Abstract
Ethyl carbamate (EC) classified as a probable human carcinogen (Group 2A) is naturally formed in alcoholic beverages and fermented foods during fermentation process and/or during storage. The objective of this study was to analyze EC in 34 food items including 14 alcoholic beverages and 20 fermented foods sold in Korea. Each food was collected from 18 supermarkets in 9 metropolitan cities in Korea, and then made into composite. According to food composition and alcohol content, samples were divided into four matrices such as apple juice, milk, Soju (liquor containing about 20% alcohol), and rice porridge. The maximum EC value of 151.06 µg/kg was found in Maesilju (liquor made from Maesil and Soju). Whisky and Bokbunjaju (Korean black raspberry wine) contained 9.90 µg/kg and 6.30 µg/kg, respectively. EC was not detected in other alcoholic beverages. Of 20 fermented foods, Japanese-style soy sauce had highest level of 15.59 µg/kg and traditional one contained 4.18 µg/kg. Soybean paste had 1.18 µg/kg, however, EC was not found in other fermented foods.
Keywords: Ethyl carbamate, Alcoholic beverage, Fermented foods
INTRODUCTION
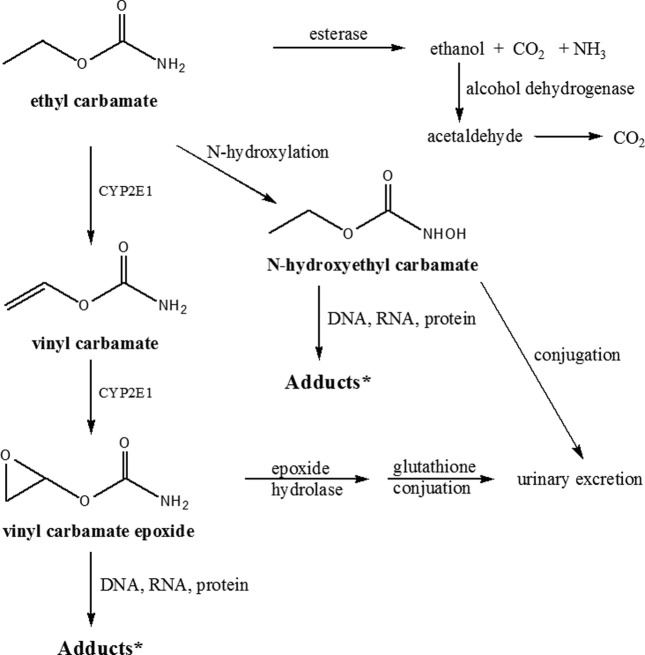
Ethyl carbamate (EC, CAS No. 51-79-6), known as urethane, is an ethyl ester of carbamic acid. It was used as a hypnotic in man and an anesthetic in animals in 1940s. However, it was found to be genotoxic and carcinogenic in 1943 (1). EC is rapidly absorbed from the gastrointestinal tract and the skin, and then distributed in the body (2). In liver, up to 90% of EC absorbed is hydrolyzed by microsomal esterase and eliminated as ethanol, carbon dioxide, and ammonia (Fig. 1). About 5% of EC is excreted in the urine after hydroxylation and conjugation. EC is also oxidized to vinyl carbamate (0.5%) by cytochrome P-450 2E1 and further converted into vinyl carbamate epoxide, which can bind covalently to DNA, RNA and proteins. The carcinogenic potential of EC includes gene mutations and DNA damage. Animal studies have shown that EC causes an increase in the incidence of tumors in several tissue sites including lung, liver, and blood vessels (3-5). Consequently, the International Agency for Research on Cancer (IARC) classified EC as a possible human carcinogen (Group 2B) in 1974 (6). Recently, IARC upgraded EC to a probable human carcinogen (Group 2A) in 2007 (7). The concern over the presence of EC and its toxicity in regularly consumed food products and alcoholic beverages has raised global interest to assess the possible risks to human health. The Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additive (JECFA) evaluated the exposure to EC in 2005 and concluded that the intake of EC from foods excluding alcoholic beverages would be of low concern (8). However, when both alcoholic beverages and foods were combined, its intake increased about 4-fold and consequently posed potential carcinogenic risks (8).
Fig. 1. Probable activation and inactivation pathways of ethyl carbamate metabolism. * Toxic metabolic pathways are indicated by bold type. * Adducts are responsible for the carcinogenic effect.
EC is naturally formed in fermented foods and alcoholic beverages during the fermentation process and/or during storage. Table 1 gives EC levels in alcoholic beverages from China, EU, Hong Kong, Japan, South Korea, and United States. The contents of EC in alcoholic beverages vary over a wide range. The levels of EC in wine ranged from 8 µg/L in white table wine to 111 µg/L in sake (9), and EC concentrations in stone-fruit brandies ranged from 100 to 22,000 µg/kg (10,11). Chinese rice wine contained nearly twice as much as EC than other Chinese alcoholic beverages (12). About 80% of EC present in spirits was formed during the distillation step and/or within the first 48 hr after distillation (13). In comparison, the contents of EC in beer were lowest, ranging from not-detected (ND) to 5.8 µg/kg. The occurrence of EC in fermented foods is shown in Table 2. The highest level of EC, up to 344 µg/kg, was found in Chinese red sufu, a fermented soybean curd (14). Toasting bread led to increases of between 3- and 8-fold in EC ranging from 3.5 to 33.8 µg/kg on a wet weight basis (15,16). Azodicarbonamide acts as a dough improver by chemically oxidizing thiol groups and rapidly forming the gluten network in the bread-making industry and as a blowing agent in beer. EC has been shown to form from azodicarbonamide breakdown during baking (17). Soy sauce had a wide range of EC from ND to 108 µg/kg. EC was also found in other fermented foods including vinegar, bread, and soybean paste. Generally, the contents of EC in fermented foods were quite lower than those of alcoholic beverages. It seems that the formation of EC is closely related to the content of ethanol.
Table 1. Concentrations (µg/kg) of ethyl carbamate in alcoholic beverages.
| Country | Product | No. of samples | Ethyl carbamate | Reference |
|---|---|---|---|---|
|
| ||||
| China | Beer | 20 | 2-3 | 12 |
| Wine | 30 | 9-34 | ||
| Rice wine | 92 | 8-515 | ||
| White spirit | 22 | 12-192 | ||
| EU members | Beer | 13 | ND-1 | 11 |
| Wine | 17 | ND-24 | ||
| Fortified wine | 15 | 14-60 | ||
| Sake | 2 | 81-164 | ||
| Stone-fruit brandy | 3,244 | ND-22,000 | ||
| Whisky | 210 | ND-1,000 | ||
| Hong Kong | Beer | 15 | ND-5.8 | 28 |
| Wine | 20 | 6.7-47 | ||
| Rice wine | 21 | 2.0-330 | ||
| Distilled spirits | 9 | 20-66 | ||
| Japan | Sake | 92 | ND-202 | 11 |
| South Korea | Distilled spirits | 5 | ND-196 | 32,56 |
| Wine | 30 | 2.64 ± 3.71 | ||
| Rice wine | 8 | 14.11 ± 9.58 | ||
| Liquor | 2 | 157-230 | ||
| Soju | 6 | ND | ||
| United States | Wine | 91 | ND-254 | 54 |
| Sake/rice wine | 34 | 10-904 | ||
| Whisky | 212 | ND-1,719 | ||
| Brandy | 26 | 9-387 | ||
Table 2. Concentrations (µg/kg) of ethyl carbamate in fermented foods.
| Country | Product | No. of samples | Ethyl carbamate | Reference |
|---|---|---|---|---|
|
| ||||
| Canada | Bread | 12 | 1.4-4.8 | 15 |
| Toast | 24 | 1.0-29.2 | ||
| China | Soy sauce | 22 | 8-108 | 12 |
| Vinegar | 11 | 2-51 | ||
| Sufu1) | 10 | 12-124 | ||
| Red sufu | 10 | 87-344 | ||
| EU members | Bakery | 50 | ND-20 | 11 |
| Fermented sauce | 44 | ND-18 | ||
| Vinegar | 10 | ND-33 | ||
| Hong Kong | Bread/rolls/buns | 15 | ND-8.6 | 28 |
| Soy sauce | 5 | 1.8-17 | ||
| Vinegar | 18 | ND-37 | ||
| Japan | Soy sauce | 26 | ND-35.2 | 38 |
| South Korea | Kimchi | 20 | ND-16.2 | 19 |
| Soy sauce | 20 | ND-73.3 | ||
| Soybean paste | 7 | ND-7.9 | ||
| Vinegar | 5 | 0.3-2.5 | ||
1)A cheese-like product that is one of the most popular fermented soybean foods in China.
Although there are currently no harmonized maximum levels for EC, some countries have established their own criteria (Table 3). Canada firstly introduced maximum limits for EC in alcoholic beverages in 1985 after high levels of EC were found in alcoholic beverages. The maximum levels were 30 µg/L for wine and 400 µg/L for fruit brandies. The United States has voluntary limits for domestic alcoholic beverages. Canadian guidelines were adopted by other countries such as Czech Republic, Brazil, France, Germany, and Switzerland. South Korea also set the maximum limit of 30 µg/L only for table wine. There are currently no guidelines governing the presence of EC in fermented foods.
Table 3. Maximum levels of ethyl carbamate for alcoholic beverages.
| Country | Wine | Fortified wine | Distilled spirits | Sake | Fruit brandy |
|---|---|---|---|---|---|
|
| |||||
| Brazil | 150 | ||||
| Canada | 30 | 100 | 150 | 200 | 400 |
| Czech Republic | 30 | 100 | 150 | 200 | 400 |
| France | 150 | 1,000 | |||
| Germany | 800 | ||||
| South Korea | 30 | ||||
| Switzerland | 1,000 | ||||
| United States | 15 | 60 | |||
In general, most of beverages and foods have various components that can hinder the analysis of EC from matrix, which affect EC analysis. Besides, beverages and foods contain low levels of EC, close to µg/kg or below detection limit. Accordingly, a number of analytical methods have been developed in various foods and beverages over the past 30 years (Table 4). Carbamate such as propyl carbamate, butyl carbamate, or 13C, 15N-EC has been used as an internal standard (18-21). However, these standards have different octanol-water partition coefficient from EC. It could lead to the difference in the recoveries between EC and internal standard. Therefore, the deuterated EC (d5-EC) is recently used to provide more precise recovery correction (12,22-24). The most traditional extraction method is a liquid-liquid extraction. Methylene chloride is commonly used as the organic solvent since EC is a weak polar compound. Solid-phase extraction (SPE) provides considerable advantages and is applied in the AOAC method (25). Extrelut or Chem-Elut has been found to be the most prevailing sorbent (15,18,22,26-28). In fatty food matrix, SPE was combined with a clean-up step such as the elution with pentane or hexane to eliminate nonpolar compounds (22). Another technique for extracting EC is a solid phase microextraction (23,29-31). It has advantages to reduce time and cost as well as to avoid EC losses. The most widespread method of EC determination is gas chromatography (GC) with polar columns. The applied detection systems include flame ionization detection (FID), electrolytic conductivity detection (ECD), and mass spectrometry (MS). The MS is the most useful and authoritative method for the quantification of EC. Recently, GC-MS-MS has been adopted to improve selectivity, sensitivity, and repeatability for EC determination (10,24,32). High performance liquid chromatography (HPLC) coupled with a fluorescence detector was employed after the derivatization with 9-xanthydrol (33). Fourier transform infrared (FTIR) spectroscopy was also used for determining EC without derivatization (10).
Table 4. Summary of the methodology of ethyl carbamate determination in alcoholic beverages and fermented foods.
| Product | Internal standard | Sample preparation | Detection | |
|---|---|---|---|---|
|
| ||||
| Liquid-liquid extraction | Solid-phase extraction | |||
|
| ||||
| Alcoholic beverages | Methyl carbamate | Extraction with CH2Cl2, CHCl3 or ethyl acetate | Chem-Elut or Extrelut | GC-MS |
| Propyl carbamate | Alumina | GC-MS/MS | ||
| n-Butyl carbamate | Addition of salts (NaCl, K2CO3 or Na2SO4) | Florisil | GC-ECD | |
| tert-Butyl carbamate | Headspace solid-phase microextraction | GC-NPD | ||
| 13C,15N-Ethyl carbamate | Dilution to 5% or 20% alcohol | GC-TEA | ||
| d5-Ethyl carbamate | Removal of ethanol | Styrenedivinylbenzene copolymer | GC-FID | |
| Isopropyl carbamate | FTIR-PLS | |||
| HPLC/FLD1) | ||||
| Fermented foods | Propyl carbamate | Extraction with CH2Cl2, ethyl acetate or petroleum ether | Chem-Elut or Extrelut | GC-MS/MS |
| n-Butyl carbamate | Deactivated alumina | GC-MS | ||
| 13C,15N-Ethyl carbamate | Removal of nonpolar compounds with n-pentane or hexane | Celite | GC-FID | |
| d5-Ethyl carbamate | C18 | |||
| Florisil | ||||
1)It was used after the precolumn derivatization with 9-xanthydrol.
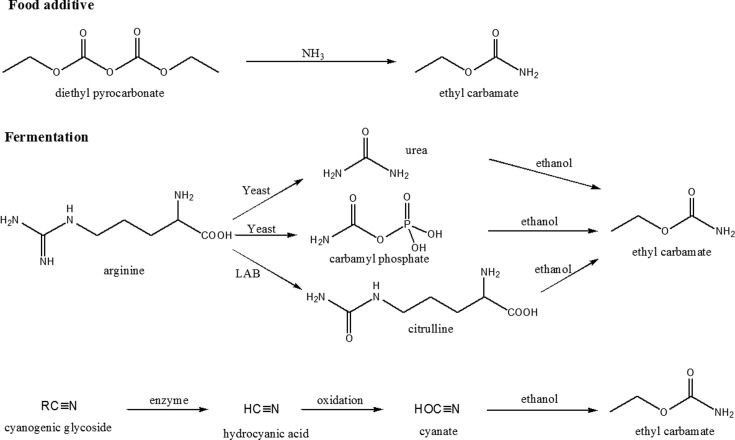
EC is formed through the reaction from ethanol and cyanate, urea, citrulline, or other N-carbamyl compounds (Fig. 2). Diethyl pyrocarbonate used as an antimicrobial agent added in beer, orange juice, and some soft drinks was found to react with ammonia to form EC in 1971 and then banned in the United States (34). Urea is the most important EC precursor in wine and sake (35,36). It is produced from the arginine metabolism through urea cycle in yeast. Carbamyl phosphate generated from arginine metabolism reacts rapidly with ethanol to form EC. In addition, citrulline produced through arginine deiminase (ADI) pathway by lactic acid bacteria is another precursor of EC in wine and soy sauce (37,38). Cyanate is a predominant precursor of EC in stone-fruit spirits and other spirits (13,39,40). Cyanogenic glycosides present mostly in the seed of stone-fruits is enzymatically or thermally degraded to hydrocyanic acid, which is oxidized to cyanate and then reacts with ethanol to form EC (2). A relatively high correlation (R = 0.597) between cyanide and EC was found in Brazilian sugar cane spirits (13). The addition of potassium metabisulfite inhibited the oxidation of hydrocyanic acid to cyanic acid, which in turn reduced the content of EC in ume, Japanese apricot, liqueur (41). On the other hand, no correlation was found between EC and cyanide in German stone-fruit spirits (10). In addition to these precursors, other factors such as the exposure to UV light, storage time, and fermenting temperature have been known to influence the formation of EC (10,12,42,43).
Fig. 2. Mechanism of ethyl carbamate formation in alcoholic beverages and fermented foods. LAB: lactic acid bacteria.
Numerous methods have been developed to reduce EC level in alcoholic beverages (Table 5). These methods include the use of refined materials, antioxidants, and genetically engineered yeasts. The reduction of EC levels has been achieved mostly by either inhibiting the production of its precursors or decomposing the precursors. Earlier preventive technologies include refining raw materials, removing seed from stone-fruits, controlling temperature and pH, filtering a fermentation product, minimizing exposure to light, and/or shortening storage time (10,42-45). Acid urease was added to hydrolyze urea into carbon dioxide and ammonia in grape wine and rice wine (45,46). Copper catalyst and potassium metabisulfite were used as cyanide catalyst and antioxidant, respectively (41,47). Genetic technologies in modifying fermentation strains provides a significant advance in inhibiting the production of urea or enhancing the metabolism of urea in yeast. The arginase encoded by the CAR1 gene in yeast degrades arginine into urea (48), which is transported and metabolized by urea amidolayse (DUR1,2) and urea permease (DUR3) in yeast. Therefore, inhibition of CAR1 expression and enhancement of DUR1,2 and DUR3 expression resulted in the decrease of urea concentrations in sake and grape wine, and consequently reduced the formation of EC (49,50). The utilization of arginine and citrulline via ADI pathway by Lactobaccilus hilgardii X1B and Oenococcus onei could avoid the possibility of EC formation in wine (51).
Table 5. Approaches to reduce the contents of ethyl carbamate in alcoholic beverages.
| Product | Method | Reference |
|---|---|---|
|
| ||
| Wine | Acid urease | 45 |
| Addition of diammonium phosphate1) | 57 | |
| Lower temperature | 43 | |
| Lower pH | 51 | |
| Increase of the expression of DUR1,2 (urea amidolyase) and DUR3 (urea permease) | 50,57 | |
| Stone-fruit spirits | De-stoning prior to mash | 10 |
| Copper catalyst | 47 | |
| Deletion of CAR1 (arginase) | 48 | |
| Automatic rinsing of the stills/Separation of tailings/non-redistilling tailings | 58 | |
| Rice wine/sake | Refining process of raw materials | 44 |
| Lower temperature | 14 | |
| Knocking out of CAR1 (arginase) | 49 | |
| Acid urease | 46 | |
| Ume liqueurs | Oxygen absorber/Addition of potassium metabisulfite | 41 |
| Spirits | Prevention of light exposure/Shorten storage time | 42 |
| Charcoal filtration | 59 | |
1)It is added to grape must fermentations to alleviate nitrogen deficiency.
EC is mainly found in fermented foods and alcoholic beverages. Korean diet includes many fermented foods such as Kimchi and soy sauce. Alcoholic beverages made from grains or fruits are also commonly consumed. For these reasons, the quantification of EC in fermented foods and beverages consumed in the Korean population was needed. Therefore, we determined EC in fermented foods and alcoholic beverages collected from 18 supermarkets in 9 metropolitan cities in Korea.
MATERIALS AND METHODS
Chemicals. EC, sodium chloride, and sodium hydroxide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Internal standard d5-EC was purchased from CDN isotopes (Pointe-Claire, Quebec, Canada). Methylene chloride and hexane were obtained from J.T. Baker (Center Valley, PA, USA). Disposable diatomite Chem Elut SPE column (50 mL) was purchased from Agilent Technology (Milwaukee, WI, USA).
Preparation of standard solution. The stock solution of EC (1000 µg/mL) was prepared by dissolving 0.1 g of EC in acetone. A working solution (400 ng/mL) was made by diluting of stock solution in acetone. The d5-EC was prepared with the same method above. For spiking in the sample, internal standard was diluted in distilled water instead of acetone.
Samples. Total 34 food items including 14 alcoholic beverages and 20 fermented foods were chosen from 734 foods appeared in the pooled intake data of 2008, 2009, 2010, and 2011 Korea National Health & Nutrition Examination Survey (KNHANES). Samples (500~800 g) were purchased from 18 supermarkets in 9 metropolitan cities according to a scheme devised by the Korea Health Industry Development Institute (KHIDI) to reflect the market share and population. Samples were pooled to make a composite, homogenized by blending, and then kept at −20℃ until analysis.
Determination of ethyl carbamate (EC). EC was determined according to the AOAC official method 994.07 with some modifications (AOAC 1997). The d5-EC was used as the internal standard instead of n-propyl carbamate. GC/MS conditions were revised to separate the d5-EC peak from EC peak and shorten a run time. To develop analytical methods depending on food composition, samples were classified into four matrices, which include non-fatty liquid (apple juice), proteinous liquid (milk), alcoholic beverage (Soju, ~20% ethanol), and non-fatty solid (rice porridge).
Apple juice matrix was firstly neutralized using 1 N sodium hydroxide solution and then 10 g of neutralized sample was mixed with 30 mL of distilled water and then 5 g of sodium chloride was dissolved in it. After spiked with 100 ng of d5-EC, it was loaded into a Chem Elut SPE column. After 4 min of equilibration, EC was eluted with 160 mL of methylene chloride at a rate of 1 drop per second. The eluent was concentrated to about 2~3 mL using a rotary evaporator, transferred into v-vial, and further concentrated to 1 mL under a gentle stream of nitrogen. Each sample was extracted and analyzed by GC-MS in triplicate.
The milk and soju were extracted using the above method with slight modification. Milk was centrifuged to remove proteins before loaded to the SPE column. Soju was diluted into 5% alcohol content to improve chromatographic resolution.
The rice porridge (20 g) was mixed with 40 mL of distilled water and then d5-EC (50 ng) was added. It was stirred for 20 min and centrifuged at 2000 rpm for 5 min.
The supernatant was loaded into the Chem Elut column after 4 min of equilibration. EC was eluted with 160 mL of methylene chloride. The eluent was concentrated to about 2~3 mL using a rotary evaporator, transferred into v-vial, and further concentrated to 1 mL under a gentle stream of nitrogen.
A 7820A GC-5977E MS (Agilent Technologies, Santa Clara, CA, USA) was used for the quantification and identification of EC. The GC conditions were as follows: capillary column 30 m length × 0.25 mm i.d., 0.25 µm film thickness DB-WAX (J&W, Folsom, CA, USA), helium carrier gas at 1 mL/min, injection volume with 2 µL in splitless mode, and injector 210℃. Oven temperature: 60℃, 10℃/min to 90℃, 2℃/min to 130℃ held for 5 min, 20℃/min to 220℃, and then held for 3 min. The MS was operated in the selected ion monitoring (SIM) with electron impact ionization (70 eV). The MS transfer line and ion source were kept at 240℃ and 230℃, respectively. Mass to charge (m/z) 62 and 64 were major fragment ions of EC and d5-EC. EC was quantified using calibration curves made from peak area ratios of EC/d5-EC (m/z 62 vs m/z 64). EC peak was identified by comparing the area ratios of m/z 62 vs m/z 74 that were major fragment ions of EC.
RESULTS AND DISCUSSION
Alcoholic beverages. The concentrations of EC in alcoholic beverages are presented in Table 6. Of 14 alcoholic beverages, the maximum value of 151.06 µg/kg was found in Maesilju, followed by whisky (9.90 µg/kg) and Bokbunjaju (6.30 µg/kg). Maesilju is a traditional Korean liqueur made out of green Maesil (Japanese apricot, a fruit of Prunus mume). The content of EC (151.06 µg/kg) in Maesilju is close to the high level (78.18 ± 63.10 µg/kg) reported previously in 7 Maesilju samples (32). Maesil contains high level of cyanogenic glycoside amygdalin (52), which is degraded to hydrocyanic acid and then oxidized to cyanate. The other ingredient of Maesilju is a Soju that is produced by diluting absolute ethanol to contain about 20% alcohol. Maesil is commonly removed after soaking in Soju for 100 days and the remaining liquid is ripened for several months or longer. It has been known that the concentrations of EC in Maesilju increased up to 216 µg/kg depending on alcohol content, soaking time of Maesil, and fermentation time (32,53). In earlier literatures, high contents of EC have been found in stone-fruit brandies (Table 1). Currently, there is no maximum EC level for Maesilju in Korea. The value of EC in Maesilju exceeds slightly the Canadian limit of 150 µg/kg for fruit brandy. This result indicates that mitigation action to reduce EC levels in Maesilju should be taken.
Table 6. Contents of ethyl carbamate in Korean alcoholic beverages.
| Product | Ethyl carbamate (μg/kg) |
|---|---|
|
| |
| Beer, canned | ND7) |
| Beer, PET bottled | ND |
| Beer, imported | N |
| Bokbunjaju1) | 6.30 |
| Cheongju2) | ND |
| Hanbangju3) | ND |
| Makgeolli4) | ND |
| Maesilju5) | 151.06 |
| Red wine | ND |
| Soju6) | ND |
| Wine, red | ND |
| Wine, white | ND |
| Wine, sparkling | ND |
| Whisky | 9.90 |
1)Korean black raspberry wine.
2)Korean rice wine similar to Japanese sake.
3)Liquor made from rice and oriental medicine.
4)Korean traditional alcoholic beverage made from rice.
5)Liquor made from Maesil and Soju.
6)Liquor containing about 20% (v/v) alcohol.
7)Not detecte.
Whisky has been reported to contain EC ranging from ND to 1,719 µg/kg (11,32,54). The level (9.9 µg/kg) of EC analyzed in this study is in the range of earlier literatures. It is quite lower than maximum permissible limit of 150 µg/kg for distilled spirits established in Canada, Czech Republic, and France. The content (6.3 µg/kg) of EC in Bokbunjaju (Korean black raspberry wine) is comparable to the value (1.66 ± 3.41 µg/kg) reported by Kim et al. (32). In other countries, wine has been known to contain a wide range of EC (ND-549 µg/kg) (11,14,28,54). Korean wines were reported to have an average of 2.64 ± 3.71 µg/kg (32). However, EC was not detected in wine analyzed in this study. Such variations in EC levels in wine are understandable due to differences in the processing conditions, raw materials, yeast, and lactic acid bacteria. EC was also not detected in beer, Soju, Cheongju (Korean rice wine), and Hanbangju (liquor made from rice and oriental medicine), which is close to the values of earlier literatures (22,32).
Fermented foods. The concentrations of EC in fermented foods are shown in Table 7. Of 20 fermented foods analyzed herein, Japanese-style soy sauce had highest content of 15.59 µg/kg and traditional one contained 4.18 µg/kg (Table 7). Soy sauce has been reported to have 8-108 µg/kg in Chinese samples, ND-35.2 µg/kg in Japanese samples, and 1.8-17 µg/kg in Hong Kong samples (Table 2). The content (1.18 µg/kg) of EC in soybean paste was in the range of ND-7.9 reported by Kim et al. (55). The concentrations of EC between 1.4 and 4.8 µg/kg were detected in 12 bread samples (15). Azodicarbonamide used as a flour bleaching agent and a dough conditioner has been reported to increase EC levels during baking (16). However, EC was not detected in 3 different breads analyzed in this study even though azodicarbonamide is allowed to use in wheat flour products with the maximum limit of 45 mg/kg in Korea. EC was not detected in cheese, Chunjang (fermented soybean product colored by caramel), Chengggukjang (soybeans fermented with Bacillus subtilis for 2~3 days), Kimchi, Maesil extract, red pepper paste, Ssamjang (mixture of soybean paste, red pepper paste, garlic, and seasoning), vinegar, and yogurt. These results indicate that most of Korean alcoholic beverages and fermented foods contain EC with low levels or below detection limit. Considering the intakes of Maesilju and soy sauce in the Korean population, preventative actions should be taken to reduce the levels of EC.
Table 7. Contents of ethyl carbamate (µg/kg) in Korean fermented foods.
| Product | Ethyl carbamate (μg/kg) |
|---|---|
|
| |
| Bread1) | ND6) |
| Milk bread1) | ND |
| Corn bread1) | N |
| Cheonggukjang2) | ND |
| Cheese | ND |
| Cheese, mozzarella | ND |
| Chunjang3) | ND |
| Kimchi, Chinese cabbage | ND |
| Maesil extract4) | ND |
| Red pepper paste, regular | ND |
| Red pepper paste with vinegar | ND |
| Ssamjang5) | N |
| Soybean paste | 1.18 |
| Soy sauce, traditional | 4.18 |
| Soy sauce, Japanese-style | 15.59 |
| Vinegar, brewed | ND |
| Vinegar, brewed with persimmon | ND |
| Yogurt, solid type | ND |
| Yogurt, liquid type | ND |
| Yogurt, diluted drink | ND |
1)Ferment their dough with yeast.
2)Korean natto.
3)Fermented soybean product colored by caramel.
4)Fermented liquid made from Japanese apricot and sugar.
5)Mixture of soybean paste, red pepper paste, garlic, and seasoning.
6)Not detected.
Acknowledgments
This research was supported by a grant (13162MFDS049) from Ministry of Food and Drug Safety in 2013-2015.
References
- 1.Nettleship A., Henshaw P.S., Meyer H.L. Induction of pulmonary tumors in mice with ethyl carbamate. J. Nat. Cancer Inst. (1943);4:309–331. [Google Scholar]
- 2.Zimmerli B., Schlatter J. Ethyl carbamate: analytical methodology, occurrence, formation, biological activity and risk assessment. Mutat. Res. (1991);259:325–350. doi: 10.1016/0165-1218(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 3.Mirvish S.S. The carcinogenic action and metabolism of urethane and N-hydroxyurethane. Adv. Cancer Res. (1968);11:1–42. doi: 10.1016/s0065-230x(08)60386-3. [DOI] [PubMed] [Google Scholar]
- 4.National Toxicology Program. [Accessed July 20, 2015];Report on Carcinogens, Thirteenth Edition: Urethane. (1983) Available from: http://ntp.niehs.nih.gov/ntp/roc/content/profiles/urethane.pdf .
- 5.Beland F.A., Benson R.W., Mellick P.W., Kovatch R.M., Roberts D.W., Fang J.L., Doerge D.R. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem. Toxicol. (2005);43:1–19. doi: 10.1016/j.fct.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 6.IARC (International Agency for Research on Cancer) Urethane. IARC Monogr. Carcinog. Risks Hum. (1974);7:111–140. [Google Scholar]
- 7.IARC (International Agency for Research on Cancer) International agency for research. 96, alcoholic beverage consumption and ethyl carbamate (urethane) 6-13 February 2007 1-5. World Health Organization; Lyon, France: (2007). [Accessed December 10 2014]. Available from: http://monographs.iarc.fr/ENG/Meetings/vol96-summary.pdf . [Google Scholar]
- 8.FAO/WHO (Food and Agriculture Organisation/World Health Organisation). Summary and conclusions of the sixtyfourth meeting of the joint meeting of the joint FAO/WHO expert committee of food additives (WHO Food Additives Series 30) world health organisation, Geneva. WHO Tech. Rep. Ser. (2005);928:1–47. Available from: http://www.who.int/ipcs/food/jecfa/summaries/en/summary_report_64_final.pdf . Accessed July 25 2013. [Google Scholar]
- 9.Jagerdeo E., Dugar S., Foster G.D., Schenck H. Analysis of ethyl carbamate in wines using solid-phase extraction and multidimensional gas chromatography/mass spectrometry. J. Agric. Food Chem. (2002);50:5797–5802. doi: 10.1021/jf025559s. [DOI] [PubMed] [Google Scholar]
- 10.Lachenmeier D.W., Schehl B., Kuballa T., Frank W., Senn T. Retrospective trends and current status of ethyl carbamate in German stone-fruit spirits. Food Addit. Contam. (2005);22:397–405. doi: 10.1080/02652030500073360. [DOI] [PubMed] [Google Scholar]
- 11.EFSA. Ethyl carbamate and hydrocyanic acid in food and beverages. EFSA J. (2007);551:1–44. [Google Scholar]
- 12.Wu H., Chen L., Pan G., Tu C., Zhou X., Mo L. Study on the changing concentration of ethyl carbamate in yellow rice wine during production and storage by gas chromatography/mass spectrometry. Eur. Food Res. Technol. (2012);235:779–782. doi: 10.1007/s00217-012-1807-7. [DOI] [Google Scholar]
- 13.Aresta M., Boscolo M., Franco D.W. Copper(II) catalysis in cyanate conversion into ethyl carbamate in spirits, and relevant reactions. J. Agric. Food Chem. (2001);49:2819–2824. doi: 10.1021/jf001346w. [DOI] [PubMed] [Google Scholar]
- 14.Wu P., Pan X., Wang L., Shen X., Yang D. A survey of ethyl carbamate in fermented foods and beverages from Zhejiang, China. Food Control. (2012);23:286–288. doi: 10.1016/j.foodcont.2011.07.014. [DOI] [Google Scholar]
- 15.Sen N.P., Seaman S.W., Boyle M., Weber D. Methyl carbamate and ethyl carbamate in alcoholic beverages and other fermented foods. Food Chem. (1993);4:359–366. doi: 10.1016/0308-8146(93)90318-A. [DOI] [Google Scholar]
- 16.Dennis M.J., Massey R.C., Ginn R., Parker I., Crews C., Zimmerli B., Zoller O., Rhyn P., Osborne B. The effect of azodicarbonamide concentrations on ethyl carbamate concentrations in bread and toast. Food Addit. Contam. (1997);14:95–100. doi: 10.1080/02652039709374502. [DOI] [PubMed] [Google Scholar]
- 17.Cañas B.J., Diachenko G.W., Nyman P.J. Ethyl carbamate levels resulting from azodicarbonamide use in bread. Food Addit. Contam. (1997);14:89–94. doi: 10.1080/02652039709374501. [DOI] [PubMed] [Google Scholar]
- 18.Xia Q., Yuan H., Wu C., Zheng J., Zhang S., Shen C., Yi B., Zhou R. An improved and validated sample cleanup method for analysis of ethyl carbamate in Chinese liquor. J. Food Sci. (2014);79:1854–1860. doi: 10.1111/1750-3841.12567. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.K., Koh E., Chung H.J., Kwon H. Determination of ethyl carbamate in some fermented Korean foods and beverage. Food Addit. Contam. (2000);17:469–475. doi: 10.1080/02652030050034055. [DOI] [PubMed] [Google Scholar]
- 20.Lim H.S., Lee K.G. Development and validation of analytical methods for ethyl carbamate in various fermented foods. Food Chem. (2011);126:1373–1379. doi: 10.1016/j.foodchem.2010.11.110. [DOI] [Google Scholar]
- 21.Canas B.J., Havery D.C., Joe F.L., Jr. Rapid gas chromatographic method for determining ethyl carbamate in alcoholic beverages with thermal energy analyzer detection. J. Assoc. Off. Anal. Chem. (1988);71:509–511. [PubMed] [Google Scholar]
- 22.Hansnip S., Crews C., Potter N., Christy J., Chan D., Bondu T., Matthews W., Walters B., Patel K. Survey of ethyl carbamate in fermented foods sold in the United Kingdom in 2004. J. Agric. Food Chem. (2007);55:2755–2759. doi: 10.1021/jf063121c. [DOI] [PubMed] [Google Scholar]
- 23.Lachenmeier D.W., Nerlich U., Kuballa T. Automated determination of ethyl carbamate in stone-fruit spirits using headspace solid-phase microextraction and gas chromatography-tandem mass spectrometry. J. Chromatogr. A. (2006);1108:116–120. doi: 10.1016/j.chroma.2005.12.086. [DOI] [PubMed] [Google Scholar]
- 24.Nóbrega I.C., Pereira G.E., Silva M., Pereira E.V., Medeiros M.M., Telles D.L., Albuquerque E.C., Jr., Oliveira J.B., Lachenmeier D.W. Improved sample preparation for GC-MS-SIM analysis of ethyl carbamate in wine. Food Chem. (2015);177:23–28. doi: 10.1016/j.foodchem.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 25.AOAC. AOAC official method 944.07: ethyl carbamate in alcoholic beverages and soy sauce. In: AOAC official methods of analysis. 17 edition. J. AOAC Int.; Gaithersburg, MD, USA: (1997). pp. 14–15. [Google Scholar]
- 26.Dennis M.J., Howarth N., Massey R.C., Parker I., Scotter M., Startin J.R. Method for the analysis of ethyl carbamate in alcoholic beverages by capillary gas chromatography. J. Chromatogr. A. (1986);369:193–198. doi: 10.1016/S0021-9673(00)90115-8. [DOI] [Google Scholar]
- 27.Fauhl C., Catsburg R., Wittkowski R. Determination of ethyl carbamate in soy sauces. Food Chem. (1993);48:313–316. doi: 10.1016/0308-8146(93)90147-8. [DOI] [Google Scholar]
- 28.Tang A.S., Chung S.W., Kwong K., Xiao Y., Chen M.Y., Ho Y.Y., Ma S.W. Ethyl carbamate in fermented foods and beverages: dietary exposure of the Hong Kong population in 2007-2008. Food Addit. Contam. Part B. (2011);4:195–204. doi: 10.1080/19393210.2011.605524. [DOI] [PubMed] [Google Scholar]
- 29.Ye C.W., Zhang X.N., Gao Y.L., Wang Y.L., Pan S.Y., Li X.J. Multiple headspace solid-phase microextraction after matrix modification for avoiding matrix effect in the determination of ethyl carbamate in bread. Anal. Chim. Acta. (2012);710:75–80. doi: 10.1016/j.aca.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Zhang J. Optimization of headspace solid-phase microextraction for analysis of ethyl carbamate in alcoholic beverages using a face-centered cube central composite design. Anal. Chim. Acta. (2008);627:212–218. doi: 10.1016/j.aca.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Perestrelo R., Petronilho S., Câmara J.S., Rocha S.M. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction as a powerful tool for quantification of ethyl carbamate in fortified wines. The case study of Madeira wine. J. Chromatogr. A. (2010);1217:3441–3445. doi: 10.1016/j.chroma.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Kim D.H., Jang H.S., Choi G.I., Kim H.J., Kim H.J., Kim H.L., Kim K.S. Determination of residue levels of ethyl carbamate in alcoholic beverages by gas chromatography/tandem mass spectrometry. J. Food Hyg. Saf. (2013);1:63–68. doi: 10.13103/JFHS.2013.28.1.063. [DOI] [Google Scholar]
- 33.Herbert P., Santos L., Bastos M., Barros P., Alves A. New HPLC method to determine ethyl carbamate in alcoholic beverages using fluorescence detection. J. Food Sci. (2002);67:1616–1620. doi: 10.1111/j.1365-2621.2002.tb08693.x. [DOI] [Google Scholar]
- 34.Solymosy F., Antoni F., Fedorcsák I. On the amounts of urethane formed in diethyl pyrocarbonate treated beverages. J. Agric. Food Chem. (1978);26:500–503. doi: 10.1021/jf60216a025. [DOI] [PubMed] [Google Scholar]
- 35.Hara S., Yoshizawa K., Nakamura K.I. Formation of ethyl carbamate in model alcoholic beverages containing urea or related compounds. Nippon Jozo Kjiokaishi. (1988);83:57–63. [Google Scholar]
- 36.Dahabieh M.S., Husnik J.I., Van Vuuren H.J. Functional enhancement of sake yeast strains to minimize the production of ethyl carbamate in sake wine. J. Appl. Microbiol. (2010);109:963–973. doi: 10.1111/j.1365-2672.2010.04723.x. [DOI] [PubMed] [Google Scholar]
- 37.Azevedo Z., Couto J.A., Hogg T. Citrulline as the main precursor of ethyl carbamate in model fortified wines inoculated with Lactobacillus hilgardii: a marker of the levels in a spoiled fortified wine. Lett. Appl. Microbiol. (2002);34:32–36. doi: 10.1046/j.1472-765x.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- 38.Matsudo T., Aoki T., Abe K., Fukuta N., Higuchi T., Sasaki M., Uchida K. Determination of ethyl carbamate in soy sauce and its possible precursor. J. Agric. Food Chem. (1993);41:352–356. doi: 10.1021/jf00027a003. [DOI] [Google Scholar]
- 39.Taki N., Imamura L., Takebe S., Kobashi K. Cyanate as a precursor of ethyl carbamate in alcoholic beverages. Jpn. J. Toxicol. Environ. Health. (1992);38:498–505. doi: 10.1248/jhs1956.38.498. [DOI] [Google Scholar]
- 40.Wucherpfennig K., Clauss E., Konja G. Formation of ethyl carbamate in alcoholic beverages based on the maraschino cherry. Dtsch. Lebensm. Rundsch. (1987);83:344–349. [Google Scholar]
- 41.Hashiguchi T., Horii S., Izu H., Sudo S. The concentration of ethyl carbamate in commercial ume (Prunus mume) liqueur products and a method of reducing it. Biosci. Biotechnol. Biochem. (2010);74:2060–2066. doi: 10.1271/bbb.100364. [DOI] [PubMed] [Google Scholar]
- 42.Baumann U., Zimmerli B. Accelerated formation of ethyl carbamate in spirits. Mitt. Geb. Lebensmittelunters. Hyg. (1988);22:175–185. [Google Scholar]
- 43.Hansnip S., Caputi A., Crews C., Brereton P. Effects of storage time and temperature on the concentration of ethyl carbamate and its precursors in wine. Food Addit. Contam. (2004);21:1155–1161. doi: 10.1080/02652030400019851. [DOI] [PubMed] [Google Scholar]
- 44.Yoshizawa K., Takahashi K., Sato K. Changes of urea content in rice and sake moromi during sake making process. Nippon Jozo Kyokaishi. (1988);83:136–141. [Google Scholar]
- 45.Arena M.E., Manca de Nadra M.C. Influence of ethanol and low pH on arginine and citrulline metabolism in lactic acid bacteria from wine. Res. Microbiol. (2005);156:858–864. doi: 10.1016/j.resmic.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Fidaleo M., Esti M., Moresi M. Assessment of urea degradation rate in model wine solutions by urease from Lactobacillus fermentum. J. Agric. Food Chem. (2006);54:6226–6235. doi: 10.1021/jf060934s. [DOI] [PubMed] [Google Scholar]
- 47.Kobashi K., Takebe S., Sakai T. Removal of urea from alcoholic beverages with an acid urease. J. Appl. Toxicol. (1988);8:73–74. doi: 10.1002/jat.2550080112. [DOI] [PubMed] [Google Scholar]
- 48.Pieper H.J., Seibold R., Luz E., Jung O. Reduction of the ethyl carbamate concentration in manufacture of Kirsch (cherry spirit). Kleinbrennerei. (1992);44:125–130. [Google Scholar]
- 49.Schehl B., Senn T., Lachenmeier D.W., Rodicio R., Heinisch J.J. Contribution of the fermenting yeast strain to ethyl carbamate generation in stone fruit spirits. Appl. Microbiol. Biotechnol. (2007);74:843–850. doi: 10.1007/s00253-006-0736-4. [DOI] [PubMed] [Google Scholar]
- 50.Kitamoto K., Oda-Miyazaki K., Gomi K., Kumagai C. Mutant isolation of non-urea producing sake yeast by positive selection. J. Ferment. Bioeng. (1993);75:359–363. doi: 10.1016/0922-338X(93)90134-T. [DOI] [Google Scholar]
- 51.Coulon J., Husnik J.I., Inglis D.L., van der Merwe G.K., Lonvaud A., Erasmus D.J., van Vuuren H.J.J. Metabolic engineering of Saccharomyces cerevisiae to minimize the production of ethyl carbamate in wine. Am. J. Enol. Vitic. (2006);57:113–124. [Google Scholar]
- 52.Arena M.E., Saguir F.M., Manca de Nadra M.C. Ariginine, citrulline and ornithine metabolism by lactic acid bacteria from wine. Int. J. Food Microbiol. (1999);52:155–161. doi: 10.1016/S0168-1605(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 53.Bolarinwa I.F., Orfila C., Morgan M.R. Amygdalin content of seeds, kernels and food products commerciallyavailable in the UK. Food Chem. (2014);152:133–139. doi: 10.1016/j.foodchem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Hwang L.H., Kim A.K., Park K.A., Kim J.Y., Hwang I.S., Chae Y.Z. The effect of raw material, alcohol con- tent, and trans-resveratrol on the formation of ethyl carbamate in plum wine. J. Food Hyg. Saf. (2009);24:194–199. [Google Scholar]
- 55.Battaglia R., Conacher H.B., Page B.D. Ethyl carbamate (urethane) in alcoholic beverages and foods: a review. Food Addit. Contam. (1990);7:477–496. doi: 10.1080/02652039009373910. [DOI] [PubMed] [Google Scholar]
- 56.Park S.K., Yoon T., Choi D. Analysis of ethyl carbamate in alcoholic beverages. Anal. Sci. Technol. (2008);21:53–57. [Google Scholar]
- 57.Adams C., van Vuuren H.J.J. Effect of timing of diammonium phosphate addition to fermenting grape must on the production of ethyl carbamate in wine. Am. J. Enol. Vitic. (2010);61:125–129. [Google Scholar]
- 58.Weltring A., Rupp M., Arzberger U., Rothenbuecher L., Koch H., Sproll C., Lachenmeier D.W. Ethyl carbamate: analysis of questionnaires about production methods of stone-fruit spirits at German small distilleries. Dtsch. Lebensm. Rundsch. (2006);102:97–101. [Google Scholar]
- 59.Park S.R., Ha S.D., Yoon J.H., Lee S.Y., Hong K.P., Lee E.H., Yeom H.J., Yoon N.G., Bae D.H. Exposure to ethyl carbamate in alcohol-drinking and nondrinking adults and its reduction by simple charcoal filtration. Food Control. (2009);20:946–952. doi: 10.1016/j.foodcont.2009.02.006. [DOI] [Google Scholar]