Abstract
Current therapies to limit kidney disease progression lack specificity and often have systemic toxicity. To approach this problem, we postulated that a human monoclonal antibody (F1.1), directed against the noncollagenous-1 domain (NC1) of α3(IV) collagen that localizes in glomeruli, could serve as a vehicle for targeted drug delivery. Given enhanced exposure of the NC1 domain of α3(IV) during glomerular diseases, with limited epitope expression in other organs, α3(IV)NC1 provides an ideal target for delivery of disease-modifying agents. As a potential disease-modifying agent, we initially took advantage of recent observations that PGE2 promoted recovery after established injury during the course of nephrotoxic nephritis. To address the general applicability of the approach, the efficacy of glomerular delivery of dexamethasone was also examined. To achieve glomerular targeted therapy, PGE2 and dexamethasone were coupled to F1.1. After confirmation of the composition and activity of the conjugates, both glomerular localization and the capacity of the conjugates to modify disease were evaluated. After injection into mice with established nephritis, resolution of disease was enhanced with both agents, with normalization of histology and improved blood urea nitrogen levels in conjugate-treated mice compared with untreated mice. The results provide a novel means of targeting glomeruli during nephritis, irrespective of cause, by providing efficient drug delivery, with the potential of limiting systemic effects.
Keywords: antibody-drug conjugates, glomeruli, nephritis, targeted delivery
chronic kidney disease, of all forms, represents a significant health burden. Current therapies to limit disease progression, modify renal injury, and/or reverse established disease are insufficient, lack specificity, and are often toxic. Development of new formulations with the capacity to specifically affect pathological processes within the kidney, with minimal effects at other sites, has many potential advantages, and we pursued this approach. General requirements for these type of agents include the ability to localize specifically within the kidney, reduce inflammation, and restore local cellular processes.
In experimental systems, other investigators have taken advantage of renal blood flow and glomerular sieving properties to deliver various agents to the kidney (e.g., using macromolecular carriers, prodrugs, liposomes, and nanoparticles) (2, 6, 16–18). By contrast, our approach involved the use of a well-defined, human monoclonal antibody (mAb) (F1.1), directed against relatively unique epitopes within the noncollagenous-1 (NC1) domain of α3(IV) collagen [i.e., those regions involved in anti-glomerular basement membrane (GBM) disease], to specifically localize in glomeruli, as a carrier for drug delivery (13). Given its proximity to glomerular cells, along with limited expression and/or availability of α3(IV)NC1 epitopes in other areas, we postulated that α3(IV)NC1 would be an ideal focus for targeting, delivering, and releasing a drug during the course of glomerular disease. Although F1.1 can be pathogenic when administered to mice in much larger doses (13), smaller doses are not nephritogenic (4), providing a rationale for initial use of intact Ab-drug conjugates to test our hypothesis. We reasoned that if successful, larger quantities of so-called “minibodies” [antibody fragments containing localizing but nonpathogenic F(ab)2 regions with linkers to specifically carry disease-modifying agents] could be created for glomerular delivery to alter the course of nephritis (9). Feasibility of the minibody approach is supported by previous studies where the V region sequences of these particular human anti-α3(IV)NC1 mAbs have been determined (13), and well-established methods to produce these type of reagents in large scale are available (e.g., for cancer therapy) (14).
METHODS
Animals, cells, and reagents.
Female C57BL/6 mice were purchased from Jackson Laboratory. All experiments were performed in compliance with federal laws and institutional guidelines. The animal protocol was approved by the Georgia Regents University Institutional Animal Care and Use Committee (no. A3307-01).
Eight- to-ten-week-old mice (18–20 g) were used for all experiments. The hepatocyte cell line AML-12 was a kind gift from Dr. M. Duncan. Established cloned immortalized mouse podocyte and mesangial cell lines were employed as described previously (1). For passage, the podocytes were grown under “growth permissive” conditions (33°C), whereas to acquire a differentiated and quiescent phenotype for use in experiments, the cells were grown under “restrictive conditions” at 37°C in 95% air-5% CO2. Anti-dexamethasone, anti-PGE2 (Abcam), anti-synaptopodin antibodies (Santa Cruz Biotechnology), EDC (Fisher Scientific), PGE2 (Sigma), and Texas red-conjugated anti-rabbit (Abcam), and Dylight 488-conjugated anti-human antibodies (Jackson ImmunoResearch) were purchased.
Isolation of F.1 antibody and production of conjugates with PGE2 and dexamethasone.
The human hybridoma cell line producing F1.1, having specificity for Ea and Eb epitopes of α3(IV) collagen, was employed, and purified human IgG was eluted from the culture supernatant as described (13). Purified antibody was chemically linked to PGE2 or dexamethasone using zero-length cross-linker 1-ethyl-3-[3-dimethylamino -propyl]carbodiimide hydrochloride (EDC) according to the manufacturer's instructions. As an isotype control, human IgG (Jackson ImmunoResearch) was linked to dexamethasone and injected into a control group of mice. In brief, F1.1 and/human IgG (5 mg) were incubated with PGE2 or dexamethasone (1 mg) and EDC (1 mg) in PBS for 2 h at room temperature. Unconjugated PGE2 or dexamethasone and EDC were removed using PD10 desalting columns (GE Healthcare), 1-ml fractions were collected and analyzed for the absorbance at 280 nm, and dot blotting was carried out using either anti-PGE2 and horseradish peroxidase (HRP)-anti-rabbit Abs or HPR-anti-human IgG Abs. Dots were visualized using precipitating DAB substrate. The amount of PGE2 linked to the antibody was calculated using a PGE2 ELISA Kit (Cayman Chemical) according to the manufacturer's instructions.
ELISA.
Recombinant α3(IV), purified from HEK 293 cells by metal affinity chromatography using HisPur Cobalt Spin Columns according to the manufacturer's instructions (Thermo Scientific), was coated at a concentration of 7 μg/ml in PBS using 96-well ELISA plates. Blocking of nonspecific binding sites was performed using 3% milk in PBS for 1 h at 37°C. F1.1 and PGE2 conjugates, F1.1 alone, and PGE2 were added to the separate wells at a range of different molarities in PBS with 0.05% Tween (2 h, duplicates). After optimizing conditions, anti-PGE2 Ab (Abcam) followed by HRP-conjugated anti-rabbit Abs were used for detection. After application of substrate buffer at the final step, the optical density of each well was read at a 600-nm wavelength. Serum cytokine levels were measured using Mouse ELISA Strips according to the manufacturer's instructions (Sygnosis).
Flow cytometry.
Podocytes, mesangial cells, and AML-12 cells were detached by mild enzymatic treatment from flasks, placed in 15-ml conical tubes (1 × 106), incubated with F1.1 and PGE2 conjugates, with or without preincubation with recombinant α3 from HEK 293 cells, for 30 min at 4°C, followed by incubation with Dylight 488 anti-human antibodies for 30 min at 4°C. The cells were then analyzed by flow cytometry.
Nephrotoxic nephritis: histological evaluation.
Sheep nephrotoxic sera (NTS) were prepared and administered as described previously (10). For disease induction, NTS was administered as a single intraperitoneal (ip) dose (13.5 μl/g body wt). PGE2-F1.1, dexamethasone-F1.1, and human IgG-dexamethasone conjugates (10 μg/g body wt) were injected ip on day 2 after NTS dosing and thereafter every 48 h. Proteinuria (BCA; Pierce) and blood urea nitrogen (BUN) levels (Stanbio Laboratory) were measured periodically (10). After 7 days, the kidneys were removed and either fixed in 10% buffered formalin or frozen in OCT medium and analyzed by light microscopy or direct immunofluorescence as described, in a blinded manner (7). Direct IF was used to determine F1.1-PGE2 conjugate localization. Synaptopodin expression was evaluated using anti-synaptopodin and Texas red-conjugated anti-rabbit Ab, whereas Dylight 488-conjugated anti-human Ab and anti-PGE2, and Texas red-conjugated anti-rabbit Ab were used to localize conjugates within organs.
Statistical analysis.
All values are presented as means ± SD. Data sets for Figs. 1B and 3, G and H, were analyzed with one-way ANOVA, using Dunnett's multiple comparisons test; data sets for Fig. 2A with two-way ANOVA, using Tukey's multiple comparisons test. P ≤ 0.05 was considered significant.
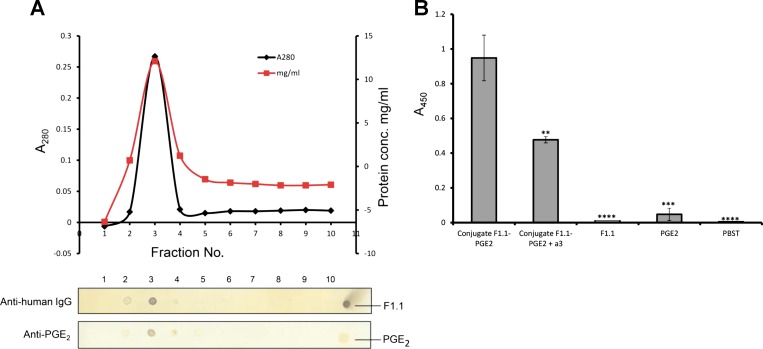
Fig. 1.
Human monoclonal anti-α3 (IV) antibodies retain antigen binding properties after conjugation with prostaglandin E2. A: elution profile of purified F1.1-PGE2 conjugate through PD-10 (G-25) column eluted with PBS. Fractions 1 ml each were collected and analyzed by dot blotting using either an anti-PGE2 antibody (Ab) followed by horseradish peroxidase (HRP)-conjugated anti-rabbit Ab or HPR-conjugated anti-human Ab; positive controls are illustrated at far end. B: ELISA. Binding of conjugates (fraction 3) to α3(IV) (7 μg/ml)-, coated wells, as detected by rabbit anti-PGE2 Ab and HRP-conjugated anti-rabbit Ab, thereby validating retention of anti-α3 activity of the conjugates. **P < 0.01. ***P < 0.001. ****P < 0.0001.
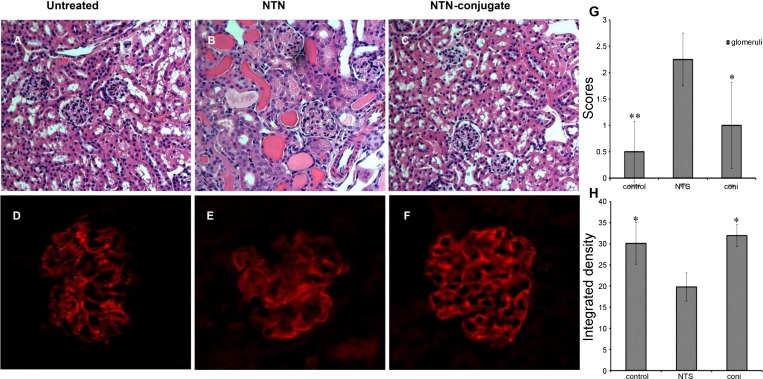
Fig. 3.
Human monoclonal anti-α3(IV)-PGE2 conjugates reduce the severity of established nephritis. A and C: normal mouse. B and E: nephrotoxic nephritis (NTN) mice. C and F: NTN mice treated with F1.1-PGE2 conjugates. Shown is histology (H&E) from day 7 kidneys (A–C) for NTS mice with severe glomerulonephritis and tubulointerstitial injury. Mice that received the F1.1-PGE2 conjugates had minimal evidence of nephritis. Quantification of H&E images by scoring supported this conclusion (G). The beneficial effect was associated with restoration of synaptopodin expression, as shown (D–F): normal glomeruli (D), NTN mice (E), and NTS/F1.1-PGE2 (F). Integrated density for synaptopodin images was quantified by using Image J software (H). The quantitative results are shown from 1 representative experiment with 5 mice/group, and they were reproducible. *P < 0.05. **P < 0.01.
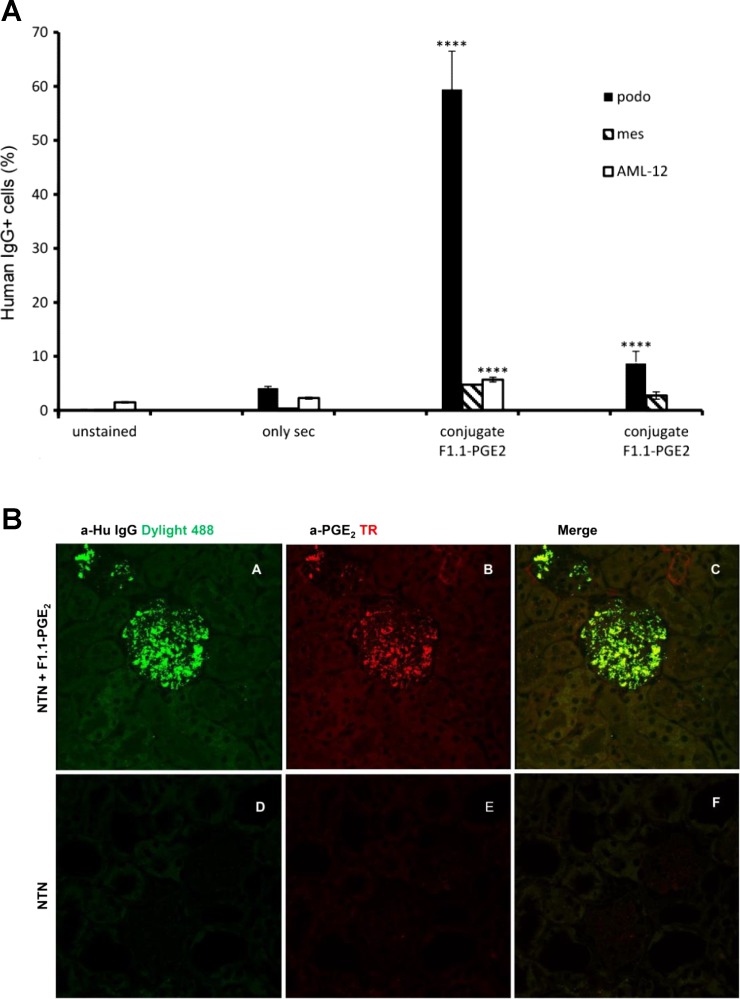
Fig. 2.
Human monoclonal anti-α3(IV) Ab conjugates bind to cultured podocytes and localize in glomeruli in vivo. A: flow cytometry revealed strong binding of conjugates to cultured podocytes with negligible interaction with other cell lines (mouse mesangial cells and hepatocyte AML-12 cells). Far right: near complete inhibition of binding to podocytes by recombinant α3(IV) confirmed antigenic specificity of conjugate binding to the cells. B: conjugates localized in glomeruli after injection into diseased mice. Frozen kidney sections from nephrotoxic sera (NTS)- and conjugate-injected mice were stained with anti-human Dylight 488 and anti-PGE2 Ab followed by anti-rabbit Texas red. Shown are kidney sections from conjugate-injected mice (A–C), kidney sections from NTS-treated mice (D–F), anti-human Dylight 488 (A and D), anti-PGE2 Ab followed by anti-rabbit Texas red (B and E), and merged images (C and F).
RESULTS AND DISCUSSION
For the present studies, the approach involved coupling of either PGE2 or dexamethasone to F1.1. After confirmation of chemical conjugation and determination that the conjugates retained antigen binding activity, the capacity of the conjugates to localize within glomeruli and modify the course of established nephrotoxic nephritis (NTN) was evaluated. PGE2 was selected, since we previously reported that systemic administration improved recovery from NTN, and this was at least in part due to a direct effect of PGE2 on renal cells (12). Dexamethasone was selected since it has well-established, potent anti-inflammatory properties. Feasibility of delivery is supported by our recent observations that when subnephritogenic doses of the human mAb were linked to a fluorophore, it localized exclusively within glomeruli, without either visualization in other organs or evidence of nephritis (4), thus providing an ideal drug delivery agent.
Human anti-α3(IV)NC1 mAb and either PGE2 or dexamethasone were coupled through formation of amide bonds between carboxylic groups of PGE2 and dexamethasone, and amide groups of the mAb, using zero-length cross-linker EDC. Unlike other cross-linkers, EDC is released as a soluble urea derivative from intermediary by-products upon completion of a reaction, so no spacer exists between the molecules being coupled (11). The conjugates were purified through G-25, and eluted fractions were analyzed by dot blotting. Only a single fraction (fraction 3) displayed strong reactivity to both anti-human IgG and anti-PGE2 Abs, confirming that PGE2 was ligated to F1.1 (Fig. 1A). The composition of conjugates was further examined by ELISA (Fig. 1B). Only the conjugates demonstrated strong binding. Specificity of binding was supported by partial inhibition of mAb binding to α3(IV), by soluble recombinant α3(IV), indicating that conjugates retained their antigen binding properties and that the cross-linking per se did not alter the antigen binding activity of conjugates. The PGE2 content in conjugates and the amount of PGE2 injected per mouse was calculated: 4.52 ± 2.11 μg/mouse (0.23 ± 0.11 μg/g body wt), which is 22 times less than when given systematically (100 μg/mouse) (9).
The functionality of conjugates was also determined by binding to podocytes by flow cytometry. Specific binding was observed with minimal reactivity with other cell lines (e.g., mouse mesangial cells and hepatocyte AML-12 cells), and the podocyte interaction was inhibited by recombinant α3(IV), confirming retention of antigenic specificity of the conjugates (Fig. 2). These properties supported further evaluation of the conjugates as candidates for therapeutic targeting in vivo.
Initially, the conjugates were administered to normal mice (10 μg/g). At these doses, the conjugates were not nephritogenic, as BUN levels (14.93 ± 2.70 mg/dl), body weight (18.45 ± 1.06 g), and histology (with scores 0–1+ for glomeruli) remained normal 7 days after injection. As a control, F1.1 was injected in a group of NTS-treated mice started on day 2. No statistically significant differences were observed in disease severity between NTS only and NTS+F1.1 mice groups (with BUN levels 117.05 ± 24.12 and 92.07 ± 13.85 mg/dl, respectively, P = 0.122), proving that an antibody alone could not contribute to recovery.
Thereafter, the capacity of the conjugates to modify disease was investigated, following administration (10 μg/g) to nephritic mice during the course of established NTN (day 2). At this time with this particular NTS preparation, the mice are proteinuric, with histological evidence of moderate to severe nephritis (7, 12). The conjugates were clearly visualized within glomeruli of diseased mice: strong and specific fluorescent staining was detected only in the glomeruli of the conjugate-treated mice (Fig. 2). Furthermore, human IgG-reactive bands were observed only in kidney lysates and not in other organ (lungs and heart) lysates, confirming that F1.1 conjugates localized predominantly in the kidneys (data not shown).
Particularly noteworthy, disease severity improved after injection of the conjugates: BUN and histology normalized compared with advancing disease in NTN mice that did not receive the conjugates (Table 1 and Fig. 3). Benefit was observed with both PGE2 and dexamethasone conjugates (Table 1). This was associated with a decrease in serum levels of multiple cytokines in conjugate-treated mice: TNF-α (55%), TGF-β (35%), MCP-1 (93%), IL-1β (25%), IL-6 (93%), IL-10 (27%), IL-12 (85%), indicating that systemic inflammation was reduced due to reduction of the local inflammatory response in the kidney. Furthermore, synaptopodin expression, which was altered during NTN, returned to a more normal localization and expression pattern (Fig. 3), similar to the restorative effect previously observed with larger systemic therapeutic doses of PGE2 (9).
Table 1.
Blood urea nitrogen and body weight changes in groups of mice
| Mice | BUN, mg/dl | Body Weight Changes, g |
|---|---|---|
| Untreated | 29.80 ± 1.81* | +0.5 ± 0.49* |
| NTS | 197.11 ± 17.16 | +4.7 ± 0.07 |
| NTS/Iso human IgG-dexamethasone | 179.30 ± 21.16 | +3.9 ± 1.85 |
| NTS +F1.1-PGE2 | 67.34 ± 0.71* | +0.8 ± 0.91* |
| NTS+F1.1-dexamethasone | 35.36 ± 18.98† | +1.2 ± 0.83* |
Values are means ± SD; n = 5 mice/group.
BUN, blood urea nitrogen; NTS, nephrotoxic sera.
P < 0.05.
P < 0.01.
In summary, we provide evidence that a systemic agent (i.e., PGE2), which was shown to reverse the course of established nephritis (12), and a steroid (i.e., dexamethasone) with presumably both local and systemic anti-inflammatory effects, can be effectively and specifically delivered to glomeruli, at much lower dosing, when coupled to human α3(IV) mAb, to enhance recovery from established nephritis. Importantly, the results provide feasibility for targeted drug delivery to glomeruli during the course of kidney disease, irrespective of etiology. In this regard, the epitopes targeted by anti-GBM antibodies are ideal, since expression is limited to the kidney and a few organs, and in sites other than glomeruli, the epitopes targeted by the anti-GBM antibodies (e.g., F1.1) are relatively inaccessible (15). Furthermore, during normal physiological conditions, within the kidney these epitopes are largely sequestered, while during the course of glomerular disease the sites become more exposed, thereby enhancing the opportunity for antibody-conjugate binding (3, 4, 15). Thus the nature of epitope exposure per se provides a perfect target during glomerular diseases.
In this context, and particularly relevant to targeting and stability for either drug release or interaction of the drug with glomerular ligands, the repetitive nature of α3(IV) epitopes within the type IV collagen network provides a stable scaffold for binding of these particular antibodies through interaction of both Fab arms with α3(IV) epitopes (13). The repetitive nature of epitopes considerably increases “effective” affinity of Ab-Ag interactions, as for monogamous bivalent binding, and thereby makes these interactions almost irreversible (5). This accounts for the typical high-affinity interactions of anti-α3(IV) Abs in nephritic patients (3), and in the setting of drug delivery provides for stability of the mAb-PGE2 or other mAb drug complexes. Indeed a strong fluorescent signal was detected in glomeruli of mice even after 48 h of injection of conjugates (Fig. 2), while there was negligible signal in kidneys after 24 h of systemic PGE2 administration, (data not shown), likely due to the rapid degradation of PGE2 that occurs when administered systematically (8), thereby requiring much larger doses to effect a local benefit. As previously mentioned, the amount of PGE2 injected per mouse (4.5 μg) as conjugate was more than 20-fold less compared with the amount administered systematically (100 μg). (In our previous studies, we have evaluated dose dependency of systemic PGE2, and even 1 μg/g body wt had no effect on nephritis.) The prolonged anchoring of the anti-α3(IV) mAb-drug complexes (Fig. 2) thus provides an ideal milieu for the subsequent interaction of an antibody-bound drug (e.g., PGE2, dexamethasone, or other agents) with surrounding glomerular or infiltrating inflammatory cells.
A major advantage of local delivery of PGE2, dexamethasone, or other drugs to glomeruli is that it has the potential for greater effects at the site of injury, without imparting significant systemic perturbations. Furthermore, given that the targeting reagent is derived from human sequences, the conjugates have the potential to be evaluated during human glomerular diseases, without generation of an immune response limiting its efficacy. In this regard, production of recombinant human reagents derived from the V region sequences of the same antibodies (13), so-called minibodies (9, 14), with linkers to attach drugs is feasible, and the approach has the potential to provide therapeutic reagents in large quantities for evaluation in many common human kidney diseases, irrespective of etiology.
In summary, the present results provide a novel means of delivery of therapeutic reagents to the kidney, and they support the notion that targeting the kidney, by taking advantage of unique epitopes within the GBM, is a feasible and promising approach for the therapy of human kidney diseases.
GRANTS
The work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK0811140 (to M. P. Madaio).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: N.K., M.M., and M.P.M. provided conception and design of research; N.K., M.M., V.I.G., and B.L. performed experiments; N.K., M.M., V.I.G., and B.L. analyzed data; N.K., M.M., V.I.G., and M.P.M. interpreted results of experiments; N.K. and V.I.G. prepared figures; N.K. drafted manuscript; N.K. and M.P.M. edited and revised manuscript; M.P.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Michael Duncan for providing the hepatocyte cell line AML-12, Dr. Kumar Vaibhav for providing additional mice, and Drs. Tracy McGaha, Sandeep Khurana, and Ravirajsinh Jadeja for helpful discussions.
REFERENCES
- 1.Akis N, Madaio MP. Isolation, culture, and characterization of endothelial cells from mouse glomeruli. Kidney Int 65: 2223–2227, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Asgeirsdottir SA, Zwiers PJ, Morselt HW, Moorlag HE, Bakker HI, Heeringa P, Kok JW, Kallenberg CG, Molema G, Kamps JA. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am J Physiol Renal Physiol 294: F554–F561, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Borza DB, Bondar O, Colon S, Todd P, Sado Y, Neilson EG, Hudson BG. Goodpasture autoantibodies unmask cryptic epitopes by selectively dissociating autoantigen complexes lacking structural reinforcement: novel mechanisms for immune privilege and autoimmune pathogenesis. J Biol Chem 280: 27147–27154, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary K, Kleven DT, McGaha TL, Madaio MP. A human monoclonal antibody against the collagen type IV alpha3NC1 domain is a non-invasive optical biomarker for glomerular diseases. Kidney Int 84: 403–408, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Chittasupho C. Multivalent ligand: design principle for targeted therapeutic delivery approach. Ther Deliv 3: 1171–1187, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Choi CH, Zuckerman JE, Webster P, Davis ME. Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci USA 108: 6656–6661, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen M, Su AW, Snyder RW, Greco A, Lipschutz JH, Madaio MP. Simvastatin protection against acute immune-mediated glomerulonephritis in mice. Kidney Int 69: 457–463, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Gomez I, Foudi N, Longrois D, Norel X. The role of prostaglandin E2 in human vascular inflammation. Prostaglandins Leukot Essent Fatty Acids 89: 55–63, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Hu S, Shively L, Raubitschek A, Sherman M, Williams LE, Wong JY, Shively JE, Wu AM. Minibody: a novel engineered anti-carcinoembryonic antigen antibody fragment (single-chain Fv-CH3) which exhibits rapid, high-level targeting of xenografts. Cancer Res 56: 3055–3061, 1996. [PubMed] [Google Scholar]
- 10.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med 203: 789–797, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khandare JJ, Chandna P, Wang Y, Pozharov VP, Minko T. Novel polymeric prodrug with multivalent components for cancer therapy. J Pharmacol Exp Ther 317: 929–937, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Kvirkvelia N, McMenamin M, Chaudhary K, Bartoli M, Madaio MP. Prostaglandin E2 promotes cellular recovery from established nephrotoxic serum nephritis in mice, prosurvival, and regenerative effects on glomerular cells. Am J Physiol Renal Physiol 304: F463–F470, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyers KE, Christensen M, Madaio MP. Modeling of human anti-GBM antibody-alpha3(IV)NC1 interactions predicts antigenic cross-linking through contact of both heavy chains with repeating epitopes on alpha3(IV)NC1. Am J Nephrol 30: 474–480, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olafsen T, Tan GJ, Cheung CW, Yazaki PJ, Park JM, Shively JE, Williams LE, Raubitschek AA, Press MF, Wu AM. Characterization of engineered anti-p185HER-2 (scFv-CH3)2 antibody fragments (minibodies) for tumor targeting. Protein Eng Des Sel 17: 315–323, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, Wilson CB, Hudson BG. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 363: 343–354, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollinger K, Hennig R, Breunig M, Tessmar J, Ohlmann A, Tamm ER, Witzgall R, Goepferich A. Kidney podocytes as specific targets for cyclo(RGDfC)-modified nanoparticles. Small 8: 3368–3375, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Scindia Y, Deshmukh U, Thimmalapura PR, Bagavant H. Anti-alpha8 integrin immunoliposomes in glomeruli of lupus-susceptible mice: a novel system for delivery of therapeutic agents to the renal glomerulus in systemic lupus erythematosus. Arthritis Rheum 58: 3884–3891, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Lin Y, Zeng Y, Sun X, Gong T, Zhang Z. Effects of mycophenolic acid-glucosamine conjugates on the base of kidney targeted drug delivery. Int J Pharm 456: 223–234, 2013. [DOI] [PubMed] [Google Scholar]