Abstract
The NLRC4 inflammasome is responsible for IL-1β processing by macrophages in response to Pseudomonas aeruginosa infection. We therefore hypothesized that mice that lack ASC, an NLRC4 inflammasome adaptor protein necessary for in vitro IL-1β production by macrophages, would be preferentially protected from a hyperinflammatory lethal challenge that is dependent on bacterial type three secretion system (T3SS) activity. We report herein that lack of ASC does not confer preferential protection in response to P. aeruginosa acute infection and that ASC−/− mice are capable of producing robust amounts of IL-1β comparable with C57BL/6 mice. We now identify that neutrophils represent the ASC-independent source of IL-1β production during the acute phases of infection both in models of acute pneumonia and peritonitis. Consequently, depletion of neutrophils in ASC−/− mice leads to a marked deficit in IL-1β production in vivo. The pulmonary neutrophil IL-1β response is predominantly dependent on caspase-1, which contrasts with data derived from ocular infection. These studies therefore identify a noncanonical mechanism of IL-1β production by neutrophils independent of ASC and demonstrate the first physiological contribution of neutrophils as an important source of IL-1β in response to acute P. aeruginosa infection during acute pneumonia and peritonitis.
Keywords: neutrophils, interleukin-1β, ASC, Pseudomonas aeruginosa, inflammation
pseudomonas aeruginosa is a gram-negative bacterium that causes acute nosocomial infections as well as chronic infections in immunocompromised individuals. P. aeruginosa is a common cause of pneumonia, sepsis, urinary tract infections, and surgical site infections (41). In cystic fibrosis patients, P. aeruginosa causes chronic pulmonary infections leading to progressive pulmonary damage (19, 23).
IL-1β is an early proinflammatory cytokine produced in response to infection with P. aeruginosa (23, 31, 43, 45). IL-1β leads to immune cell recruitment and subsequent amplification of cytokine and chemokine responses leading to eventual bacterial clearance. The murine data support the proposed clinical role of the IL-1 pathway, in that regulated IL-1R signaling is important for clearance of P. aeruginosa but sustained signaling through IL-1R contributes to inflammation-driven host pathology (12, 13, 39). The IL-1β response to P. aeruginosa is induced by the NLRC4 (nucleotide-binding domain, leucine-rich repeat-containing family, caspase-associated recruitment domain 4) inflammasome (16, 28, 43). In macrophages, believed to be the predominant in vivo cellular source of IL-1β (16, 28, 29, 43, 45), this inflammasome complex contains NLRC4, ASC (apoptosis associated speck-like protein containing a caspase recruitment domain), and caspase-1 (5, 25). The NLRC4 inflammasome is activated in response to bacterial type-three secretion system (T3SS)-driven injection of effector ligands into the host cell cytosol (2, 16, 28, 30, 43). This leads to pro-IL-1β processing and release as mature IL-1β. Loss of a functional bacterial T3SS or any of the NLRC4 inflammasome components, namely NLRC4, caspase-1, and ASC, leads to a deficit in IL-1β production by macrophages in response to P. aeruginosa (16, 28, 35, 43).
Since loss of ASC leads to a deficit in IL-1β release from macrophages in vitro (5, 6, 16, 35, 43), we hypothesized that ASC−/− mice would exhibit reduced IL-1β production and inflammation-driven pathology in vivo, resulting in reduced mortality. Here we report that loss of ASC expression in vivo does not phenocopy the effects previously derived in vitro with macrophages. Surprisingly, we observed no deficit for IL-1β production in ASC−/− mice in vivo during acute P. aeruginosa infection. We resolve this discrepancy with the identification of a noncanonical mechanism for IL-1β production in vivo in response to acute P. aeruginosa infection. We demonstrate that neutrophils, which are recruited to the site of infection, have a differential ASC requirement from macrophages for IL-1β release and contribute to IL-1β production in vivo. Neutrophils are responsible for the ASC-independent but caspase-1-dependent IL-1β production to P. aeruginosa, which contrasts with previously published data on neutrophils and P. aeruginosa (22). Importantly, IL-1β production by neutrophils is sufficient to overcome loss of ASC expression by macrophages during acute infection with P. aeruginosa and depletion of neutrophils leads to reduced in vivo IL-1β production in the absence of ASC. Our results reveal a novel mechanism by which neutrophils function as a critical source of IL-1β and have broad relevance to IL-1β-driven immunity and pathology in response to other pathogens.
MATERIALS AND METHODS
Mice.
Age-matched mice on the C57BL/6 background were used for all experiments. C57BL/6 WT mice were obtained from the National Cancer Institute (Bethesda, MD) or Charles River Laboratories (Frederick, MD). ASC−/−, NLRC4−/−, and NLRP3−/− mice have been described previously (25, 26) and were obtained from Dr. V. Dixit (Genentech, CA). Caspase-1−/− and neutrophil elastase (NE)−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME).
Ethics statement.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council (33). Animal use was approved by the Dartmouth Institutional Animal Care and Use Committee (Protocol berw.bl.2). Procurement and use of human cells were approved by the Dartmouth Committee for the Protection of Human Subjects (CPHS; Protocol 21738). Samples were obtained from adult volunteers with informed consent.
Materials.
The mouse IL-1β DuoSet ELISA kit was purchased from R&D Systems (Minneapolis, MN). The EasySep mouse neutrophil enrichment kit for negative selection of neutrophils was purchased from Stemcell Technologies (Vancouver, BC, Canada). The mouse neutrophil isolation kit for positive selection of neutrophils was purchased from Miltenyi Biotec (Auburn, CA). PE-conjugated anti-mouse pro-IL-1β (clone NJTEN3), PE-conjugated anti-mouse CD11b (clone M1/70), and PerCP Cy5.5-conjugated anti-mouse CD45 (clone 30-F11) were purchased from eBioscience (San Diego, CA). FITC-conjugated anti-mouse F4/80 (clone BM8), FITC-conjugated anti-mouse CD11c (clone N418), PE-conjugated anti-mouse Gr1 (clone RB6-8C5), and APC-conjugated anti-mouse Ly6G (clone 1A8) were purchased from BioLegend (San Diego, CA). Polyclonal anti-mouse IL-1β (AF-401-NA and GTX74034) was purchased from R&D Systems and GeneTex (Irvine, CA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Z-VAD-fmk was purchased from Invivogen (San Diego, CA). 3,4-Dichloroisocoumarin (DCIC) was purchased from Sigma-Aldrich (St. Louis, MO). Propidium iodide was purchased from MP Biomedicals (Solon, OH). FITC-conjugated anti-human CD66b (clone 80H3) was purchased from Beckman Coulter (Marseille, France). Rat anti-mouse Ly6G (clone 1A8) used for neutrophil depletion and rat IgG2a isotype control (clone 2A3) were purchased from BioXCell (West Lebanon, NH). Endotoxin-free Ficoll-Paque Plus and Dextran T500 were purchased from Amersham Biosciences/GE (Pittsburgh, PA).
Cells.
Bone marrow-derived dendritic cells (BMDC) were cultured using a modification of the protocol of Inaba et al. (21) as previously described (1). Six- to seven-day-old BMDC were used for infection. For isolation of murine macrophages, naïve mice were intraperitoneally injected with 1 ml of 4% thioglycollate solution. Macrophages were harvested by peritoneal lavage 4–5 days later. Following erythrocyte lysis and being washed with PBS, cells were resuspended in RPMI 1640 containing 10% FBS. For isolation of murine neutrophils, naïve mice were intraperitoneally injected with 1 ml of 4% thioglycollate solution. Peritoneal cells were harvested by peritoneal lavage 12–18 h later and neutrophils were enriched using negative selection (described above) for most neutrophil experiments or positive selection (described above) per manufacturer's instructions. Enriched neutrophils were resuspended in RPMI 1640 containing 10% FBS or DMEM. Purity of the negative-selection enriched neutrophils was confirmed by surface-staining for CD11b+Ly6G+ cells.
Human cells.
Peripheral blood was collected from healthy volunteers by venipuncture into heparinized tubes. Neutrophils were isolated as previously described (38) with some modifications. Briefly, fresh blood was diluted with PBS, overlaid on Ficoll-Paque Plus and centrifuged for 30 min at 400 g. After all layers above the erythrocytes were removed, 3 ml of 3% Dextran T500 in PBS and 15 ml of 37°C PBS were added. Erythrocytes were sedimented at 37°C for 45 min. The buffy coat was centrifuged at 448 g for 5 min to pellet the neutrophils. Residual erythrocytes were lysed and washed with excess PBS. Neutrophils were resuspended in RPMI 1640 containing 10% FBS. Purity of the isolated neutrophils was confirmed by surface staining for CD11b+CD66b+ cells and by microscopy.
Bacteria.
P. aeruginosa strains on the PA14 background and wild-type (WT) Salmonella enterica serovar Typhimurium strain LT2 were provided by Drs. G. O'Toole and D. Hogan (Dartmouth Medical School, Hanover, NH). The popB mutant has been previously described (35).
In vivo bacterial infection.
For lethal challenge experiments, mice were infected intratracheally or intraperitoneally with subcultured and washed 5 × 106 colony-forming units (CFU) of WT or popB mutant PA14 bacteria. For pulmonary sublethal infection, mice were infected intratracheally with subcultured and washed 3 × 106 CFU of WT PA14 bacteria in a total volume of 50 μl and euthanized 4 h postinjection (hpi), and bronchoalveolar lavage (BAL) fluid was collected using 800 μl of 5 mM EDTA in PBS. Cell-free BAL fluid was used for analyses of IL-1β production. For analysis of infiltrating cells, BAL cells were pelletted by centrifugation at 311 g for 5 min and surface stained with the indicated antibodies after Fc receptor block with monoclonal antibody 2.4G2. The cells were washed, fixed, and permeablized to intracellularly stain with PE-conjugated anti-mouse pro-IL-1β. Fluorescence-minus-one controls were used for flow cytometry gating and BAL cells were surface stained with the indicated antibodies and intracellularly stained with PE-conjugated anti-mouse pro-IL-1β as described above.
For intraperitoneal sublethal infection, mice were injected with 3 × 106 CFU of WT PA14 and euthanized 4 hpi, and peritoneal lavage fluid was collected using 1.5 ml PBS. Blood samples were collected from the inferior vena cava. Serum and cell-free peritoneal lavage samples were analyzed for IL-1β by ELISA. For analysis of infiltrating cells, peritoneal lavage cells were pelletted by centrifugation at 311 g for 5 min and surface stained with the indicated antibodies after Fc receptor block with monoclonal antibody 2.4G2 and intracellularly stained with PE-conjugated anti-mouse pro-IL-1β as described above. Fluorescence-minus-one controls were used for flow cytometry gating.
In vivo neutrophil depletion.
Naive mice were injected with 200 μg/mouse of anti-Ly6G or rat IgG2a isotype control both 3 days and 1 day before infection. Mice were then injected intraperitoneally with 3 × 106 CFU of WT PA14 and euthanized 4 hpi, and peritoneal lavage fluid was collected using 1.5 ml PBS. Blood was collected from the inferior vena cava. Cell-free peritoneal lavage fluid and serum were analyzed for IL-1β production. Recovered peritoneal CFU were determined by plating 10-fold serial dilutions of the peritoneal lavage fluid.
In vitro bacterial infection.
Peritoneal macrophages or BMDC or neutrophils were harvested, washed, and resuspended in RPMI 1640 containing 10% FBS. A total of 2.5 × 105 cells were aliquoted/well in a 24-well plate. Subcultured and washed bacteria of the indicated genotype of PA14 or Salmonella enterica serovar Typhimurium were added at the indicated multiplicity of infection (MOI). Bacteria were coincubated for 3 h at 37°C in 5% CO2. Cell-free supernatants were analyzed for IL-1β production by ELISA. For DCIC and Z-VAD-fmk inhibition experiments, cells were treated with either 20 μM of DCIC, 20 μM of Z-VAD-fmk, or an equal volume of DMSO control as indicated for 1 h at 37°C in 5% CO2 before infection with bacteria. For cell death analyses, enriched C57BL/6 neutrophils were treated with either 20 μM Z-VAD-fmk or an equal volume of DMSO for 1 h at 37°C in 5% CO2 before infection with bacteria for 3 h. Cells were then scraped, washed twice, stained with propidium iodide, and analyzed by flow cytometry. Bacteria were gated out based on scatter controls, and fluorescence-minus-one controls were used to determine gating for propidium iodide-positive cells.
Ex vivo bacterial infection.
C57BL/6 mice were injected with 1 ml thioglycollate/mouse, and peritoneal cells were harvested at 4 hpi or 3 days postinjection by peritoneal lavage. Uninjected C57BL/6 mice were used to collect resident peritoneal cells at 0 h. A total of 2.5 × 105 cells were aliquoted per well in a 24-well plate. Subcultured and washed WT or popB mutant PA14 bacteria were added at MOI of 0.2. Bacteria were coincubated for 3 h at 37°C in 5% CO2. Cells were washed and surface-stained with FITC-conjugated anti-mouse F4/80 and APC-conjugated anti-mouse Ly6G after Fc receptor block with monoclonal antibody 2.4G2 and intracellularly stained with PE-conjugated anti-mouse pro-IL-1β as described above.
Immunoblotting.
For in vitro immunoblotting, enriched neutrophils were resuspended in serum-free DMEM. A total of 2 × 106 cells were aliquoted/well in a 12-well plate. Subcultured and washed bacteria were added at the indicated MOI. Bacteria were coincubated for 5 h at 37°C in 5% CO2. Cell-free supernatants were precipitated with methanol. Proteins were separated by electrophoresis on a 15% polyacrylamide gel. For the DCIC and Z-VAD inhibitor experiment, cells were treated with 20 μM DCIC or 20 μM Z-VAD for 1 h at 37°C in 5% CO2 before infection with bacteria. ImageJ (version 10.2) was used to quantify immunoblots from the DCIC and Z-VAD inhibitor experiment. Data are representative of at least two independent experiments.
For in vivo immunoblotting of peritoneal lavage, 500 μl of peritoneal lavage from infected C57BL/6 or ASC−/− mice were treated with methanol to precipitate the proteins. Proteins were separated by electrophoresis on a 15% polyacrylamide gel. Data are representative of multiple independent biological experiments. For immunoblotting of peritoneal lavage cell lysates, peritoneal lavage was collected as described above using 1.5 ml PBS/mouse from infected C57BL/6 or caspase-1−/− mice. Peritoneal lavage cells were pelletted by centrifugation at 311 g for 5 min and used for preparing whole cell lysates. Proteins were separated by electrophoresis on a 15% polyacrylamide gel. Data are representative of three independent biological experiments. For IL-1β detection, blots were probed with either anti-mouse IL-1β (AF-401-NA) as shown or with anti-mouse IL-1β (GTX74034) as confirmation (data not shown).
Statistical analyses.
Means ± SD are shown for each graph, derived from multiple independent biological experiments. Unless otherwise specified in the figure legend, unpaired Student's t-test with Welch's correction was performed to analyze statistical significance within the different groups using Prism 4.0a (GraphPad Software,). Statistical significance is represented in figures by asterisks.
RESULTS
In vitro vs. in vivo outcomes reveal a discrepancy for the requirement of ASC expression to IL-1β production in response to P. aeruginosa infection.
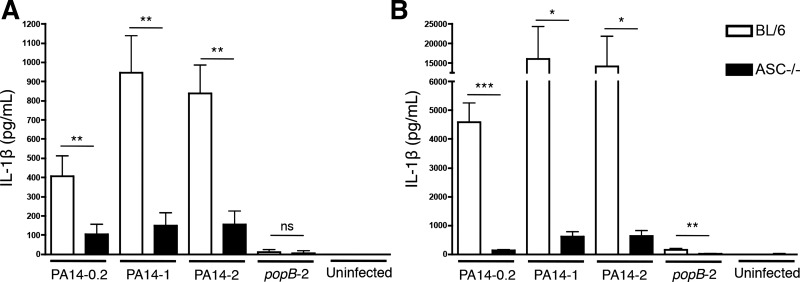
Macrophages are considered to be the predominant source of IL-1β during the early phases of P. aeruginosa infection (16, 28, 43, 45). Consistent with previous studies on the NLRC4 inflammasome (6, 16, 28, 35, 43), we found that both murine macrophages (Fig. 1A) and BMDC (Fig. 1B) required ASC expression for robust in vitro IL-1β production in response to PA14 infection. This response was dose dependent and required bacterial T3SS function (Fig. 1, A and B). We therefore hypothesized that ASC-knockout mice may be protected from hyper-inflammatory IL-1β responses following an acute P. aeruginosa infection. In support of this were the previous observations that exacerbated or sustained IL-1 responses to P. aeruginosa generate immunopathology and even IL-1β-associated lethality (12, 39), while loss or blockade of the IL-1 pathway in similar circumstances is protective. Accordingly, we and others have found in established murine models of pneumonia and peritonitis (35, 43, 45) that mice challenged with popB bacteria, which are deficient in T3SS activity requisite for inflammasome activation, do not elicit IL-1β responses (35, 45).
Fig. 1.
ASC expression is required for IL-1β production by macrophages and dendritic cells in response to Pseudomonas aeruginosa infection. Peritoneal macrophages (A) or bone marrow-derived dendritic cells (BMDCs; B) from C57BL/6 or ASC−/− mice were infected with PA14 [wild-type (WT)] or with the popB mutant of PA14 at the indicated multiplicity of infection (MOI). Culture supernatants were collected 3 h postinfection and analyzed by ELISA for IL-1β production. Data are expressed as means ± SD accumulated from 2 independent biological experiments (n = 4 for all samples except uninfected; n = 3). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005.
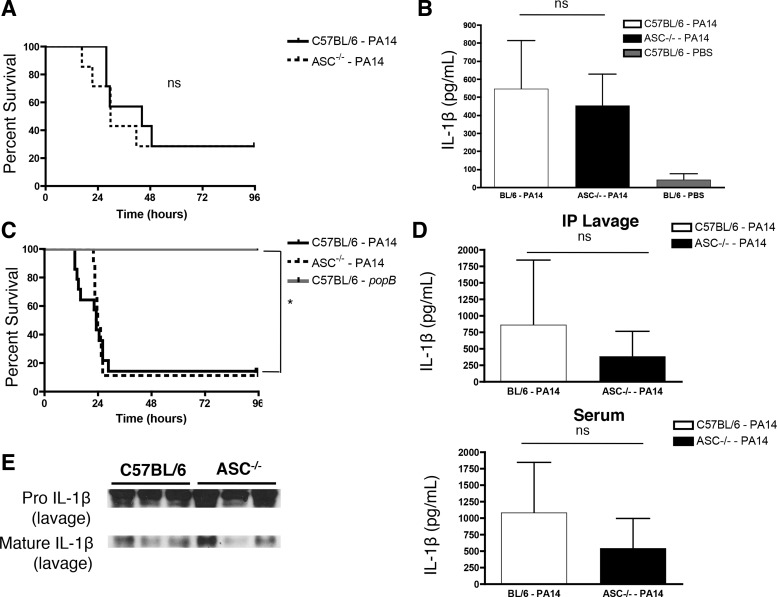
When C57BL/6 mice and ASC−/− mice were given intratracheal challenges with WT PA14, ASC−/− mice unexpectedly did not preferentially survive compared with C57BL/6 mice (Fig. 2A). To resolve this surprising outcome, we tested if in vivo loss of ASC recapitulated our in vitro data (Fig. 1) and led to reduced IL-1β production. Strikingly, ASC−/− mice given an intratracheal infection with a sublethal dose of WT PA14 displayed no marked deficit in pulmonary IL-1β production in vivo at 4 hpi by ELISA (Fig. 2B), which was dependent on the instillation of WT PA14. Consistent with our in vivo pulmonary data, a model of acute peritonitis recapitulated these data, in which we observed that ASC−/− mice challenged with WT PA14 did not preferentially survive compared with C57BL/6 mice (Fig. 2C). However, bacterial T3SS activity was required for inducing lethality, as mice challenged with the T3SS-deficient popB mutant of PA14 survived (Fig. 2C). ASC−/− mice injected intraperitoneally with a sublethal dose of WT PA14 displayed no marked deficit in IL-1β production in vivo at 4 hpi by both ELISA (Fig. 2D) and by Western analyses (cleaved IL-1β shown in Fig. 2E). Our in vivo data using models of acute pneumonia and peritonitis are in contrast to the in vitro observations presented in Fig. 1. This comparable in vivo IL-1β release by C57BL/6 and ASC−/− mice (Fig. 2) prompted us to ascertain the ASC-independent source of IL-1β.
Fig. 2.
ASC−/− mice are not protected from P. aeruginosa lethal challenge and produce comparable levels of elicited in vivo IL-1β. A: C57BL/6 and ASC−/− (n = 7/group) were infected intratracheally with 5 × 106 colony-forming units (CFU) of WT PA14 and survival was monitored up to 96 h postinfection. Survival curves were compared using the log-rank test. B: C57BL/6 and ASC−/− (n = 7/group) mice were infected intratracheally with a sublethal dose (3 × 106 CFU) of WT PA14. C57BL/6 mice instilled with PBS (n = 5) were used as a control. Mice were euthanized 4 h postinfection and bronchoalveolar lavage (BAL) samples were collected. BAL samples were analyzed by ELISA for IL-1β production. C: C57BL/6 (n = 14) and ASC−/− (n = 9) mice were infected intraperitoneally with 5 × 106 CFU of WT PA14 and survival was monitored up to 96 h postinfection. C57BL/6 mice were challenged with 5 × 106 CFU of popB mutant PA14 (n = 8) as a control. Survival curves were compared using the log-rank test. D: C57BL/6 and ASC−/− mice were infected intraperitoneally (IP) with a sublethal dose (3 × 106 CFU) of WT PA14. Mice were euthanized 4 h postinfection and peritoneal lavage and blood samples were collected. Peritoneal lavage (top; n = 15/group) and blood serum (bottom; n = 11/group) samples were analyzed by ELISA for IL-1β production. Data are expressed as means ± SD. E: representative Western blot for pro- and the biologically active cleaved mature IL-1β released in the peritoneum at 4 h postinfection. Results are representative of, or accumulated from, 2–5 independent experiments.
Temporally recruited peritoneal leukocytes upregulate pro-IL-1β production upon infection.
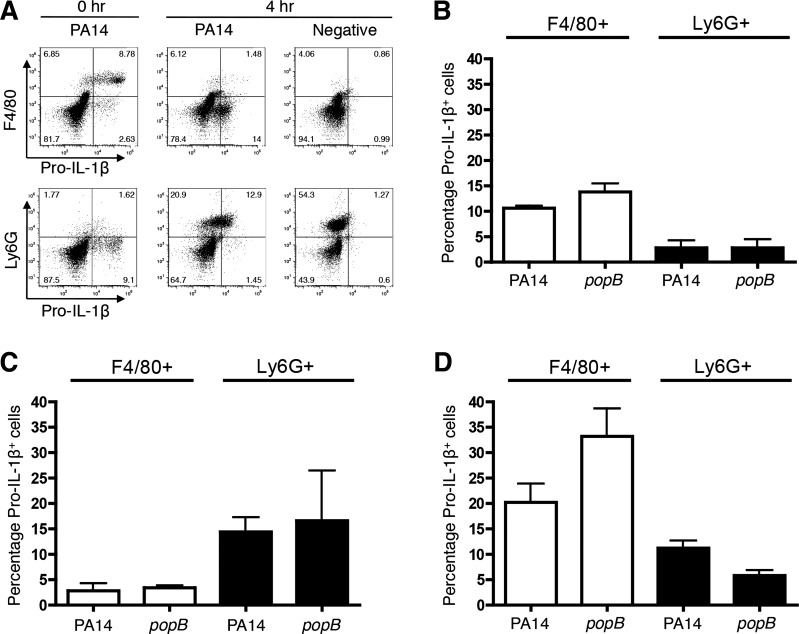
To assess the temporal contributions of IL-1β by recruited cells, we implemented an ex vivo system of analyses. C57BL/6 mice were injected with thioglycollate intraperitoneally and recruited cells isolated at temporal intervals were infected with P. aeruginosa ex vivo; thioglycollate injection alone does not lead to pro-IL-1β upregulation (Fig. 3A and data not shown). We found that resident F4/80+ macrophages were the predominant source of pro-IL-1β in naïve mice (Fig. 3, A and B). Upon thioglycollate injection, Ly6G+ neutrophils were rapidly recruited to the peritoneum (Fig. 3A) and were the predominant source of pro-IL-1β upon infection rather than F4/80+ macrophages (Fig. 3, A and C). At 3 days postthioglycollate injection, the majority of the cells that were pro-IL-1β+ were F4/80+ macrophages (Fig. 3D). These data indicate that, following an initial stimulus, both recruited neutrophils and subsequent macrophages upregulate pro-IL-1β expression and likely contribute to the IL-1β response.
Fig. 3.
Macrophages and neutrophils temporally recruited to the peritoneum produce pro-IL-1β upon ex vivo infection. C57BL/6 mice were injected with thioglycollate and peritoneal cells were collected by lavage at 0 h (naïve uninjected), 4 h, or 3 days. The harvested cells were infected with WT or popB PA14 at MOI = 0.2 and analyzed 3 h postinfection. Bacteria were excluded from analyses by gating based on scatter controls, and the percentage of pro-IL-1β+F4/80+ and pro-IL-1β+Ly6G+ cells was determined within the populations of peritoneal cells. A: representative dot plot for pro-IL-1β+ cells at 0 or 4 h. B–D: percentage of pro-IL-1β+F4/80+ and pro-IL-1β+Ly6G+ peritoneal cells from 0 h (B), 4 h (C), or 3 days (D) upon ex vivo infection. Data are expressed as means ± SD. Results are representative A, or compiled from independent biological experiments (A–D; n = 3).
Neutrophils are the predominant population of pro-IL-1β+ cells upon acute pulmonary challenge in vivo.
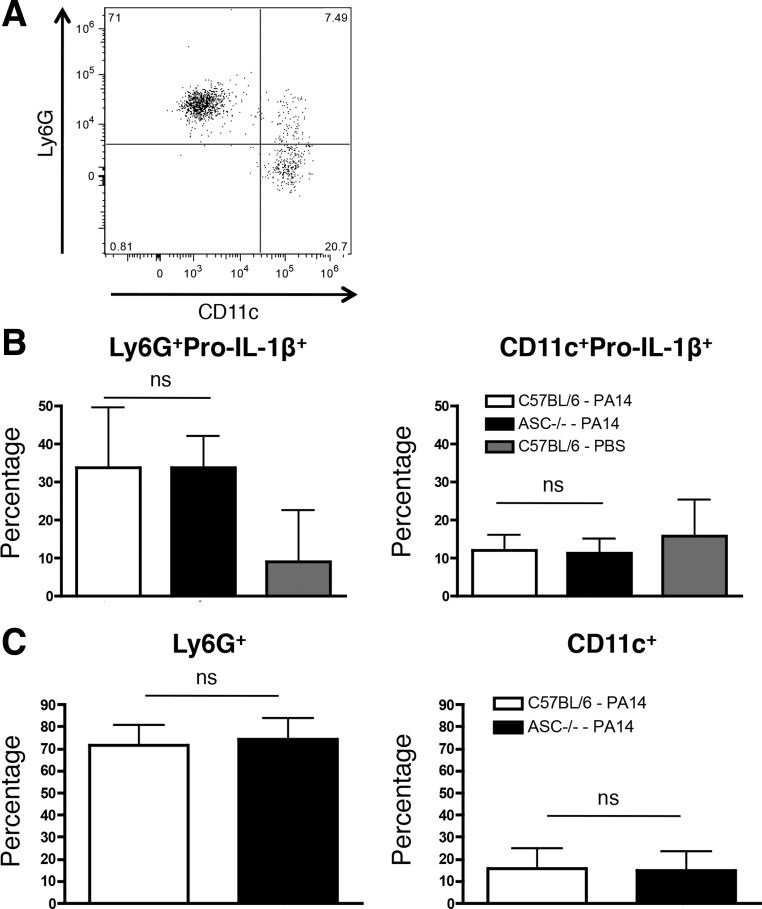
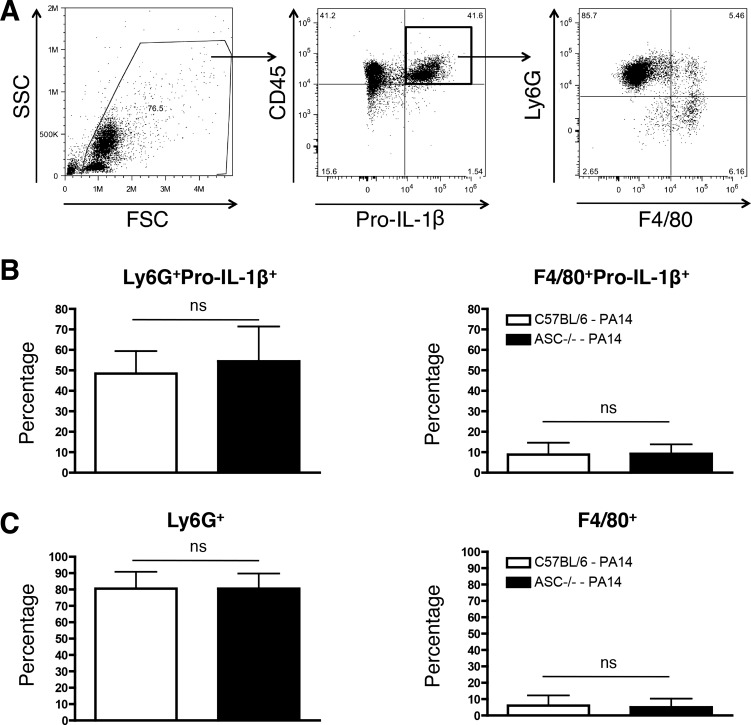
Loss of ASC did not lead to an in vivo defect in IL-1β production in the BAL fluid at 4 hpi (Fig. 2B). To assess if neutrophils might resolve the discrepancy observed for the requirement of ASC for IL-1β production in vitro and in vivo (Figs. 1 and 2), we intratracheally infected mice with a sublethal dose of WT PA14. We observed that all pro-IL-1β+ cells were CD45+ (data not shown), consistent with our data that recruited peritoneal leukocytes upregulate pro-IL-1β production (Fig. 3). While there was a fraction of pro-IL-1β+ cells that was Ly6G−CD11c+ alveolar macrophages, the majority of the pro-IL-1β+ cells were Ly6G+CD11c− neutrophils (Fig. 4, A–C). Robust neutrophil recruitment required PA14 instillation and was not observed in control mice (Fig. 4B and data not shown), and there were no differences in either the pro-IL-1β+ recruited neutrophils or macrophages between C57BL/6 and ASC−/− mice (Fig. 4, B and C). Altogether, our data demonstrate that neutrophils likely contribute to early in vivo IL-1β production (Fig. 2B) and may account for the discrepancy observed for the requirement of ASC for IL-1β production in vitro and in vivo.
Fig. 4.
ASC is not required for pulmonary IL-1β production in response to an acute PA14 infection. C57BL/6 (n = 7) and ASC−/− (n = 7) mice were infected intratracheally with a sublethal dose (3 × 106 CFU) of WT PA14. C57BL/6 mice instilled with PBS (n = 5) were used as a control. Mice were euthanized 4 h postinfection and BAL samples were collected. A: representative dot plot quantifying Ly6G+ and CD11c+ cells within a CD45+pro-IL-1β+ gate that excludes bacteria at 4 h postinfection. B: percentage of pro-IL-1β+Ly6G+ (left) and pro-IL-1β+CD11c+ (right) cells out of the total BAL cells at 4 h postinfection. C: percentage of Ly6G+CD11c− (left) and Ly6G−CD11c+ (right) within the gated CD45+pro-IL-1β+ population as depicted in A. Data are expressed as means ± SD from 4 independent biological experiments.
Neutrophils are the predominant population of peritoneal pro-IL-1β+ cells upon acute challenge in vivo.
To address the possibility that neutrophils may directly contribute to IL-1β production by an ASC-independent mechanism during peritoneal infection (Fig. 2, D and E), we first verified if peritoneal neutrophils are capable of upregulating pro-IL-1β expression upon infection. We infected mice with a sublethal dose of WT PA14 and analyzed the peritoneal cells at 4 hpi for pro-IL-1β production. Consistent with the pulmonary infection and our data using recruited peritoneal leukocytes for pro-IL-1β production, we observed that all pro-IL-1β+ cells were CD45+ (Fig. 5A), Upon in vivo infection, the majority of the pro-IL-1β+ cells were Ly6G+F4/80low neutrophils, with <10% of the pro-IL-1β+ cells being Ly6G−F4/80+ macrophages (Fig. 5, A–C). Notably, the percentage of pro-IL-1β+ recruited neutrophils and macrophages in C57BL/6 and ASC−/− mice were comparable (Fig. 5, B and C). Our data demonstrate that ASC is not required for acute in vivo pulmonary and peritoneal IL-1β production to P. aeruginosa infection (Fig. 2, B, D, and E) and suggest that neutrophils could directly contribute to IL-1β production (Figs. 3, 4, and 5).
Fig. 5.
Neutrophils are the predominant peritoneal cellular subset that express pro-IL-1β+ at early time points following P. aeruginosa infection. C57BL/6 (n = 15) and ASC−/− (n = 15) mice were infected intraperitoneally with a sublethal dose (3 × 106 CFU) of WT PA14. Mice were euthanized 4 h postinfection and peritoneal lavage was collected. A: representative dot plots depicting the gating scheme for pro-IL-1β+ cells at 4 h postinfection. Bacteria were excluded by gating. B: percentage of pro-IL-1β+Ly6G+ (left) and pro-IL-1β+F4/80+ (right) cells out of the total peritoneal cells at 4 h postinfection. C: percentage of Ly6G+F4/80− (left) and Ly6G−F4/80+ (right) within the gated CD45+pro-IL-1β+ population as depicted in A above. Data are expressed as means ± SD from 5 independent biological experiments.
Purified neutrophils secrete IL-1β in an ASC-independent manner in response to P. aeruginosa.
To test if neutrophils directly release IL-1β upon P. aeruginosa infection and to assess their molecular requirements for this response, enriched murine neutrophils were infected in vitro. Since neutrophils expressed pro-IL-1β both in the lung as well as the peritoneum (Figs. 4 and 5) and could account for ASC-independent IL-1β production (Fig. 2, B, D, and E), we enriched murine neutrophils from the peritoneum to obtain a higher yield of cells compared with those obtained from the BAL fluid. Neutrophils were recruited in response to thioglycollate and subjected to further enrichment (as described in materials and methods). Enriched neutrophils released IL-1β in response to PA14, in a manner that was dose dependent and required bacterial T3SS function (Fig. 6A). To assess if cell death was triggered in response to PA14 infection and was concomitant with IL-1β production, we performed propidium iodide staining of infected cells. WT PA14 infection triggered robust amounts of cell death in neutrophils (Fig. 6B). The cell death was largely dependent on T3SS activity by the bacteria: T3SS-deficient bacteria triggered lower amounts of cell death compared with cell death in response to WT PA14, which is consistent with necrotic/pyroptotic death observed in macrophages and dendritic cells (16, 35, 43). However, pharmacologic blockade of caspase activity by Z-VAD-fmk did not lead to a reduction in cell death triggered by PA14 infection (Fig. 6B) suggesting that most of the observed cell death was due to T3SS-induced necrosis.
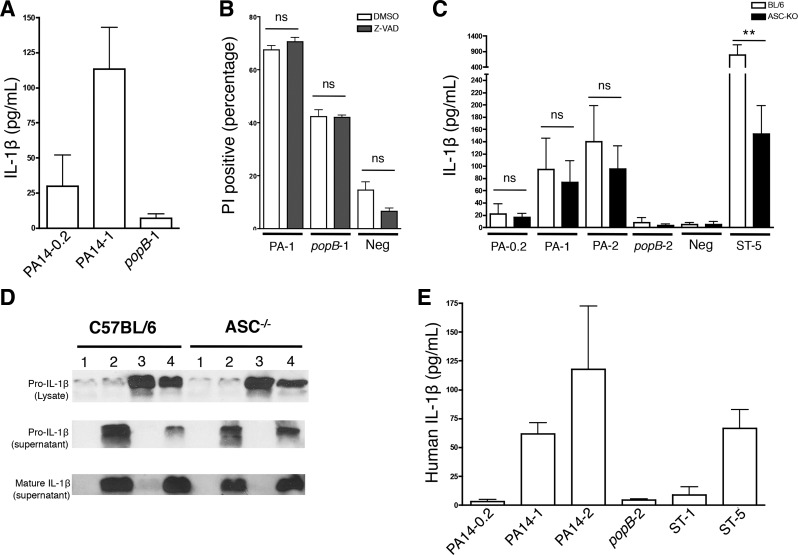
Fig. 6.
Purified neutrophils secrete IL-1β in response to P. aeruginosa. Murine neutrophils of the indicated genotype were enriched by positive selection (A) or negative selection (B, C, and D) and were infected with PA14 (WT), popB PA14, or Salmonella Typhimurium at the indicated MOI. Culture supernatants were collected 3 h postinfection and analyzed by ELISA for IL-1β production (A and C). B: to assess specificity and magnitude of cell death upon P. aeruginosa infection, neutrophils were infected with the indicated genotype of PA14 at a MOI=1 in the presence or absence of the caspase inhibitor Z-VAD. D: for Western blotting, whole cell lysates and culture supernatants were collected at 5 h postinfection and analyzed for pro- and the biologically active cleaved mature IL-1β. The lanes correspond to neutrophils infected with bacteria as follows: lane 1, negative control; lane 2, WT PA14 MOI = 2; lane 3, popB PA14 MOI = 2; and lane 4, S. Typhimurium MOI = 5. E: enriched human neutrophils were infected with PA14 (WT), popB mutant PA14 or S. Typhimurium at the indicated MOI. Culture supernatants were collected 3 h postinfection and analyzed by ELISA for IL-1β production. Data are expressed as means ± SD. Results are representative of 2 (A, n = 5; B, n ≥ 3; D and E, n = 4) and 4 independent biological experiments (C, n ≥ 6). **P ≤ 0.01.
We next tested if ASC was required for IL-1β production in response to P. aeruginosa. The IL-1β response to P. aeruginosa strain PA14 was ASC independent at all the tested MOI (Fig. 6C) and led to the production of cleaved IL-1β (Fig. 6D); S. Typhimurium, which is an ASC-dependent pathogen under the conditions we employed for our studies, was used as a positive control for IL-1β responses (Fig. 6, C and D). Studies employing purified human neutrophils validated the murine studies, with the demonstration that human neutrophils also produced IL-1β in a dose-dependent and T3SS-dependent fashion in response to P. aeruginosa (Fig. 6E). These data demonstrate that purified murine and human neutrophils represent a measurable source of IL-1β in response to P. aeruginosa infection (Figs. 2, B, D, and E, 4, and 5) in addition to the IL-1β released by resident macrophages, and the ASC independence of in vitro IL-1β responses by murine neutrophils supports the in vivo outcome with the use of a purified system.
S. Typhimurium activates both NLRP3- and NLRC4-dependent responses, which converge to the ASC-dependent pathway and lead to the subsequent release of IL-1β (5). We reasoned that in the absence of NLRP3, the IL-1β response to S. Typhimurium would be predominantly dependent on the NLRC4- and ASC-dependent inflammasome. We therefore hypothesized that if neutrophils do not require ASC in the absence of NLRP3-driven responses for IL-1β release in response to S. Typhimurium, NLRP3−/− and ASC−/− neutrophils will have comparable IL-1β production. Consistent with previous reports that NLRP3 is not required by macrophages to produce IL-1β in response to P. aeruginosa (16, 28, 43), NLRP3−/− and ASC−/− neutrophils produced comparable amounts of IL-1β in response to P. aeruginosa infection. Interestingly ASC−/− neutrophils produced significantly lower amounts of IL-1β compared with NLRP3−/− neutrophils in response to S. Typhimurium at all the tested MOI (data not shown). These data indicate that ASC is still a critical component and required by neutrophils for IL-1β production in response to S. Typhimurium but not P. aeruginosa. Our data suggest that IL-1β is produced in response to P. aeruginosa in an ASC-independent fashion and is likely representative of a specific response to P. aeruginosa rather than a broad mechanism for IL-1β secretion.
The neutrophil IL-1β response to P. aeruginosa is dependent on caspase-1.
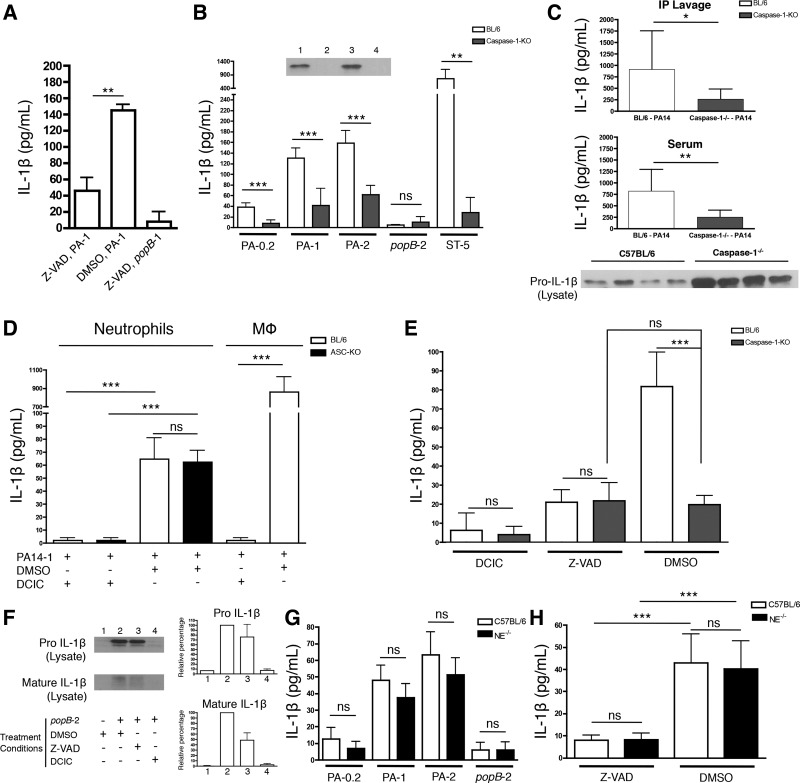
Both caspase-1-dependent and serine protease-dependent mechanisms of pro-IL-1β cleavage have been reported (3, 10, 11, 14, 20, 22). However, we find that neutrophils are dependent on caspase-1 expression for an efficient IL-1β response, which contrasts with findings derived from ocular infection in which the IL-1β response was attributed to NE expression (22). Firstly, caspase inhibition by Z-VAD-fmk, a pan-caspase inhibitor (17, 37, 42, 44, 47), led to significantly reduced IL-1β responses by neutrophils (Fig. 7A). Secondly, caspase-1−/− neutrophils were substantially defective for IL-1β production compared with C57BL/6 neutrophils in response to P. aeruginosa (Fig. 7B). To control that caspase-1−/− and WT neutrophils are equally capable of upregulating pro-IL-1β expression, they were infected with the popB mutant of PA14 that induces pro-IL-1β expression and minimizes the release of pro-IL-1β from cells and cell death. No difference in pro-IL-1β upregulation was observed (Fig. 7B, inset), suggesting that caspase-1 activity was required for efficient cleavage and release of IL-1β and the decreased amounts of IL-1β released by caspase-1−/− neutrophils were not a consequence of reduced pro-IL-1β upregulation. The in vitro results for IL-1β production were validated in vivo: caspase-1−/− mice (Fig. 7C), but not ASC deficient mice (Fig. 2), released significantly lower amounts of IL-1β compared with C57BL/6 mice in response to an intraperitoneal WT PA14 infection. These results are further supported by the observation that, following infection with WT PA14, peritoneal cells from caspase-1−/− mice were capable of upregulating pro-IL-1β production but had a defect in releasing it as mature IL-1β and therefore accumulated it intracellularly (Fig. 7C, bottom).
Fig. 7.
Caspase-1 dependence for neutrophil secretion of IL-1β in response to P. aeruginosa. Neutrophils were enriched by negative selection, treated with either 20 μM Z-VAD or 20 μM DCIC where indicated, and infected with WT PA14, popB PA14 or Salmonella Typhimurium at the indicated MOI. Culture supernatants were collected 3 h postinfection and analyzed by ELISA for IL-1β production (A, B, D, E, G, and H). A: C57BL/6 neutrophils were infected with WT PA14 or popB PA14 at a MOI = 1 in the presence or absence of Z-VAD. B: C57BL/6 and caspase-1−/− neutrophils were infected with WT PA14, popB PA14, or S. Typhimurium at the indicated MOI and IL-1β was analyzed by ELISA. Inset: WT C57BL/6 (lanes 1 and 2) or caspase-1−/− (lanes 3 and 4) neutrophils were infected with popB PA14 at a MOI = 2 (lanes 1 and 3) or uninfected (lanes 2 and 4), and cells were collected at 5 h postinfection and lysates analyzed for pro-IL-1β by Western analyses. C: C57BL/6 and caspase-1−/− mice were infected intraperitoneally with a sublethal dose (3 × 106 CFU) of WT PA14. Mice were euthanized 4 h postinfection and peritoneal lavage and blood samples were collected. Peritoneal lavage (top) and blood serum (middle) samples were analyzed by ELISA for IL-1β production (n ≥ 9). Pro-IL-1β was assayed from whole cell lysates of recruited peritoneal cells at 4 h postinfection (bottom). D: murine neutrophils or macrophages of the indicated genotype were infected with PA14 at a MOI of 1 in the presence or absence of DCIC. Data for neutrophils were compared using one-way ANOVA with the Tukey-Kramer posttest. E: C57BL/6 and caspase-1−/− neutrophils were infected with WT PA14 at MOI = 1 in the presence or absence of DCIC or Z-VAD. Data were compared using one-way ANOVA with the Tukey-Kramer posttest. F: C57BL/6 neutrophils were infected with popB mutant PA14 at MOI = 2 in the presence or absence of Z-VAD or DCIC, and subsequently analyzed by Western blot with the same protocol used in B, inset. G: C57BL/6 and neutrophil elastase−/− neutrophils were infected with WT PA14 or popB mutant PA14 at the indicated MOI. H: C57BL/6 and neutrophil elastase−/− neutrophils were infected with WT PA14 at MOI = 1 in the presence or absence of Z-VAD. Data were compared using one-way ANOVA with the Tukey-Kramer posttest. Results are representative of 2 (A, n = 4; D, n = 6; E, n = 6; F and G, n = 6; H, n = 6) and 3 independent biological experiments (B, n = 6; C, n ≥ 9). Data are expressed as means ± SD. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005.
To test the contribution of serine proteases to the residual, caspase-1-independent IL-1β production by neutrophils in response to PA14, we infected C57BL/6 or ASC−/− neutrophils in the presence of the serine protease inhibitor DCIC. Treatment of neutrophils with DCIC did not induce measureable neutrophil or bacterial cytotoxicity (data not shown). Surprisingly, pretreatment of neutrophils with DCIC led to a complete loss of IL-1β release (Fig. 7D). Strikingly, DCIC treatment also led to a complete loss of caspase-1- and ASC-dependent IL-1β production by macrophages (Fig. 7D). Therefore, we suspected that DCIC was blocking both caspase-1 and serine protease-dependent IL-1β responses either directly or indirectly, and so we tested if DCIC treatment was affecting IL-1β production by blocking protease activity either downstream or upstream of pro-IL-1β production. DCIC- or Z-VAD-treated C57BL/6 neutrophils were infected with the popB mutant of PA14 to induce pro-IL-1β production while preventing its T3SS-dependent release from the cells. As expected, Z-VAD treatment still led to pro-IL-1β production (Fig. 7F). However, DCIC treatment blocked upregulation of pro-IL-1β expression. Infection of neutrophils with popB bacteria led to the cleavage of small amounts of pro-IL-1β to mature IL-1β that remained in the cytosol but was not released outside the cell (Fig. 7F and data not shown). While DCIC treatment led to a complete loss of cytosolic cleaved IL-1β (Fig. 7F) due to the upstream loss of pro-IL-1β, cytosolic cleaved IL-1β was partially observed upon Z-VAD treatment (Fig. 7F), suggesting that while caspase-1 is important for release of IL-1β outside the cell (Fig. 7, A and B), serine proteases could partially contribute to cytosolic IL-1β maturation.
To delineate the specific contributions of caspase-1 and NE to neutrophil IL-1β production, we utilized WT, NE−/−, and caspase-1−/− neutrophils in combination with either DCIC or Z-VAD treatment before infection with P. aeruginosa. IL-1β production was largely independent of NE expression in response to P. aeruginosa infection on a per-cell basis (Fig. 7G). However, when NE−/− neutrophils were treated with Z-VAD, the IL-1β response was significantly reduced and comparable to that observed with Z-VAD-treated WT neutrophils (Fig. 7H). Z-VAD treatment reduced elicited IL-1β from WT neutrophils to the level of caspase-1−/− neutrophils, while caspase-1−/− neutrophils were relatively insensitive to Z-VAD treatment. These data support that caspase-1 activity by neutrophils is the dominant source of mature IL-1β in response to P. aeruginosa infection (Fig. 7E), with negligible contributions made by NE on a per-cell basis.
In vivo depletion of neutrophils results in reduced IL-1β production in ASC−/− mice.
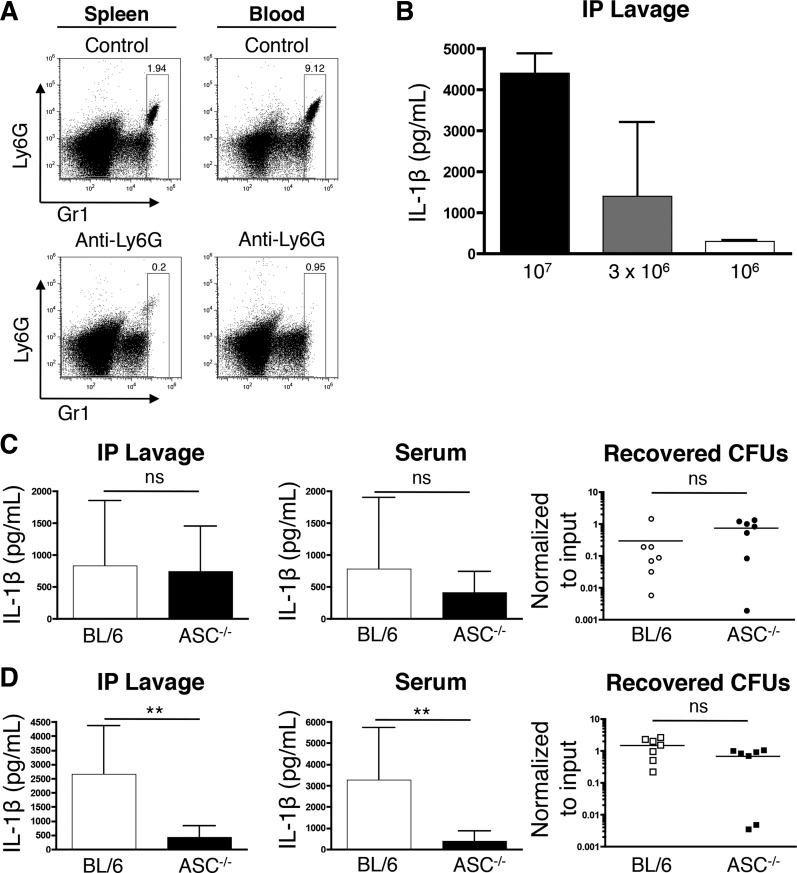
Since ASC-independent IL-1β production was observed using both pulmonary and peritoneal models of infection (Figs. 2, 4, and 5), we employed the acute peritonitis model to study the effects of neutrophil depletion to IL-1β production in vivo. We hypothesized that if neutrophils are contributing to in vivo IL-1β production in an ASC-independent manner, and if macrophages require ASC for IL-1β production, then the depletion of neutrophils will lead to reduced IL-1β production in response to P. aeruginosa infection in ASC−/− mice. To test this, we depleted mice of neutrophils with anti-Ly6G antibody (Fig. 8A). When mice were challenged with 3 × 106 CFU/mouse (the dose also used in (Figs. 2, D and E, and 5), it led to a consistent and measurable IL-1β response and was within a range in which modest changes in MOI result in measurable differences to in vivo IL-1β output ((Fig. 8). Consistent with our previous results, isotype control-treated ASC−/− and WT mice produced comparable amounts of IL-1β (Fig. 8C). However, upon Ly6G-mediated depletion of neutrophils, the ASC−/− mice exhibited a significantly reduced in vivo IL-1β response to P. aeruginosa infection compared with neutrophil-depleted WT mice (Fig. 8D). Intriguingly, depletion of neutrophils led to an exacerbated response in WT mice (Fig. 8, C and D). This increased IL-1β response is likely a consequence of reduced clearance of bacteria due to neutropenia, which is empirically supported by our observation that greater peritoneal bacterial CFU were recovered from the neutrophil depleted WT mice compared with the isotype control-treated WT mice (P = 0.0177, WT anti-Ly6G vs. WT isotype control treated) and consistent with our data on peritoneal IL-1β production that show that even incremental increases in bacterial load can affect the IL-1β response (Fig. 8B). Reduced bacterial clearance from neutropenic mice then leads to higher IL-1β production by WT peritoneal macrophages, whereas ASC−/− peritoneal macrophages have a significant defect in IL-1β production which is consistent with our in vitro results (Fig. 1A). These data provide the first demonstration that neutrophils support ASC-independent IL-1β production in vivo in response to P. aeruginosa infection and provide a demonstration that neutropenia leads to reduced IL-1β production in ASC−/− mice in comparison to WT mice.
Fig. 8.
Neutropenia leads to reduced peritoneal IL-1β production in response to P. aeruginosa. C57BL/6 and ASC−/− mice were depleted of neutrophils with anti-Ly6G IgG or treated with isotype control antibody (A, C, and D). A: representative analyses of efficacy of neutrophil depletion in circulating blood and spleens from the treatment groups. B: C57BL/6 mice were infected intraperitoneally with the indicated CFU of WT PA14. IL-1β content within the peritoneal lavage samples were subsequently assayed by ELISA(n = 2/group). C and D: C57BL/6 and ASC−/− mice were treated with isotype control antibody or depleted of neutrophils with anti-Ly6G antibody as described above. Mice were subsequently infected intraperitoneally with 3 × 106 CFU of WT PA14, euthanized 4 h postinfection, and peritoneal lavage and blood samples were collected for IL-1β analysis by ELISA. The peritoneal lavage fluid was analyzed for the total number of recovered CFU normalized to CFU of input bacteria. C: IL-1β from peritoneal lavage (left), blood serum (middle; BL/6, n = 10; ASC−/−, n = 7) samples and recovered peritoneal CFU from isotype IgG treated mice (right; n = 7/group). D: IL-1β from peritoneal lavage (left), blood serum (right; n = 10/group) and recovered peritoneal CFU from anti-Ly6G treated mice (right; n = 7/group). Data in A, C, and D are expressed as means ± SD and are derived from at least 3 independent biological experiments. **P ≤ 0.05.
DISCUSSION
In vitro studies using macrophages and dendritic cells have demonstrated that the NLRC4-, caspase-1-, and ASC-dependent inflammasome is critical for IL-1β production in response to P. aeruginosa (2, 16, 28, 43). We reasoned that since ASC is required for in vitro IL-1β production in response to P. aeruginosa, the loss of ASC will lead to reduced IL-1β production in vivo and acute pathological phenotypes. The discrepancy between our in vitro macrophage and in vivo IL-1β data led to two novel insights: neutrophils utilize a noncanonical, ASC-independent mechanism to produce mature IL-1β, and that neutrophils functionally contribute to acute in vivo IL-1β responses to P. aeruginosa infection.
Macrophages have been classically thought to be the predominant contributors to IL-1β production through the canonical caspase-1- and ASC-dependent inflammasome (5, 6, 16, 28, 29, 35, 43, 45). It was initially proposed that neutrophils lack the inflammasome components required to trigger IL-1β production in the context of infection (9, 29). However, while recent reports have emerged that human and murine neutrophils can produce IL-1β (3, 8, 10, 22, 24), the contributions and impact of neutrophils to overall IL-1β production, and their mechanisms, are largely unclear.
In both our pulmonary and peritonitis infection models, neutrophils were highly enriched at the site of infection at 4 hpi. Based on previously published data in response to P. aeruginosa (22) and our findings, we propose that neutrophils temporally contribute to IL-1β production during different phases of infection. IL-1R-driven signaling leads to increased recruitment of neutrophils to the site of infection (31, 45). Once neutrophils arrive at the site of infection, our data support that they enhance the in vivo IL-1β response. In tandem, neutrophils can exert antibacterial responses such as phagocytosis and production of reactive oxygen species, leading to eventual bacterial clearance (32). In the case of ASC-deficiency, it is possible that the residual IL-1β produced by ASC−/− macrophages is sufficient to recruit neutrophils, which can then amplify IL-1β levels in an ASC-independent fashion. Alternatively, inflammatory cytokine/chemokine responses to pathogen-associated molecular patterns (PAMPs) by the resident leukocytes could recruit neutrophils to the site of infection where they contribute to IL-1β production. Since T3SS activity is required for IL-1β production in vivo (35, 45) and IL-1R signaling amplifies neutrophil recruitment (31, 45), we believe that the initial trigger for IL-1β production is dependent on the leukocyte host-cytosol access of an inflammasome ligand, whereas the recruitment of neutrophils is likely dependent on PAMPs, and further amplified by IL-1R signaling. Since both murine and human neutrophils are capable of IL-1β production in response to P. aeruginosa in vitro, we propose that neutrophils are an important cell type contributing to the IL-1β response to P. aeruginosa.
The ASC-independent mechanism of IL-1β production by neutrophils contrasts the canonical caspase-1- and ASC-dependent mechanism of macrophages (5, 6, 16, 28, 29, 35, 43). Robust levels of IL-1β production by neutrophils have previously been reported to be either both ASC and caspase-1 dependent (3, 10, 24) or serine protease dependent (3, 11, 20, 22). This is the first report to demonstrate an ASC-independent but caspase-1-dependent role for neutrophil IL-1β production in response to P. aeruginosa.
Purified serine proteases have been described to cleave pro-IL-1β (11, 20), although their effects have primarily been described using pharmacological approaches (3, 22). We observed that DCIC treatment led to a complete loss of IL-1β production in neutrophils and, unexpectedly, in macrophages following P. aeruginosa infection. Furthermore, DCIC treatment led to a drastic reduction in pro-IL-1β production. While DCIC has been shown to biochemically block human caspases at high concentrations (27), our data strongly support that DCIC primarily blocks upregulation of pro-IL-1β, consistent with previous reports that DCIC can block NF-κB-dependent activity (15, 34, 36). Thus DCIC treatment can have effects via blocking either serine proteases that cleave pro-IL-1β or catalytic events that lead to pro-IL-1β production. NE inhibitor IV, another inhibitor employed for studies with P. aeruginosa (22), has been shown to block NF-κB-dependent signaling at high concentrations (7, 18), potentially leading to downstream effects on IL-1β production. Our data support that care should be taken in interpreting data derived from use of these inhibitors. Using a combination of genetic knockout cells and pharmacological inhibitors, we showed that majority of IL-1β production by neutrophils is dependent on caspase-1 and a small fraction of it may be dependent on serine proteases but is independent of NE. NE−/− mice were reported to have in vivo defects in IL-1β production in a P. aeruginosa corneal infection model (22). However, interpretation of this outcome is confounded since NE−/− mice have defects in intracellular Gram-negative bacterial killing (4), cellular adhesion, transmigration, and inflammatory cytokine production (46). Notably, NE−/− mice have reduced accumulation of neutrophils in the lung following exposure to cigarette smoke (40). We clearly demonstrate that WT and NE−/− neutrophils infected with P. aeruginosa produce comparable amounts of IL-1β on a per-cell basis. This shows that neutrophils can cleave IL-1β independent of NE expression. Another possibility is that while IL-1β is produced in a caspase-1-independent manner following corneal infection (22), IL-1β production requires caspase-1 in the peritonitis model in response to P. aeruginosa as supported by our results at 4 hpi as well as at 15 hpi (Fig. 7 and unpublished data, Y. R. Patankar and B. Berwin).
Although this study provides new insights into IL-1β production by neutrophils, several aspects of this process remain to be fully elucidated. The comparison to ASC-dependent macrophages suggests that neutrophils produce IL-1β independent of full NLRC4 inflammasome complex formation. Since in vitro IL-1β production by neutrophils was largely caspase-1 and partially NLRC4-dependent (Fig. 7 and data not shown), one possibility is that a caspase-1/NLRC4 complex without ASC is sufficient to act as a site for IL-1β maturation. Another possibility is that cytosolic active caspase-1 can cleave pro-IL-1β to secrete it as mature IL-1β in an ASC-complex-independent fashion. Additionally, neutrophils undergoing pyroptosis or necrosis could release pro-IL-1β that could then be cleaved by proteases targeted for release or caspase-1 released during cell death. However, while blocking caspase-1 activity led to a significant reduction in IL-1β released outside the cell, it had modest effects on Pseudomonas-induced cell death. This suggests that while pro-IL-1β released outside the cell upon cell death can potentially be cleaved by proteases outside the cell upon pharmacological inhibition of caspase-1, blockade of cellular caspase-1 activity significantly blunts the IL-1β response. Thus, while alternative proteolytic mechanisms of IL-1β release are not mutually exclusive, our data support that caspase-1 is more important than any other individual protease component for IL-1β release in response to P. aeruginosa. There is likely another mechanistic nuance that has yet to be revealed, based on our observation that neutrophils produce IL-1β in response to P. aeruginosa by an ASC-independent mechanism distinct from the ASC-dependent IL-1β produced in response to S. Typhimurium. These differential mechanisms are worth investigation in future studies as they may reveal fundamental differences in responses to extracellular and intracellular bacteria.
While neutrophils are unequivocally important in bacterial clearance, our data indicate that they are also a critical cell type involved in IL-1β production. Although neutrophils produce lower IL-1β than macrophages, they represent the most prevalent leukocyte that are rapidly recruited to the site of pulmonary infection. In this regard, we provide evidence that there is a differential temporal contribution by various leukocytes for IL-1β production and there is likely an early window during which neutrophil IL-1β responses are relevant and sufficient to propagate the host immune response. While this is clearly important to the successful host response to a bacterial infection, this also has relevance to hyper-inflammatory responses that induce immune pathology. In this respect, our findings reveal the basis for why ASC−/− and WT mice show comparable susceptibility to a P. aeruginosa lethal challenge (Fig. 2, A and C). Lethality in these models is due to hyper-inflammatory responses resulting in septic shock, and our data dovetail with those of others in showing that lethality was not due to systemic toxicity due to P. aeruginosa PAMPs such as endotoxin since a functional bacterial T3SS was required to confer lethality following intraperitoneal infection (Fig. 2C).
In conclusion, using murine models of pneumonia and peritonitis, we demonstrate that ASC−/− mice have no defect in IL-1β production in response to P. aeruginosa infection in vivo. Neutrophils contribute to IL-1β production in vivo, and importantly, we identify a noncanonical mechanism by which they can produce IL-1β in response to P. aeruginosa infection in an ASC-independent but caspase-1-dependent fashion.
GRANTS
This work was supported by National Institutes of Health (NIH) COBRE Grants P30- RR-032136-01 and P30-GM-106394, the Dartmouth Lung Biology Translational Research Core, Cystic Fibrosis Foundation Research Development Program (STANTO11R0 to B. L. Berwin), NIH Grants P42-ES-007373 and UL1-TR-001086, and the Copenhaver and Thomas Fellowship (to Y. R. Patankar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.R.P. and B.L.B. conception and design of research; Y.R.P., R.M., and B.L.B. performed experiments; Y.R.P. and B.L.B. analyzed data; Y.R.P. and B.L.B. interpreted results of experiments; Y.R.P. and B.L.B. prepared figures; Y.R.P. and B.L.B. drafted manuscript; Y.R.P. and B.L.B. edited and revised manuscript; Y.R.P., R.M., and B.L.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. George O' Toole, Deborah Hogan, David Leib, William Green, Yina Huang, Edward Usherwood, and Robert Cramer (Geisel School of Medicine at Dartmouth) and Dr. Ralph Budd (University of Vermont) for reagents and discussion.
REFERENCES
- 1.Amiel E, Alonso A, Uematsu S, Akira S, Poynter ME, Berwin B. Pivotal Advance: Toll-like receptor regulation of scavenger receptor-A-mediated phagocytosis. J Leukoc Biol 85: 595–605, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlehamn CS, Evans TJ. Pseudomonas aeruginosa pilin activates the inflammasome. Cell Microbiol 13: 388–401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakele M, Joos M, Burdi S, Allgaier N, Poschel S, Fehrenbacher B, Schaller M, Marcos V, Kummerle-Deschner J, Rieber N, Borregaard N, Yazdi A, Hector A, Hartl D. Localization and functionality of the inflammasome in neutrophils. J Biol Chem 289: 5320–5329, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med 4: 615–618, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med 207: 1745–1755, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8: 471–483, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao J, Liu Q. Protective effects of sivelestat in a caerulein-induced rat acute pancreatitis model. Inflammation 36: 1348–1356, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Cassel SL, Janczy JR, Bing X, Wilson SP, Olivier AK, Otero JE, Iwakura Y, Shayakhmetov DM, Bassuk AG, Abu-Amer Y, Brogden KA, Burns TL, Sutterwala FS, Ferguson PJ. Inflammasome-independent IL-1beta mediates autoinflammatory disease in Pstpip2-deficient mice. Proc Natl Acad Sci USA 111: 1072–1077, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog 7: e1002452, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen KW, Gross CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, Schroder K. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep 8: 570–582, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci USA 96: 6261–6266, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest 123: 1630–1637, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol 25: 469–484, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fantuzzi G, Ku G, Harding MW, Livingston DJ, Sipe JD, Kuida K, Flavell RA, Dinarello CA. Response to local inflammation of IL-1 beta-converting enzyme- deficient mice. J Immunol 158: 1818–1824, 1997. [PubMed] [Google Scholar]
- 15.Finco TS, Beg AA, Baldwin AS Jr.. Inducible phosphorylation of I kappa B alpha is not sufficient for its dissociation from NF-kappa B and is inhibited by protease inhibitors. Proc Natl Acad Sci USA 91: 11884–11888, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol 37: 3030–3039, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem 273: 32608–32613, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Hagiwara S, Iwasaka H, Hidaka S, Hasegawa A, Noguchi T. Neutrophil elastase inhibitor (sivelestat) reduces the levels of inflammatory mediators by inhibiting NF-κB. Inflamm Res 58: 198–203, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, Greene C, McElvaney G, Mall M, Doring G. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros 11: 363–382, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem 265: 6318–6322, 1990. [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci USA 90: 3038–3042, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karmakar M, Sun Y, Hise AG, Rietsch A, Pearlman E. Cutting edge: IL-1beta processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and caspase-1. J Immunol 189: 4231–4235, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 306: L591–L603, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mankan AK, Dau T, Jenne D, Hornung V. The NLRP3/ASC/caspase-1 axis regulates IL-1beta processing in neutrophils. Eur J Immunol 42: 710–715, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430: 213–218, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228–232, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Mesner PW, Bible KC Jr, Martins LM, Kottke TJ, Srinivasula SM, Svingen PA, Chilcote TJ, Basi GS, Tung JS, Krajewski S, Reed JC, Alnemri ES, Earnshaw WC, Kaufmann SH. Characterization of caspase processing and activation in HL-60 cell cytosol under cell-free conditions. Nucleotide requirement and inhibitor profile. J Biol Chem 274: 22635–22645, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci USA 105: 2562–2567, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11: 1136–1142, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA 107: 3076–3080, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mijares LA, Wangdi T, Sokol C, Homer R, Medzhitov R, Kazmierczak BI. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol 186: 7080–7088, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6: 173–182, 2006. [DOI] [PubMed] [Google Scholar]
- 33.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press, 1996. [Google Scholar]
- 34.Orlowski M, Michaud C. Pituitary multicatalytic proteinase complex. Specificity of components and aspects of proteolytic activity. Biochemistry 28: 9270–9278, 1989. [DOI] [PubMed] [Google Scholar]
- 35.Patankar YR, Lovewell RR, Poynter ME, Jyot J, Kazmierczak BI, Berwin B. Flagellar motility is a key determinant of the magnitude of the inflammasome response to Pseudomonas aeruginosa. Infect Immun 81: 2043–2052, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powers JC, Asgian JL, Ekici OD, James KE. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem Rev 102: 4639–4750, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Rauber P, Angliker H, Walker B, Shaw E. The synthesis of peptidylfluoromethanes and their properties as inhibitors of serine proteinases and cysteine proteinases. Biochem J 239: 633–640, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson JP, Carter WO, Narayanan P. Functional assays by flow cytometry. In: Manual of Clinical Laboratory Immunology, edited by Rose NR, de M, acario E, Folds JD, Lane HC, Nakamura R. Washington, DC: American Society for Microbiology, 1997, p. 245. [Google Scholar]
- 39.Schultz MJ, Rijneveld AW, Florquin S, Edwards CK, Dinarello CA, van der Poll T. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol 282: L285–L290, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol 163: 2329–2335, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S; National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34: 1–14, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Slee EA, Zhu H, Chow SC, MacFarlane M, Nicholson DW, Cohen GM. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J 315: 21–24, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med 204: 3235–3245, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW. Substrate specificities of caspase family proteases. J Biol Chem 272: 9677–9682, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Wangdi T, Mijares LA, Kazmierczak BI. In vivo discrimination of type 3 secretion system-positive and -negative Pseudomonas aeruginosa via a caspase-1-dependent pathway. Infect Immun 78: 4744–4753, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young RE, Thompson RD, Larbi KY, La M, Roberts CE, Shapiro SD, Perretti M, Nourshargh S. Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. J Immunol 172: 4493–4502, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Zhu H, Fearnhead HO, Cohen GM. An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells. FEBS Lett 374: 303–308, 1995. [DOI] [PubMed] [Google Scholar]