Abstract
Aldosterone affects fluid retention in the body by affecting how much salt and water that the kidney retains or excretes. There is limited information about the effect of prolonged aldosterone excess and treatment on body fluid in primary aldosteronism (PA) patients. In this study, body composition changes of 41 PA patients with unilateral aldosterone producing adenoma (APA) were assessed by a bio-impedance spectroscopy device. Patients with APA receiving adrenalectomy, as compared with those treated with spironolactone, had significantly lower relative overhydration (OH) and urine albumin excretion, and significantly higher urine sodium excretion four weeks after treatment. These differences dissipated 12 weeks after the initial treatment. Independent factors to predict decreased relative OH four weeks after treatment were male patients and patients who experienced adrenalectomy. Patients who underwent adrenaelctomy had significantly decreased TNF-α and increased serum potassium level when compared to patients treated with spironolactone 4 and 12 weeks after treatment. In this pilot study, we found that adrenalectomy leads to an earlier increase in renal sodium excretion and decreases in body fluid content, TNF-α, and urine albumin excretion. Adrenalectomy yields a therapeutic effect more rapidly, which has been shown to ameliorate overhydration in PA patients.
Primary aldosteronism (PA), characterized by inappropriate production of aldosterone, affects 5–13% of patients with hypertension1. Mineralocorticoid receptor (MR) antagonists (spironolactone) usually bring about target blood pressure (BP) control in patients with bilateral adrenal hyperplasia (BAH)2 with life-long medication. Aldosterone producing adenoma (APA) is the most common subtype that can be cured by adrenalectomy. The results of short-term, follow-up studies showed that unilateral adrenalectomy or treatment with MR antagonists is effective in correcting hypertension and hypokalemia in PA patients. However, normalization of blood pressure and correction of hypokalemia are not the only goals in managing PA. The excessive production of aldosterone in PA patients may lead to a higher rate of cardiovascular events and renal damage disproportionate to BP levels3,4.
Aldosterone affects fluid retention in the body by way of salt and water that the kidney retains or excretes. In PA, there is an increase in plasma volume due to increased sodium reabsorption5. A previous study demonstrated that patients with PA had greater values for plasma volume and extracellular fluid volume than did patients with essential hypertension6. There is limited information about the effect of prolonged aldosterone excess and treatment on body fluid. The clinical assessment of fluid status is relatively difficult because physical signs of edema are of limited value in diagnosing excess intravascular volume7. Bioimpedance spectroscopy is a simple and effective approach for the assessment of fluid status and for evaluating fat mass and lean mass8,9. The accuracy of fluid status and body composition measured by spectroscopy has been validated against available gold standard reference methods10,11. The main objective of this work was to compare the effect of adrenalectomy and spironolactone on body composition. Therefore, we performed a prospective study to evaluate the body composition changes as assessed by bio-impedance spectroscopy device in patients with APA treated with either adrenalectomy or spironolactone. We also assessed the changes of cardiac dysfunction, fluid overload, and renal function by novel biomarkers, and of the levels urine sodium and potassium excretion before and after treatment.
Patients and methods
Ethics Statement
The study complied with the Declaration of Helsinki and was approved by the institutional review board of National Taiwan University Hospital (Taipei, Taiwan) in addition to obtaining informed consent. All participants signed the informed consent form before inclusion in the study.
Patient Selection
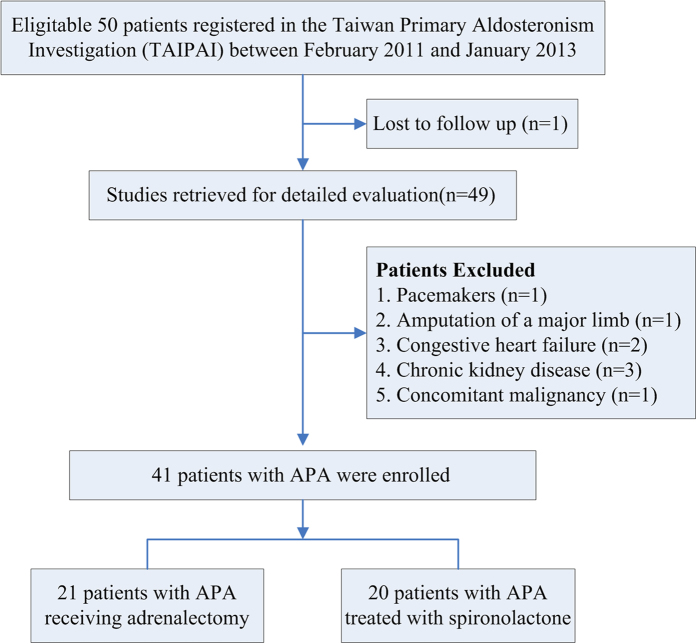
All patients were registered in the Taiwan Primary Aldosteronism Investigation (TAIPAI) between February 2011 and January 2013. This prospective study enrolled 21 patients diagnosed as APA and received adrenalectomy. Another 20 patients with APA treated with spironolactone were enrolled as a control group. It was the patient’s choice to receive adrenalectomy or medical therapy. The database was constructed for quality assurance at two medical centers and their four affiliated hospitals in different cities in Taiwan. Before confirmatory tests, all antihypertensive medications were discontinued for at least 21 days. Diltiazem and/or doxazosin were administered to control markedly high blood pressure when required12. The study excluded patients who had pacemakers/implanted defibrillators or amputation of a major limb, an acute disease, an obvious body weight change before enrollment (>5 kg in 3 months), pregnancy, or use of oral contraceptives. Additional exclusion criteria were clinically significant comorbid conditions such as uncontrolled hypertension, New York Heart Classification (NYHC) III-IV congestive heart failure, stage 3 or worse chronic kidney disease ([GFR] <60 mL/[min · 1.73 m2], or diagnosed with cancer within the previous 5 years (Fig. 1). We suggested a low salt diet with sodium intake less than 3,000 mg per day to all patients beginning 21 days before treatment and continuing throughout the study period. Medications that might interfere with the renin-aldosterone axis, including steroids, sex hormones, licorice, or non-steroidal anti-inflammatory drugs were also withheld. No patient took mineralocorticoid receptor antagonists (MRA) prior to participation in the study.
Figure 1. Trial profile.
*Abbreviations: APA, aldosterone producing adenoma.
PA Confirmation
Patients with an abnormal aldosterone-renin ratio (ARR) were confirmed to have PA by saline infusion tests and subsequent imaging studies for subtype identification (Figure S1). The diagnosis of APA was established in hypertensive patients with elevated ARR, TAIPAI score more than 60% and evidence for lateralized disease by adrenal CT (n = 41), NP59 scintigraphy (n = 13) or AVS (n = 22) (Text S1 and Figure S1).
Study Protocol
All patients underwent measurements of BCM, blood pressure (BP), plasma N-terminal pro-brain natriuretic peptide (NT-proBNP), C reactive protein (CRP), tumor necrosis factor (TNF)-α, Cystatin C, and biochemistry before starting spironolactone (50 mg daily) treatments or receiving adrenalectomy. For patients treated with spironolactone, measurements were repeated after 4 and 12 weeks of medication. Patients who underwent unilateral adrenalectomy for adenoma took the second and third measurement at 4 and 12 weeks after the operation.
Blood pressure determinations were taken in the right arm after being seated for 5 min by a trained research assistant with a mercury sphygmomanometer with brachial cuff adjusted to the patient’s brachial circumference. The blood pressure was measured thrice at 5-minute intervals. The average value was used for analysis.
BCM Measurements
Measurements of the fluid status were performed by a single well-trained nurse, using a portable whole-body bioimpedance spectroscopy device, the BCM (Fresenius Medical Care, Bad Homburg, Germany). The BCM measures the electrical responses at 50 different frequencies between 5 and 1000 kHz. Electrodes were attached to the hand and foot on the non-dominant side of the body after the patient had been in recumbent position for at least 5 minutes. Only one BCM measurement was performed for each individual patient because this method had good interobserver and intraobserver reproducibility10.
Fluid status assessed by the BCM is represented by overhydration (OH), which was derived from the impedance data based on a three compartment model13. The three compartments are lean tissue mass, adipose tissue mass, and OH. OH is the difference between the amount of extracellular water (ECW) in the tissue actually detected by the BCM and the amount of water present in tissue predicted using physiological models under normal (euvolemic) conditions. OH index defined as OH normalized to ECW and expressed as a percentage (%). All values of OH were compared with those obtained from an age and sex-matched healthy population. Fluid overload was defined as a relative OH index ≥7%, corresponding to the 90th percentile value for the reference cohort14. Relative lean tissue mass (LTM) and relative fat mass were presented as a percentage.
Laboratory Measurements
The concentration of aldosterone was measured by radioimmunoassays with commercial kits (Aldosterone Maia Kit, Adaltis Italia S.p.A., Bologna, Italy). The lowest detectable concentration of aldosterone is 10.0 pg/mL. The normal range of aldosterone is 70–350 pg/mL in the upright position. PRA was measured as the generation of angiotensin I in vitro using a commercially available radioimmunoassay kit (Stillwater, MN, USA). The normal range of PRA was 2.63 ± 1.32 ng/mL/ h in the upright position. The mean (standard deviation [SD]) intra and interassay coefficients of variation (CVs) for the PRA assay were 1.9 (5.0%) and 4.5 (5.2%) respectively.
The plasma levels of TNF-α, CRP (R&D Systems, Minneapolis, MN) and NT-proBNP (USCN Life Science, Wuhan, P.R. China) were measured using the commercially available enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions. Urine albumin excretion was defined as the urinary albumin-to-creatinine ratio (mg/mg) determined using the first morning void. Cystatin C was measured using a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Siemens, Berlin, Germany) with a nephelometer (BNII; Siemens).
Statistical Analysis
The data were presented as the mean values ± standard deviation. We used a logistic regression analysis to explore the factors for early decreased OH. The Mann-Whitney U test was used to determine the difference between the two treatment groups. The study assessed univariate correlations between relative OH and potential explanatory variables using Pearson’s correlation analyses.
To examine the effect of different treatment strategies on various time-sequential variables, we fitted the marginal linear regression models to the repeatedly measured responses using the generalized estimating equations (GEE) method15. We then calculated standardized regression coefficients and their 95% confidence intervals (CIs). GEE is efficient in achieving higher power with smaller sample size or lower number of repeated measurements in both complete and missing data scenarios16,17. We performed statistical analyses with SPSS for Windows, version 18.0 (SPSS Inc., Chicago, IL, U.S.A.) and R software, version 2.8.1 (Free Software Foundation, Inc., Boston, MA, U.S.A.). A normal distribution was attained by appropriate transformations of skewed variables such as aldosterone and ARR. The p-values <0.05 were considered statistically significant.
Result
Patient Characteristics
Twenty-one patients with APA receiving adrenalectomy (men, 12 (57.1%); age, 58.25 ± 21.92 years) and 20 patients with APA treated with spironolactone (men, 11 (55.0%); age, 57.25 ± 4.95 years) completed the BCM measurement and laboratory evaluation. Table 1 summarizes baseline subject characteristics before treatment. The operative group had significantly lower serum potassium and higher TNF-α level than those treated with spironolactone at enrollment. Baseline BCM profile was similar between patients in the two study groups.
Table 1. Clinical and biochemical characteristics of study patients.
| Adrenalectomy | Spironolactone | p | |
|---|---|---|---|
| Patients(n) | 21 | 20 | |
| Demography | |||
| Male (%) | 12(57.1%) | 11(55.0%) | 0.766 |
| Age (y/o) | 58.25 ± 21.92 | 57.25 ± 4.95 | 0.754 |
| BMI(kg/m2) | 24.9 ± 4.1 | 24.5 ± 3.1 | 0.897 |
| MAP(mmHg) | 107.7 ± 14.2 | 103.9 ± 14.2 | 0.421 |
| DM, n (%) | 2 (9.5%) | 3 (15%) | 0.643 |
| Aldosteronism profile | |||
| Sodium (mmol/L) | 139.1 ± 1.9 | 140.8 ± 3.5 | 0.157 |
| Potassium (mmol/L) | 3.62 ± 0.52 | 4.34 ± 0.88 | 0.004 |
| PAC (ng/dL) * | 38.1(33.3–54.7) | 53.3(36.5–80.7) | 0.149 |
| Log ARR | 2.42 ± 0.68 | 1.92 ± 0.86 | 0.082 |
| UNa (mmol/L) | 59.3 ± 34.8 | 73.1 ± 44.5 | 0.347 |
| UK (mmol/L) | 31.6 ± 20.8 | 33.1 ± 13.6 | 0.443 |
| Body composition | |||
| Relative OH (%) | 2.51 ± 5.45 | 5.12 ± 4.63 | 0.176 |
| Relative fat (%) | 28.8 ± 8.5 | 28.9 ± 6.9 | 0.897 |
| Relative LTM (%) | 59.7 ± 11.2 | 59.0 ± 8.8 | 0.988 |
| Other variables | |||
| HOMA-IR (mU/L·mmol/L) | 39.4 ± 40.0 | 45.8 ± 62.7 | 0.664 |
| C-reactive protein (mg/L) | 1.52 ± 2.13 | 1.93 ± 2.16 | 0.433 |
| TNF-α (pg/ml) | 8.47 ± 4.23 | 5.18 ± 1.56 | 0.009 |
| NT-proBNP (pg/ml) | 2.83 ± 2.82 | 6.07 ± 9.78 | 0.165 |
| ACR (mg/mg) | 0.47 ± 0.12 | 0.81 ± 0.21 | 0.393 |
| Creatinine (mg/dl) | 1.09 ± 0.59 | 0.93 ± 0.26 | 0.574 |
| Cystatin C (mg/dl) | 0.78 ± 0.14 | 0.82 ± 0.22 | 0.779 |
| Categories of hypertensive drugs before recruitment | |||
| β- blockers (%) | 7 (33.3) | 5 (25) | 0.789 |
| ACEI/ ARB (%) | 6 (28.6) | 7 (35) | 0.542 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ACR, Urine albumin-to-creatinine ratio ; APA, aldosterone producing adenoma; ARB, angiotensin II receptor blockers; ARR, aldosterone-renin ratio (ng/dL per ng/mL/h); BMI, body mass index; DM, Diabetes mellitus; HOMA-IR, homeostasis model assessment for insulin resistance; MAP, mean arterial blood pressure; NT-proBNP, N-terminal pro-brain natriuretic peptide; LTM, lean total mass; OH, overhydration; PAC, plasma aldosterone concentration ; TNF-α, Tumor necrosis factor-alpha; UK, urine potassium; UNa, urine sodium.
Data were provided as the mean values ± standard deviation, Significance was determined by Wilcoxon signed rank test in nonparametric distribution.
*Median (interquartile range).
Note: To convert potassium in mmol/L to mEq/L, multiple by 1; PAC in ng/dL to nmol/L, multiple by 0.02774; PRA in ng/mL/hr to ng/(Lxs), multiple by 0.2778.
Factors Associated with Relative OH at Enrollment
Correlations between baseline relative OH and other variables in the overall sample were assessed. Baseline relative OH was positively correlated with baseline serum Cystatin C (r = 0.456, p = 0.005) and urine albumin excretion (r = 0.336, p = 0.042) (Fig. 2).
Figure 2. Factors associated with relative overhydration (OH) at baseline.
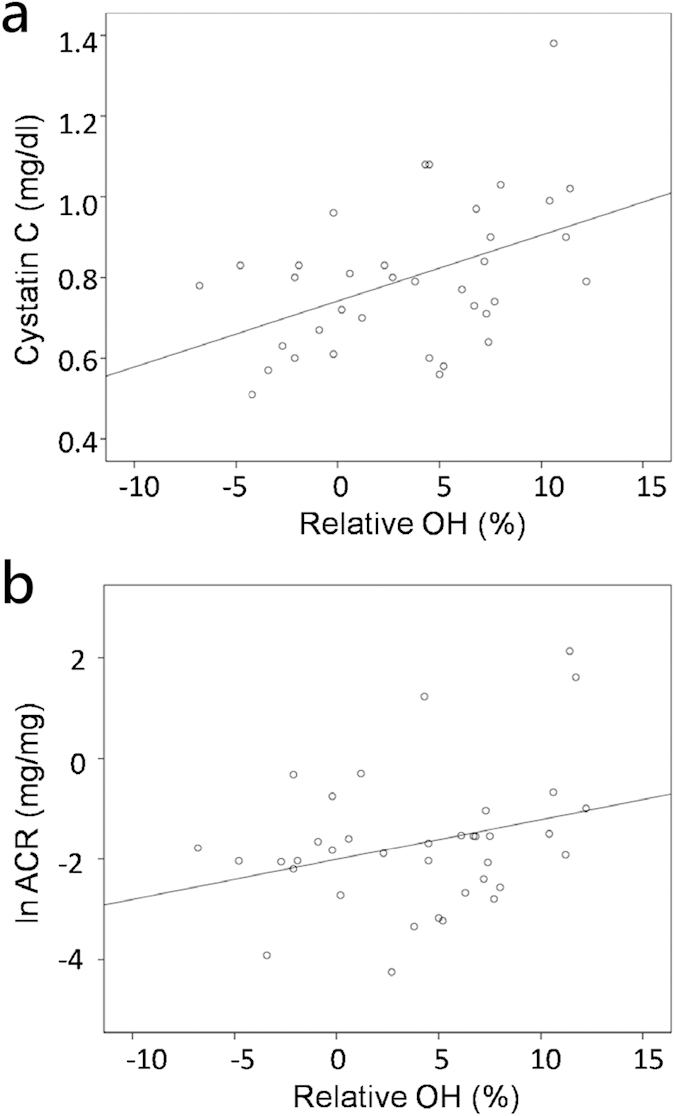
Univariate analysis of correlations of baseline relative OH with the (a) cystatin C, (b) ln urine albumin-to-creatinine ratio (ACR)(a) cystatin C vs. relative OH at baseline r = 0.456, p = 0.005. (b) ln urine albumin-to–creatinine ratio (ACR) vs. relative OH at baseline r = 0.336, p = 0.042.
Time Dependent Variables after Treatment
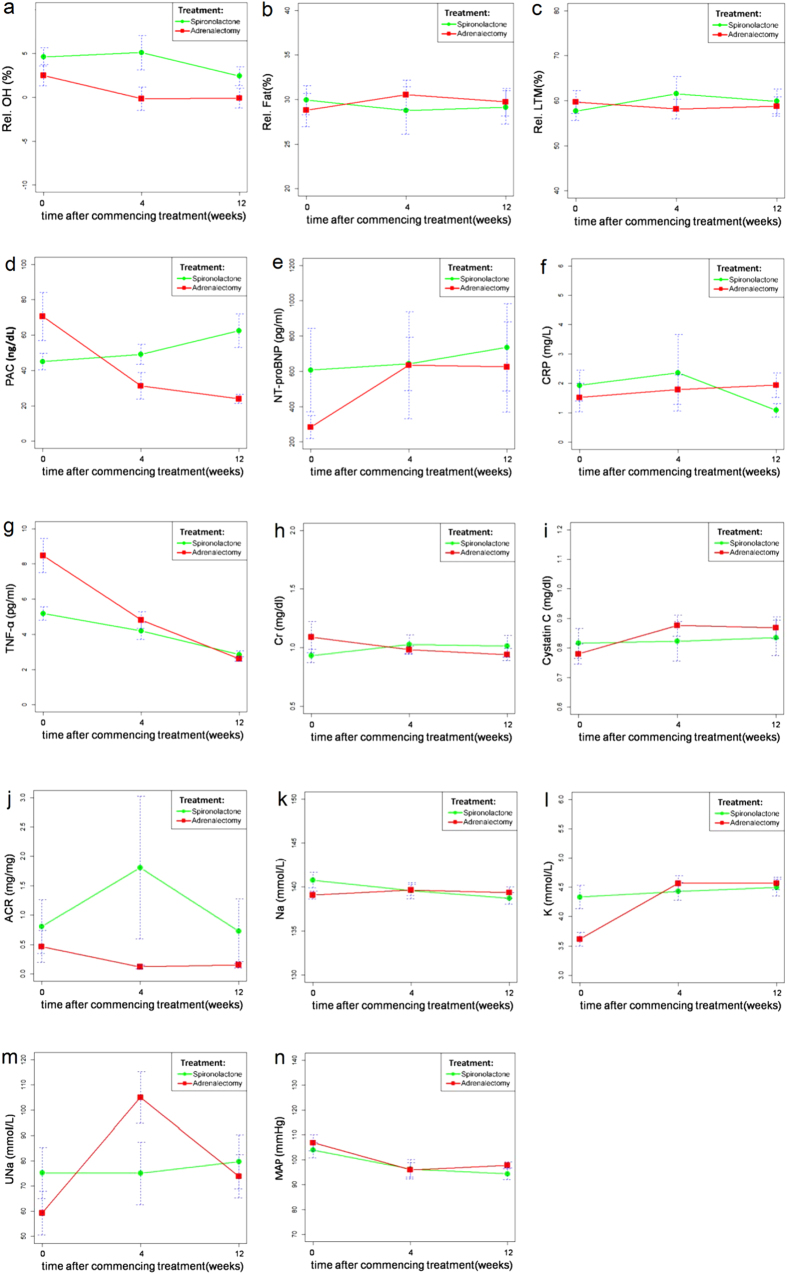
Compared with the data at enrollment, relative LTM, serum creatinine, Cystatin C, sodium and CRP did not significantly change during the study period. Plasma aldosterone concentration (PAC) in operative group decreased over time and that of spironolactone group increased over time. In both groups, relative OH, TNF-α, and MAP (mean arterial blood pressure) showed a time-dependent decrease (p = 0.008, <0.001, and <0.001, respectively) after treatment (Fig. 3).
Figure 3. Generalized estimating equations to examine the effect of different treatment strategies on various time-sequential variables.
Panels a-c: BCM parameters; Panels d-g: hormones and biomarkers; and Panels h-n: renal parameters. (a) The Relative overhydration (Rel. OH) between group, p = 0.062. Changes in time period contributed to changes in Rel. OH after 4 weeks (p = 0.011). Rel. OH was significantly different at 12 weeks (p = 0.008). (b) The Relative fat (Rel. Fat) between group, p = 0.034. Changes in time period contributed to changes in Rel. Fat after 4 weeks (p = 0.0105). (c) The Relative lean tissue mass (Rel. LTM) between group, p = 0.73. (d) The plasma aldosterone concentration (PAC) between group, p = 0.1942. PAC was significantly different at 4 weeks (p = 0.0034) and 12 weeks (p = 0.001). (e) The N-terminal pro-brain natriuretic peptide (NT-proBNP) between group, p = 0.448. (f) The C-reactive protein (CRP) between groups, p = 0.89. (g) The Tumor necrosis factor-alpha (TNF-α) between groups, p < 0.001. Changes in time period contributed to changes in TNF-α after 4 weeks (p < 0.001). TNF-α was significantly different at 12 weeks (p < 0.001). Changes in time period contributed to changes in TNF-α after 12 weeks (p < 0.001). (h) The serum creatinine (Cr) between groups, p = 0.46. (i)The cystatin C between groups, p = 0.570. (j) The urine albumin-to-creatinine ratio (ACR) between groups, p = 0.607. Changes in time period contributed to changes in ACR after 4 weeks (p = 0.003). (k) The serum sodium (Na) between groups, p = 0.49. (l) The serum potassium (K) between groups, p < 0.001. Changes in time period contributed to changes in K after 4 weeks (p < 0.001). Changes in time period contributed to changes in K after 12 weeks (p < 0.001). (m) The urine sodium (UNa) between groups, p = 0.26674. Changes in time period contributed to changes in UNa after 4 weeks (p < 0.001). (n) The mean arterial blood pressure (MAP) between groups, p = 0.7761. MAP was significantly different at 4 weeks (p = 0.003) and 12 weeks (p < 0.001).
Using GEE model, relative OH, relative fat, urine sodium excretion, and urine albumin excretion change over time differed significantly between patients in the two study groups at four weeks after treatment. Patients in operative group, as compared with those in spironolactone group, had significantly lower relative OH (p = 0.011), urine albumin excretion (p = 0.003), and significantly higher urine sodium excretion (p < 0.001), relative fat (p = 0.034). Nonetheless, these differences dissipated at 12 weeks after the commencing treatment. Relative OH at 12 weeks was significantly decreased in both modalities as compared with that at baseline. A significant difference of serum potassium was observed between the two groups, especially the augmentation from baseline to 12 weeks (p < 0.001) in operative group. TNF-α attenuation over time differed significantly between patients in the two study groups at 4 (p < 0.001) and 12 weeks (p < 0.001) after treatment. Patients in operative group had significantly lower TNF-α than spironolactone group after treatment.
Factor Predicted to Improve Relative OH after One Month
A larger proportion of patients in adrenalectomized group had decreased relative OH (p = 0.003) at four weeks. However, these kinds of trends were not significantly different at 12 weeks (Table 2).
Table 2. Relative Risks and 95% Confidence Intervals of Independent Factors to predict decreased overhydration 4 weeks after treatment by Multivariate Logistic Regression Model.
| Variable | β | Wald | p | Odds ratio | 95% CI |
|---|---|---|---|---|---|
| Adrenalectomy | 3.431 | 4.382 | 0.036 | 30.9 | 1.244 767.7 |
| Male sex | 4.444 | 4.072 | 0.044 | 85.1 | 1.136 6374 |
*All covariates listed in Table 1 are put into multivariate logistic regression model.
In multivariate regression models using demographics, aldosterone profile, BCM profile, and other variables listed in Table 1, patients who received adrenalectomy (OR 30.9, p = 0.036) and were males (OR 85.1, p = 0.044) could predict decreased relative OH at four weeks after treatment.
Discussion
To our knowledge, this is the first study to determine the body composition change after treatment in APA patients. We found that patients receiving adrenalectomy, as compared with those treated with spironolactone, had significantly lower relative OH, decreased TNF-α, urine albumin excretion, and higher urine sodium excretion at four week after treatment. Adrenalectomy might yield a therapeutic effect more rapidly, which has been shown to ameliorate cardiac hypertrophy in PA patients18.
On Target Treatment of PA
Long-term exposure to high aldosterone levels, independent of blood pressure level, could eventually lead to cardiovascular and renal structural and functional damage3. Previous studies showed that cardiovascular and renal damages seem to benefit from treatment, yet the relative efficacy of adrenalectomy compared with spironolactone have not been clearly evaluated19,20. Our present study further suggests that relative hyperfiltration in PA improved soon after adrenalectomy as reflected by decreasing microalbuminuria. Factors for reversed hyperfiltration after treatment include decreased extracellular fluid volume and postoperative aldosterone suppression, both of which were found to occur earlier in adrenalectomized patients in the current study.
There are several concerns about the treatment with spironolactone. Findings regarding benefits of spironolactone use are limited partly due to frequent occurrence of dose-dependent side-effects, especially gynecomastia, among Asian patients21 and subsequent decrease in efficacy and medication compliance. As shown in an Italian study, 28% of PA patients discontinued MR antagonists treatment 22. Spironolactone treatment may also induce an increase in aldosterone, and subsequently hyperaldosterone trigger a vicious cycle with nongenomic effects that leads to an insufficient effect of prescribed spironolactone on blocking MR21. Another concern about spironolactone treatment is the slow onset of efficacy of spironolactone treatment, which is in agreement with our finding in this study. Regression of LV hypertrophy requires a longer time to occur in patients treated with spironolactone than in those treated with surgery, regardless of the level of BP changes23. As demonstrated by our previous prospective study, proteinuria dropped off soon after adrenalectomy but still failed to significantly decrease one year after initiation of spironolactone treatment20. Using data of the German population-based survey, Reincke et al. investigated the risk factors associated with morality in PA patients. As compared with medical treatment of PA, adrenalectomy was associated with reduced all-cause mortality and proved to be protective24. In a long-term follow-up study, Catena et al. found that both surgical and medical treatment of PA effectively reduce left ventricular mass. However, the effects of adrenalectomy occur earlier and are greater than those of spironolactone25. A prospective study investigating blood pressure control demonstrated that the adrenalectomized patients required significantly less drugs than medically treated26. Among Japanese patients with APA, amelioration of hypertension significantly associated with surgical treatment, while medical treatment showed no relationship27. This disparity might be explained by the differing duration, severity of disease, and spironolactone dose.
Aldosteronism and Proinflammation
Most importantly, patients in the operative group had significantly attenuated TNF-α compared with patients in the spironolactone group. These results may be attributed directly to rapidly decreased PAC and possibly indirectly attributed to an earlier decrease in relative OH after adrenalectomy. Previous studies have demonstrated that PA is not merely an independent risk factor for CVD and cerebrovascular events28, but PA is also associated with increased level of oxidative stress and inflammatory biomarkers29,30. Aldosterone excess is detrimental not only through increased blood pressure but also through blood pressure independent pro-inflammatory effects on the heart and vessel walls24. A study has previously revealed that TNF-α mRNA and protein biosynthesis augmented in an adult feline myocardium resulted in volume overload31. The OH index was shown to be related to inflammation32 and a prediction to patients mortality33. Studies of Left Ventricular Dysfunction (SOLVD) have shown that circulating levels of TNF-α were elevated in patients with congestive heart failure, and that the level of TNF-α, leading to progressive cardiac dilatation and failure34, had a direct relationship with heart failure35. Adrenalectomy ameliorated aldosterone excess and led to an earlier improvement of volume overload, thus resulting in a more rapid decrease in TNF-α when compared to MR antagonist treatment.
Aldosteronism and Fluid Overload
Our data showed volume overload, a process that might be contribute to high blood pressure, cardiovascular structural damage, and poor patient outcome, might be effectively ameliorated in patients who underwent adrenalectomy for APA during the study period. The increase in blood pressure and volume in PA patients caused glomerular hyperfiltration and microalbuminuria20,36. Sechi et al. showed that PA is characterized by partially reversible kidney dysfunction37 and that albuminuria is a marker of a hemodynamic rather than a structural renal defect. Renal insufficiency has been reported to occur in 7%38 to 29%39 and proteinuria in 8%40 to 24%38 of PA patients. Subtle kidney impairment may be masked by hyperfiltration before treatment and intrarenal hemodynamic adaptation to the effects of aldosterone excess35. However, after a prolonged period of exposure, hyperaldosteronism eventually results in loss of glomerluar function. Our study revealed that fluid overload might become evident in patients who had kidney function impairment. Serum Cystatin C, a marker of kidney function, and urine albumin excretion were positively correlated with baseline relative OH.
Study Limitations
Our study has some limitations that should be acknowledged. We were unable to evaluate dietary fluid intake during the treatment period. Patients were over-hydrated if one’s sodium and water intake was excessive despite of treatment modality. Regarding medication treatment, we could not examine the dose-dependent effects of spironolactone. Furthermore, our study had a relatively small sample size. When there was no prior information on which to base a sample size, we were unable to present power analyses. However, the results of this pilot study provide evidence and guidance in the planning and design of further large scale trials.
Conclusions
In this pilot study, we found that adrenalectomy leads to the normalization of plasma aldosterone concentration followed by an earlier increase in renal sodium excretion and decreases in overhydration, TNF-α, and urine albumin excretion than patients who received spironolactone. Though spironolactone achieves a similar therapeutic effect 12 weeks after treatment, the long-term effects of surgery and medication on cardiovascular and renal outcome remain unclear. Large-scale investigation of this finding will be necessary, and timely identification of PA is important to maximize the benefits of target treatment.
Additional Information
How to cite this article: Wu, C.-H. et al. Effect of Treatment on Body Fluid in Patients with Unilateral Aldosterone Producing Adenoma: Adrenalectomy versus Spironolactone. Sci. Rep. 5, 15297; doi: 10.1038/srep15297 (2015).
Supplementary Material
Acknowledgments
The authors would like to thank the staff of the Second Core Lab in the Department of Medical Research in National Taiwan University Hospital for technical assistance. We thank the Taiwan Kidney consortium, TR15, National Research Program for Biopharmaceuticals. Clinical Trial Registration Number, NCT00746070; Date of registration: September 1, 2008
Footnotes
Author Contributions K.D.W. and V.C.W. designed research; C.H.W., Y.W.Y., Y.C.T., Y.H.H., T.S.C. and V.C.W. conducted research; S.C.H., Y.H.L. and V.C.W. analyzed data and performed statistical analysis; C.H.W. wrote the main manuscript text; all authors reviewed the manuscript.
References
- Young W. F. Jr. Minireview: primary aldosteronism–changing concepts in diagnosis and treatment. Endocrinology 144, 2208–2213 (2003). [DOI] [PubMed] [Google Scholar]
- Nomura K. et al. Plasma aldosterone response to upright posture and angiotensin II infusion in aldosterone-producing adenoma. J Clin Endocrinol Metab 75, 323–327 (1992). [DOI] [PubMed] [Google Scholar]
- Milliez P. et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45, 1243–1248 (2005). [DOI] [PubMed] [Google Scholar]
- Group T. S. et al. Association of kidney function with residual hypertension after treatment of aldosterone-producing adenoma. Am J Kidney Dis 54, 665–673 (2009). [DOI] [PubMed] [Google Scholar]
- Fardella C. E., Mosso L. M. & Carvajal C. A. [Primary aldosteronism]. Rev Med Chil 136, 905–914 (2008). [PubMed] [Google Scholar]
- Ichikawa S. et al. Effect of spironolactone on fluid volumes and adrenal steroids in primary aldosteronism. Jpn Circ J 48, 1184–1196 (1984). [DOI] [PubMed] [Google Scholar]
- Agarwal R., Andersen M. J. & Pratt J. H. On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol 3, 153–158 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthie J. R. Bioimpedance measurements of human body composition: critical analysis and outlook. Expert Rev Med Devices 5, 239–261 (2008). [DOI] [PubMed] [Google Scholar]
- Jaffrin M. Y. & Morel H. Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys 30, 1257–1269 (2008). [DOI] [PubMed] [Google Scholar]
- Moissl U. M. et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 27, 921–933 (2006). [DOI] [PubMed] [Google Scholar]
- Wabel P., Chamney P., Moissl U. & Jirka T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif 27, 75–80 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. D. et al. Preoperative diagnosis and localization of aldosterone-producing adenoma by adrenal venous sampling after administration of metoclopramide. J Formos Med Assoc 100, 598–603 (2001). [PubMed] [Google Scholar]
- Chamney P. W. et al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr 85, 80–89 (2007). [DOI] [PubMed] [Google Scholar]
- Wieskotten S. et al. Bioimpedance-based identification of malnutrition using fuzzy logic. Physiol Meas 29, 639–654 (2008). [DOI] [PubMed] [Google Scholar]
- Zeger S. L. & Liang K. Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42, 121–130 (1986). [PubMed] [Google Scholar]
- Ma Y., Mazumdar M. & Memtsoudis S. G. Beyond repeated-measures analysis of variance: advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med 37, 99–105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu V. C. et al. Effect of diuretic use on 30-day postdialysis mortality in critically ill patients receiving acute dialysis. PLoS One 7, e30836 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catena C. et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med 168, 80–85 (2008). [DOI] [PubMed] [Google Scholar]
- Sechi L. A., Colussi G., Di Fabio A. & Catena C. Cardiovascular and renal damage in primary aldosteronism: outcomes after treatment. Am J Hypertens 23, 1253–1260 (2010). [DOI] [PubMed] [Google Scholar]
- Wu V. C. et al. Primary aldosteronism: changes in cystatin C-based kidney filtration, proteinuria, and renal duplex indices with treatment. J Hypertens 29, 1778–1786 (2011). [DOI] [PubMed] [Google Scholar]
- Strauch B. et al. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens 21, 1086–1092 (2008). [DOI] [PubMed] [Google Scholar]
- Mulatero P. et al. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J Clin Endocrinol Metab 93, 1366–1371 (2008). [DOI] [PubMed] [Google Scholar]
- Catena C. et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension 50, 911–918 (2007). [DOI] [PubMed] [Google Scholar]
- Reincke M. et al. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension 60, 618–624 (2012). [DOI] [PubMed] [Google Scholar]
- Catena C. et al. Mineralocorticoid antagonists treatment versus surgery in primary aldosteronism. Horm Metab Res 42, 440–445 (2010). [DOI] [PubMed] [Google Scholar]
- Rossi G. P. et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension 62, 62–69 (2013). [DOI] [PubMed] [Google Scholar]
- Miyake Y. et al. Prognosis of primary aldosteronism in Japan: results from a nationwide epidemiological study. Endocr J 61, 35–40 (2014). [DOI] [PubMed] [Google Scholar]
- Mulatero P. et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab 98, 4826–4833 (2013). [DOI] [PubMed] [Google Scholar]
- Stehr C. B. et al. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J Hypertens 28, 2120–2126 (2010). [DOI] [PubMed] [Google Scholar]
- Vogt B. & Burnier M. Aldosterone and cardiovascular risk. Curr Hypertens Rep 11, 450–455 (2009). [DOI] [PubMed] [Google Scholar]
- Kapadia S. R. et al. Hemodynamic regulation of tumor necrosis factor-alpha gene and protein expression in adult feline myocardium. Circ Res 81, 187–195 (1997). [DOI] [PubMed] [Google Scholar]
- Vega A., Quiroga B., Abad S., Ruiz C. & Lopez-Gomez J. M. Study on overhydration in dialysis patients and its association with inflammation. Nefrologia 34, 579–583 (2014). [DOI] [PubMed] [Google Scholar]
- O’Lone E. L., Visser A., Finney H. & Fan S. L. Clinical significance of multi-frequency bioimpedance spectroscopy in peritoneal dialysis patients: independent predictor of patient survival. Nephrol Dial Transplant 29, 1430–1437 (2014). [DOI] [PubMed] [Google Scholar]
- Feldman A. M. et al. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol 35, 537–544 (2000). [DOI] [PubMed] [Google Scholar]
- Torre-Amione G. et al. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 27, 1201–1206 (1996). [DOI] [PubMed] [Google Scholar]
- Wu V. C. et al. Kidney impairment in primary aldosteronism. Clin Chim Acta 412, 1319–1325 (2011). [DOI] [PubMed] [Google Scholar]
- Sechi L. A. et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA 295, 2638–2645 (2006). [DOI] [PubMed] [Google Scholar]
- Nishimura M. et al. Cardiovascular complications in patients with primary aldosteronism. Am J Kidney Dis 33, 261–266 (1999). [DOI] [PubMed] [Google Scholar]
- Reincke M. et al. Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab 94, 869–875 (2009). [DOI] [PubMed] [Google Scholar]
- Danforth D. N. Jr., Orlando M. M., Bartter F. C. & Javadpour N. Renal changes in primary aldosteronism. J Urol 117, 140–144 (1977). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.