK stimulation, parallels previous studies using S256D and S256A mutations (17, 23). Van Balkom et al. (23) had also shown that S229A and T244A mutations had no deleterious effect on the response to VP/FK when the S256 residue is present. We and others (13, 22) have shown that AQP2 S261D is intracellular in the absence of constitutive S256 phosphorylation. We previously reported that the S256D mutation in a wild-type background was highly resistant to endocytosis, as revealed using a cold-block strategy to accumulate and trap internalized AQP2 in a perinuclear patch, representing at least in part, the trans-Golgi network (20). Our observation that S256D in the SallA/G background was similarly unaffected by cold block indicates that phosphorylation of other residues in the C terminus is not required for membrane accumulation of AQP2.
We previously observed that in contrast to S256D, the S269D mutant in a WT background did not constitutively accumulate in the plasma membrane in LLC-PK1 cells. Using the all SallA/G background, we again did not observe plasma membrane accumulation of the S269D mutant under baseline conditions or in response to VP/FK. Results using the SallA/G S256S mutation eliminated the possibility that pS269 and other potential phosphorylation sites are required for AQP2 membrane accumulation in response to VP/FK. This result is consistent with data from Hoffert et al. (7), who expressed S269A MDCK cells and showed that they still accumulated on the plasma membrane after VP stimulation. They also showed that S269D MDCK cells exhibited increased retention of AQP2 on the apical membrane, but, their findings were performed in the presence of functional S256, which could have contributed to the observed effect of the S269D mutant. Indeed, we (and others) have detected a substantial level of phosphorylation of AQP2 at S256 under baseline, non-stimulated condition in cultured cells (24). Our results now clearly show that VP-mediated AQP2 accumulation on the plasma membrane is independent of the phosphorylation of S269. In vivo, pS269 is undetectable in vasopressin deficient Brattleboro (BB) rats, and it is found exclusively in apical membranes of normal and BB rat CD principal cells after VP treatment (16). While our data suggest that in vitro modifications of S269 cannot overcome the requirement of intact S256 for regulated trafficking of AQP2, a role of S269 phosphorylation in prolonging the cell surface expression of AQP2 seems a likely possibility and has been suggested previously (7, 15).
The SallA/G S264D mutant failed to traffic in response to VP/FK, consistent with the observation that phosphorylation at this site seems to be dependent on prior phosphorylation of S256 (7). In vivo, pS264 is observed in apical and early endosomal compartments in the normal rat and mouse kidney (4). Much like S269, this apical sorting is probably dependent on intact S256. Tamma et al. (22) found that both S261A and S261D mutations in MDCK cells had deleterious effects on apical sorting of AQP2 after FK stimulation, due to increased ubiquitination. Further studies need to be performed to determine whether these phosphorylation mutations affect AQP2 ubiquitinylation and degradation over time.
The response of all cell lines in this study to MβCD treatment shows that none of the phosphorylation sites of the AQP2 C terminus are absolutely vital for AQP2 constitutive recycling. MβCD blocks endocytosis and traps constitutively recycling AQP2 at the plasma membrane (12). Similarly, the accumulation of endocytosed AQP2 in a perinuclear patch, which occurs by blocking the exit of AQP2 from a preexocytotic compartment that includes the trans-Golgi network (20), also does not require AQP2 C-terminal phosphorylation. Previous observations that examined these events used AQP2 constructs that contained one or more functional C-terminal phosphorylation sites (12). Only the S256 phosphorylation mimic (S256D) was sufficient to inhibit endocytosis and abrogate the formation of a cold block-induced perinuclear patch. However, we did not examine the speed of recovery from the cold block in this study, and it is possible that some mutants used here may affect the kinetics of AQP2 release from the perinuclear compartment and/or passage through the Golgi.
In summary, our data provide strong evidence that S256 phosphorylation alone is necessary and sufficient for regulated membrane accumulation of AQP2. We confirm that S256 phosphorylation plays a major role in inhibiting endocytosis, consistent with previous reports (13, 20). Furthermore, we show that pS264 and pS269, two phosphorylation events previously observed to increase after VP/FK stimulation, are not critical for this regulated pathway to occur. Finally and importantly, we show that constitutive recycling of AQP2 does not depend on phosphorylation of any of the known putative C-terminal phosphorylation sites.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant DK096586 (D. Brown) and NIH grant minority supplement DK-096586-01 (J. Arthur). H. A. Lu is supported by NIH Grants DK092619, DK096586, and DK096015, the Nephcure Foundation, and MGH/ECOR. Additional support for the Program in Membrane Biology Microscopy Core comes from the Boston Area Diabetes and Endocrinology Research Center (DK57521) and the MGH Center for the Study of Inflammatory Bowel Disease (DK43351).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.A., J.H., and H.J.L. contributed conception and design of research; J.A., J.H., N.N., W.W.J., and W.L. performed experiments; J.A., J.H., N.N., W.W.J., W.L., X.C., D.B., and H.J.L. analyzed data; J.A., J.H., N.N., and H.J.L. interpreted results of experiments; J.A. and J.H. prepared figures; J.A. and H.J.L. drafted manuscript; J.A., J.H., W.L., D.B., and H.J.L. edited and revised manuscript; J.A., J.H., N.N., W.W.J., W.L., D.B., and H.J.L. approved final version of manuscript.
REFERENCES
- 1.Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown D, Hasler U, Nunes P, Bouley R, Lu HA. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr Opin Nephrol Hypertens 17: 491–498, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Rice W, Gu Z, Li J, Huang J, Brenner MB, Van Hoek A, Xiong J, Gundersen GG, Norman JC, Hsu VW, Fenton RA, Brown D, Lu HA. Aquaporin 2 promotes cell migration and epithelial morphogenesis. J Am Soc Nephrol 23: 1506–1517, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA 105: 3134–3139, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Gustafson CE, Katsura T, McKee M, Bouley R, Casanova JE, Brown D. Recycling of AQP2 occurs through a temperature- and bafilomycin-sensitive trans-Golgi-associated compartment. Am J Physiol Renal Physiol 278: F317–F326, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F817–F822, 1997. [PubMed] [Google Scholar]
- 11.Katsura T, Verbavatz JM, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci USA 92: 7212–7216, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Lu HJ, Matsuzaki T, Bouley R, Hasler U, Qin QH, Brown D. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am J Physiol Renal Physiol 295: F290–F294, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matlin KS, Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell 34: 233–243, 1983. [DOI] [PubMed] [Google Scholar]
- 15.Moeller HB, Aroankins TS, Slengerik-Hansen J, Pisitkun T, Fenton RA. Phosphorylation and ubiquitylation are opposing processes that regulate endocytosis of the water channel aquaporin-2. J Cell Sci 127: 3174–3183, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Moeller HB, Knepper MA, Fenton RA. Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424–429, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nejsum LN, Zelenina M, Aperia A, Frokiaer J, Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol 288: F930–F938, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen S, Terris J, Andersen D, Ecelbarger C, Frokiaer J, Jonassen T, Marples D, Knepper MA, Petersen JS. Congestive heart failure in rats is associated with increased expression and targeting of aquaporin-2 water channel in collecting duct. Proc Natl Acad Sci USA 94: 5450–5455, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice WL, Zhang Y, Chen Y, Matsuzaki T, Brown D, Lu HA. Differential, phosphorylation dependent trafficking of AQP2 in LLC-PK1 cells. PLoS One 7: e32843, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodal SK, Skretting G, Garred Ø, Vilhardt F, Van Deurs B, Sandvig K. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell 10: 961–974, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamma G, Robben JH, Trimpert C, Boone M, Deen PM. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am J Physiol Cell Physiol 300: C636–C646, 2011. [DOI] [PubMed] [Google Scholar]
- 23.van Balkom BW, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, van der Sluijs P, Deen PM. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem 277: 41473–41479, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Yui N, Lu HA, Chen Y, Nomura N, Bouley R, Brown D. Basolateral targeting and microtubule-dependent transcytosis of the aquaporin-2 water channel. Am J Physiol Cell Physiol 304: C38–C48, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]