Abstract
Pulmonary fibrosis is a common and dose-limiting side-effect of ionizing radiation used to treat cancers of the thoracic region. Few effective therapies are available for this disease. Pulmonary fibrosis is characterized by an accumulation of myofibroblasts and excess deposition of extracellular matrix proteins. Although prior studies have reported that ionizing radiation induces fibroblast to myofibroblast differentiation and collagen production, the mechanism remains unclear. Transforming growth factor-β (TGF-β) is a key profibrotic cytokine that drives myofibroblast differentiation and extracellular matrix production. However, its activation and precise role in radiation-induced fibrosis are poorly understood. Recently, we reported that lactate activates latent TGF-β through a pH-dependent mechanism. Here, we wanted to test the hypothesis that ionizing radiation leads to excessive lactate production via expression of the enzyme lactate dehydrogenase-A (LDHA) to promote myofibroblast differentiation. We found that LDHA expression is increased in human and animal lung tissue exposed to ionizing radiation. We demonstrate that ionizing radiation induces LDHA, lactate production, and extracellular acidification in primary human lung fibroblasts in a dose-dependent manner. We also demonstrate that genetic and pharmacologic inhibition of LDHA protects against radiation-induced myofibroblast differentiation. Furthermore, LDHA inhibition protects from radiation-induced activation of TGF-β. We propose a profibrotic feed forward loop, in which radiation induces LDHA expression and lactate production, which can lead to further activation of TGF-β to drive the fibrotic process. These studies support the concept of LDHA as an important therapeutic target in radiation-induced pulmonary fibrosis.
Keywords: lactate, lactate dehydrogenase, myofibroblast, pulmonary fibrosis, ionizing radiation
pulmonary fibrosis is characterized by progressive and often irreversible accumulation of matrix proteins that lead to impairment of lung function. Radiation-induced lung injury can result in pulmonary fibrosis, which occurs in up to 20% of patients who receive thoracic radiation therapy (5, 23). The pathophysiology surrounding radiation-induced pulmonary fibrosis is poorly understood with few effective therapies available.
One of the hallmarks of pulmonary fibrosis, regardless of the cause, is the accumulation of excess extracellular matrix proteins such as collagen. These extracellular matrix proteins are primarily produced by scar-forming cells called myofibroblasts, which are differentiated lung fibroblasts that exhibit a myocyte-like phenotype. In normal wound healing, myofibroblasts produce extracellular matrix proteins that are essential for wound contracture. During the final phases of normal wound healing, myofibroblasts undergo apoptosis. However, during the pathologic process of fibrosis, myofibroblasts persist and contribute to prolonged matrix protein generation, deposition, and accumulation (26, 27), eventually leading to loss of normal tissue architecture and function.
Transforming growth factor-β (TGF-β) is a potent profibrotic cytokine that induces myofibroblast differentiation. Latent TGF-β exists in the extracellular matrix in the lung and must be activated by dissociation of the latency-associated peptide (LAP) to exhibit its biological functions. There are several known activation routes including proteolysis, mechanical stretch, integrin binding, and decreases in pH (1).
Recently, we reported that lactate activates TGF-β via a pH-dependent mechanism (17). Importantly, this activation occurs with physiologic levels of lactate and physiologically attainable pH. We found that lactate is increased in the lungs of patients with idiopathic pulmonary fibrosis (IPF) compared with healthy controls and induces myofibroblast differentiation in primary human lung fibroblast cultures (17). Furthermore, the enzyme responsible for lactate production, lactate dehydrogenase-A (LDHA), is increased in IPF lung tissue, and the expression of LDHA in primary human lung fibroblast cultures is regulated by TGF-β (17). Thus we have proposed a positive feed-forward loop in which TGF-β upregulates LDHA, leading to increased lactate production and further activation of TGF-β. Alternatively, this feed-forward loop could be initiated by increased production of lactate, leading to local or transient decreases in pH and activation of latent TGF-β in the extracellular matrix (ECM). This active TGF-β can then signal fibroblasts and myofibroblasts to differentiate, increase ECM production, and increase LDHA expression and lactate production. We hypothesize that this feed-forward loop leads to persistent myofibroblast differentiation thereby promoting the progression of fibrosis.
Although it is well documented that thoracic radiation induces collagen expression and causes late-stage fibrosis in the lung (9, 25, 31), few studies have explored the cellular mechanisms surrounding radiation-induced myofibroblast differentiation. TGF-β has been implicated in the development of radiation-induced fibrosis in several organs including the skin, lung, and liver (24, 30, 40) and TGF-β levels in the lung increase in the weeks following thoracic radiation (9, 31, 40). However, the mechanism of TGF-β activation in radiation-induced fibrosis remains poorly understood. Given our recent findings demonstrating the role of LDHA expression in IPF lung tissue, we sought to examine whether ionizing radiation regulates LDHA and if inhibition of LDHA may represent a novel therapeutic target in radiation-induced fibrosis. We report here that LDHA is upregulated in radiation-induced fibrosis lung tissue and in irradiated lung fibroblasts, that lactate is required for radiation-induced myofibroblast differentiation, and that inhibition of LDHA activity prevents radiation-induced myofibroblast differentiation in primary human lung fibroblast cultures. These studies implicate LDHA as a possible therapeutic target for radiation-induced pulmonary fibrosis.
MATERIALS AND METHODS
Human cell culture and tissue samples.
Primary human lung fibroblast strains were derived from tissue explants as previously described (8). All donors gave informed written consent. Tissue sections from patients with radiation-induced lung fibrosis or control (nonfibrotic) tissue sections were obtained from the Department of Pathology at the University of Rochester and were de-identified. All human subjects research was performed under the supervision of the University of Rochester Research Subjects Review Board.
Cell culture and irradiations.
Fibroblasts were cultured in Eagle's minimum essential media (Life Technologies, Gaithersburg, MD) supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2 mM l-glutamine, antibiotic, and antimycotic (GIBCO, Carlsbad, CA) at 37°C with 7% CO2. Cells were irradiated with a 137Cs γ-ray source at ∼2.70 cGy/min dose rate at indicated doses. Human recombinant TGF-β1 (R&D Systems, Minneapolis, MN) was used at 1 ng/ml. LDHA was genetically inhibited using Smart Pool ONTARGET siRNA or Smart Pool Non-targeting Control Pool (Thermo Scientific, Waltham, MA) and siImporter transfection reagent (Upstate Cell Signaling Solutions, Charlottesville, VA) according to the manufacturer's specifications. Cells were transfected 18 h before irradiation. TGF-β1 receptor 1 was inhibited using SB431542 (Sigma-Aldrich) at 2.5 μM added 30 min before irradiations, and then added daily on days 2–4. Cell viability was measured using Alamar Blue reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's specifications. LDHA was pharmacologically inhibited with Gossypol (Sigma-Aldrich) at indicated doses starting 1 h before irradiations.
TGF-β activity.
A TGF-β bioassay was performed as previously described (17). Briefly, Mv1Lu mink lung epithelial cells were cultured with conditioned media from irradiated human lung fibroblasts for 24 h before proliferation rates were measured using a 3H labeled thymidine incorporation assay. The inverse of proliferation rates are expressed as a fold change from 0-Gy controls.
TGF-β bioactivity was also measured using a previously described Smad-dependent TGF-β luciferase reporter containing 4 tandem smad binding elements (SBE) upstream of the minimal thymidine kinase (TK) promoter (38). Primary human lung fibroblasts were transfected with pSBEx4-TK-luc and a CMV-Renilla luciferase (Promega, Madison, WI) via electroporation with an Amaxa nucleofector. Transfected cells were plated and grown for 24 h before being irradiated or treated with TGF-β (5 ng/ml). After 36 h, cells were lysed with Dual-glow luciferase assay buffer (Promega) and luciferase was measured using a Varioskan luminescence plate reader (Thermo Scientific). Luciferase readings were normalized to nonirradiated controls.
Western blotting.
Cell lysates were run on SDS-PAGE under reducing conditions and probed for expression of α-smooth muscle actin (α-SMA; Sigma-Aldrich), LDHA (Abcam, Cambridge, MA). Glyceraldehyde 3-phosphate dehydrogenase (Abcam) was used as a loading control. Densitometry was performed as previously described (8).
Immunohistochemistry.
Paraffin-embedded lung tissue sections from de-identified patients with radiation-induced fibrosis or from C57BL/6 mice exposed to 5-Gy total body plus 10-Gy thoracic radiation as previously described (22) were stained for α-SMA (Sigma-Aldrich) and LDHA (Abcam) as previously described (17).
Immunofluorescence.
Primary human lung fibroblasts were irradiated in T-25 flasksand and then trypsinized the next day and subcultured for 48 h in glass chamber slides for immunofluorescence staining. Cells were then fixed in 4% paraformaldehyde and stained with an antibody to α-SMA (Sigma-Aldrich) followed by an anti-mouse AlexaFluor 568 (Invitrogen). Slides were mounted with Prolong Gold (Invitrogen) supplemented with DAPI to visualize nuclei and imaged using a Zeiss Axio Imager Z.I Microscope.
Quantitative real-time polymerase chain reaction.
RNA was isolated from primary human lung fibroblast and mouse lungs as previously described (8). Reverse transcription was performed using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Real-time PCR was performed using SsoAdvanced SYBR Green (Bio-Rad). The following primer sequences were used: human 18S: forward, GGTCGCTCGCTCCTCTCCCA; reverse, AGGGGCTGACCGGGTTGGTT; human Col1a1: forward, TTGAAGGAGGATGTTCCCATCT; reverse, ACAGACACATATTTGGCATGGTT; Col3a1: forward, TTGAAGGAGGATGTTCCCATCT; reverse, ACAGACACATATTTGGCATGGTT; mouse 18S: forward, GCTTGCTCGCGCTTCCTTACCT; reverse, TCACTGTACCGGCCGTGCGTA; and mouse LDHA: forward, TGGCGACTCCAGTGTGCCTG; reverse, AGGCACTGTCCACCACCTGCT.
Soluble collagen slot blot.
Soluble collagen was measured in cell supernatants as previously described (20). Briefly, 5 μl of cell supernatants were applied to a PVDF membrane using a vacuum manifold (Schleicher and Schuell, Keene, NH) under nondenaturing conditions. Membranes were probed using an antibody to collagen 1 (Santa Cruz Biotechnology, Dallas, TX).
Seahorse bioanalyzer extracellular acidification rate measurement.
Primary human lung fibroblasts were irradiated in T-25 flasks and then trypsinized the next day and subcultured into XF96 well plates at 5,000 cells per well (Seahorse Bioscience, North Billerica, MA). The extracellular acidification rate (ECAR) was measured using a Seahorse Bioscience XF96 Flux Analyzer according to company specifications. Briefly, ECAR was measured every 5 min for 1 h. Data was analyzed once cells equilibrated to calibration fluid and extracellular acidification rates reached a plateau (after ∼20 min).
Lactate measurements.
Lactate levels were measured in cell supernatants using a Nova BioProfile Automated Analyzer (Nova Biomedical, Waltham, MA) according to company specifications. Lactate levels are expressed as fold change from 0-Gy controls.
Statistical analysis.
All data are expressed as means ± SD. One-way ANOVA and t-test with Tukey's posttest were used to establish statistical significance using Graph Pad Prism software (La Jolla, CA). Results were considered significant if the P < 0.05.
RESULTS
LDHA expression is increased in radiation-induced pulmonary fibrosis.
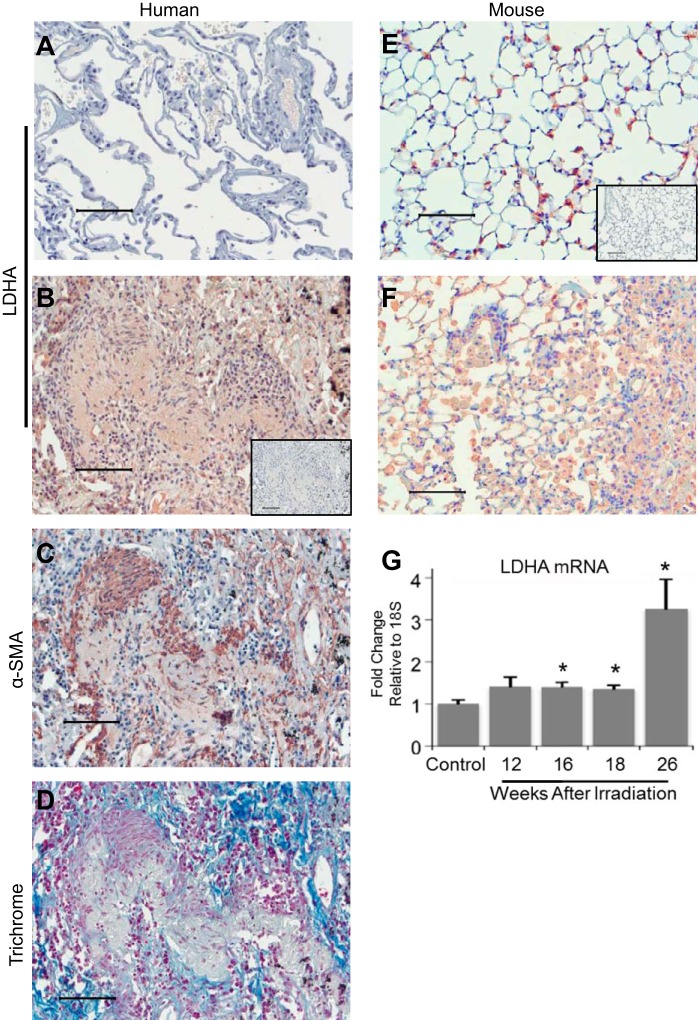
We previously reported that LDHA expression is increased in the lung tissue from patients with IPF (17). To investigate whether LDHA is also increased in radiation-induced pulmonary fibrosis, immunohistochemical staining for LDHA and α-SMA was performed on serial paraffin-embedded tissue sections from patients with radiation-induced pulmonary fibrosis and nonirradiated controls. (Fig. 1, A–C). Fibrotic lung tissue was also stained with Gomori's trichrome to visualize collagen deposition (Fig. 1D). Lung tissue from patients with radiation-induced pulmonary fibrosis had increased cellular content in the interstitium compared with healthy lung tissue (Fig. 1, B and C), with increased staining for α-SMA, a marker of myofibroblast differentiation. There was increased staining for LDHA (Fig. 1C) corresponding to areas of intense α-SMA expression (Fig. 1, B and C).
Fig. 1.
Lactate dehydrogenase A (LDHA) expression is increased in radiation-induced fibrotic lung tissue. Lung biopsies were obtained from patients who received thoracic radiation for cancer treatment and from nonirradiated controls, immunostained for α-smooth (α-SMA) or LDHA, and developed with Nova red. (A) Nonfibrotic lung sections were stained for LDHA. B and C: serial paraffin-embedded tissue sections from radiation-induced fibrotic lung tissue were stained for LDHA and α-SMA. D: radiation-induced fibrotic lung tissue was stained with Gomori trichrome. E–G: C57BL/6 mice were exposed to 5-Gy total-body plus 10-Gy thoracic radiation and were harvested at 12–26 wk postradiation. Paraffin-embedded lung tissue sections from control (E) and irradiated (F) mice at 26 wk postradiation were stained for LDHA. G: RNA was isolated from whole lung homogenates and mRNA levels of LDHA were measured by quantitative real-time PCR and normalized to GAPDH. Isotype controls for immunohistochemical staining are B and E, insets. Scale bars represent 100 μm. *P ≤ 0.05 by ANOVA compared with nonirradiated control; n = 3–8 mice per group.
LDHA expression was also increased in the lung tissue in C57BL/6 mice 26 wk after exposure to 5-Gy total-body plus 10-Gy thoracic irradiation (Fig. 1, E and F). In this model, pulmonary fibrosis is evident beginning at 20 wk postradiation and is progressive thereafter (22, 37). Nonirradiated control mice showed some LDHA staining, which was primarily localized in the alveolar epithelium (Fig. 1E). However, mice exposed to radiation had increased cellular content in the interstitium and increased LDHA staining localized to the interstitium compared with nonirradiated controls. LDHA mRNA expression was measured with quantitative real-time PCR (qRT-PCR) in total lung homogenates harvested between 12–26 wk postradiation. Compared with nonirradiated control mice, mice exposed to radiation had increased LDHA mRNA levels starting at 16 wk postradiation (Fig. 1G). By peak fibrosis at 26 wk, mice exposed to radiation had a threefold increase in LDHA mRNA levels compared with control mice (Fig. 1G).
Ionizing radiation induces LDHA expression and lactate production in primary human lung fibroblasts.
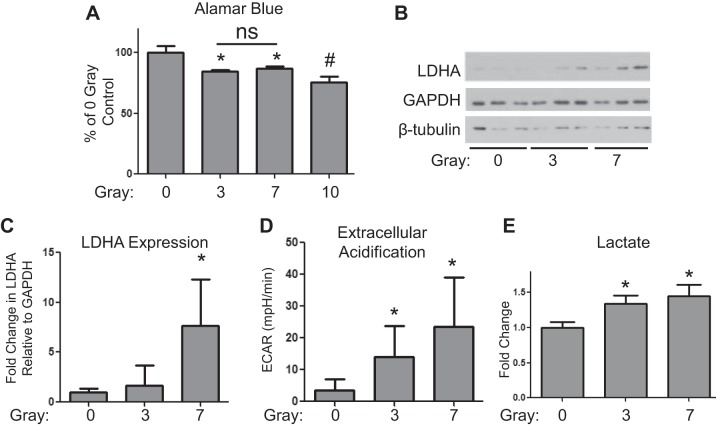
To investigate the mechanisms involved in radiation-induced pulmonary fibrosis, we next determined whether ionizing radiation induces the expression of LDHA in primary human lung fibroblast cultures. Lung fibroblasts were irradiated with 0, 3, 7, and 10 Gy of ionizing radiation, and viability was assessed after 5 days using the Alamar blue assay. Because 10 Gy resulted in a significant decrease in cell viability compared with 3 and 7 Gy (Fig. 2A), we used 3 and 7 Gy for our remaining experiments.
Fig. 2.
Ionizing radiation induces LDHA expression, lactate production, and extracellular acidification in lung fibroblasts. Primary human lung fibroblasts were exposed to 0, 3-, 7-, and 10-Gy radiation from a 137Cs γ-ray source and cell lysates, and supernatants were collected at 5 days postradiation. A: cell viability was measured using an Alamar blue assay of mitochondrial activity (n = 3). B: LDHA protein expression was analyzed by Western blot. One representative experiment is shown (n = 3). C: LDHA expression relative to GAPDH was determined by densitometry and normalized to 0-Gy control. D: extracellular acidification rates (ECAR) were measured using a Seahorse Bioscience XF96 Flux Analyzer (n = 10–12). E: lactate levels were measured in the supernatants using a Nova BioProfile Automated Analyzer (n = 3). *P ≤ 0.05, compared with 0-Gy control by ANOVA. #P ≤ 0.05, compared with 3 and 7 Gy; ns = no statistical significance.
Ionizing radiation induced LDHA expression in primary human lung fibroblasts in a dose-dependent manner, with a greater than fivefold induction at 7 Gy (Fig. 2, B and C). Importantly, radiation exposure also significantly increased the rate at which irradiated fibroblasts released acid into the media (ECAR). At least some of this extracellular acid release was in the form of lactate, as cell supernatants from irradiated fibroblasts contained significantly higher levels of lactate (Fig. 2E).
Ionizing radiation induces myofibroblast differentiation.
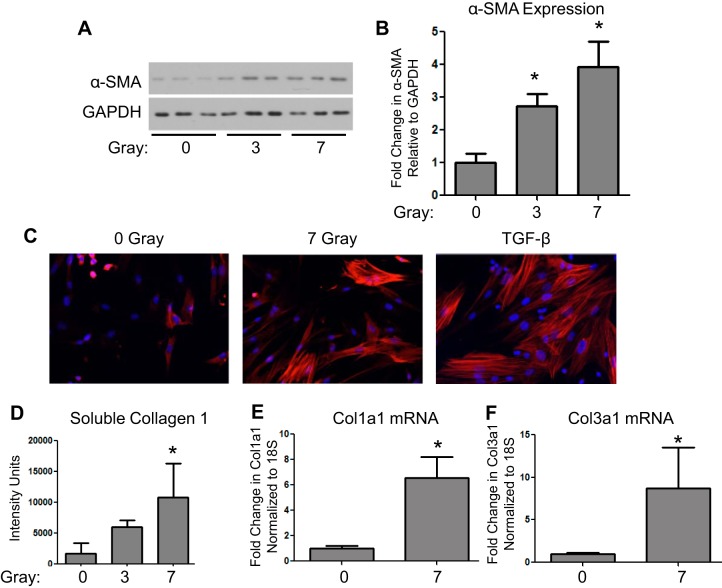
Irradiated lung tissue expresses high levels of α-SMA, a marker of myofibroblast differentiation (Fig. 1). To determine whether ionizing radiation induces myofibroblast differentiation, primary human lung fibroblasts were exposed to 3- and 7-Gy ionizing radiation and cultured for 5 days. Radiation induced α-SMA expression in a dose-dependent manner (Fig. 3, A and B). α-SMA was also analyzed using immunofluorescence staining. Seven-gray radiation caused a significant increase in actin fiber staining following radiation in a similar manner to the induction of α-SMA by TGF-β (Fig. 3C). In addition, 7 Gy induced extracellular soluble collagen 1 protein (Fig. 3D) and in collagen 1 and 3 mRNA expression (Fig. 3, E and F).
Fig. 3.
Ionizing radiation induces myofibroblast differentiation. Primary lung fibroblasts were exposed to 0, 3, and 7 Gy, and cell lysates and supernatants were collected at 5 days postradiation. A and B: α-SMA protein expression was analyzed by Western blot and densitometry. One representative experiment is shown (*P ≤ 0.05 by ANOVA compared with 0 Gy; n = 3). C: immunofluorescence staining for α-SMA (red) was performed on cell cultures exposed to 7-Gy radiation or transforming growth factor-β (TGF-β). Cell nuclei were stained with DAPI. D: Soluble collagen was measured in supernatants from irradiated cells cultures at 5 days postradiation using a Slot Blot Assay (*P ≤ 0.05 by ANOVA compared with 0 Gy; n = 3). E and F: total RNA was isolated from cells exposed to 7-Gy radiation at 3 days postradiation and quantitative real-time PCR was performed for Col1a1 and Col3a1 (*P ≤ 0.05 by t-test; n = 3).
Ionizing radiation activates TGF-β.
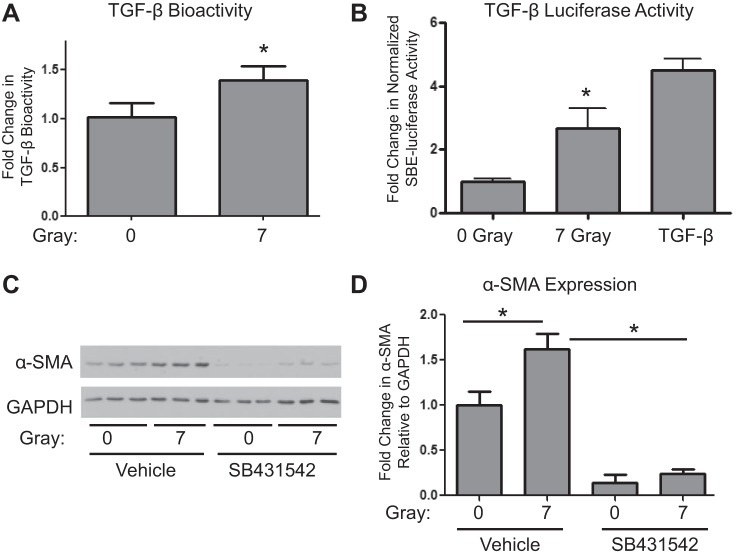
TGF-β has been reported to play an important role in radiation-induced tissue injury in multiple organs, including skin and lung. Furthermore, we have demonstrated that lactate can activate latent TGF-β in human lung fibroblast cultures. To test whether radiation leads to activation of latent TGF-β in human lung fibroblast cultures, a mink lung epithelial cell bioassay was performed with cell supernatants from control and irradiated cultures to determine levels of TGF-β bioactivity. Seven-gray radiation significantly increased TGF-β bioactivity in 5-day cell culture media from irradiated cells (Fig. 4A). Additionally, we transfected the lung fibroblasts with a highly specific Smad-dependent TGF-β reporter construct before irradiation, and determined lucerifase activity after 36 h. Irradiation caused a 2.5-fold increase in TGF-β luciferase activity compared with 0-Gy controls (Fig. 4B), and this was comparable to the effect of addition of exogenous active TGF-β. To demonstrate that myofibroblast differentiation is driven by this activation of TGF-β and not some other effect of ionizing radiation, we incubated fibroblasts with a specific TGF-β receptor 1 inhibitor SB431542 (15). SB431542 significantly attenuated radiation-induced α-SMA expression (Fig. 4, C and D), confirming that radiation-driven activation of latent TGF-β plays a key role in radiation-induced myofibroblast differentiation.
Fig. 4.
Ionizing radiation activates TGF-β. A: TGF-β bioactivity was measured using a mink lung cell proliferation assay. Mink lung cells were incubated with supernatants from primary human lung fibroblast cell cultures exposed to 0- or 7-Gy radiation harvested at day 5, and proliferation was measured using [3H]thymidine incorporation. Inverse of proliferation rates relative to 0-Gy controls were plotted (*P ≤ 0.05 by t-test, n = 3). B: lung fibroblasts were transfected with a Smad-dependent TGF-β luciferase reporter assay before irradiation or addition of TGF-β and harvested 36 h after treatment (n = 6). Fold changes in normalized luciferase activity from 0-Gy controls were plotted (*P ≤ 0.05 by t-test, n = 6). C and D: lung fibroblasts were treated daily with 2.5 μM TGF-β receptor 1 inhibitor (SB431542), starting 30 min before exposure to 0- or 7-Gy radiation. Cell lysates were collected 5 days postradiation and were analyzed for α-SMA protein expression levels by Western blot and densitometry (*P ≤ 0.05 by ANOVA; n = 3).
LDHA is required for radiation-driven myofibroblast differentiation.
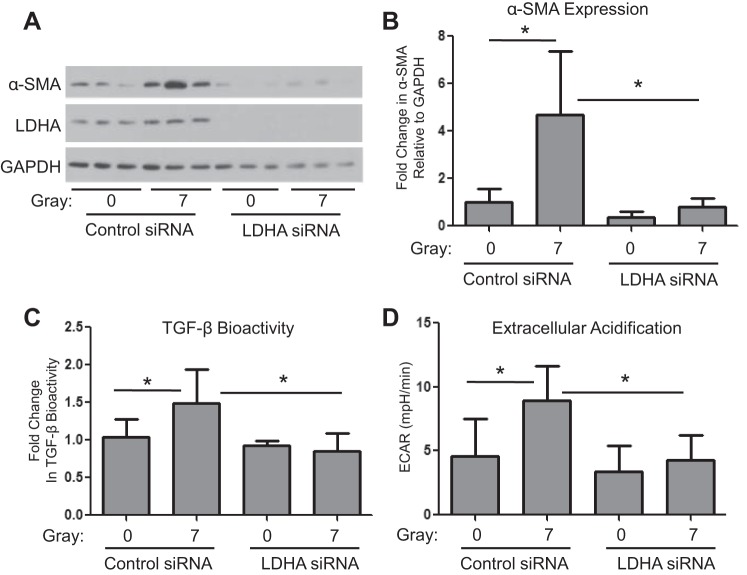
To determine whether increased lactate production is required for irradiation-driven myofibroblast differentiation, we used an siRNA approach to silence LDHA before irradiation. We achieved a high degree of LDHA knockdown (Fig. 5A). Ionizing radiation was unable to drive myofibroblast differentiation, as determined by α-SMA expression, in LDHA knockdown fibroblasts (Fig. 5, A and B). LDHA siRNA also prevented radiation-induced TGF-β activation (Fig. 5C). Compared with control siRNA transfected cells, cells transfected with LDHA siRNA before radiation produced less lactate (data not shown) and had lower ECAR than cells transfected with control siRNA (Fig. 5D).
Fig. 5.
LDHA is required for radiation-driven myofibroblast differentiation. Primary human lung fibroblasts were transfected with either an LDHA siRNA pool or a nontargeting control siRNA pool 18 h before exposure to 7 Gy irradiation. A and B: cell lysates were collected 5 days postradiation and were analyzed for LDHA and α-SMA protein expression levels by Western blot and densitometry. (n = 3). C: TGF-β bioactivity was measured in cell supernatants using a mink lung cell bioassay (n = 6). D: ECAR was measured using a Seahorse Bioscience XF96 Flux Analyzer (n = 20–22). *P ≤ 0.05 by ANOVA.
Gossypol, an LDH inhibitor, inhibits radiation-induced myofibroblast differentiation.
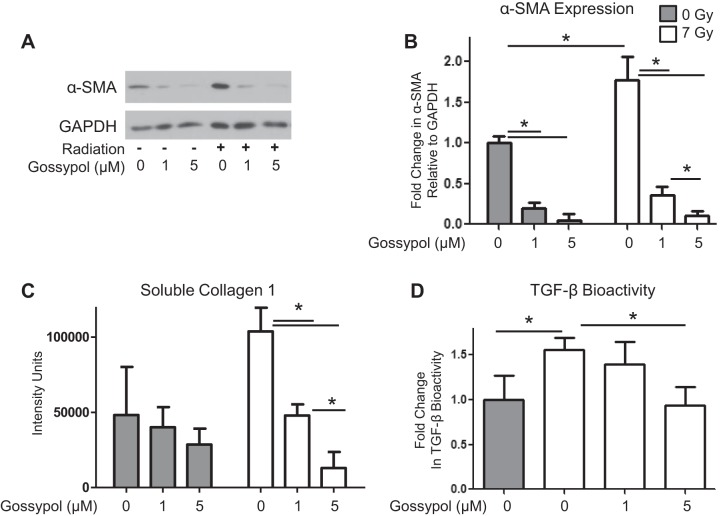
We next determined whether pharmacologic inhibition of LDHA would also prevent radiation-induced myofibroblast differentiation. Pretreatment of lung fibroblasts with Gossypol, an LDH inhibitor derived from cottonseed, before irradiation with 7 Gy strongly prevented radiation-induced myofibroblast differentiation in a dose-dependent manner (P ≤ 0.001; Fig. 6, A and B). Interestingly, Gossypol also reduced baseline levels in α-SMA expression (Fig. 6, A and B). Additionally, Gossypol inhibited radiation-induced collagen production (Fig. 6C) and radiation-induced TGF-β bioactivity (Fig. 6D).
Fig. 6.
Gossypol, an LDH inhibitor, inhibits radiation-induced myofibroblast differentiation. A and B: primary human lung fibroblasts were pretreated with Gossypol at 1 and 5 μM starting 1 h before irradiation with 0 or 7 Gy. Cell lysates were collected 5 days postradiation and were analyzed for α-SMA protein expression levels by Western blot and densitometry. One representative set of conditions is shown (n = 3). C: soluble collagen 1 was measured in culture supernatants using a Slot Blot Assay (n = 3). D: TGF-β bioactivity was measured in cell supernatants using a mink lung cell bioassay (n = 6). *P ≤ 0.05 by ANOVA.
DISCUSSION
We have previously reported that lactate is increased in lung tissue from patients with IPF and that lactate promotes fibroblast to myofibroblast differentiation via pH-dependent activation of latent TGF-β (17). Here, we show for the first time that lactate also plays a central role in radiation-induced pulmonary fibrosis. Ionizing radiation induces the expression of LDHA in lung fibroblasts, which leads to increased extracellular acidification, increased extracellular lactate, and increased TGF-β bioactivity (Figs. 2–4). Expression of LDHA is required for efficient TGF-β bioactivity and fibroblast to myofibroblast differentiation, as siRNA knockdown of LDHA expression largely ablates the profibrotic effect of irradiation (Fig. 5). LDHA expression is also strongly upregulated in human and mouse lung radiation fibrosis tissue (Fig. 1), supporting that this is a clinically important finding.
For our mechanistic studies, we used a single 7-Gy dose of radiation. This dose is consistent with what lung tissue could encounter during radiation therapy for primary or metastatic tumors. The recently initiated stereotactic body radiation therapy protocol uses high doses of radiation over several days (21). For example, patients with lung cancer could receive anywhere from 5 to 30 Gy in a single fractionated dose and up to 60 Gy total (6, 21, 32).
LDHA preferentially converts pyruvate to lactate and is upregulated during hypoxia when oxygen is in limited supply for oxidative phosphorylation (36). LDHA is also highly upregulated in tumor cells regardless of oxygen tension, a phenomenon termed the Warburg effect (14, 36). The Warburg effect describes a highly glycolytic phenotype with a high production of lactate, regardless of whether oxygen is available for oxidative phosphorylation. Here, we report characteristics consistent with the Warburg effect in primary human lung fibroblasts exposed to radiation, suggesting that similar changes in metabolic pathways may be seen in radiation-induced fibrosis and cancer.
Other metabolic similarities related to tumor growth are evident in pulmonary fibrosis, including uncontrolled cell proliferation and tissue invasion (35). Interestingly, consistent with highly metabolic tumors, patients with IPF have increased 18F-FDG metabolism in lung parenchyma when visualized with high-resolution positron emission tomography/computerized tomography, indicating that areas of fibrosis have increased glucose uptake (12). Increased glucose uptake can contribute to accelerated growth and proliferation, a characteristic that is also consistent with fibroblasts from fibrotic lung tissue (16, 29). Taken together with our results of increased LDHA expression in myofibroblasts in radiation-induced fibrosis (Fig. 1), we hypothesize that radiation causes changes in cellular metabolism in fibroblasts that is similar to changes seen in tumor cells to allow for increased proliferation to drive fibrosis.
Ionizing radiation drives activation of TGF-β through upregulation of LDH and lactate, and this active TGF-β in turn drives myofibroblast differentiation and collagen production. While the importance of myofibroblasts to lung fibrosis is well-understood, it is certainly possible that radiation-induced upregulation of lactate and TGF-β will upregulate profibrotic functions of fibroblasts without inducing myofibroblast differentiation. It should also be noted that while we have examined the direct effects of ionizing radiation on lung fibroblasts, it is possible that other lung cell types contribute to lactate production, extracellular acidification, and TGF-β activation in lung fibrosis. For example, we observed some epithelial LDHA staining in lung tissue from patients with radiation-induced pulmonary fibrosis (Fig. 1), as well as in IPF, sarcoidosis, and nonspecific interstitial pneumonia (17). Epithelial cells may upregulate LDHA and lactate production, which then exerts a profibrotic bystander effect on nearby fibroblasts. We plan to investigate other cellular sources of lactate during fibrogenesis in future studies.
We demonstrate here that ionizing radiation leads to increased TGF-β bioactivity (Fig. 4) and that silencing LDHA with siRNA dampens this bioactivity and prevents radiation-induced extracellular acidification (Fig. 5). We have previously shown that lactate activates TGF-β through a pH-dependent mechanism (17). It is interesting that even though irradiation strongly induces myofibroblast differentiation and collagen, there was only a relatively modest increase in active TGF-β in the culture medium at day 5 (Fig. 4A). It should be noted that the amount of active TGF-β in the medium at any given point is a balance among expression, activation, and uptake and may not reflect the total amount available to the cells over time. A second bioassay indicated that the amount of active TGF-β available to the cells during the first 36 h is comparable to addition of exogenous TGF-β (Fig. 4B).
It is well-established that radiation causes induction of TGF-β (3, 24, 40), although the mechanisms of radiation-induced TGF-β activation are poorly understood. One suggested mechanism is oxidation dependent activation through redox-related mechanisms (4). Activation through redox mechanisms would likely occur very quickly following radiation, given that reactive oxygen species induced by ionizing radiation are short lived (18). We hypothesize that radiation also causes TGF-β activation via extracellular acidification from increased lactate production, which would provide a mechanism for long-term and sustained TGF-β bioactivity during fibrosis. While our in vitro mechanistic studies were performed on a short time scale, we believe that lactate-induced acidification following radiation is a slow-rolling, feed-forward mechanism of TGF-β activation. Given that we have reported that TGF-β can induce expression of LDHA (17), we propose a positive feed-forward loop in which radiation causes extracellular acidification that activate latent TGF-β, which can further induce LDHA. Over time, this feed-forward cycle can result in amplification of fibrotic processes to give rise to tissue fibrosis. Therefore, it is critical to interrupt this fibrotic feed-forward loop when designing therapies for radiation-induced pulmonary fibrosis.
Inhibition of LDHA is an area of active research in the oncology field since LDHA overexpression is associated with cancer cell growth, poor prognosis and drug resistance (2, 7, 39, 42). Here, genetic knockdown of LDHA expression prevented radiation-induced myofibroblast differentiation (Fig. 5). However, therapeutic gene silencing in patients presents several technical hurdles. Gossypol, a potent LDHA inhibitor derived from cottonseed (11, 19) has been explored as a novel pharmaceutical therapy in breast cancer (34) and has been shown to have anti-tumorigenic effects in breast and colon cancer cells (10, 33, 34). Here, we show that Gossypol potently inhibits myofibroblast differentiation, collagen production, and TGF-β bioactivity with similar efficacy to genetic knockdown.
It should be noted that Gossypol may have other effects in addition to LDH inhibition including induction of apoptosis, modulation of cell cycle regulatory proteins and/or inhibition of other enzymatic activities (28). These so-called off-target effects may in fact be beneficial in the context of fibrosis. For example, resistance to apoptosis by myofibroblasts is hypothesized to contribute to the pathogenesis of fibrosis in vivo (13, 41). While Gossypol itself may not ultimately be adopted as an antifibrotic therapy, new and more specific LDHA inhibitors are under development. Our results are the first proof of principle that inhibition of LDHA activity may have therapeutic benefit in lung fibrosis.
GRANTS
This research was supported in part by National Institutes of Health Grants T32-ES-007026, T32-HL-066988, and U19-AI-091036, the Greg Chandler and Guy F. Solimano Pulmonary Fibrosis Research Fund, and the Davis Endowment. R. M. Kottmann was supported in part by a Parker B. Francis Fellowship. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.J., T.H.T., J.P.W., R.P.P., P.J.S., and R.M.K. conception and design of research; J.L.J., K.M.O., S.J.P., C.F.W., and R.M.K. performed experiments; J.L.J., S.J.P., C.F.W., and R.M.K. analyzed data; J.L.J., T.H.T., J.P.W., R.P.P., P.J.S., and R.M.K. interpreted results of experiments; J.L.J. and C.F.W. prepared figures; J.L.J. drafted manuscript; J.L.J., T.H.T., R.P.P., P.J.S., and R.M.K. edited and revised manuscript; J.L.J., C.F.W., R.P.P., P.J.S., and R.M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Eric Hernady and Jennie Miller for technical help with mouse radiation experiments and tissue collection, Amali Epa for help with collagen slot blot assay, Dr. Hsi-Min Hsiao for help with real-time PCR, Dr. Joshua Munger for use of the Nova BioProfile Analyzer, and Dr. Paul Brooks for use of the Seahorse Bioanalyzer.
REFERENCES
- 1.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci 116: 217–224, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett 358: 1–7, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest 93: 892–899, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10: 1077–1083, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration 71: 301–326, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, Timmerman R. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 75: 677–682, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9: 425–434, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson HE, Kulkarni A, Lehmann GM, Garcia-Bates TM, Thatcher TH, Huxlin KR, Phipps RP, Sime PJ. Electrophilic peroxisome proliferator-activated receptor-gamma ligands have potent antifibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol 41: 722–730, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein JN, Johnston CJ, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor β gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys 28: 621–631, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert NE, O'Reilly JE, Chang CJ, Lin YC, Brueggemeier RW. Antiproliferative activity of gossypol and gossypolone on human breast cancer cells. Life Sci 57: 61–67, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Granchi C, Bertini S, Macchia M, Minutolo F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr Med Chem 17: 672–697, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Groves AM, Win T, Screaton NJ, Berovic M, Endozo R, Booth H, Kayani I, Menezes LJ, Dickson JC, Ell PJ. Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. J Nucl Med 50: 538–545, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, Cui Z, Thannickal VJ. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem 279: 1359–1367, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 134: 703–707, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-B superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62–74: 65–74, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Jordana M, Schulman J, McSharry C, Irving LB, Newhouse MT, Jordana G, Gauldie J. Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. Am Rev Respir Dis 137: 579–584, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, Honnons S, Jones C, Isern NG, Hu JZ, Nathan SD, Grant G, Phipps RP, Sime PJ. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am J Respir Crit Care Med 186: 740–751, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res 61: 3894–3901, 2001. [PubMed] [Google Scholar]
- 19.Lee CY, Moon YS, Yuan JH, Chen AF. Enzyme inactivation and inhibition by Gossypol. Mol Cell Biochem 47: 65–70, 1982. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann GM, Xi X, Kulkarni AA, Olsen KC, Pollock SJ, Baglole CJ, Gupta S, Casey AE, Huxlin KR, Sime PJ, Feldon SE, Phipps RP. The aryl hydrocarbon receptor ligand ITE inhibits TGFbeta1-induced human myofibroblast differentiation. Am J Pathol 178: 1556–1567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, Teh BS, McGarry RC, Cardenes HR, Timmerman RD. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 7: 44–54, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Manning CM, Johnston CJ, Reed CK, Lawrence BP, Williams JP, Finkelstein JN. Lung irradiation increases mortality after influenza A virus challenge occurring late after exposure. Int J Radiat Oncol Biol Phys 86: 128–135, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks L, Yu X, Vujaskovic Z, Smalljr W, Folz R, Anscher M. Radiation-induced lung injury. Semin Radiat Oncol 13: 333–345, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Martin M, Lefaix JL, Delanian S. TGF-β1 and radiation fibrosis: a master switch and the specific therapeutic target? Int J Radiat Oncol Biol Phys 47: 277–290, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 63: 5–24, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol 29: 5–17, 1997. [DOI] [PubMed] [Google Scholar]
- 27.O'Kane S, Ferguson MW. Transforming growth factor βs and wound healing. Int J Biochem Cell Biol 29: 63–78, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE. (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-XL-mediated apoptosis resistance. Mol Cancer Ther 4: 23–31, 2005. [PubMed] [Google Scholar]
- 29.Raghu G, Chen Y, Rusch V, Rabinovitch PS. Differential proliferation of fibroblasts cultured from normal and fibrotic human lungs. Am Rev Respir Dis 138: 703–708, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Rübe CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, Willich N, Rüce C. Dose-dependent induction of transforming growth factor β (TGF-β) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys 47: 1033–1042, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys 33: 99–109, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Timmerman RD, Paulus R, Galvin J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, Fowler J, Gore E, Choy H. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuszynski GP, Cossu G. Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Res 44: 768–771, 1984. [PubMed] [Google Scholar]
- 34.Van Poznak C, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van Zee K, Borgen P, Gollub M, Bacotti D, Yao TJ, Bloch R, Ligueros M, Sonenberg M, Norton L, Hudis C. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat 66: 239–248, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J 35: 496–504, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason KA, Medhora MM, Moulder JE, Okunieff P, Otterson MF, Robbins ME, Smathers JB, McBride WH. Animal models for medical countermeasures to radiation exposure. Radiat Res 173: 557–578, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woeller CF, O'Loughlin CW, Roztocil E, Feldon SE, Phipps RP. Salinomycin and other polyether ionophores are a new class of antiscarring agent. J Biol Chem 290: 3563–3575, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P, Signoretti S, Billiard J, Duffy KJ, Grant A, Wang X, Lorkiewicz PK, Schatzman S, Bousamra M, Lane AN 2nd, Higashi RM, Fan TW, Pandolfi PP, Sukhatme VP, Seth P. Targeting lactate dehydrogenase-a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab 19: 795–809, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi ES, Bedoya A, Lee H, Chin E, Saunders W, Kim SJ, Danielpour D, Remick DG, Yin S, Ulich TR. Radiation-induced lung injury in vivo: Expression of transforming growth factor-beta precedes fibrosis. Inflammation 20: 339–352, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida K, Kuwano K, Hagimoto N, Watanabe K, Matsuba T, Fujita M, Inoshima I, Hara N. MAP kinase activation and apoptosis in lung tissues from patients with idiopathic pulmonary fibrosis. J Pathol 198: 388–396, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis 4: e532, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]