Abstract
Phenotypic divergence in diet-induced obesity (DIO) and hepatic steatosis has been reported in two independently generated lines of L-Fabp−/− mice [New Jersey (NJ) L-Fabp−/− vs. Washington University (WU) L-Fabp−/− mice]. We performed side-by-side studies to examine differences between the lines and investigate the role of genetic background, intestinal microbiota, sex, and diet in the divergent phenotypes. Fasting-induced steatosis was attenuated in both L-Fabp−/− lines compared with C57BL/6J controls, with restoration of hepatic triglyceride levels following adenoviral L-Fabp rescue. Both lines were protected against DIO after high-saturated-fat diet feeding. Hepatic steatosis was attenuated in WU but not NJ L-Fabp−/− mice, although this difference between the lines disappeared upon antibiotic treatment and cohousing. In contrast, there was phenotypic divergence in L-Fabp−/− mice fed a high cocoa butter fat diet, with WU L-Fabp−/− mice, but not NJ L-Fabp−/− mice, showing protection against both DIO and hepatic steatosis, with some sex-dependent (female > male) differences. Dense mapping revealed no evidence of unintended targeting, duplications, or deletions surrounding the Fabp1 locus in either line and only minor differences in mRNA expression of genes located near the targeted allele. However, a C57BL/6 substrain screen showed that the NJ L-Fabp−/− line contains ∼40% C57BL/6N genomic DNA, despite reports that these mice were backcrossed six generations. Overall, these findings suggest that some of the phenotypic divergence between the two L-Fabp−/− lines may reflect unanticipated differences in genetic background, underscoring the importance of genetic background in phenotypic characterization.
Keywords: triglyceride, fatty acid binding protein, Jax mice, divergent phenotypes, C57BL/6 substrain, lipid trafficking, microbiome, gene knockout
fatty acid binding proteins (FABPs) are abundant cytosolic lipid binding proteins, expressed in a tissue-specific pattern (33, 35) and thought to facilitate intracellular fatty acid (FA) trafficking, from uptake to storage or oxidation, or from lipid droplets for secretion (31, 33, 35). Over the past decade, two groups have generated and characterized the phenotypes of mice with germline deletion of the dominant hepatic FABP, i.e., L-Fabp (3, 17–24, 26–30, 39). However, conclusions regarding L-Fabp function inferred from these knockout lines are complicated because of some divergent phenotypes, particularly related to high-fat diet-induced obesity (DIO). One line of L-Fabp−/− mice, initially generated by Binas and colleagues (Fabp1tm1Bin), demonstrated increased body weight in both male and female knockouts compared with control mice, both on a chow diet and following high-fat feeding (3, 12, 19, 21, 24). The other line of L-Fabp−/− mice (Fabp1tm1Ddsn), generated by our laboratory, showed reduced weight gain in female mice compared with controls when fed either chow or high-saturated-fat diets (26–28, 30). Some of the phenotypes associated with these conflicting studies are summarized in Table 1. It is also worth noting that even the phenotypes of Fabp1tm1Bin mice are somewhat variable, with both sex and age-based differences in obesity and hepatic steatosis (Table 1). In addition to differences in obesity, there were also divergent findings between the L-Fabp−/− lines with regard to hepatic cholesterol content, bile acid metabolism, and biliary lipids following dietary supplementation with cholesterol and high-fat diets (18, 20, 28, 39). Other observations were generally consistent between the two lines, including reduced hepatic steatosis and reduced FA oxidation, likely due to decreased FA transport and availability (2–4, 9, 21, 23, 27–29).
Table 1.
Summary of published studies documenting obesity or hepatic lipid phenotypes in L-Fabp−/− mouse lines
| Authors | Ref. No. | KO Line | Sex | Age | Diet | Background | Control | KO Phenotype |
|---|---|---|---|---|---|---|---|---|
| Studies showing increased obesity or hepatic lipid | ||||||||
| Martin et al.,J Biol Chem, 2003 | 22 | Fabp1tm1Bin | F | 13–15 mo | Chow | Mixed | Littermate | Increased liver chol, CE, PL |
| Martin et al., Biochemistry, 2003 | 23 | Fabp1tm1Bin | F | 13–15 mo | Chow | Mixed | Littermate | Increased liver CE, PL; decreased liver TG |
| Martin et al., Am J Physiol Gastrointest Liver Physiol, 2006 | 18 | Fabp1tm1Bin | F | 7–12 wk | 1.25% Chol/control | Mixed | Littermate | Increased BW on control and chol. Increased liver chol |
| Martin et al., Mol Cell Biochem, 2009 | 21 | Fabp1tm1Bin | M | 3–18 mo | Chow | N6 to C57BL/6? | C57BL/6? | Increased BW by 9 mo; liver TG decreased at 3 mo, not 18 mo |
| Martin et al., J Nutr, 2008 | 19 | Fabp1tm1Bin | F | 3–18 mo | Chow | N6/C57BL/6? | C57BL/6? | Increased BW by 9 mo; liver TG decreased at 3 mo, not 18 mo |
| Martin et al., Am J Physiol Gastrointest Liver Physiol, 2009 | 17 | Fabp1tm1Bin | Both | >8 mo | Chow | From N2 to N10 to C57BL/6NCr | Littermate | Increased liver chol in F; Decreased liver TG in M |
| Atshaves et al., Lipids, 2010 | 3 | Fabp1tm1Bin | Both | 7–20 wk | Control, HFD, pair fed | N6 C57Bl/6NCr | C57BL/6NCr | Increased BW in HFD-fed F; no Δ in M. No Δ in liver TG |
| McIntosh et al., Lipids, 2013 | 24 | Fabp1tm1Bin | F | 8–20 wk | Control, HFD | N11 C57Bl/6NCr | C57BL/6NCr | Increased BW on HFD, not control |
| Gajda et al., J Biol Chem, 2013 | 12 | Fabp1tm1Bin | M | 8–20 wk | High SF, UF diets | N6 C57BL/6J | C57BL/6J | Increased BW and weight gain on both HFD |
| Studies showing decreased/unchanged obesity or hepatic lipid | ||||||||
| Newberry et al., J Biol Chem, 2003 | 29 | Fabp1tm1Ddsn | M | 2–4 mo | Chow, fasted | Mixed | Littermate | Decreased liver TG after fasting |
| Atshaves et al., J Biol Chem, 2004 | 4 | Fabp1tm1Bin | M | 2–4 mo | Chow, AIN76A | Mixed | Littermate | Decreased liver TG, FA, total lipid |
| Atshaves et al., Am J Physiol Cell Physiol, 2005 | 2 | Fabp1tm1Bin | Both | 2 mo | Chow, phytanic acid | Mixed | Littermate | Decreased liver TG in M |
| Martin et al., Biochem J, 2005 | 20 | Fabp1tm1Bin | M | 7–12 wk | 1.25% Chol/control | Mixed | Littermate | No Δ in BW; liver TG decreased on control diet |
| Newberry et al., Hepatology, 2006 | 30 | Fabp1tm1Ddsn | F | 12–30 wk | Western HFD | N5 to C57BL/6J | C57BL/6J | Decreased BW on HFD. Decreased liver TG |
| Newberry et al., Am J Physiol Gastrointest Liver Physiol, 2008 | 28 | Fabp1tm1Ddsn | F | 2–8 mo | 1.25% Chol, SF, and PUFA | N8 to C57BL/6J | C57BL/6J | Decreased obesity/liver TG on SF and chol, but not PUFA diet |
| Newberry et al., Mol Cell Biochem, 2009 | 27 | Fabp1tm1Ddsn | F | 10–24 wk | High chol, SF, and Western | N7 to C57BL/6J | C57BL/6J | Reduced weight gain on HF and chol diets |
| Lagakos et al., Am J Physiol Gastrointest Liver Physiol, 2011 | 15 | Fabp1tm1Bin | M | 0–5 mo | Chow | N6 C57BL/6J | Littermate | No difference in BW at 5 mo |
| Newberry et al., J Lipid Res, 2012 | 26 | Fabp1tm1Ddsn | F | 3–13 mo | Chow | N7 to C57BL/6J | C57BL/6J | Reduced BW by 5 mo; liver TG lower at 3 and 13 mo |
| Chen et al., Hepatology, 2013 | 7 | Fabp1tm1Ddsn | F | 8–20 | HF, high fructose | N8–9 to C57BL/6J | C57BL/6J | Reduced hepatic TG |
KO, knockout; F, female; M, male; HFD, high-fat diet (fat source not indicated); SF, high-saturated fat diet; UF, unsaturated fat diet; chol, cholesterol; CE, cholesterol ester; PL, phospholipid; TG, triglyceride; BW, body weight; Δ, change; chow, rodent chow diet; FA, fatty acid; PUFA, high polyunsaturated fat diet. “C57BL/6?” indicates that the specific substrain was not reported.
Several hypotheses have been proposed to explain the divergent obesity phenotype of the two L-Fabp−/− lines (knockout targeting strategy, sex, diet composition, genetic background, microbiome), yet no satisfactory explanation has emerged. The initial gene targeting of both knockouts was undertaken in 129-derived embryonic stem (ES) cells. Subsequently, both lines were backcrossed into the C57BL/6 background, although different C57 substrains were used (See Fig. 1A). These details are important in light of recent studies demonstrating genetic, physiological, and behavioral differences between C57BL/6J and C57BL/6N substrains (13, 14, 25, 32, 37), differences that could potentially contribute to the phenotypic differences observed between the L-Fabp−/− lines. Fabp1tm1Ddsn mice, referred to hereafter as WU (Washington University) L-Fabp−/− mice, were backcrossed to C57BL/6J mice purchased from Jackson Laboratory using a speed congenic breeding strategy, as described in materials and methods (30). Fabp1tm1Bin mice were backcrossed to C57BL/6NCr mice for at least six generations (19, 21). More recently, C57BL/6NCr congenic Fabp1tm1Bin mice, backcrossed an additional six generations to C57BL/6J (12, 15), were shown to exhibit an obesity phenotype in males fed high-fat diet (12). These Fabp1tm1Bin mice (12) were used in the present study and will be referred to hereafter as NJ (New Jersey) L-Fabp−/− mice. Information on the specific genetic background of mice used in each of the previous studies is included in Table 1.
Fig. 1.
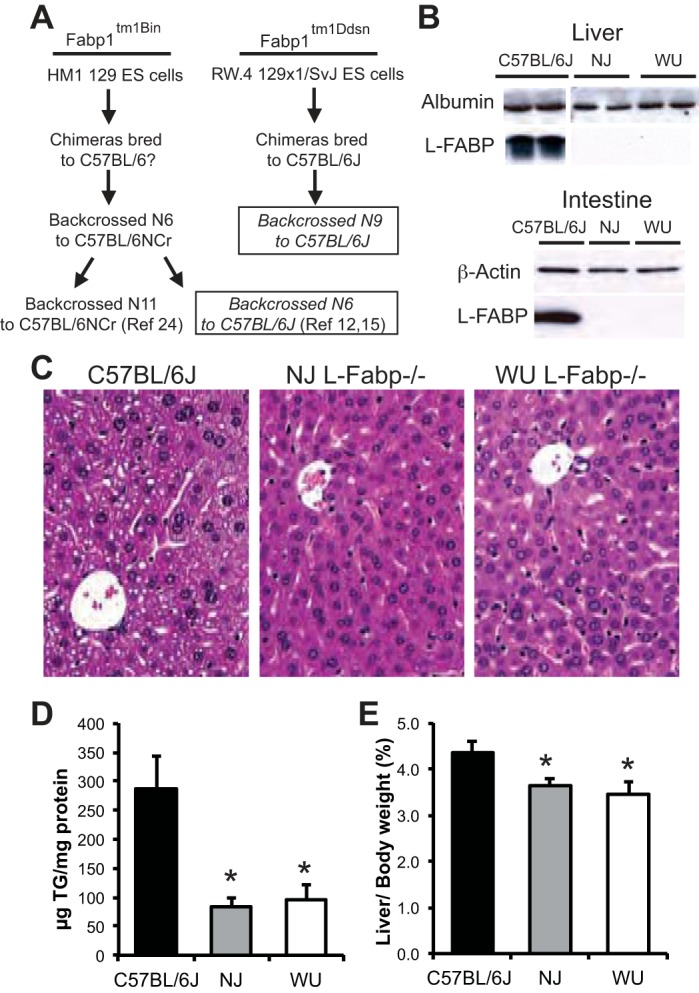
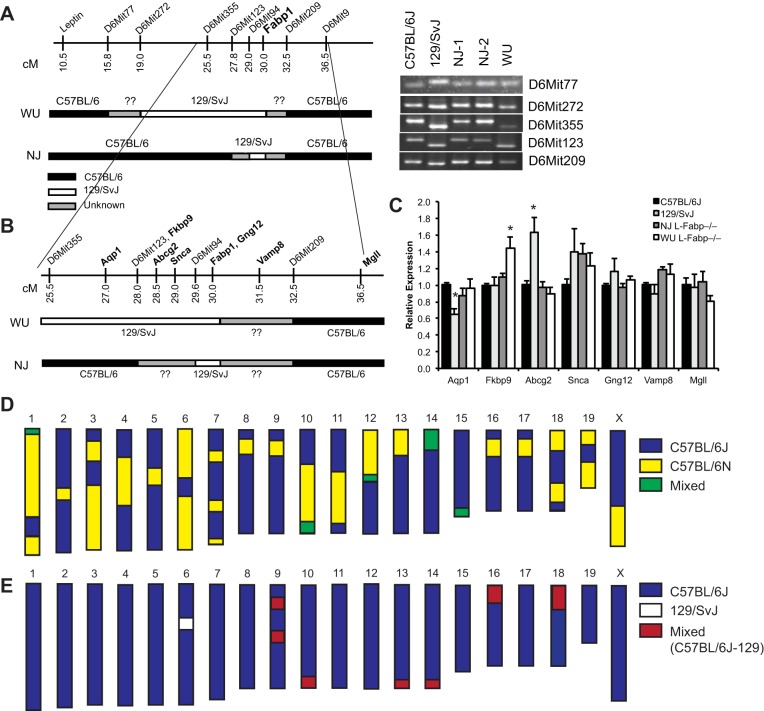
Reduced hepatic steatosis in fasted New Jersey (NJ) and Washington University (WU) L-Fabp−/− mice. A: flow diagram outlining historical genetic background information for each of the L-Fabp−/− lines. The designation of chimeras bred to C57BL/6? in the Fabp1tm1Bin mice indicates that the substrain used (i.e., N or J) was not specified. Boxes identify the two lineages used in the current comparison. B: Western blot analyses showing absence of liver-fatty acid binding protein (L-FABP) in liver (top) and proximal small intestine (bottom) in both lines of L-Fabp−/− mice. Each well contains 10 μg of protein. Levels of albumin (A) and β-actin (B) are shown as loading controls. C: representative images (×200) of hematoxylin- and eosin-stained liver tissue from C57BL/6J and NJ and WU L-Fabp−/− mice fasted 48 h. D: hepatic triglyceride (TG) content in mice fasted 48 h. E: liver weight in fasted mice, expressed as percentage of body weight. NJ Fabp−/− mice for this experiment were F1 and F2; WU Fabp−/− mice were N9, F3. Values are means ± SE; n = 5–7 male mice per group. *P < 0.05 vs. C57BL/6J. ES, embryonic stem.
To clarify the basis for the phenotypic differences between the lines of L-Fabp−/− mice on a C57BL/6J genetic background, we performed detailed side-by-side studies of WU and NJ L-Fabp−/− mice using two model systems of altered hepatic FA flux. First, we examined the response to prolonged fasting, a model of acute FA uptake and oxidation in which reduced ketogenesis and hepatic steatosis were observed previously in WU L-Fabp−/− mice (29). Second, we examined obesity and hepatic steatosis in L-Fabp−/− mice fed two distinct high-saturated-fat diets, since susceptibility to DIO, particularly in female mice, appears to be the most divergent phenotype between the two L-Fabp−/− lines. Specifically, we studied female L-Fabp−/− mice fed a medium-chain, saturated FA diet, containing 20% hydrogenated coconut oil (referred to as SF diet). In addition, we examined obesity and steatosis in both male and female L-Fabp−/− mice fed a long-chain, saturated FA diet containing 20% cocoa butter as a fat source (CB diet). The rationale for including male mice in CB study was because of a recent report showing that NJ L-Fabp−/− mice fed a CB diet exhibit DIO (12). Finally, in view of the phenotypic divergence we observed, we undertook dense mapping of the Fabp1 locus and performed a C57BL/6 substrain array using markers that distinguish between C57BL/6J and C57BL/6N genomic DNA (gDNA). Those findings revealed surprising and unanticipated differences in the genetic background of the two lines, which we believe are critically important to understanding the phenotypes observed.
MATERIALS AND METHODS
Animals.
Fabp1tm1Ddsn mice (referred to as WU L-Fabp−/−) were generated as described previously using RW.4 129SvJ ES cells (29), and backcrossed seven generations to C57BL/6J mice (purchased from Jackson Laboratory, Bar Harbor, ME) using an SSLP-based (simple sequence length polymorphism) speed congenic screening strategy (Fig. 1A). SSLP markers were chosen based on their ability to distinguish differences in repeat length between 129/SvJ and C57BL/6J gDNA. Mice were screened at each backcross generation, and mice containing the least amount of 129/SvJ gDNA were selected as breeders, using mice of both sexes. A list of SSLP markers is available upon request. Since then, WU L-Fabp−/− mice have been backcrossed to C57BL/6J two additional times (2007, 2012) to maintain fecundity, but were otherwise maintained as homozygote knockouts. Where possible, both backcross generation (N, number of backcrosses to C57BL/6J) and filial generation (F, brother × sister breeding) information of WU L-Fabp−/− mice is indicated in the figure legend and/or method for each experiment.
Fabp1tm1Bin mice were generated in HM1 ES cells by Martin et al. (22) and backcrossed to C57BL/6NCr mice (Fig. 1A). These mice were then backcrossed for six generations to C57BL/6J, as detailed (12, 15), and were generously provided by Storch and colleagues [line referred to hereafter as NJ L-Fabp−/− mice]. All NJ L-Fabp−/− mice used in experiments were bred in our facility from homozygote knockout crosses, and filial (F) breeding generation (since arrival in our facility) is indicated in the method or figure legend for each experiment. C57BL/6J mice were purchased at 5–8 wk of age (no. 000664, Jackson Laboratory) for direct use as controls, as previously described (30). Each experiment reported used a freshly obtained cohort of C57BL/6J mice from Jackson Laboratory. No C57BL/6J mice bred in our facility were used in experiments. 129/SvJ mice were purchased from Jackson Laboratory (no. 000691) for direct use as controls (26) for the gene expression studies (see Fig. 8). Mice were maintained on a 12:12-h light-dark cycle, fed standard rodent chow (PicoLab 20, no. 5053), and housed with littermates of same sex, three to five mice per cage, in standard isolator cages with corncob bedding, unless otherwise noted. All animal protocols followed National Institutes of Health (NIH) guidelines and were approved by the Washington University Animal Studies Committee.
Fig. 8.
Differences in C57BL/6 substrain may contribute to phenotypic differences between NJ and WU L-Fabp−/− mice. A: schematic diagram of simple sequence length polymorphism (SSLP) markers near Fabp1 locus, with genetic background of WU and NJ L-Fabp−/− mice, as determined by SSLP mapping. Regions found to be C57BL/6 are black, regions of 129/SvJ gDNA are white, and undetermined areas are gray. Note that SSLP primers do not distinguish between C57BL/6 substrains. PCR products from several informative markers are shown at right, with C57BL/6J and 129/SvJ gDNA used as controls. A list of additional markers and mapping results is provided in Table 7. B: expanded diagram showing the location of several genes near Fabp1 locus, with genetic background of the respective L-Fabp−/− genotypes shown below. C: relative expression of genes near Fabp1 in the livers of SF diet-fed C57BL/6J, 129/SvJ (26), and NJ and WU L-Fabp−/− mice. Values are means ± SE; n = 3–5 samples per genotype. D and E: schematic diagrams depicting results of C57BL/6 substrain single-nucleotide polymorphism (SNP) panel in NJ (D) and WU (E) L-Fabp−/− mice. For D, chromosomal regions identified as C57BL/6J are blue, regions identified as C57BL/6N are yellow, and regions that are variable (animal to animal or heterozygous) are green. For E, C57BL/6J regions are blue, non-C57BL/6J regions (129/SvJ) are white, and mixed regions are red. Actual SNP mapping data are provided in Supplemental Table S1.
Fasting and adenoviral rescue.
All fasting studies used male mice, age 11–16 wk, fed standard rodent chow before fasting, and maintained as detailed above. Mice were fasted for 48 h, starting at ∼10 AM, with water provided ad libitum. Serum and tissues (liver and gonadal fat pad) were collected, weighed, and snap frozen in liquid nitrogen or fixed in 10% formalin for histological evaluation. On average, mice lost ∼5 g body wt during the 48-h fast. For some studies, adenoviral FLAG-L-Fabp adenovirus (7) or Ad-βGal (Iowa Gene Transfer Core, Iowa City, IA) was injected intravenously (∼6.7 × 108 plaque-forming units/mouse) into the tail vein 5 days before fasting. When possible, littermates were used for side-by-side injections of Ad-L-Fabp or Ad-βGal.
High-SF diet study.
Female mice were fed a diet containing 20% hydrogenated coconut oil (no. 960242, MP Biomedicals, Solon, OH), a fat source that is primarily medium-chain FA. Diet composition and FA profile (as provided by the manufacturer) are shown in Tables 2 and 3. Mice were fed SF diet starting at 12 wk of age and weighed weekly. After 15 wk, serum and tissues were collected following a 4-h fast and snap frozen in liquid nitrogen or fixed in 10% formalin for histological analysis. Liver and gonadal fat pad weights were recorded. For the 2007 SF feeding study, female WU L-Fabp−/− and C57BL/6J mice were ∼10 wk of age at start of SF feeding. Experiments involving SF-fed 129/SvJ mice (gene expression studies, see Fig. 8) were published previously (26). Food consumption studies were performed after 7–8 wk on SF diet, in individual mice housed on wire metabolic cage racks. After a 5-day acclimation period, food consumption was measured, and feces were collected for 72 h. Fecal fat was measured gravimetrically using a modification of our laboratory's previous protocol (28). Briefly, an aliquot (∼0.2 g) of dried feces was solubilized overnight in 1.6 ml water, and feces were disrupted using 1 mM glass beads in a Bullet Blender (Next Advance, Averill Park, NY). Lipid was extracted with 2 ml chloroform-methanol (2:1), and the organic phase was transferred to preweighed glass vials, dried under nitrogen, and reweighed to determine lipid mass. Fecal fat was expressed as percentage of feces mass extracted and used to calculate fat absorption based on total fecal mass, total food consumption, and diet fat content.
Table 2.
Composition of high-fat diets
| SF Diet | CB Diet | Western Diet | |
|---|---|---|---|
| Ingredient, g/kg | |||
| Casein | 200 | 200 | 195 |
| dl-Methionine | 3 | 3 | 3 |
| Sucrose | 305.8 | 305.8 | 341.5 |
| Corn starch | 200 | 200 | 150 |
| Cocoa butter | 200 | ||
| Coconut oil | 200 | ||
| Anhydrous milkfat | 210 | ||
| Cholesterol | 1.5 | ||
| Alphacel | 50 | 50 | |
| Cellulose | 50 | ||
| dl-α-Tocopherol (250 IU/g) | 1.2 | 1.2 | 0 |
| Vitamin mix, Teklad | 10 | ||
| AIN-76 mineral mix | 40 | 40 | 35 |
| Calcium carbonate | 4 | ||
| kcal/g | 4.39 | 4.39 | 4.50 |
| Composition % by mass | |||
| Protein | 20.3 | 20.3 | 17.3 |
| Carbohydrate | 50.7 | 50.7 | 48.5 |
| Fat | 20.0 | 20.0 | 21.2 |
| %kcal from | |||
| Protein | 17.5 | 17.5 | 15.2 |
| Carbohydrate | 43.7 | 43.7 | 42.7 |
| Fat | 38.8 | 38.8 | 42.0 |
CB, cocoa butter diet. Data were provided by the manufacturer.
Table 3.
Fatty acid profile of fat sources in high-fat diets
| Chain Length | SF Diet, %total FA | CB Diet, %total FA | Western Diet, %total FA |
|---|---|---|---|
| C4:0 | 2.1 | ||
| C6:0 | 0.6 | 1.5 | |
| C8:0 | 7.7 | 1.1 | |
| C10:0 | 5.8 | 2.6 | |
| C12:0 | 45.7 | 3.3 | |
| C14:0 | 17.3 | 10.6 | |
| C16:0 | 8.2 | 24.4 | 28.9 |
| C16:1 | 1.5 | ||
| C18:0 | 12.0 | 35.4 | 12.5 |
| C18:1 | 1.0 | 38.1 | 24.9 |
| C18:2 | 2.1 | 3.6 | |
| C18:3 | 0.6 | 0.7 | |
| C20:0 | 0.6 |
Data were provided by diet manufacturer.
High-CB diet study.
Male and female mice were fed a diet containing 20% cocoa butter (custom diet no. CSMD761, MP Biomedicals), containing predominantly long-chain FA (Tables 2 and 3). Note that the CB and SF diets differ only in fat source. Mice were fed CB diet starting at 10 wk of age and weighed weekly for 12 wk. Tissue and serum were collected following a 4-h fast and snap frozen in liquid nitrogen. Liver and gonadal fat pad weights were recorded. For the 2007 CB feeding study, mice were fed SF diet for 18 wk, starting at ∼10 wk of age.
Western diet study.
Female C57BL/6J and WU L-Fabp−/− mice were fed high-cholesterol, high-SF Western diet (WD) (TD88137, Harlan Teklad) for 18 wk, as described (30). Composition and FA profile are shown in Tables 2 and 3. Serum and tissues were obtained at death following a 4-h fast, as described above.
Antibiotic treatment.
Chow-fed, female NJ and WU L-Fabp−/− mice were given antibiotics (Abx) in drinking water (1 g/l ampicillin, 1 g/l metronidazole, 1 g/l neomycin, 0.5 g/l vancomycin) for 10 days. Following treatment, mice from the two L-Fabp−/− lines were co-housed (3–5 mice per cage, NJ and WU together) to assimilate intestinal microbiota. SF feeding was initiated ∼2 wk after removal of Abx. NJ L-Fabp−/− mice used for this study were F6; WU L-Fabp−/− mice were N9, F6. Mice were weighed weekly, and tissue/serum samples were collected after 11 wk on the diet, as described above.
Tissue and serum biochemical analyses.
For analysis of lipid levels, tissue was homogenized in PBS using a Bullet Blender (∼40 mg tissue, 1-mm glass beads) and extracted as described previously (30). Lipid levels were measured using enzymatic assay kits from Wako Chemicals (Richmond, VA) and normalized to protein content (30). For Western blots, equal amounts of protein [extracts prepared as described previously, (30)] were separated by SDS-PAGE (4–20% Tris-glycine, Invitrogen) and probed with antibodies to albumin (no. ab83465, Abcam, Cambridge, MA), β-actin (A2066, Sigma, St. Louis, MO), or L-Fabp [generously provided by Dr. Jeff Gordon (29)]. For gene expression, RNA was isolated (Trizol, Invitrogen), and cDNA prepared (ABI High Capacity cDNA, Life Technologies, Grand Island, NY). Real-time qPCR was performed with Fast SYBR Green Master mix (Life Technologies) on a Step One Plus instrument (Life Technologies), using primers described previously (26). Osmium stained liver tissue was prepared as described previously (7). Serum β-hydroxybutyric acid levels were measured using Autokit 3-HB kit from Wako Chemicals. Serum insulin levels were analyzed using a Singulex Erenna Immunoassay (Singulex, Alameda, CA) in the Diabetes Research Center core facility. Serum lipid levels were measured using kits from Wako Chemicals.
For analysis of total serum FA by mass spectrometry, serum was hydrolyzed and extracted using a modified Bligh-Dyer protocol in the presence of an internal standard, d4-7,7,8,8-palmitic acid (Cambridge Isotope Laboratories) and FA were derivatized by amino methyl phenyl pyridium into FA-amino methyl phenyl pyridium species to obtain high sensitivity. Sample analysis was performed with a Shimadzu 10A HPLC system coupled to a TSQ Quantum Ultra triple quadrapole mass spectrometer (Thermo Scientific) in selected reaction monitoring mode under electrospray ionization(+). Data were analyzed using Xcalibur software (Thermo), and values are reported as peak area ratios of analytes per 20 μl of serum to the internal standard. Triene-to-tetraene ratio and essential FA (EFA) index [ratio of (n-3 + n-6) to (n-7 + n-9)] are biochemical markers of EFA deficiency (16).
SSLP mapping and single-nucleotide polymorphism analysis.
Genotyping reactions were performed and analyzed as described previously (26). As mentioned previously, SSLP markers were selected based on their ability to distinguish between 129/SvJ and C57BL/6 strains and do not discriminate between C57BL/6 substrains. See Table 7 for chromosome 6 SSLP markers and genotyping results. The C57BL/6 substrain characterization single-nucleotide polymorphism (SNP) panel (Jax Genome Scanning service) was used to identify regions of C57BL/6N, C57BL/6J, and 129/SvJ gDNA in WU and NJ L-Fabp−/− mice (n = 2–3/genotype). For both Fabp−/− lines, gDNA from offspring of two different breeding pairs was analyzed. NJ L-Fabp−/− samples were F7/8; WU L-Fabp−/− samples were N9, F8.
Table 7.
Informative chromosome 6 simple sequence length polymorphism markers (C57BL/6 vs. 129/SvJ)
| Marker | Position, Mb | Position, cM | WU Mice | NJ Mice |
|---|---|---|---|---|
| D6Mit138 | 4.45 | 1.8 | C57 | C57 |
| D6Mit268 | 34.68 | 15.6 | C57 | C57 |
| D6Mit272 | 44.38 | 19.0 | SvJ | C57 |
| D6Mit33 | 50.84 | 25.5 | SvJ | C57 |
| D6Mit355 | 53.42 | 26.5 | SvJ | C57 |
| D6Mit123 | 56.88 | 27.8 | SvJ | C57 |
| D6Mit94 | 62.62 | 29.6 | SvJ | SvJ |
| Fabp1 | 71.15 | 32.1 | ||
| D6Mit209 | 75.59 | 32.5 | C57 | C57 |
| D6Mit188 | 75.39 | 32.5 | n/d | C57 |
| D6Mit9 | 87.38 | 38.0 | C57 | C57 |
| D6Mit36 | 104.45 | 48.9 | C57 | C57 |
| D6Mit199 | 138.76 | 68 | C57 | C57 |
| D6Mit373 | 147.00 | 77.7 | C57 | C57 |
Approximate position of each marker is listed in both Mb and cM. Genotyping result in L-Fabp−/−mice is indicated for each marker. n/d, Not detected.
Statistical analysis.
All data are presented as means ± SE, unless otherwise noted. Statistical analyses (one-way ANOVA, with Bonferroni post hoc test on all groups) were performed using Graph Pad prism software (version 4.0, Graph Pad Software, La Jolla, CA).
RESULTS
Reduced fasting-induced hepatic steatosis in both lines of L-Fabp−/− mice.
Our laboratory's earlier characterization of male WU L-Fabp−/− mice demonstrated a marked reduction in hepatic steatosis after a prolonged fast, compared with littermate (mixed genetic background) controls, consistent with a defect in hepatic FA trafficking (29). Here we examined the response of males from both L-Fabp−/− lines to a 48-h fast, using male C57BL/6J mice from Jackson Laboratory as controls. Western blot analysis confirmed robust L-Fabp expression in the liver and proximal small intestine of C57BL/6J mice, as well as the absence of L-FABP protein in both knockout strains (Fig. 1B). Hematoxylin- and eosin-stained liver tissue from fasted mice showed both macrovesicular and microvesicular steatosis in hepatocytes of C57BL/6J mice (Fig. 1C, left), with minimal lipid accumulation in the L-Fabp−/− lines (Fig. 1C). Quantification of hepatic lipid showed an approximately fourfold reduction in triglyceride (TG) content in both L-Fabp−/− lines (Fig. 1D), with a corresponding decrease in liver size (Fig. 1E). The present data from WU L-Fabp−/− mice are similar to historical data with mice on a mixed genetic background (L-Fabp−/−, 56 ± 20 μg TG/mg protein; L-Fabp+/+, 222 ± 43 μg TG/mg protein, P < 0.001) generated more than 10 yr ago (29). We also observed reduced ketogenesis in both L-Fabp−/− lines, as evidenced by a nonsignificant trend toward lower serum β-hydroxybutyric acid levels compared with C57BL/6J controls (Table 4).
Table 4.
Characterization of fasted C57BL/6J and L-Fabp−/−mice
| C57BL/6J | NJ L-Fabp−/− | WU L-Fabp−/− | |
|---|---|---|---|
| n | 5 | 8 | 5 |
| Final BW, g | 21.6 ± 0.8a | 25.1 ± 0.8b | 20.2 ± 0.4a |
| Liver, g | 0.9 ± 0.1a | 0.9 ± 0.04a | 0.7 ± 0.1a,b |
| Liver/body, % | 4.2 ± 0.2a | 3.6 ± 0.1b | 3.5 ± 0.3b |
| Fat, g | 0.12 ± 0.06a | 0.41 ± 0.05b | 0.10 ± 0.04a |
| Fat/body, % | 0.5 ± 0.3a | 1.6 ± 0.2b | 0.5 ± 0.2a |
| Hepatic lipid | |||
| TG, μg/mg | 286.6 ± 56.7a | 83.7 ± 14.2b | 95.8 ± 25.1b |
| Chol, μg/mg | 17.5 ± 1.1 | 17.2 ± 1.1 | 17.8 ± 1.3 |
| FFA, nmol/mg | 101.8 ± 7.9a | 44.1 ± 5.4b | 86.7 ± 5.7a |
| Serum | |||
| TG, mg/dl | 31.6 ± 6.4 | 29.2 ± 6.7 | 32.8 ± 7.8 |
| Chol, mg/dl | 51.2 ± 4.2 | 48.2 ± 6.0 | 55.7 ± 7.8 |
| FFA, mmol/l | 0.35 ± 0.05 | 0.32 ± 0.07 | 0.38 ± 0.06 |
| Glucose, mg/dl | 135 ± 14 | 137 ± 19 | 137 ± 20 |
| β-HBA, μmol/l | 650 ± 155a | 362 ± 100a | 339 ± 89a |
Values are means ± SE; n, no. of mice. NJ, New Jersey; WU, Washington University; FFA, free fatty acid; β-HBA, β-hydroxybutyric acid. a,bDifferent superscript letters indicate significant differences between genotypes: P < 0.05.
Restoration of hepatic TG accumulation with adenoviral expression of L-FABP.
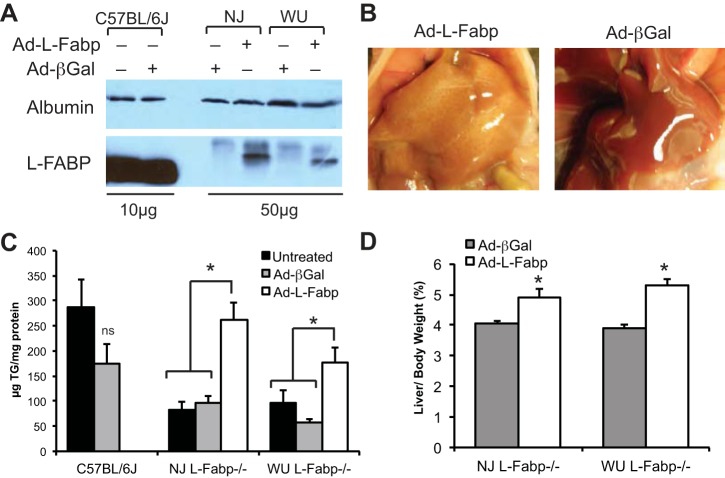
We used Ad-L-Fabp to transiently express L-FABP in liver of both lines of L-Fabp−/− mice, with Ad-βGal as a control (Fig. 2A). Although expression of exogenous L-FABP was not equivalent to expression levels in C57BL/6J mice (Fig. 2A), livers of Ad-L-Fabp-treated L-Fabp−/− mice were paler and contained significantly more TG compared with both untreated and Ad-βGal controls (Fig. 2, B and C). Liver size (normalized to body weight) was also significantly increased in Ad-L-Fabp injected L-Fabp−/− mice (Fig. 2D). Together, these data show decreased hepatic TG accumulation in both lines of fasted L-Fabp−/− mice that can be corrected by restoration of L-FABP expression. It is worth noting that reversal of the reduced hepatic steatosis phenotype was accomplished with even modest levels (<1/5 wild type) of exogenous L-FABP protein (Fig. 2A).
Fig. 2.
Restoration of fasting-induced steatosis by adenoviral L-Fabp. A: expression of L-FABP protein in livers of C57BL/6J mice with or without Ad-βGal (10 μg protein/lane) and in L-Fabp−/− mice injected with Ad-βGal or Ad-L-Fabp (50 μg protein/lane). Exogenous L-Fabp (from Ad-L-Fabp transduction) migrates slower than endogenous L-Fabp (C57BL/6J) because of the addition of a FLAG epitope tag. Expression of albumin is shown as a loading control. B: livers of WU L-Fabp−/− mice injected with either Ad-L-Fabp (left) or Ad-βGal (right) following a 48-h fast. C: hepatic TG in untreated (solid bars), Ad-βGal injected (shaded bars), and Ad-L-Fabp (open bars) injected C57BL/6J and NJ and WU L-Fabp−/− mice following a 48-h fast. D: liver weight in fasted Ad-βGal and Ad-L-Fabp mice expressed as percentage of final body weight. All NJ Fabp−/− mice for this experiment are F1; WU Fabp−/− mice are N8, F9 or N8, F10. Values are means ± SE; no. of mice per group: untreated controls, n = 5–7/genotype, Ad-βGal, n = 3–5/genotype; Ad-L-Fabp, n = 5–6/genotype. *P < 0.05 vs. Ad-βGal and untreated controls.
DIO and steatosis in L-Fabp−/− mice fed high-SF diet.
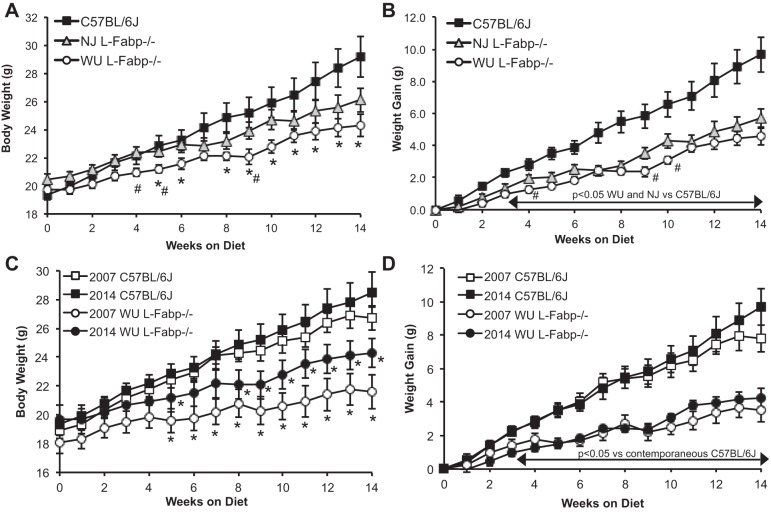
We next performed a side-by-side comparison of DIO in WU and NJ L-Fabp−/− mice, with C57BL/6J mice as controls, using female mice to align with our laboratory's previous studies (27, 28). Mice were fed a high-SF diet (20% hydrogenated coconut oil, see Tables 2 and 3 for details) starting at 12 wk of age and weighed weekly. WU L-Fabp−/− mice were significantly smaller than C57BL/6J mice after 5 wk on the SF diet (Fig. 3A), consistent with our previous observations. NJ L-Fabp−/− mice were slightly larger at the start of the study, but overall exhibited a trend toward reduced body weight compared with C57BL/6J controls. Differences in body weight between the L-Fabp−/− lines were minimal, with WU mice weighing slightly less than NJ mice throughout the experiment, reaching statistical significance at several time points (Fig. 3A). Importantly, average weight gain in both lines of L-Fabp−/− mice was significantly attenuated compared with C57BL/6J, with the NJ line exhibiting similar weight gain as WU L-Fabp−/− mice (Fig. 3B).
Fig. 3.
Reduced body weight and weight gain in L-Fabp−/− mice fed a high-saturated fat (SF) diet. A: average body weight of mice fed high-SF diet. B: average weight gain of mice fed a high-SF diet. Weight gain is significantly reduced in both NJ and WU L-Fabp−/− lines vs. C57BL/6J starting at 2 wk, as shown by the arrow. *P < 0.05 vs. C57BL/6J mice. #P < 0.05 between WU and NJ L-Fabp−/− genotypes. n = 10 C57BL/6J, 15 NJ L-Fabp−/− mice, 11 WU L-Fabp−/− mice. NJ Fabp−/− mice for this experiment are F1; WU Fabp−/− mice (2014) are N8, F14-15. C and D: comparison of current (2014) and historical (2007) data in WU L-Fabp−/− and C57BL/6J mice fed high-SF diet (n = 11–13/genotype). C: *Significant differences in body weight between WU L-Fabp−/− and contemporaneous C57BL/6J cohorts. D: differences in weight gain are significant after 3 wk on SF diet. 2007 WU L-Fabp−/− mice for this experiment were N7, F12-14. Values are means ± SE.
To examine the reproducibility of these observations, we compared data obtained from C57BL/6J and WU L-Fabp−/− mice in the present study (2014) to data generated more than 7 yr ago (28) and found that average body weight and weight gain of C57BL/6J mice were nearly identical in the two studies (Fig. 3, C and D). These data suggest that the obesity phenotype of C57BL/6J mice is reproducible, despite some animal-to-animal variability in both experiments. Moreover, body weight and weight gain of SF-fed WU L-Fabp−/− mice were also reproducible, although the average initial body weight of mice in the present study was slightly greater than in the previous study because mice were started on the diet 2 wk earlier (Fig. 3, C and D).
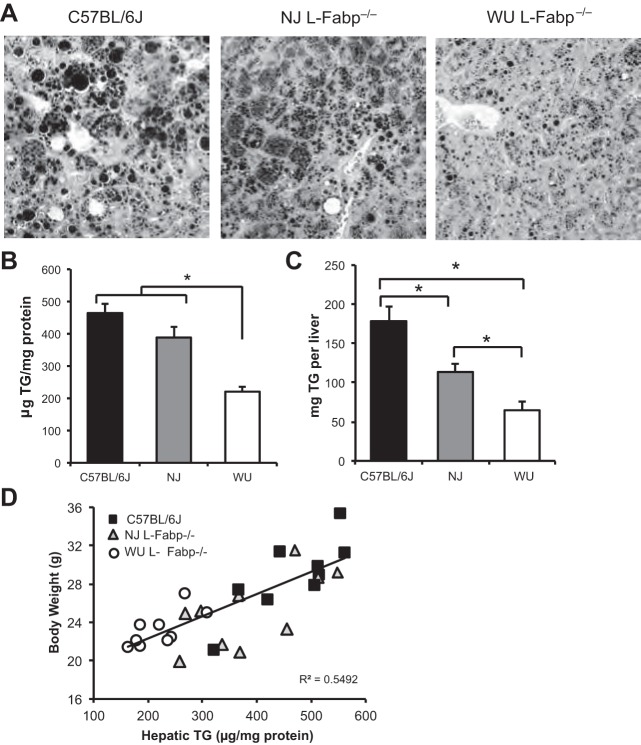
Average liver size was reduced in both L-Fabp−/− lines compared with C57BL/6J controls, including when normalized to body weight (Table 5). Osmium tetroxide-stained liver tissue shows the presence of large lipid droplets in C57BL/6J mice (Fig. 4A), but fewer, smaller droplets in both lines of L-Fabp−/− mice. Hepatic TG content was reduced in WU L-Fabp−/− livers compared with both C57BL/6J and NJ L-Fabp−/− mice (Fig. 4B). NJ L-Fabp−/− mice showed a trend toward reduced hepatic TG vs. C57BL/6J mice (Fig. 4B), and a significant decrease when corrected for liver size (Fig. 4C). In general, hepatic TG content correlated strongly with body weight over a threefold range of TG content (Fig. 4D), with reduced hepatic steatosis in WU L-Fabp−/− animals paralleling their reduced body weight.
Table 5.
Characterization of high-SF diet-fed mice
| C57BL/6J | NJ L-Fabp−/− | WU L-Fabp−/− | |
|---|---|---|---|
| Initial BW, g | 19.5 ± 0.4 (9) | 20.6 ± 0.4 (15) | 19.7 ± 0.4 (11) |
| Final BW, g | 28.8 ± 1.3a (9) | 26.4 ± 0.9a (15) | 24.3 ± 0.9b (11) |
| Weight gain, g | 9.4 ± 1.0a (9) | 5.8 ± 0.7b (15) | 4.6 ± 0.6b (11) |
| Liver, g | 1.6 ± 0.1a (9) | 1.3 ± 0.1b (15) | 1.2 ± 0.1b (11) |
| Liver/body, % | 5.5 ± 0.2a (9) | 4.9 ± 0.2b (15) | 5.0 ± 0.2b (11) |
| Fat weight, g | 1.0 ± 0.2 (9) | 0.8 ± 0.1 (15) | 0.5 ± 0.1 (11) |
| Fat/body, % | 3.3 ± 0.4 (9) | 3.0 ± 0.3 (15) | 2.2 ± 0.3 (11) |
| Food consumed, g/day | 3.34 ± 0.1a (9) | 2.91 ± 0.05b (15) | 3.11 ± 0.13a,b (9) |
| Fat absorption, % | 98.8 ± 0.1 (5) | 98.5 ± 0.1 (5) | 98.6 ± 0.1 (5) |
| Serum | |||
| TG, mg/dl | 18.3 ± 3.8 (9) | 17.8 ± 3.2 (10) | 11.6 ± 0.8 (7) |
| Chol, mg/dl | 67.4 ± 2.0 (9) | 70.4 ± 2.6 (10) | 77.7 ± 7.5 (7) |
| FFA, mmol/l | 0.77 ± 0.04 (9) | 0.80 ± 0.06 (10) | 0.67 ± 0.03 (7) |
| Glucose, mg/dl | 382 ± 15a (9) | 302 ± 31a (10) | 251 ± 20b (7) |
| Insulin, pg/ml | 69.5 ± 14 (6) | 95.8 ± 24 (6) | 95.0 ± 13.4 (5) |
| Hepatic lipid | |||
| TG, μg/mg | 466 ± 28a (9) | 388 ± 33a (10) | 221 ± 16b (9) |
| Chol, μg/mg | 35.7 ± 1.5 (9) | 36.9 ± 3.2 (10) | 30.5 ± 2.4 (9) |
| FFA, nmol/mg | 107.4 ± 4.0a (9) | 96.2 ± 3.1a (10) | 85.2 ± 2.9a,b (9) |
| PL, μg/mg | 103.2 ± 9.2 (9) | 87.7 ± 9.0 (10) | 94.3 ± 5.9 (9) |
| Intestinal lipid | |||
| TG, μg/mg | 267 ± 33 (5) | 354 ± 90 (6) | 335 ± 87 (5) |
| Chol, μg/mg | 22.4 ± 1.8 (5) | 24.3 ± 3.9 (6) | 25.8 ± 4.1 (5) |
| FFA, nmol/mg | 190 ± 18a (5) | 232 ± 31a,b (6) | 183 ± 32a (5) |
Values are means ± SE; nos. in parentheses, no. animals studied for each parameter. a,bDifferent superscript letters indicate a significant difference between the groups: P < 0.05.
Fig. 4.
Liver weight and steatosis in C57BL/6J and L-Fabp−/− mice fed high-SF diet. A: osmium tetroxide-stained liver tissue from mice fed SF for 15 wk. Representative fields were photographed at ×200 magnification. B: hepatic TG content in high-SF diet-fed mice, expressed as μg TG per mg protein. *Hepatic TG is reduced in WU L-Fabp−/− mice compared with C57BL/6J and NJ L-Fabp−/− mice. n = 9–10 mice/genotype. C: hepatic TG expressed as mg TG per liver, to correct for differences in liver mass. Values are means ± SE. D: correlation between final body weight and hepatic TG content. Overall correlation coefficient is 0.798. Correlation of individual groups is as follows: C57BL/6J, 0.801; NJ L-Fabp−/− mice, 0.684; WU L-Fabp−/− mice, 0.709. NJ Fabp−/− mice for this experiment are F1; WU Fabp−/− mice (2014) are N8, F14-15.
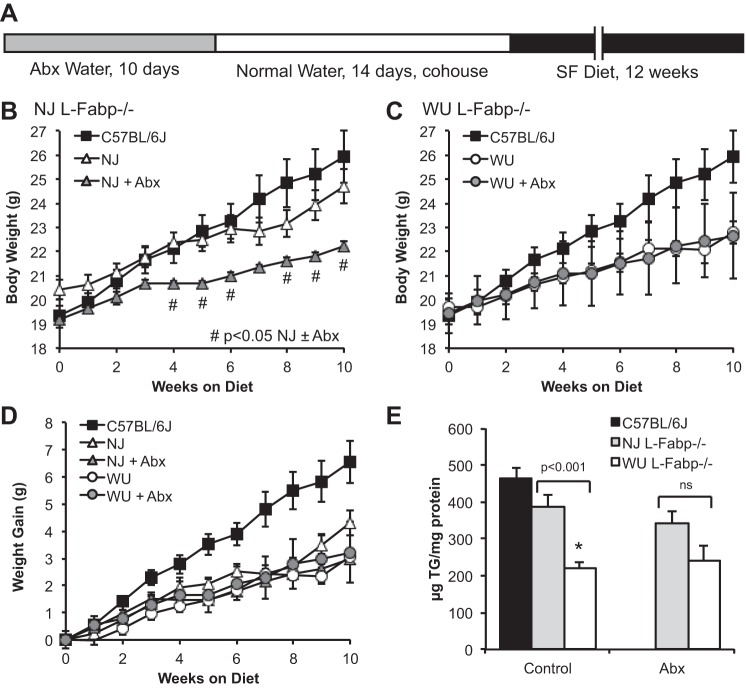
Abx treatment abrogates differences in SF-fed L-Fabp−/− lines.
We next examined the possibility that environmental modifiers, such as facility-specific microbiota, might account for some of the phenotypic divergence noted in previous work. Female mice from both L-Fabp−/− lines were treated with Abx for 10 days, then cohoused to assimilate the intestinal microbiome. SF-diet feeding was initiated after 2 wk (Fig. 5A). Average body weight (both initial and during SF feeding) of Abx-treated NJ L-Fabp−/− mice was reduced compared with that of both untreated NJ mice and C57BL/6J controls (Fig. 5B). In contrast, Abx treatment had no effect on body weight of WU L-Fabp−/− mice (Fig. 5C). Abx pretreatment did not alter average weight gain of WU or NJ L-Fabp−/− mice (Fig. 5D). Interestingly, Abx treatment reduced steatosis in NJ L-Fabp−/− mice, abrogating differences in hepatic TG content between the two L-Fabp knockout lines (Fig. 5E), with differences vs. C57BL/6J controls now reaching statistical significance. Together these data suggest that some of the phenotypic distinctions between WU and NJ L-Fabp−/− mice fed SF diet may reflect differences in intestinal microbiota. Because the intent of this experiment was to determine whether Abx treatment would alter the response to SF feeding between the two L-Fabp−/− lines (rather than vs. wild-type, C57BL/6J controls), we did not include a separate group of Abx-treated C57BL/6J mice in parallel. The intestinal microbiota of C57BL/6J mice from Jackson Laboratory is likely distinct, although in our hands these mice generally display a consistent phenotype from experiment to experiment and over time.
Fig. 5.
Antibiotic (Abx) treatment before SF feeding reduces differences in body weight and steatosis between NJ and WU L-Fabp−/− mice. A: schematic diagram showing Abx treatment protocol. B and C: average body weight of SF-fed NJ (B) and WU (C) L-Fabp−/− mice, with and without Abx pretreatment, shown relative to data from untreated C57BL/6J mice (from Fig. 3A) presented as a reference. #Significant differences between treated and untreated NJ L-Fabp−/− mice. D: weight gain of mice fed high-SF diet for 10 wk, with differences significant vs. untreated C57BL/6J for all L-Fabp−/− groups (±Abx) after 5 wk on the diet. E: hepatic TG content in Abx-treated mice. Note that differences in steatosis between NJ and WU L-Fabp−/− mice were reduced by Abx treatment. For Abx groups, n = 8 NJ L-Fabp−/− mice (F6) and 6 WU L-Fabp−/− mice (N9, F6). Values are means ± SE. *P < 0.001. ns, Nonsignificant.
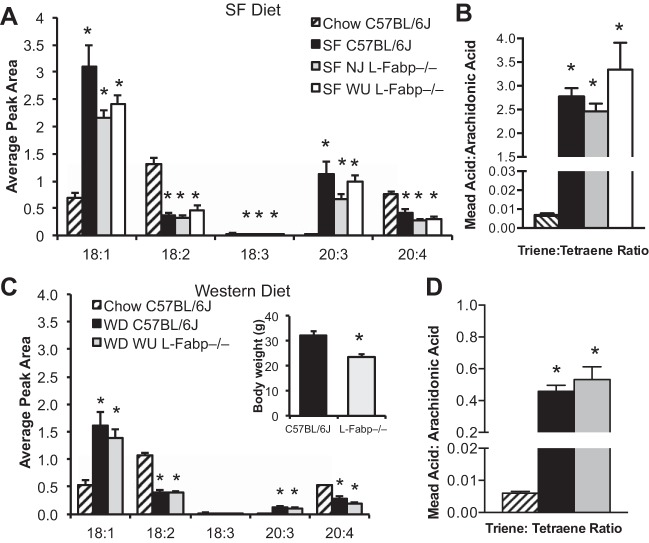
Reduced weight gain in L-Fabp−/− mice is not due to EFA deficiency.
The hydrogenated coconut oil present in SF diet contains primarily medium-chain FA, with low levels of EFAs. It has been suggested that reduced weight gain and steatosis observed in WU L-Fabp−/− mice, both in this study and previous studies, may reflect EFA deficiency (specifically in L-Fabp−/− mice) following prolonged SF feeding and thus explain their reduced weight gain (11, 12). Although SF-fed L-Fabp−/− mice did not exhibit any of the physiological characteristics of EFA deficiency [decreased dietary fat absorption, increased steatosis, and hair loss (6, 8, 16, 36, 38)], we assessed EFA deficiency biochemically by looking for alterations in the ratio of serum FAs. All SF-fed mice, regardless of genotype, showed increased serum oleic [18:1 (n-9)] and mead acid [20:3 (n-9)] and a corresponding decrease in linoleic [18:2 (n-6)] and arachidonic acid [20:4 (n-6)] levels compared with age-matched, chow-fed C57BL/6J controls (Fig. 6A). The triene-to-tetraene ratio in all SF-fed mice was increased >200-fold relative to chow-fed C57BL/6J (Fig. 6B), with a corresponding decrease in EFA index (chow C57, 3.16 ± 0.31; SF C57, 0.18 ± 0.01; SF NJ, 0.24 ± 0.06; SF WU, 0.21 ± 0.01), confirming biochemical EFA deficiency in all genotypes fed SF diet for 16 wk. Importantly, the degree of EFA deficiency did not differ between SF-fed C57BL/6J and L-Fabp−/− genotypes, indicating that differences in body weight cannot be attributed to EFA deficiency. We also examined the serum FA profile in C57BL/6J and WU L-Fabp−/− mice fed a high-cholesterol, long-chain FA WD (30). Significant differences in FA profile and triene-to-tetraene ratios were observed between chow-fed C57BL/6J controls and both WD-fed genotypes (Fig. 6, C and D), suggesting mild EFA deficiency. Again however, although WD-fed WU L-Fabp−/− mice weighed significantly less than WD-fed controls (Fig. 6C), the serum EFA profile did not differ by genotype, strongly suggesting that EFA deficiency cannot explain differences in DIO and hepatic steatosis between C57BL/6J and L-Fabp−/− mice.
Fig. 6.
Essential fatty acid (EFA) deficiency in L-Fabp−/− mice fed high-SF diet cannot account for decreased obesity and steatosis. A: average peak area of specific fatty acids species in serum of chow C57BL/6J and SF-fed mice. n = 4 animals per group. For all FA species shown, levels are significantly different in chow-fed C57BL/6J mice compared with each of the SF-fed groups. B: ratio of mead acid [20:3 (n-9)] to arachidonic acid [20:4 (n-6)]. Triene-to-tetraene ratios >0.2 indicate EFA deficiency. C: average peak area of serum fatty acids in chow and Western diet (WD) fed mice. n = 3–4 animals/genotype. Inset shows final body weight of C57BL/6J and WU L-Fabp−/− mice fed WD (30). WU L-Fabp−/− mice fed WD are from 2004, generation N7, F3. D: triene-to-tetraene ratio of serum fatty acids in chow- and WD-fed mice. Values are means ± SE. *P < 0.05 vs. chow C57BL/6J controls.
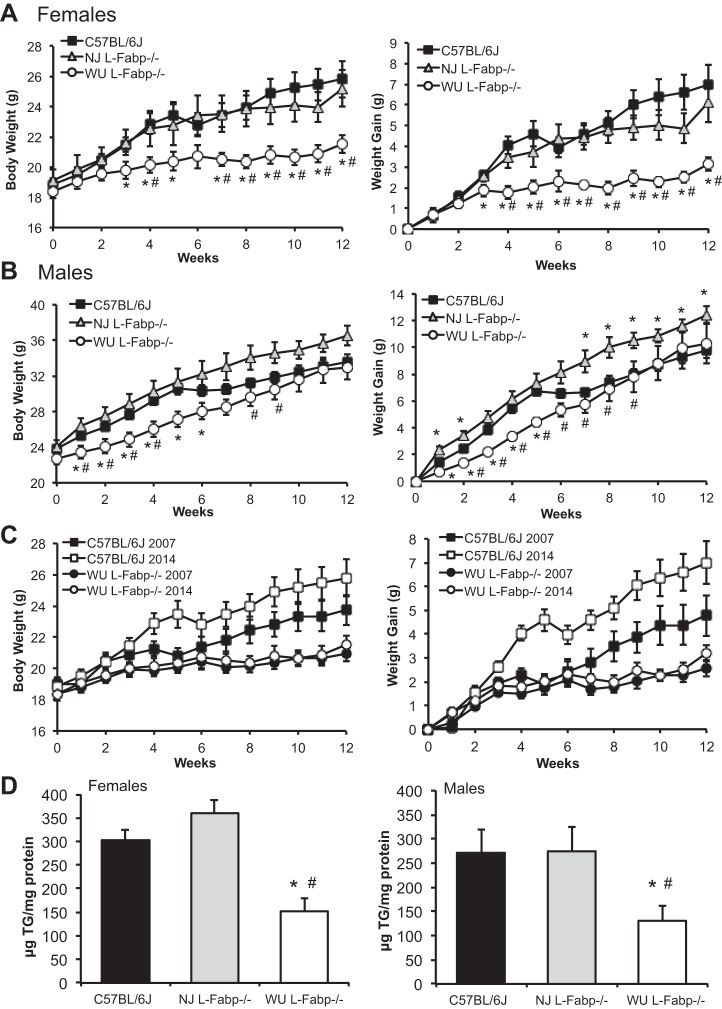
DIO and steatosis in L-Fabp−/− mice fed high-CB diet.
One challenge in resolving phenotypic differences between the two lines of L-Fabp−/− mice has been that the studies have involved different high-fat diets, different sexes, and different facilities. As an example, Gajda et al. (12) recently described an obese phenotype in male L-Fabp−/− mice fed a 20% CB diet. Because the L-Fabp−/− mice used in that study were from the same line as the NJ mice used in the present study [provided by Gajda and collaborators (12), reported as backcrossed 6 generations into C57BL/6J] and were performed relatively recently, we examined the effects of CB feeding on DIO and steatosis, using a side-by-side comparison in C57BL/6J and NJ and WU L-Fabp−/− mice. In addition, we expanded the comparison to include both sexes.
In general, WU L-Fabp−/− mice exhibit reduced body weight and weight gain vs. both C57BL/6J and NJ L-Fabp−/− mice (Fig. 7, A and B), paralleling what we have observed in previous studies (27, 28, 30), although the protection against DIO in male WU L-Fabp−/− mice was less dramatic than that in females. Body weight and weight gain in NJ L-Fabp−/− mice was similar to that of CB-fed C57BL/6J controls, with neither a protective nor an obese phenotype, yet clearly different from that of WU L-Fabp−/− mice (Fig. 7, A and B). Importantly, the present findings in female WU L-Fabp−/− mice aligns well with unpublished data generated more than 7 yr ago in our laboratory, using female L-Fabp−/− and C57BL/6J controls fed a 20% CB diet (Fig. 7C). It is worth pointing out that, although the weight curves of C57BL/6J mice in 2007 and 2014 show some variation, the WU L-Fabp−/− mice were protected against DIO in both studies (Fig. 7C). Hepatic TG content was significantly reduced in female and male WU L-Fabp−/− mice compared with both C57BL/6J and NJ L-Fabp−/− mice (Fig. 7D), despite only minor differences in final body weight in the male genotypes (Table 6). A similar reduction in steatosis was previously observed in CB-fed WU L-Fabp−/− mice compared with C57BL/6J controls (unpublished data).
Fig. 7.
Reduced obesity and steatosis in WU L-Fabp−/− mice but not NJ L-Fabp−/− mice fed a high cocoa butter (CB) fat diet. A: body weight (left) and weight gain (right) of female mice fed CB diet for 12 wk. n = 6–10/genotype. B: body weight (left) and weight gain (right) of male mice fed CB diet for 12 wk. n = 6–11/genotype. C: comparison of current and historical data in WU L-Fabp−/− mice fed CB diet, showing both body weight (left) and weight gain (right). In 2007 study, differences in body weight of L-Fabp−/− and C57BL/6J mice (n = 14–15/genotype) were significant (P < 0.05) after 7 wk on the diet. In the present study, differences in body weight between the genotypes were significant after 3 wk. D: hepatic TG content, expressed as μg TG/mg protein, in female (left) and male (right) mice fed 20% CB diet (2014 samples). n = 4–6 samples/genotype. For 2014 CB studies, NJ L-Fabp−/− mice were F7; WU L-Fabp−/− mice were N9, F7. For 2007 CB samples, WU L-Fabp−/− mice were N8, F3-4. Values are means ± SE. *P < 0.05 vs. C57BL/6J. #P < 0.05 vs. NJ L-Fabp−/− mice.
Table 6.
Characterization of mice fed 20% cocoa butter diet
| C57BL/6J | NJ L-Fabp−/− | WU L-Fabp−/− | |
|---|---|---|---|
| Females | |||
| n | 10 | 6 | 10 |
| Final BW, g | 25.3 ± 1.2a | 24.6 ± 1.1a | 21.2 ± 0.6b |
| Weight gain, g | 7.0 ± 1.0a | 6.1 ± 1.0a | 3.2 ± 0.3b |
| Liver, g | 1.1 ± 0.1 | 1.0 ± 0.03 | 0.9 ± 0.1 |
| Liver/body, % | 4.3 ± 0.2 | 4.0 ± 0.2 | 4.3 ± 0.2 |
| Fat, g | 0.8 ± 0.1a | 0.6 ± 0.1a | 0.3 ± 0.02b |
| Fat/body, % | 2.8 ± 0.4a | 2.4 ± 0.2a | 1.3 ± 0.1b |
| Serum | |||
| TG, mg/dl | 12.9 ± 2.9 | 18.5 ± 3.4 | 21.2 ± 5.4 |
| Chol, mg/dl | 69.4 ± 3.4 | 56.8 ± 4.7 | 56.5 ± 4.9 |
| FFA, mmol/l | 0.32 ± 0.05a | 0.64 ± 0.1b | 0.50 ± 0.06a |
| Glucose, mg/dl | 337 ± 27a | 241 ± 30b | 251 ± 29a |
| Hepatic lipid | |||
| TG, μg/mg | 302 ± 23a | 361 ± 28a | 152 ± 28b |
| Chol, μg/mg | 20.8 ± 1.7a | 18.4 ± 1.3a | 12.3 ± 3.5b |
| FFA, nmol/mg | 80 ± 7 | 67 ± 5 | 60 ± 4 |
| Males | |||
| n | 9 | 11 | 6 |
| Final BW, g | 32.9 ± 0.8a | 36.0 ± 1.0b | 32.8 ± 1.6a |
| Weight gain, g | 9.8 ± 0.6a | 12.5 ± 0.6b | 10.3 ± 1.5a |
| Liver, g | 1.2 ± 0.04 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| Liver/body, % | 3.7 ± 0.1 | 3.5 ± 0.2 | 3.8 ± 0.1 |
| Fat, g | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.2 |
| Fat/body, % | 4.3 ± 0.2a | 3.8 ± 0.1a,b | 4.5 ± 0.4a |
| Serum | |||
| TG, mg/dl | 28.3 ± 2.9 | 23.2 ± 3.1 | 37.5 ± 6.5 |
| Chol, mg/dl | 106.8 ± 4.9 | 90.7 ± 9.5 | 105.7 ± 8.9 |
| FFA, mmol/l | 0.30 ± 0.3a | 0.49 ± 0.09a,b | 0.54 ± 0.06b |
| Glucose, mg/dl | 349 ± 12a | 239 ± 35b | 434 ± 41a |
| Hepatic lipid | |||
| TG, μg/mg | 272 ± 48a | 274 ± 50a | 131 ± 32b |
| Chol, μg/mg | 18.0 ± 1.3 | 19.2 ± 1.4 | 17.2 ± 0.8 |
| FFA, nmol/mg | 98 ± 8 | 87 ± 4 | 96 ± 4 |
Values are means ± SE; n, no. of mice. a,bDifferent superscript letters indicate significant differences between genotypes: P < 0.05. Hepatic lipid was assayed in 5–7 animals per genotype. Serum was assayed in 5–6 animals per genotype.
Differences in genetic background in lines of L-Fabp−/− mice.
The high-CB diet feeding study in particular revealed clear phenotypic differences between the two L-Fabp−/− lines, including differences in body weight and hepatic steatosis. One possible explanation for this divergence is a difference in their genetic background. We first examined whether there was differential carryover of residual, non-C57BL/6 gDNA from 129/SvJ-derived ES cells in the region near the Fabp1 gene. This is relevant because a number of quantitative trait loci linked to differences in body weight and obesity between mouse strains have been mapped to the 40 Mb surrounding the Fabp1 locus (26). Using SSLP markers (Table 7), we surveyed the amount of non-C57BL/6 gDNA near the Fabp1 locus in both lines and found that NJ L-Fabp−/− mice contain ∼12 cM less 129/SvJ gDNA than WU L-Fabp−/− mice (Fig. 8, A and B). We monitored the expression of genes near the Fabp1 locus in the livers of both lines of L-Fabp−/− mice, as well as in 129/SvJ and C57BL/6J mice. Only minor differences in mRNA expression of these genes were observed, including slightly increased expression of Fkbp9 in WU L-Fabp−/− mice (Fig. 8C). However, it bears emphasis that minor changes in Fkbp9 mRNA observed in our laboratory's previous studies did not correlate with changes in Fkbp9 protein expression (7). Moderate differences in expression of two genes (Aqp1, Abcg2) were seen in 129/SvJ mice compared with C57BL/6J, although similar changes were not observed in either of the L-Fabp−/− lines, despite the fact that these genes are likely encoded by 129/SvJ gDNA, particularly in WU L-Fabp−/− mice. Importantly, we saw no difference in the expression of Snca mRNA, which is relevant in light of recent studies showing that a subpopulation of commercially available C57BL/6 mice contain a deletion of the Snca locus (34).
As a separate consideration, we recalled the findings from recent studies showing that there are phenotypic differences between C57BL/6N and C57BL/6J substrains, including susceptibility to DIO (13, 25, 32, 37). Because NJ L-Fabp−/− mice were initially backcrossed to C57BL/6N, it was possible that some residual C57BL/6N gDNA might potentially influence the phenotype. We, therefore, used a whole genome, C57BL/6 substrain-specific SNP array to map chromosomal regions derived from each substrain in NJ and WU L-Fabp−/− mice. To our surprise, only ∼60% of SNP markers (90/150) in NJ L-Fabp−/− mice were identified as C57BL/6J gDNA (Fig. 8D, Supplemental Table S1; Supplemental material for this article is available online at the Journal website), with the rest identical to C57BL/6N. This finding suggests that the NJ L-Fabp−/− line was not backcrossed six generations as stated (12, 15), but more likely only one to two generations from 6N to 6J, or vise versa, since six backcross generations should produce mice with only minor amounts of original strain gDNA. Moreover, the chromosomal regions identified as 6N or 6J gDNA were similar in each of the 3 NJ L-Fabp−/− samples analyzed (Supplemental Table S1), indicating that the differences were static and consistent with filial (brother × sister) breeding for multiple generations. In contrast, in WU L-Fabp−/− mice, >95% of the SNP markers were C57BL/6J, with ∼5% of SNPs (8/150) identified as C57BL/6N. Because WU L-Fabp−/− mice have never been bred to C57BL/6N mice (nor do we have this strain in our colony), we repeated the substrain analysis with 129/SvJ gDNA and found that 129/SvJ and C57BL/6N gDNA contained the same nucleotide in seven of the non-C57BL/6J SNPs found in WU L-Fabp−/− mice (Supplemental Table S1). Thus these SNPs likely represent residual carryover of 129/SvJ gDNA in WU L-Fabp−/− mice, despite many generations of backcrossing. It is worth noting that only one of the non-C57BL/6J SNPs was homozygous in both samples (i.e., both chromosomes are 129/SvJ). This SNP is located on chromosome 6, near the Fabp1 locus, consistent with a region of 129/SvJ gDNA persisting around the targeted locus. In comparison, in NJ L-Fabp−/− mice, only 39 of 60 non-C57BL/6J SNPs have the same nucleotide in 129/SvJ and C57BL/6N mice, while the rest are unique to C57BL/6N. Together these data indicate that large regions of C57BL/6N gDNA are still present in NJ L-Fabp−/− mice.
Because of the differences in genetic background between the two L-Fabp−/− lines, we examined the expression of several genes near informative SNPs whose expression level has been shown to vary based on genetic background (13). However, we found no differences in the expression of these particular genes in WU vs. NJ L-Fabp−/− mice of either sex (data not shown). Together these data show that significant differences in substrain genetic background exist between the two L-Fabp−/− lines, a finding that could potentially explain some of their phenotypic divergence, particularly since C57BL/6N mice are more susceptible to DIO (13, 32, 37).
DISCUSSION
The primary objective of this study was to understand fundamental discrepancies in the phenotype attributed to L-Fabp deletion, particularly phenotypes related to DIO and hepatic steatosis in mice on a C57BL/6J background. We reasoned that undertaking a side-by-side comparison of both L-Fabp−/− lines in parallel, using mice that we believed were on a similar genetic background (i.e., C57BL/6J), would permit us to directly assess whether there were consistent phenotypic differences (as outlined in Table 1) under the same environmental and dietary conditions. We used diets and mice of the same sex that we had studied previously and always compared the phenotype of L-Fabp−/− mice to C57BL/6J mice purchased directly from Jackson Laboratory.
Overall, we found that the phenotype of the two L-Fabp knockout lines was more similar than previous studies would suggest, although some marked differences remain. (See Table 8 for summary of side-by-side studies performed herein.) As observed previously (29), hepatic TG content and ketogenesis were reduced in both L-Fabp−/− lines following a prolonged fast compared with C57BL/6J controls (Fig. 1). Importantly, “rescue” of L-Fabp−/− mice with exogenous L-FABP via adenoviral delivery reversed the defect in hepatic FA trafficking and restored hepatic lipid accumulation in both knockout lines (Fig. 2). In the high-SF feeding study, female L-Fabp−/− mice from both lines displayed significantly reduced weight gain compared with C57BL/6J mice, although the average body weight of NJ L-Fabp−/− mice was slightly greater than that of WU L-Fabp−/− mice (Fig. 3, A and B). Pretreatment with Abx abrogated the subtle differences in initial body weight, resulting in nearly identical body weight and weight gain (Fig. 5, B–D). Differences in hepatic steatosis between SF-fed NJ and WU L-Fabp−/− mice were also reduced by Abx treatment, although there was still a trend to increased steatosis in NJ animals (Fig. 5E). These results suggest that some of the phenotypic differences in the two L-Fabp−/− lines may be linked to intestinal microbiota. Although it was not our intent in the present studies, it will be of future interest to understand the role of microbial communities in the protection of L-Fabp−/− mice from SF- and CB-induced DIO and hepatic steatosis, but using an appropriate side-by-side comparison of mice on the same genetic background.
Table 8.
Summary of observations in our side-by-side comparison of two L-Fabp−/− lines
| Experiment | Sex | NJ L-Fabp−/− | WU L-Fabp−/− |
|---|---|---|---|
| 48-h Fast | Male | Reduced hepatic TG vs. C57BL/6J | Reduced hepatic TG vs. C57BL/6J |
| High-SF diet | Female | Reduced weight gain vs. C57BL/6J | Reduced weight gain, BW, and steatosis vs. C57BL/6J |
| No change in BW or steatosis | |||
| High-CB diet | Male | Increased weight gain vs. C57BL/6J | Trend to reduced BW |
| No change in steatosis | Reduced hepatic steatosis vs. C57BL/6J | ||
| High-CB diet | Female | No change in BW or steatosis vs. C57BL/6J | Reduced weight gain, BW, and steatosis vs. C57BL/6J |
In contrast, in high-CB diet study, both male and female WU L-Fabp−/− mice were consistently smaller, with decreased weight gain, compared with both NJ L-Fabp−/− and C57BL/6J mice (Fig. 7, A and B), although the differences were less dramatic in males. Hepatic steatosis was reduced in both sexes of WU L-Fabp−/− mice compared with both NJ L-Fabp−/− and C57BL/6J mice (Fig. 7D), consistent with previous studies and our laboratory's unpublished data (27, 28, 30). It is worth noting that, in this study, male NJ L-Fabp−/− mice did not exhibit obesity compared with C57BL/6J controls, findings at odds with recent studies in NJ L-Fabp−/− mice studied in the NJ facility (12). These findings further support the suggestion that environmental effects may play a role in the DIO phenotype. Our findings also illustrate an important divergence between the L-Fabp−/− lines in high-fat diet-induced hepatic steatosis. WU L-Fabp−/− mice exhibited reduced steatosis compared with C57BL/6J mice on both SF and CB diets, data that are consistent (in both phenotype and magnitude) with our historical data (27, 28, 30), and likely linked in part to reduced DIO. In contrast, hepatic TG in NJ L-Fabp−/− mice was not significantly different compared with that in C57BL/6J controls in females fed the SF diet, or in males or females fed the CB diet (Figs. 4B and 7D).
We, as well as Storch, Schroeder, and colleagues, previously outlined a number of possible explanations for the discrepant phenotypes in the L-Fabp−/− lines, which we have now tried to address directly in this study (3, 12, 17). Our laboratory previously addressed concerns that the phenotype of WU L-Fabp−/− mice was linked to the green fluorescent protein “knock-in” targeting strategy by using antisense oligonucleotides (ASO) to produce liver-specific L-Fabp knockdown in C57BL/6J mice, and we found that L-Fabp ASO treatment reduced obesity and steatosis (26). To address the role of genetic background, we also performed ASO knockdown studies in 129/SvJ and C57BL/6J mice, again showing both protection against and reversal of DIO (as well as protection/reversal of hepatic steatosis) following L-Fabp knockdown (26). To address concerns about EFA deficiency, we examined serum FA from mice fed high-fat diets and showed similar diet-induced changes in FA profile in C57BL/6J and L-Fabp−/− mice (Fig. 6), indicating that the differences in obesity cannot be attributed to EFA deficiency. Finally, studies presented here attempted to minimize environmental variability (diet, housing, microbiota) by using mice bred and studied side by side to assimilate the intestinal microbiome.
The most important implication of our study is that phenotypic differences between the L-Fabp−/− lines likely reflect unanticipated differences in genetic background. As outlined in Fig. 1A, the L-Fabp−/− lines were each generated in 129-derived ES cells, then backcrossed to either C57BL/6J or C57BL/6NCr. However, large regions of non-C57 gDNA often remain around a targeted locus, despite repeated backcrosses (1), because the targeted allele is selected in each generation. We found differences in the amount of residual 129/SvJ gDNA near the Fabp1 locus between the L-Fabp−/− lines (4.5 cM and 16.7 cM non-C57 gDNA in NJ and WU L-Fabp−/− mice, respectively), but no dramatic change in the expression of nearby genes, and no evidence of gene duplication or locus-specific effects of gene targeting (Fig. 8). Interestingly, several genes showed differential expression between 129/SvJ and C57BL/6J livers, emphasizing the potential implication of differences in genetic background. Finally, the substrain-specific SNP array data showed that significant regions of C57BL/6N gDNA are present in NJ L-Fabp−/− mice, with ∼40% of SNP markers shown to be non-C57BL/6J (Fig. 8, Supplemental Table S1). Although we did not observe any change in the expression of genes identified by Heiker et al. (13), as linked to 6N/6J SNPs, we did observe differences in expression of Nnt between NJ and WU L-Fabp−/− mice (data not shown).
We believe that this unexpected difference in genetic background is relevant not only to the phenotypic divergence noted, but also adds to a growing literature showing genetic and phenotypic differences between C57BL/6N and C57BL/6J substrains of mice (13, 25, 32, 37). The C57BL/6N line was separated from the C57BL/6J line in 1951 and has been maintained separately. Known genetic differences include deletion of Snca gene, altered expression of Nnt, which has been linked to changes in glucose intolerance (10), and a point mutation in Cyfip2 gene that alters cocaine responsiveness (14). Documented phenotypic differences between 6J and 6N substrains include both metabolic (fat mass, energy expenditure, gas exchange, food consumption) and cardiovascular (blood pressure, pulse rate) parameters (32). Recently it was shown that there are differences in intestinal microbiome of C57BL/6N and C57BL/6J mice, as well as distinct differences in the hepatic metabolome between the strains (37). Body composition data from the Mouse Phenome database shows that both male and female C57BL/6N mice are significantly larger than C57BL/6J mice at 12 wk, with increased bone mineral density (http://phenome.jax.org). It is worth noting that divergent responses to acetaminophen-induced liver injury were observed in two lines of JNK2−/− mice and found to correlate with differences in C57BL/6 substrain (5). Importantly, this group identified several knockout lines, sold by Jackson Laboratory and listed as C57BL/6J, that did not have deletion of Nnt gene, indicating that they were not C57BL/6J congenic. Going forward, substrain differences will become more of an issue, as the NIH sponsored Knock Mouse Project (KOMP, https://www.komp.org) is using ES cells from the C57BL/6N line. Knockout mice generated by this project must be compared with C57BL/6N, not to the traditional C57BL/6J “gold standard” reference line. Together these findings emphasize that researchers must be cautious when attributing a particular phenotype to deletion of a specific gene, and that phenotypes should also include strain details (vendor, stock number) with meticulous documentation of backcrossing.
Overall, our studies demonstrate significant overlap in the phenotype of the two lines of L-Fabp−/− mice and provide progress toward understanding its function in hepatic FA metabolism. While environmental effects may underlie some of the phenotypic divergence of the L-Fabp−/− lines, we suggest that unanticipated genetic differences that were not controlled for play a critical role.
GRANTS
This work was supported by National Institutes of Health Grants DK-56260, HL-38180, and DK-52574 (Morphology and Murine Models Core) to N. O. Davidson. The Washington University Diabetes Research Center and the Washington University Metabolomics Facility are supported by Grant P30 DK-020579.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.P.N. conception and design of research; E.P.N., S.K., Y.X., J.L., and H.J. performed experiments; E.P.N., D.S.O., and N.O.D. analyzed data; E.P.N., Y.X., D.S.O., and N.O.D. interpreted results of experiments; E.P.N. prepared figures; E.P.N. and N.O.D. drafted manuscript; E.P.N., D.S.O., and N.O.D. edited and revised manuscript; E.P.N., D.S.O., and N.O.D. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Almodovar AJ, Luther RJ, Stonebrook CL, Wood PA. Genomic structure and genetic drift in C57BL/6 congenic metabolic mutant mice. Mol Genet Metab 110: 396–400, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Atshaves BP, McIntosh AL, Payne HR, Mackie J, Kier AB, Schroeder F. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am J Physiol Cell Physiol 288: C543–C558, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Atshaves BP, McIntosh AL, Storey SM, Landrock KK, Kier AB, Schroeder F. High dietary fat exacerbates weight gain and obesity in female liver fatty acid binding protein gene-ablated mice. Lipids 45: 97–110, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atshaves BP, McIntosh AM, Lyuksyutova OI, Zipfel W, Webb WW, Schroeder F. Liver fatty acid-binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem 279: 30954–30965, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bourdi M, Davies JS, Pohl LR. Mispairing C57BL/6 substrains of genetically engineered mice and wild-type controls can lead to confounding results as it did in studies of JNK2 in acetaminophen and concanavalin A liver injury. Chem Res Toxicol 24: 794–796, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardot P, Chambaz J, Thomas G, Rayssiguier Y, Bereziat G. Essential fatty acid deficiency during pregnancy in the rat: influence of dietary carbohydrates. J Nutr 117: 1504–1513, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Chen A, Tang Y, Davis V, Hsu FF, Kennedy SM, Song H, Turk J, Brunt EM, Newberry EP, Davidson NO. Liver fatty acid binding protein (L-Fabp) modulates murine stellate cell activation and diet-induced nonalcoholic fatty liver disease. Hepatology 57: 2202–2212, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark SB, Ekkers TE, Singh A, Balint JA, Holt PR, Rodgers JB Jr. Fat absorption in essential fatty acid deficiency: a model experimental approach to studies of the mechanism of fat malabsorption of unknown etiology. J Lipid Res 14: 581–588, 1973. [PubMed] [Google Scholar]
- 9.Erol E, Kumar LS, Cline GW, Shulman GI, Kelly DP, Binas B. Liver fatty acid binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPARα in fasting mice. FASEB J 18: 347–349, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 55: 2153–2156, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Gajda AM, Storch J. Enterocyte fatty acid-binding proteins (FABPs): different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot Essent Fatty Acids 93: 9–16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gajda AM, Zhou YX, Agellon LB, Fried SK, Kodukula S, Fortson W, Patel K, Storch J. Direct comparison of mice null for liver or intestinal fatty acid-binding proteins reveals highly divergent phenotypic responses to high fat feeding. J Biol Chem 288: 30330–30344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiker JT, Kunath A, Kosacka J, Flehmig G, Knigge A, Kern M, Stumvoll M, Kovacs P, Bluher M, Kloting N. Identification of genetic loci associated with different responses to high-fat diet-induced obesity in C57BL/6N and C57BL/6J substrains. Physiol Genomics 46: 377–384, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Kumar V, Kim K, Joseph C, Kourrich S, Yoo SH, Huang HC, Vitaterna MH, de Villena FP, Churchill G, Bonci A, Takahashi JS. C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science 342: 1508–1512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagakos WS, Gajda AM, Agellon L, Binas B, Choi V, Mandap B, Russnak T, Zhou YX, Storch J. Different functions of intestinal and liver-type fatty acid-binding proteins in intestine and in whole body energy homeostasis. Am J Physiol Gastrointest Liver Physiol 300: G803–G814, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le HD, Fallon EM, Kalish BT, de Meijer VE, Meisel JA, Gura KM, Nose V, Pan AH, Bistrian BR, Puder M. The effect of varying ratios of docosahexaenoic acid and arachidonic acid in the prevention and reversal of biochemical essential fatty acid deficiency in a murine model. Metabolism 62: 499–508, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BJ, Pai PJ, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein gene-ablated mice. Am J Physiol Gastrointest Liver Physiol 297: G1053–G1065, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice. Am J Physiol Gastrointest Liver Physiol 290: G36–G48, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid-binding protein gene-ablated female mice exhibit increased age-dependent obesity. J Nutr 138: 1859–1865, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty-acid-binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem J 391: 549–560, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin GG, Atshaves BP, McIntosh AL, Payne HR, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation enhances age-dependent weight gain in male mice. Mol Cell Biochem 324: 101–115, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J Biol Chem 278: 21429–21438, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Martin GG, Huang H, Atshaves BP, Binas B, Schroeder F. Ablation of the liver fatty acid binding protein gene decreases fatty acyl CoA binding capacity and alters fatty acyl CoA pool distribution in mouse liver. Biochemistry 42: 11520–11532, 2003. [DOI] [PubMed] [Google Scholar]
- 24.McIntosh AL, Atshaves BP, Landrock D, Landrock KK, Martin GG, Storey SM, Kier AB, Schroeder F. Liver fatty acid binding protein gene-ablation exacerbates weight gain in high-fat fed female mice. Lipids 48: 435–448, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulligan MK, Ponomarev I, Boehm SL 2nd Owen JA, Levin PS, Berman AE, Blednov YA, Crabbe JC, Williams RW, Miles MF, Bergeson SE. Alcohol trait and transcriptional genomic analysis of C57BL/6 substrains. Genes Brain Behav 7: 677–689, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Newberry EP, Kennedy SM, Xie Y, Luo J, Crooke RM, Graham MJ, Fu J, Piomelli D, Davidson NO. Decreased body weight and hepatic steatosis with altered fatty acid ethanolamide metabolism in aged L-Fabp−/− mice. J Lipid Res 53: 744–754, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newberry EP, Kennedy SM, Xie Y, Luo J, Davidson NO. Diet-induced alterations in intestinal and extrahepatic lipid metabolism in liver fatty acid binding protein knockout mice. Mol Cell Biochem 326: 79–86, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newberry EP, Kennedy SM, Xie Y, Sternard BT, Luo J, Davidson NO. Diet-induced obesity and hepatic steatosis in L-Fabp−/− mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am J Physiol Gastrointest Liver Physiol 294: G307–G314, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem 278: 51664–51672, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology 44: 1191–1205, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqi S, Saleem U, Abumrad NA, Davidson NO, Storch J, Siddiqi SA, Mansbach CM 2nd. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J Lipid Res 51: 1918–1928, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, Sorg T, Wong K, Bedu E, Cartwright EJ, Dacquin R, Djebali S, Estabel J, Graw J, Ingham NJ, Jackson IJ, Lengeling A, Mandillo S, Marvel J, Meziane H, Preitner F, Puk O, Roux M, Adams DJ, Atkins S, Ayadi A, Becker L, Blake A, Brooker D, Cater H, Champy MF, Combe R, Danecek P, di Fenza A, Gates H, Gerdin AK, Golini E, Hancock JM, Hans W, Holter SM, Hough T, Jurdic P, Keane TM, Morgan H, Muller W, Neff F, Nicholson G, Pasche B, Roberson LA, Rozman J, Sanderson M, Santos L, Selloum M, Shannon C, Southwell A, Tocchini-Valentini GP, Vancollie VE, Westerberg H, Wurst W, Zi M, Yalcin B, Ramirez-Solis R, Steel KP, Mallon AM, de Angelis MH, Herault Y, Brown SD. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol 14: R82, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smathers RL, Petersen DR. The human fatty acid-binding protein family: evolutionary divergences and functions. Hum Genomics 5: 170–191, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci 2: 11, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem 285: 32679–32683, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi R, Manku MS, Horrobin DF. Impaired platelet aggregation and thromboxane generation in essential fatty acid-deficient rats. J Nutr 117: 1520–1526, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Walker A, Pfitzner B, Neschen S, Kahle M, Harir M, Lucio M, Moritz F, Tziotis D, Witting M, Rothballer M, Engel M, Schmid M, Endesfelder D, Klingenspor M, Rattei T, Castell WZ, de Angelis MH, Hartmann A, Schmitt-Kopplin P. Distinct signatures of host-microbial meta-metabolome and gut microbiome in two C57BL/6 strains under high-fat diet. ISME J 8: 2380–2396, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werner A, Minich DM, Havinga R, Bloks V, Van Goor H, Kuipers F, Verkade HJ. Fat malabsorption in essential fatty acid-deficient mice is not due to impaired bile formation. Am J Physiol Gastrointest Liver Physiol 283: G900–G908, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Xie Y, Newberry EP, Kennedy SM, Luo J, Davidson NO. Increased susceptibility to diet-induced gallstones in liver fatty acid binding protein knockout mice. J Lipid Res 50: 977–987, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.