Abstract
Flow-mediated dilation (FMD) is a noninvasive technique used to measure conduit artery vascular function. Limited information is available on normative FMD values in healthy children and adolescents. The objective of this study was to assess relationships between age and sex with FMD across childhood and adolescence. Nine hundred and seventy-eight asymptomatic children (12 ± 3 yr, range 6–18 yr, 530 male) underwent ultrasonic brachial artery assessment before and after 5 min of forearm ischemia. Sex differences in FMD and baseline artery diameter were assessed using mixed linear models. Baseline artery diameter was smaller in females than males [2.96 mm (95% CI: 2.92–3.00) vs. 3.24 mm (3.19–3.28), P < 0.001] and increased with age across the cohort (P < 0.001). Diameter increased between ages 6 and 17 yr in males [from 2.81 mm (2.63, 3.00) to 3.91 mm (3.68, 4.14)] but plateaued at age 12 yr in females. Males had a lower FMD [7.62% (7.33–7.91) vs. 8.31% (7.95–8.66), P = 0.024], specifically at ages 17 and 18 yr. There was a significant effect of age on FMD (P = 0.023), with a reduction in FMD apparent postpuberty in males. In conclusion, the brachial artery increases structurally with age in both sexes; however, there are sex differences in the timing and rate of growth, in line with typical sex-specific adolescent growth patterns. Males have a lower FMD than females, and FMD appears to decline with age; however, these findings are driven by reductions in FMD as males near maturity. The use of age- and sex-specific FMD data may therefore not be pertinent in childhood and adolescence.
Keywords: endothelial function, aging, pediatrics, vascular health
although the clinical manifestations of cardiovascular disease (CVD) become apparent in mid to late life, atherosclerosis has its origins in childhood (26, 37), with early stages apparent during fetal development (5, 36). Effective early detection in individuals at increased risk of future CVD is critical for CVD prevention, yet commonly used cardiovascular risk algorithms based on “traditional” risk factors, such as the Framingham risk score, often fail to accurately predict the presence of atherosclerosis (19) and are unsuitable for use in children. These findings emphasize the need for alternative methods of risk stratification in young asymptomatic individuals.
Endothelial dysfunction is considered a manifestation of the cumulative impact of traditional risk factors and an early sign of atherosclerotic disease (42). It is present in young children who have CVD risk factors (8) and is reversed by interventions known to diminish CV risk (21, 43, 44). Assessment of endothelial function may potentially represent a quantitative method to assess the presence and extent of childhood CV risk before the development of overt disease. Ultrasound imaging during flow-mediated dilation (FMD) (8) is the noninvasive assessment of peripheral conduit artery diameter response to elevated flow and shear stress following a brief period (usually 5 min) of limb ischemia (39). FMD is primarily a nitric oxide-mediated, endothelium-dependent response (14) that strongly and independently predicts CV events among adults (15, 18).
Despite the widespread use of FMD in pediatric research to identify endothelial dysfunction, to our knowledge there are currently no published normative values from childhood to adolescence. As a result, endothelial dysfunction can only be defined in a study-specific context, rendering between-study comparisons of “abnormal” FMD problematic. The primary aim of the current study was to determine the differences in, and relationships between, FMD and age, and FMD and sex in a large group of healthy children and adolescents, and to provide normative reference values for FMD across childhood and adolescence.
METHODS
Nine hundred and seventy-eight asymptomatic children (12 ± 3 yr old, 530 male; Table 1) were included in data analysis. Data were generated by the Vascular Research Group at Liverpool John Moores University (n = 421), The Laboratory of Integrative Human Physiology at University of Minnesota (n = 526), and University of Western Australia (n = 31) from vascular research studies that occurred between 2008 and 2012. Via parental completion of a health screening questionnaire, all subjects were deemed healthy, normotensive, and not suffering from known CV or metabolic conditions. Subjects were not using any vasoactive medications, and participants with body mass index (BMI) >25 kg/m2 based on age- and sex-specific cut points were excluded (9). Ethics approvals were obtained from Liverpool John Moores University, University of Minnesota, and University of Western Australia Ethics Committees. Written and verbal informed consent was obtained from parents/guardians and children before participation in the study.
Table 1.
Descriptive statistics
| Cohort | Females | Males | P Value | |
|---|---|---|---|---|
| n | 978 | 448 | 530 | |
| Age, yr | 12.2 (12.0–12.4) | 12.3 (12.1–12.6) | 12.2 (11.9–12.4) | 0.241 |
| Height, m | 1.52 (1.52–1.54) | 1.52 (1.51–1.53) | 1.53 (1.52–1.55) | 0.286 |
| Weight, kg | 50.5 (49.41–51.98) | 50.01 (48.36–51.66) | 51.29 (49.36–53.21) | 0.369 |
| BMI, kg/m2 | 20 (20.6–21.2) | 21 (20.6–21.5) | 21 (20.4–21.2) | 0.412 |
| FMD, % | 7.94 (7.71–8.16) | 8.31 (7.95–8.66) | 7.62 (7.33–7.91) | 0.024 |
| Adjusted FMD, % | 8.44 (6.67–10.24) | 8.74 (6.06–11.05) | 8.15 (5.89–10.46) | 0.261 |
| Baseline artery diameter, mm | 3.11 (0.308–0.314) | 2.96 (2.92–3.00) | 3.24 (3.19–3.28)* | <0.001 |
| Time to peak, s | 60.7 (59.0–62.5) | 57.3 (55.8–59.8) | 63.6 (61.2–66.1)* | 0.001 |
Values are means (95% CI); n = no. of subjects. P value denotes significant sex difference. BMI, body mass index; FMD, flow-mediated dilation; time to peak, time to peak diameter post-cuff deflation during FMD test.
Subjects were required to attend a laboratory to complete anthropometric and vascular testing. All assessments were made in a quiet temperature-controlled room. Participants and their parents/guardians were informed that children must undertake a 4-h fast prior to assessment and avoid any strenuous physical activity for 24 h prior to testing. Height and weight were determined using a wall-mounted stadiometer and an electronic scale, respectively. BMI was calculated as the body weight in kilograms divided by the height in meters squared.
Assessment of conduit vessel function.
Brachial artery internal diameter was measured using high-resolution ultrasonography (Sequoia 512, Siemens, New York, NY or Terason t3000, Teratech) with an 8- to 12-MHz probe to visualize the brachial artery in longitudinal section. B-mode images were obtained at a reproducible point in the distal third of the upper arm. Ultrasonic parameters were set to optimize longitudinal, two-dimensional B-mode images of the luminal-arterial wall interface with the focal zone set to the near wall. Once set, these parameters remained constant throughout the session and the probe was held in a constant position. Endothelium-dependent vasodilation was assessed by measuring FMD after 5 min of forearm ischemia (28). Briefly, a 1-min baseline measurement was taken, and then a pneumatic rapid cuff inflator (Hokanson, Bellevue, WA) placed around the forearm distal to the humeral epicondyle was inflated to 220 mmHg for 5 min (39). Recording of the image ceased on inflation of the cuff and recommenced 30 s before deflation. Recording continued for a period of 3 min post-cuff deflation (6, 39). All laboratories involved in the study demonstrate good reproducibility for the FMD technique; when FMD was measured 7 days apart, the coefficient of variation was 11.1% for University of Minnesota, 14.7% for University of Western Australia (47), and 10.9% for Liverpool John Moores University (40).
Posttest analysis of brachial artery diameter.
Data were analyzed with the use of custom-designed automated edge-detection and wall-tracking software, the validity and reproducibility of which have been previously demonstrated (11, 13, 40, 47). B-mode frames were assessed by automated edge-detection software through the use of pixel density and frequency distribution algorithm. An optimal region of interest was initially selected by the sonographer from the first B-mode image on the basis of the quality of the image and discrimination between the intima and lumen, and then all subsequent analysis was conducted via the automated edge detection. Although the initial selection of the area of interest is operator determined based on optimizing image quality, all subsequent analysis is operator independent and carried out without investigator bias (16). Peak dilation during the each study was defined as the greatest percent change from resting baseline brachial artery diameter.
Statistical analysis.
All statistical analyses were performed using SPSS software (21.0; Chicago, IL). After visual inspection of the Q-Q plot for residuals created for all variables, residual data were deemed normally distributed. Sex differences were assessed using independent groups t-test or analysis of covariance. Pearson's correlation coefficients (two tailed) were used to describe the strength of relationships between the dependent variables (FMD and baseline artery diameter) and potential covariates [shear rate area under the curve (SRAUC), height, weight, and BMI]. Although significant associations existed between baseline artery diameter and height, weight, and BMI, collinearity diagnostics indicated that there were issues between age (independent variable), height, weight, and BMI [variance inflation factor (VIF) mean >10 and tolerance <0.2] (7, 29); as a result, it was deemed inappropriate to include these variables as covariates in the model. In line with previous findings (3), SRAUC was also omitted from the covariate analysis because no relationship was apparent between FMD and SRAUC (subsample with SRAUC data available, n = 580, r = 0.039, P = 0.348).
Baseline artery diameter and FMD were then analyzed for differences by age, sex, and age × sex interaction using mixed linear modeling. In addition, FMD data were analyzed with covariate control for baseline artery diameter (adjusted FMD); this approach allows FMD to be scaled for changes in artery diameter (2, 4). Statistically significant differences were followed up with the least significant difference (LSD) approach to multiple comparisons (31). Regression analysis by sex was performed to determine the relationship between age and primary outcomes. Finally a three-way (laboratory × age × sex) mixed linear model was employed to determine any between laboratory differences across the cohort. Data are means (95% CI) unless stated otherwise, and statistical significance was taken as P < 0.05.
RESULTS
Differences between laboratories.
There were no significant differences in FMD (P = 0.160) or baseline artery (P = 0.398) diameter across ages or sex between laboratories.
Descriptive data.
Descriptive characteristics for the whole cohort and by sex are presented in Table 1. The distribution of children in each age group and descriptive data by age are presented in Table 2. There were no significant differences between males and females for age, height, weight, or BMI (P > 0.05; Table 1). Across the cohort and in both sexes, height, weight, and BMI increased with age (P < 0.05; Tables 2, 3, and 4). However, males had a significantly larger baseline brachial artery diameter (3.24 ± 0.56 vs. 2.96 ± 0.43 mm, P < 0.001; Table 1) and took longer to reach peak diameter following cuff release (63.6 ± 28.2 vs. 57.3 ± 26.4 s, P < 0.001).
Table 2.
Descriptive statistics by age in yearly decrements
| Age, yr | n | Height, m | Weight, kg | BMI, kg/m2 | FMD, % | Adjusted FMD, % | Baseline artery diameter, mm | Time to peak, s |
|---|---|---|---|---|---|---|---|---|
| 6 | 29 | 1.18 (1.16–1.20) | 22.21 (20.88–23.55) | 15.9 (15.0–16.7) | 8.72 (7.36–10.08) | 10.07 (7.54–12.66) | 2.62 (2.45–2.79) | 64 (55–75) |
| 7 | 32 | 1.25 (1.23–1.26) | 25.65 (23.97–27.32) | 16.5 (15.6–17.4) | 7.93 (6.44–9.42) | 6.15 (3.65–8.70) | 2.80 (2.65–2.49) | 75 (65–87) |
| 8 | 40 | 1.29 (1.27–1.31) | 38.20 (26.21–30.19) | 16.9 (15.9–17.9) | 7.30 (6.23–8.37) | 12.05 (9.59–14.56) | 2.84 (2.69–2.99) | 62 (53–71) |
| 9 | 66 | 1.36 (1.34–1.38) | 34.59 (32.26–36.92) | 18.6 (17.7–19.5) | 7.99 (7.21–8.77) | 8.66 (6.90–10.45) | 2.93 (2.82–3.04) | 64 (57–70) |
| 10 | 133 | 1.41 (1.40–1.42) | 37.48 (36.08–38.88) | 18.8 (18.3–19.4) | 8.75 (8.02–9.47) | 9.57 (7.67–9.90) | 2.90 (2.83–2.97) | 67 (62–72) |
| 11 | 150 | 1.47 (1.46–1.48) | 43.20 (41.11–45.28) | 19.8 (19.1–20.6) | 8.28 (7.66–8.89) | 8.78 (5.86–9.38) | 2.90 (2.84–2.96) | 64 (60–69) |
| 12 | 106 | 1.53 (1.51–1.54) | 49.07 (46.46–51.68) | 20.8 (20.0–21.6) | 7.69 (7.00–8.38) | 7.96 (6.64–9.29) | 3.07 (2.99–3.15) | 56 (51–61) |
| 13 | 91 | 1.61 (1.59–1.63) | 58.01 (55.38–60.63) | 22.4 (21.5–23.2) | 7.95 (7.27–8.62) | 8.31 (6.86–9.78) | 3.17 (3.09–3.25) | 56 (52–61) |
| 14 | 79 | 1.66 (1.64–1.68) | 64.36 (59.41–65.30) | 22.5 (21.6–23.5) | 7.38 (6.65–8.10) | 7.11 (5.56–8.68) | 3.33 (3.23–3.43) | 61 (55–67) |
| 15 | 77 | 1.69 (1.67–1.71) | 65.52 (61.99–69.06) | 22.9 (21.9–23.9) | 7.14 (6.47–7.81) | 7.31 (5.73–8.92) | 3.37 (3.26–3.47) | 54 (48–60) |
| 16 | 70 | 1.69 (1.66–1.71) | 69.41 (64.59–74.23) | 24.2 (22.8–25.7) | 8.11 (7.14–9.08) | 8.24 (6.60–9.90) | 3.27 (3.15–3.40) | 56 (50–63) |
| 17 | 51 | 1.73 (1.70–1.75) | 72.22 (67.17–77.28) | 24.1 (22.7–25.5) | 7.99 (6.89–9.09) | 7.26 (5.27–9.28) | 3.60 (3.43–3.78) | 60 (51–70) |
| 18 | 54 | 1.76 (1.74–1.79) | 77.80 (72.04–83.55) | 25.0 (23.2–26.7) | 7.11 (6.31–7.92) | 8.26 (6.35–10.21) | 3.68 (3.52–3.84) | 50 (44–57) |
Values are means (95% CI); n = no. of subjects in each age group.
Table 3.
Descriptive data by age in females
| Age, yr | n | Height, m | Weight, kg | BMI, kg/m2 | FMD, % | Adjusted FMD, % | Baseline artery diameter, mm | Time to peak, s |
|---|---|---|---|---|---|---|---|---|
| 6 | 13 | 1.19 (1.16–1.22) | 21.32 (19.88–22.77) | 15 ± 1 (14.4–15.8) | 8.24 (6.25–10.22) | 8.09 (4.42–11.89) | 2.38 (0.21–0.26) | 65.5 (49.4–81.5) |
| 7 | 10 | 1.24 (1.20–1.27) | 24.95 (22.35–27.55) | 16 (14.9–17.5) | 8.91 (6.46–11.36) | 5.21 (1.14–9.43) | 2.76 (0.24–0.31) | 69.1 (59.9–88.3) |
| 8 | 11 | 1.30 (1.26–1.34) | 29.10 (23.65–34.54) | 17 (14.2–20.2) | 6.29 (4.40–8.18) | 13.89 (9.70–18.25) | 2.63 (0.24–0.28) | 52.9 (33.9–71.8) |
| 9 | 23 | 1.37 (1.35–1.40) | 33.72 (31.04–36.39) | 18 (16.8–19.1) | 8.15 (6.77–9.53) | 9.30 (6.43–12.24) | 2.88 0.26–0.31) | 60.5 (51.5–69.5) |
| 10 | 68 | 1.41 (1.39–1.43) | 37.44 (35.68–39.20) | 19 (18.1–19.5) | 9.15 (8.07–10.23) | 9.57 (7.89–11.28) | 2.82 (0.27–0.29) | 70.7 (62.9–78.6) |
| 11 | 72 | 1.47 (1.46–1.49) | 44.80 (41.41–48.19) | 20 (19.2–21.7) | 8.49 (7.50–9.48) | 9.25 (7.65–10.86) | 2.78 (0.27–0.29) | 61.5 (54.7–68.4) |
| 12 | 53 | 1.54 (1.52–1.56) | 51.06 (47.29–54.83) | 21 ± 4 (20.1–22.4) | 7.95 (6.94–8.96) | 7.79 (5.96–9.65) | 3.06 (0.30–0.32) | 48.2 (2.7–53.6) |
| 13 | 51 | 1.60 (1.58–1.63) | 58.58 (55.08–62.07) | 23 (21.6–23.9) | 8.02 (6.99–9.05) | 8.52 (6.61–10.47) | 3.09 (0.30–0.32) | 51.8 (45.9–57.6) |
| 14 | 33 | 1.64 (1.62–1.67) | 60.89 (56.59–65.19) | 23 (21.0–24.2) | 7.27 (6.06–8.48) | 6.25 (3.90–8.65) | 3.21 (0.31–0.34) | 55.6 (45.1–66.2) |
| 15 | 35 | 1.65 (1.63–1.67) | 61.23 (58.38–64.08) | 23 (21.5–23.9) | 7.45 (6.39–8.50) | 7.84 (5.49–10.24) | 3.15 (0.30–0.33) | 46.7 (49.8–53.6) |
| 16 | 36 | 1.62 (1.58–1.66) | 63.07 (6.78–69.37) | 24 (21.8–25.6) | 8.71 (7.33–10.09) | 9.43 (7.12–11.80) | 3.03 (0.29–0.32) | 58.3 (47.8–68.7) |
| 17 | 21 | 1.65 (1.61–1.70) | 62.70 (56.23–69.17) | 23 (20.8–24.8) | 9.82 (7.92–11.73) | 8.55 (5.41–11.79) | 3.17 (0.30–0.33) | 47.2 (39.0–55.4) |
| 18 | 22 | 1.69 (1.65–1.73) | 71.74 (63.36–80.13) | 25 (22.2–28.1) | 8.64 (7.47–9.81) | 9.94 (6.99–12.97) | 3.22 (0.31–0.33) | 50.9 (42.3–59.5) |
Values are means (95% CI); n = no. of female subjects in each age group.
Table 4.
Descriptive by age in males
| Age, yr | n | Height, m | Weight, kg | BMI, kg/m2 | FMD, % | Adjusted FMD, % | Baseline artery diameter, mm | Time to peak, s |
|---|---|---|---|---|---|---|---|---|
| 6 | 16 | 1.17 (1.14–1.20) | 22.99 (20.74–25.25) | 16.6 (15.1–18.1) | 9.11 (7.06–11.17) | 12.09 (8.65–15.64) | 2.81 (2.63–3.00) | 64.3 (50.6–77.9) |
| 7 | 22 | 1.25 (1.23–1.27) | 25.98 (23.70–28.25) | 16.7 (15.4–17.9) | 7.48 (5.52–9.44) | 7.90 (4.29–9.98) | 2.81 (2.646–2.98) | 78.9 (64.4–93.3 |
| 8 | 29 | 1.29 (1.26–1.31) | 27.86 (25.78–29.94) | 16.8 (15.8–17.8) | 7.90 (6.64–9.15) | 10.23 (7.66–12.85) | 2.92 (2.73–3.11) | 64.9 (54.5–75.4) |
| 9 | 43 | 1.35 (1.32–1.37) | 35.07 (31.70–38.43) | 18.9 (17.7–20.1) | 7.90 (6.92–8.89) | 8.03 (6.00–10.11) | 2.95 (2.85–3.05) | 65.2 (55.8–74.6) |
| 10 | 65 | 1.41 (1.39–1.42) | 37.52 (35.27–39.77) | 18.9 (18.1–19.7) | 8.33 (7.35–9.30) | 9.56 (7.88–11.27) | 2.99 (2.88–3.11) | 63.5 (57.0–70.0) |
| 11 | 78 | 1.47 (1.45–1.49) | 41.72 (39.17–44.26) | 19.2 (18.4–20.1) | 8.08 (7.31–8.86) | 8.31 (6.77–9.87) | 3.01 (0.93–3.09) | 66.9 (60.1–73.8) |
| 12 | 53 | 1.52 (1.49–1.54) | 47.15 (43.49–50.81) | 20.3 (19.1–21.5) | 7.42 (6.46–8.39) | 8.13 (6.24–10.06) | 3.09 (2.97–3.21) | 63.0 (54.9–71.1) |
| 13 | 40 | 1.61 (1.59–1.64) | 57.28 (53.15–61.41) | 21.9 (20.6–23.2) | 7.86 (7.01–8.72) | 8.10 (5.93–10.30) | 3.27 (3.12–3.41) | 62.7 (55.3–70.1) |
| 14 | 46 | 1.67 (1.64–1.69) | 63.43 (59.29–67.57) | 22.5 (21.3–23.7) | 7.46 (6.53–8.38) | 7.98 (4.67–8.93) | 3.42 (3.29–3.56) | 64.5 (57.6–71.5) |
| 15 | 42 | 1.72 (1.70–1.75) | 69.19 (63.21–75.17) | 23.1 (21.4–24.8) | 6.89 (6.00–7.78) | 6.78 (6.22–20.35) | 3.55 (3.41–3.70) | 59.3 (50.5–68.2) |
| 16 | 34 | 1.75 (1.73–1.77) | 76.11 (69.17–83.06) | 24.8 (22.6–27.1) | 7.47 (6.08–8.87) | 7.05 (4.76–9.40) | 3.54 (3.37–3.70) | 54.3 (45.4–63.1) |
| 17 | 30 | 1.78 (1.75–1.80) | 78.89 (72.37–85.41) | 25.0 (23.1–26.9) | 6.71 (5.52–7.90) | 5.98 (3.56–8.46) | 3.91 (3.68–4.14) | 70.6 (55.0–86.1) |
| 18 | 32 | 1.82 (1.79–1.84) | 82.09 (74.23–89.95) | 24.9 (22.6–27.2) | 6.06 (5.08–7.05) | 6.61 (4.17–9.10) | 3.99 (3.80–4.19) | 50.9 (41.2–61.5) |
Values are means (95% CI); n = no. of male subjects in each age group.
Differences in baseline artery diameter.
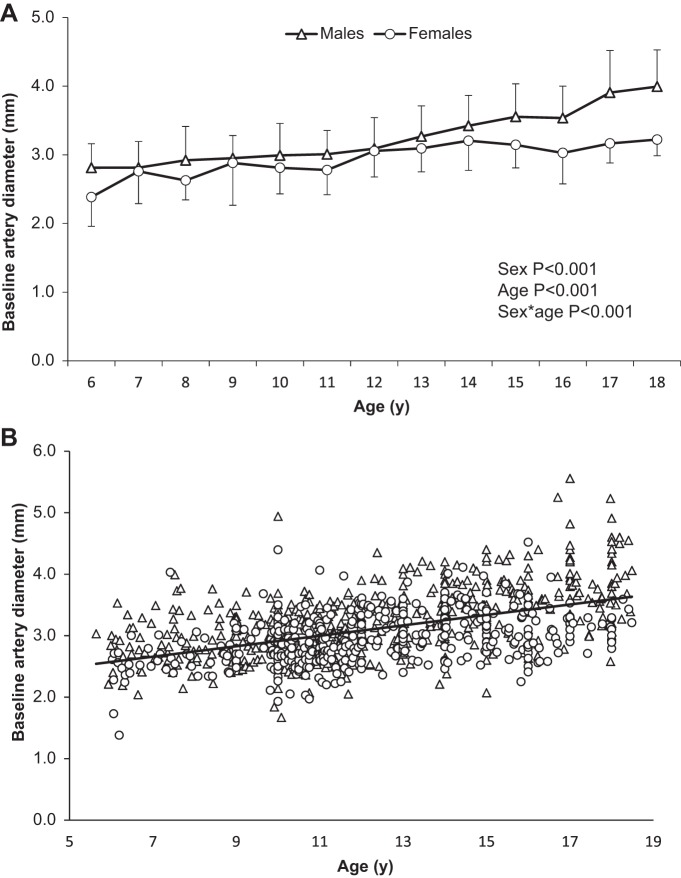
Baseline artery diameter was smaller in females than males [2.96 mm (2.92–3.00) vs. 3.24 mm (3.19–3.28), P < 0.001; Table 1], with significant differences apparent in males and females at all ages except 7, 8, and 12 yr (P < 0.05). Artery diameter increased with age across the cohort (P < 0.001), and there was a significant interaction between age and sex (P < 0.001; Fig. 1A); whereas diameter significantly increased year to year between ages 6 and 17 yr in males [from 2.81 mm (2.63–3.00) to 3.91 mm (3.68–4.14)], artery diameter size increased yearly before plateauing at age 12 yr in females [from 2.38 mm (0.21–0.26) to 3.06 mm (0.30–0.32); Fig. 1A]. Baseline brachial artery diameter was significantly predicted by age in both males (β = 0.103, P < 0.001) and females (β = 0.061, P < 0.001; Fig. 1B).
Fig. 1.
A: mean baseline brachial artery diameter data by age in females and males. B: distribution of baseline brachial artery diameter by age and sex across cohort, with a trend line for whole cohort data.
Differences in FMD.
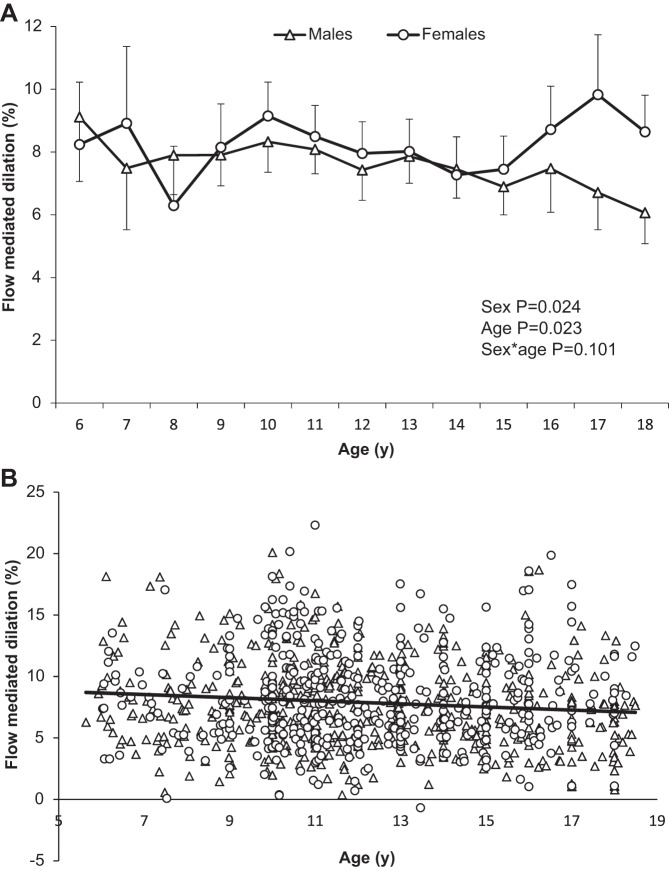
Females had a significantly larger FMD than males (P = 0.024; Table 1), although pairwise comparisons indicate that sex differences were apparent at ages 17 and 18 yr only (P < 0.01). FMD declined with age across the whole cohort (P = 0.023; Table 2 and Fig. 2A). Pairwise comparisons indicate that FMD at age 18 yr was significantly lower than at ages 6, 10, 11, 13, and 16 yr; in addition, FMD at age 8 yr was significantly lower compared with ages 6, 10, and 11 yr (P < 0.05). There was no interaction between age and sex for FMD (P = 0.101; Fig. 2A). Age significantly predicted FMD in the males (β = −0.202, P < 0.001), but not in females (β = −0.018, P = 0.770; Fig. 2B).
Fig. 2.
A: mean flow-mediated dilation (FMD) data by age in females and males. B: distribution of FMD values by age and sex across cohort, with a trend line for whole cohort data.
When FMD was adjusted for baseline artery diameter, sex differences were attenuated (P = 0.261; Table 1); however, the impact of age remained (P = 0.024; Table 2), with post hoc analysis revealing FMD was significantly higher at age 8 yr compared with all other groups except for ages 6 and 10 yr, and a significantly higher FMD at age 10 yr compared with ages 7, 14, and 15 yr (P < 0.05; Table 2). There was no age × sex interaction (P = 0.396). Regression models, including baseline artery diameter, indicate that age no longer affects FMD in males (β = 0.016, P < 0.166) but does affect FMD in females (β = 0.024, P = 0.014).
DISCUSSION
The primary aims of this cross-sectional study were 1) to determine the relationship between conduit artery endothelial function and age in healthy children and adolescents, 2) to assess the relationship between sex and FMD in these age groups, and 3) to generate age- and sex-specific reference data that can be used as a means of defining normal FMD values in young people. The results from our analysis indicate that FMD was significantly related to age in the whole cohort and that FMD is larger in females than males, although these sex differences are diminished when covariate control for baseline artery diameter is applied and, furthermore, appear to be driven primarily by differences in FMD at 17 and 18 yr; thus FMD does not appear to be age and sex dependent between the ages of 6 and 16 yr. Males had a larger baseline brachial artery diameter compared with females, and baseline brachial artery diameter increased with age in both sexes, although sex differences in the timing and rate of this growth were apparent, implying that age- and sex-specific data are more important for artery size than for endothelial function.
A previous review proposed the hypothesis that FMD may not alter during healthy childhood and adolescence (12). The basis of this hypothesis was derived from interpolation of data collected from multiple laboratories using different protocols (20, 27, 41, 46) in small samples of healthy children across a limited age range. However, it is accepted that the validity and reliability of FMD depends largely on consistency of methodology. We therefore aimed to address these limitations by pooling large sets of pediatric data collected in well-established vascular research laboratories (Liverpool John Moores University, University of Western Australia, and University of Minnesota), which followed the same experimental protocol and complied with current best practice guidelines (39). In line with the Fernhall and Agiovlasitis (12) hypothesis, and Marlatt et al. (23), who recently reported no differences in FMD across Tanner stages, we observed similar FMD across the younger ages but no differences in older adolescents, indicating that there is little difference in FMD throughout childhood and adolescence.
Females had a larger FMD than males (Table 1), specifically at ages 17 and 18 yr (Fig. 2A), and there was a small but significant decrement in FMD with age in males (Fig. 2B, β = −0.202, P < 0.001) but not females (Fig. 2B, β = −0.018, P = 0.770). As one may expect, when FMD is adjusted to account for baseline artery diameter, the decline in male FMD with age is attenuated (β = 0.016, P < 0.166). Interestingly though, this analysis revealed a very small but significant increase in FMD in females with age (β = 0.024, P = 0.014), which appears to be driven by a year-to-year increase in FMD from age 15 yr (Table 3). Taken together, these data indicate that the sex differences observed in our study are actually driven by improvements in female FMD that occur at ∼15–16 yr of age and imply that the end of puberty may therefore be a critical point for sex-specific changes in endothelial function. This finding is consistent with several studies indicating that higher estrogen levels are associated with enhanced vascular function (34). These data also suggest that the use of age- and sex-specific reference values for FMD may not be necessary in all pediatric age groups; instead, a single reference value may be suitable for children ages 6–16 yr. Accordingly, when the 17- and 18-yr-old were removed from the analyses, 95% CI data indicate a normal FMD would be in the range 7.7–8.2%.
Similarly to age, the impact of height and weight on FMD throughout childhood appears to be negligible. In agreement with our previous findings (17), we did not observe an association between FMD and weight in our cohort of healthy children; it should be noted, however, that children with BMI > 25 kg/m2 were excluded from the current study. We are therefore unable to comment on the impact of obesity on FMD throughout childhood and adolescents. Associations between FMD and height were of the same magnitude between the current and our previous observations (r = −0.10, P = 0.002 and r = −0.14, P = 0.11, respectively) but reached statistical significance in the current study due to the much larger sample size. Despite a significant relationship, the coefficient for height and FMD was weak and is arguably mediated via the much stronger relationship between height and baseline artery diameter (r = 0.553, P < 0.001). We observed moderate associations between baseline artery diameter and height and weight; however, these variables are also very strongly related to age (r = 0.871, P < 0.001 and r = 0.750, P < 0.001, respectively). Taking these findings together, there are clearly issues of collinearity to consider when choosing the most appropriate scaling methods, if any, for this type of study. With these issues in mind, and in line with our previous recommendations (17), we chose not to scale data for height or weight, and instead chose to perform allometric scaling of our FMD data for baseline artery diameter (2, 4).
Overall, baseline brachial artery diameter was smaller in females than males [2.96 mm (2.92–3.00) vs. 3.24 mm (3.19–3.28), P < 0.001], and differences were particularly apparent as males neared biological maturity (Fig. 1A). Age was a significant predictor of brachial artery diameter in both males and females. Furthermore, significant associations between baseline artery diameter and height and weight were also apparent in both sexes, which further supports the hypothesis that the size of the artery diameter may be mediated by maturation and growth. A steady age-related increase in brachial artery diameter is apparent in both males and females; however, the rate of increase becomes more marked as males reach the age of 13–14 yr and continues at this rate until peak at age 18 yr. Conversely, in the females, changes are subtle and peak brachial artery diameters appear much earlier, at around age 14 yr. Interestingly, the changes observed appear to coincide somewhat with the timing and changes in the tempo of growth expected in normal development (24, 25); however, due to having insufficient numbers of children with the necessary growth and maturational status data (Tanner stage or time from peak height velocity) in our cohort, we are unable to definitively comment on the developmental effects on vascular structure and function.
A critical explanation for our findings most likely relates to the different effects of estrogen and testosterone during puberty and between sexes. Estrogen modulates molecular pathways that improve vascular endothelial function (1) and may confer direct protection to the vessel wall (45). Accordingly, previous findings indicate that adult females have greater carotid artery compliance and distensibility than males; however, there were no sex differences apparent in children (22). Our findings support this previous study; despite finding an overall sex difference in FMD, this difference appeared to be primarily driven by changes in FMD in from ages 16–18 yr (Fig. 2A). Conversely, sex differences in baseline brachial artery diameter are apparent throughout childhood (Fig. 1A), but there is an upsurge in the rate at which males' artery diameter increases at age 12 yr. These changes appear to correspond to the onset of puberty in males (33, 38), when prolonged excretion of pituitary luteinizing hormone increases concentrations of testosterone and dihydrotestosterone, which in turn promote growth in muscle and other tissues (32), and may also influence the rate of growth of the vasculature. In females, recent data indicate that age at menarche is related to adult brachial artery diameter (35) and suggest that the hormonal changes, specifically in estrogen, brought about by the onset of menarche in females play a key role in regulating arterial diameter. This would appear to fit with the occurrence of the plateau in artery diameter in the females in the current study; however, because we do not have data related to the onset of menarche, we are unable to specifically test this hypothesis.
A key limitation that has prevented cross study/laboratory comparison of normal FMD data has been the lack of consistency in experimental protocols used between groups. One of the major strengths of the current analysis is the use of a clear protocol that has been set out as “standard” by the most recent methodological guidelines for the technique (39). Furthermore, compared with previous studies in which FMD data were collected across a limited age range of children, we have been able to provide data in healthy children from age 6 to 18 yr. Despite these strengths, there are some key limitations that should be taken into account when interpreting the data. First, we did not utilize a within-subject longitudinal study design, increasing the likelihood of between-subject variability influencing results. Nonetheless, previous data (30) imply that, even within subjects, exogenous factors can have a large impact on FMD. Second, we were unable to account for maturation in the current study; however, previous findings indicate that endothelial function does not differ by Tanner stage and thus imply that accounting for pubertal stage when reporting vascular data in children and adolescents may be unnecessary (23). Third, because we did not have sufficient artery wall or smooth muscle function data to assess their relationship with age and sex, we are unable to comment on the impact of age on these structural and functional parameters; however, Marlatt et al. (23) have previously demonstrated no changes in smooth muscle function over stages of pubertal development. Fourth, due to the retrospective nature of the current study, we were unable to collate SRAUC data for the whole sample (398 children without SR data); however, data from those with complete SRAUC data (n = 580) indicate no relationship between SRAUC and FMD (r = 0.039, P = 0.348), in line with previous studies in children which have found no relationship between SR and FMD. Although the protocol used to collect the vascular data was identical between laboratories, different analysis software was utilized in each laboratory; nonetheless, all laboratories used automated wall-tracking and edge-detection software programs that demonstrate good validity and reproducibility (10, 47), and analyses revealed no systematic difference in FMD by cohort, age group, or sex according to the analysis approach or specific laboratory, thus providing further support for the stringent adherence to the current best practice guidelines (39). Finally, we were unable to access age in months for all children in the cohort, so age in years was used in data analyses. We accept that this may have some impact on the findings given that there may be large chronological and maturational differences between children in the same age group; furthermore, although we made every effort to maximize participant numbers in each age group, there are some ages where sample size is relatively low, which may also potentially impact results.
Conclusions.
This is the first study, to our knowledge, that aimed to empirically determine the relationship between FMD, age, and sex in healthy children and adolescents. The results from our analysis indicate that there does appear to be age and sex differences in FMD, but that these differences are only apparent as adolescents near the mature state (>16 yr old). These data suggest that age- and sex-specific FMD data are not needed in pediatric populations. Additionally, we demonstrate a sex-specific increase in baseline artery diameter with age that appears to be associated with the timing and tempo of pubertal development.
GRANTS
This work was partially supported by the Liverpool Neighbourhood Renewal Fund, Liverpool City Council, and National Institutes of Health Grants R01 DK-072124-01A3 (to J. Steinberger), R01 CA-113930-01A1 (to J. Steinberger), M01 RR-00400, 1UL1 RR-033183, and UL1 TR-000114 (Clinical and Translational Science Institute, University of Minnesota-Twin Cities).
D. J. Green is supported by National Health and Medical Research Council of Australia Grant 1045204.
DISCLAIMERS
The views expressed in the submitted article are the authors' and not an official position of the named institutions or funders.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.D.H., A.S.K., J.S., H.Z., K.M., D.P., and L.H.N. performed experiments; N.D.H., A.S.K., J.S., H.Z., K.M., D.P., and L.H.N. analyzed data; N.D.H., D.R.D., G.S., A.S.K., and D.J.G. interpreted results of experiments; N.D.H. prepared figures; N.D.H. drafted manuscript; N.D.H., D.R.D., G.S., A.S.K., J.S., H.Z., K.M., D.P., L.H.N., and D.J.G. edited and revised manuscript; N.D.H., D.R.D., G.S., A.S.K., J.S., H.Z., K.M., D.P., L.H.N., and D.J.G. approved final version of manuscript; D.R.D., G.S., A.S.K., and D.J.G. conception and design of research.
REFERENCES
- 1.Arora S, Veves A, Caballaro AE, Smakowski P, LoGerfo FW. Estrogen improves endothelial function. J Vasc Surg 27: 1141–1146; discussion 1147, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis 226: 425–427, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson G, Batterham AM, Black MA, Cable NT, Hopkins ND, Dawson EA, Thijssen DH, Jones H, Tinken TM, Green DJ. Is the ratio of flow-mediated dilation and shear rate a statistically sound approach to normalization in cross-sectional studies on endothelial function? J Appl Physiol 107: 1893–1899, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson G, Batterham AM, Thijssen DH, Green DJ. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens 31: 287–291, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ. Fetal origins of coronary heart disease. BMJ 311: 171–174, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time-course of flow-mediated dilation (FMD) in humans. Hypertension 51: 203–210, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Bowerman BL, O'Connell RT. Linear Statistical Models: An Applied Approach. Pacific Grove, CA: Duxbury, 1990. [Google Scholar]
- 8.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Cole TJ, Bellizzi MC, Flegal KM, Deitz WM. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320: 1240–1243, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dengel DR, Jacobs DR, Steinberger J, Moran AM, Sinaiko AR. Gender differences in vascular function and insulin sensitivity in young adults. Clin Sci (Lond) 120: 153–160, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dengel DR, Kelly AS, Zhang L, Hodges JS, Baker KS, Steinberger J. Signs of early sub-clinical atherosclerosis in childhood cancer survivors. Pediatr Blood Cancer 61: 532–537, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Fernhall B, Agiovlasitis S. Arterial function in youth: window into cardiovascular risk. J Appl Physiol 105: 325–333, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Green DJ, Cheetham C, Reed C, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during lower limb exercise. J Appl Physiol 93: 361–368, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated? A meta-analysis. Hypertension 63: 376–382, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Green DJ, Reed C. Novel methods for simultaneous assessment of peripheral conduit and resistance vessel function in vivo. In: “ In vivo assessment of vascular function in humans”, edited by Duffy SD, and Chin-Dusting J. New York, NY: Nova Science Publications, 2006. [Google Scholar]
- 17.Hopkins ND, Green DJ, Tinken TM, Sutton L, McWhannell N, Cable NT, Stratton G, George K. Does brachial artery flow-mediated dilation scale to anthropometric characteristics? Eur J Appl Physiol 110: 171–176, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Johnson KM, Dowe DA, Brink JA. Traditional clinical risk assessment tools do not accurately predict coronary atherosclerotic plaque burden: a CT angiography study. AJR Am J Roentgenol 192: 235–243, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman CL, Kaiser DR, Steinberger J, Dengel DR. Relationships between heart rate variability, vascular function, and adiposity in children. Clin Auton Res 17: 165–171, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Kelly AS, Wetzsteon RJ, Kaiser DR, Steinberger J, Bank AJ, Dengel DR. Inflammation, insulin, and endothelial function in overweight children and adolescents: The role of exercise. J Pediatr 145: 731–736, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Marlatt KL, Kelly AS, Steinberger J, Dengel DR. The influence of gender on carotid artery compliance and distensibility in children and adults. J Clin Ultrasound 41: 340–346, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marlatt KL, Steinberger J, Dengel DR, Sinaiko A, Moran A, Chow LS, Steffen LM, Zhou X, Kelly AS. Impact of pubertal development on endothelial function and arterial elasticity. J Pediatr 163: 1432–1436, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 44: 291–303, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 45: 13–23, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGill HC Jr, McMahan CA, Herderick EE, Tracy RE, Malcom GT, Zieske AW, Strong JP. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol 20: 836–845, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Meyer A, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol 48: 1865–1870, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Myers RH. Classical and Modern Regression with Applications. Pacific Grove, CA: Duxbury/Thompson Learning, 1990. [Google Scholar]
- 30.Pahkala K, Heinonen OJ, Simell O, Viikari JS, Ronnemaa T, Niinikoski H, Raitakari OT. Association of physical activity with vascular endothelial function and intima-media thickness. Circulation 124: 1956–1963, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Perneger TV. What's wrong with Bonferroni adjustments. BMJ 316: 1236–1238, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogol AD. Androgens and puberty. Mol Cell Endocrinol 198: 25–29, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health 31: 192–200, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res 53: 597–604, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Schnabel RB, Biener MP, Wilde S, Sinning CR, Ojeda FM, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Espinola-Klein C, Blankenberg S, Munzel T, Wild PS. Sex differences in noninvasive vascular function in the community. J Hypertens 31: 1437–1446; discussion 1446, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Skilton MR, Evans N, Griffiths KA, Harmer JA, Celemajer DS. Aortic wall thickness in newborns with intraauterine growth restriction. Lancet 365: 1484–1486, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis 9: 19–32, 1989. [PubMed] [Google Scholar]
- 38.Tanner JM, Whitehouse RH, Marshall WA, Carter BS. Prediction of adult height from height, bone age, and occurrence of menarche, at ages 4 to 16 with allowance for midparent height. Arch Dis Child 50: 14–26, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-grand B, Sidi D, Girardet J, Bonnet D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 358: 1400–1404, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Vita JA, Keaney JF. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Watts K, Beye P, Siafarikas A, Davis EA, Jones TW, O'Driscoll G, Green DJ. Exercise training normalises vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol 43: 1823–1827, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Watts K, Beye P, Siafarikas A, Jones T, Davis E, Green DJ. Exercise training in obese children: effects on vascular function and body composition. J Pediatr 144: 620–625, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab 86: 5389–5395, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation 109: 1981–1986, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Green D. Improved analysis of brachial artery ultrasound images using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]