Abstract
We previously reported reduced limb muscle fiber succinate dehydrogenase (SDH) activity and capillarity density and increased cross-sectional areas (CSAs) of all fiber types in maintenance hemodialysis (MHD) patients compared with matched controls that may contribute to their effort intolerance and muscle weakness. This study evaluated whether endurance training (ET), strength training (ST), or their combination (EST) alters these metabolic and morphometric aberrations as a mechanism for functional improvement. Five groups were evaluated: 1) controls; 2) MHD/no training; 3) MHD/ET; 4) MHD/ST; and 5) MHD/EST. Training duration was 21.5 ± 0.7 wk. Vastus lateralis muscle biopsies were obtained after HD at baseline and at study end. Muscle fibers were classified immunohistochemically, and fiber CSAs were computed. Individual fiber SDH activity was determined by a microdensitometric assay. Capillaries were identified using antibodies against endothelial cells. Type I and IIA fiber CSAs decreased significantly (10%) with EST. In the ET group, SDH activity increased 16.3% in type IIA and 19.6% in type IIX fibers. Capillary density increased significantly by 28% in the EST group and 14.3% with ET. The number of capillaries surrounding individual fiber type increased significantly in EST and ET groups. Capillary-to-fiber ratio increased significantly by 11 and 9.6% in EST and ET groups, respectively. We conclude that increments in capillarity and possibly SDH activity in part underlie improvements in endurance of MHD patients posttraining. We speculate that improved specific force and/or neural adaptations to exercise underlie improvements in limb muscle strength of MHD patients.
Keywords: chronic renal failure, exercise training, muscle fiber size, muscle fiber oxidative capacity, muscle capillaries
patients with end stage kidney failure (ESKF) exhibit limb skeletal muscle dysfunction that culminates in impaired muscle strength and endurance capacity and that significantly curtails effort tolerance (9, 14, 24). Impaired exercise capacity in ambulatory patients with ESKF and, in particular, reduced skeletal muscle strength also may be potent predictors of mortality (11, 32).
We recently reported in vastus lateralis muscle biopsies from patients on maintenance hemodialysis (MHD) compared with normal controls, matched for age, sex, and ethnicity, that succinate dehydrogenase (SDH), a key mitochondrial oxidative enzyme, was significantly reduced in all muscle fiber types (21). In addition, there were marked ultrastructural changes in muscle fiber mitochondria in the MHD patients (21). We postulated that these findings, coupled with reduced capillary density (a decrease in number of capillaries per unit area of contractile muscle tissue), could at least partly explain the impaired muscle endurance capacity in MHD patients (21). We also noted that the cross-sectional areas (CSA) of all fiber types were increased in MHD patients compared with controls (21). We believe that this reflects individual fiber edema that, together with reduced contractile muscle tissue [caused by enhanced proteolysis and reduced fractional synthesis rate of mixed muscle proteins and myosin heavy chains (1, 4, 28)], may account for the decreased specific force (i.e., force per unit area of functional contractile muscle proteins).
Our group previously reported that endurance exercise training of MHD patients improved not only parameters of cardiovascular fitness but also the strength, power, and fatigability of the lower limbs over a 9-wk period (34). More recently, we examined the influences of 21 wk of endurance training (ET), strength training (ST), and their combination (EST) in a large cohort of MHD patients compared with MHD patients and appropriately matched normal healthy controls (CTL) who received no training (NT). Significant increments in exercise time (+96% and +80%), work rate (+35% and +71%), and total work (+113% and +138%) were observed in ET and EST groups, respectively (18). As might be expected, increased strength was noted in the ST (+286%) and EST (+149%) patients (19).
We hypothesized that the significant improvement in strength and endurance capacity of the MHD patients after training (19) would be at least in part accounted for by muscle cellular changes induced by the interventions. The aim of this study was therefore to evaluate changes in muscle fiber morphometry, oxidative capacity, and capillarity after ET, ST, or their combination (EST) in muscle biopsies before (21) and after training. It should be emphasized that the patients reported in this study are the same cohort of patients reported in previous publications pertaining to this expansive clinical trial (18, 19, 21, 37).
METHODS
Subjects.
Subjects studied were 59 patients (40 men and 19 women) undergoing maintenance hemodialysis (MHD) for a mean of 54.7 ± 8.6 (SE) mo (range, 6 to 297 mo) and 22 normal control (CTL) subjects (17 men and 5 women). The mean age was 42.1 ± 1.5 yr for HD and 40.9 ± 2.6 yr for CTL (see also Refs. 18, 19, and 21 for additional demographics). Five groups of subjects were evaluated: 1) CTL (n = 22); 2) MHD with no training (NT; n = 15); 3) MHD with endurance training (ET; n = 13); 4) MHD with strength training (ST; n = 17); and 5) MHD with a combination of endurance and strength training (EST; n = 14). The EST patients spent half as much time undergoing ST as the ST patients and half as much time undergoing ET as the ET patients. Training duration was 21.5 ± 0.7 wk. Additional details regarding the specific exercise training regimens and randomization procedures were previously reported (19).
Because MHD patients are less active physically, we sought to enroll CTL subjects who exhibited similar levels of a sedentary lifestyle. Other controlling criteria included: similar age, sex distribution, and racial/ethnic mix. For the healthy CTL, there was no evidence of chronic illnesses or acute inflammatory processes. The MHD patients were clinically stable during the study, with no evidence of acute or chronic inflammatory states, and absence of severe heart, lung, or liver failure or muscle or joint diseases. No patients had insulin-dependent diabetes, vasculitis, or a functioning renal transplant. None received corticosteroids or took excessive alcohol or illicit drugs. Both MHD patients and healthy CTL subjects were training naive. MHD was performed 3 times/wk, with each session lasting 4 h. This study was approved by the Human Subjects Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and by the Institutional Review Board of the Burns & Allen Research Institute at Cedars-Sinai Medical Center. Written informed consent was obtained from all subjects.
Skeletal muscle biopsies.
Biopsies were obtained from the right vastus lateralis muscle at baseline and at the end of the exercise training for MHD patients and again after an interval of 9.6 ± 0.2 wk in CTL subjects. These were obtained at a consistent site. Biopsies were performed on the right vastus lateralis muscle ∼10 cm cephalad to the superior border of the patella, 1-2 cm anterior to the midlateral line of the right leg, as previously described (19, 21, 37). All muscle samples were processed by a single experienced investigator (M.F.) in an identical fashion. The sample was viewed under a dissecting scope, rapidly blotted of extra fluid and cleaned of fat and blood if present, and the fiber orientation was verified. With small muscle biopsies, it is not feasible to control for fiber length as one would with a muscle strip or whole muscle. The muscle specimen was therefore prepared at resting length, mounted on cork with optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura Finetek U.S.A., Torrance, CA), oriented for transverse sectioning (i.e., with fibers perpendicular to cork surface), and then rapidly frozen in isopentane cooled to its melting point by liquid nitrogen. The fresh frozen muscle samples were stored at −80°C until analysis. All histochemical, quantitative histochemical, and immunohistochemical studies, including initial sectioning of muscle specimens, were performed by a single experienced investigator to minimize variation in sample processing and analysis.
Immunohistochemistry: muscle fiber types and capillaries.
For the assessment of muscle fiber classification and capillarity, serial cross-sections of the muscle sample were cut (10-μm thickness) using a cryostat (Reichert-Jung, model 2800E, Nussloch, Germany) kept at −20°C. Muscle cryosections were dried at room temperature, fixed in cold acetone for 5 min, washed with phosphate-buffered saline (PBS) for 5 min and incubated in 5% goat serum for 15 min at room temperature. For the identification of capillaries, muscle sections were exposed to a monoclonal mouse antibody (MAb; IgG1) against human endothelial cells, specifically platelet/endothelial adhesion molecule (PECAM-1) or CD31 (clone JC/70A; DAKO North America, Carpinteria, CA) for 1 h at room temperature. Various anti-myosin heavy chain (MyHC) MAbs were used for the indirect immunoperoxidase identification of MyHC isoforms within single fibers. Our aim in this study was to classify muscle fibers into the major fiber types only, i.e., based on the MyHC isoform mainly expressed. We did not specifically attempt to explore coexpressing/hybrid fibers. Serial muscle sections were incubated for 1 h at room temperature in one the following MAbs: A4.951, reacting with human MyHC I (β/slow); N2.261, reacting with human MyHCs I+IIA; and A4.74, reacting with human fast MyHCs (i.e., IIA+IIX). These mouse MAbs (IgG1) were raised from hybridoma cell lines obtained from the American Type Culture Collection.
Muscle morphometry.
Muscle fiber proportions (into types I, IIA, and IIX), fiber cross-sectional areas (CSAs), and capillaries were determined from microscopic images of digitized muscle sections, using a computer-based imaging-processing system. A microscope stage micrometer was used to calibrate the imaging system for morphometry. With a ×20 microscope objective, we determined that each pixel had an area of 0.15 μm2. Muscle fiber proportions, CSAs, and capillaries were determined from samples of 150–200 fibers for each subject. Group means for each fiber type were computed from the average values obtained from each individual subject (i.e., no pooling of fibers). Individual fiber CSAs were determined from the number of pixels within manually outlined fiber boundaries. Muscle capillarity was determined by 1) the capillary density, defined as the number of capillaries per square millimeter of muscle area; 2) the number of capillaries per fiber (and per fiber type), i.e., the average number of capillaries in physical contact with each fiber or the mean number of capillaries surrounding each fiber; and 3) the capillary-to-fiber ratio, i.e., the total number of capillaries divided by the total number of fibers within a muscle section.
Single fiber succinate dehydrogenase activity.
Fiber oxidative capacity was determined by quantifying the activity of succinate dehydrogenase (SDH; a key mitochondrial enzyme in the Krebs cycle) in individual muscle fibers. The methodology employed to quantitate SDH activity has been described in detail in previous reports (2, 3, 21). Briefly, in the histochemical reaction for SDH, the progressive reduction of nitroblue tetrazolium (NBT) to an insoluble colored compound (a diformazan) is used as a reaction indicator. The reduction of NBT is mediated by H+ ions released in the conversion of succinate to fumarate. In a series of 6-μm-thick sections, the incubation medium contained a large quantity of succinate (60 mM) and, thus, the SDH reaction was not substrate limited. In other sections, succinate was absent from the incubation medium, so that the reduction of NBT in these sections was nonspecific. These sections are referred to as tissue blanks.
The concentration of NBT diformazan (NBT-dfz) deposited within a muscle fiber was calculated using the Beer Lambert equation:
where OD was the optical density of the muscle fiber measured at 570 nm (the peak absorbance wave length for NBT-dfz), k was the molar extinction coefficient for NBT-dfz (26,478 mol/cm), and l was the path length (i.e., 6 μm section thickness) for light absorbance. The OD of muscle fibers was determined using a microdensitometric procedure implemented on the computer-based image processing system. The video image was then digitized into a matrix of 1,024 × 1,024 pixels (picture elements). The gray levels of the video scanner were calibrated for photometry (OD units) using a series of neutral density filters (0.004 to 2.00 OD units, Melles Griot, Rochester, NY). During the SDH reaction, the formation of NBT-dfz in muscle fibers increases linearly over a period of at least 7–9 min. In reactions where succinate was absent from the reaction medium, there was measurable staining (i.e., reduction of NBT), but the OD did not change significantly across the same time periods. The tissue blank OD also corresponded to the OD measured at time zero in reactions where succinate was present in the medium. On the basis of these data, we justified the use of a single end-point measurement of OD, with a reaction time of 5 min. The reaction was stopped at 5 min by immediately rinsing sections (both tissue blanks and those with succinate) with distilled water. Sections were dried, mounted, and were kept in the dark until images were scanned and digitized (∼2 to 3 h). In previous experiments, we have determined that there were no significant changes in individual muscle fiber OD values if sections were scanned/digitized within 10 h after the end of the SDH reaction (2, 3).
From these end-point measurements, a rate of SDH reaction was interpolated. Mean SDH activity of individual muscle fibers was determined by averaging the OD of all pixels within outlined muscle fibers. To correct for the nonspecific formation of NBT-dfz, the tissue blank OD for each fiber was subtracted from the OD measured when substrate was added to the incubation medium. From the Beer-Lambert equation, the mean SDH activity of each fiber was expressed as millimoles of fumarate per l of tissue per minute. Approximately 150–200 fibers (i.e., same fibers sampled for the measurement of cross-sectional area) were analyzed for SDH from each specimen. The SDH activity of each individual fiber was used to determine the mean SDH activity for each fiber type. Group means for each fiber type were computed from the average values obtained from each individual subject (i.e., no pooling of fibers).
Statistical analysis.
The distribution of data was tested for normality. Statistical analysis on MHD patients was performed using a mixed factorial (4 × 2, for training group and time) ANOVA with repeated measures on the pre-post data (SigmaStat v. 2.0, Jandel, Richmond, CA). No treatment interaction between ET and ST could be assessed because, as described above, the EST group is not equivalent to the combination of ET and ST, in that the EST group received only half the ET volume and half the ST volume, compared with either the ET or ST groups. Post hoc analysis (Bonferroni) was used to compare differences in independent groups. An α level of 0.05 was used to determine significance. Values are presented as means ± SE. Data from healthy sedentary control subjects are not included in the statistical analysis because their comparison with the entire MHD cohort prior to training was previously published (21). We, however, still incorporate the CTL data in the figures to allow a visual assessment of the relative changes after training in the MHD subgroups to the CTL subjects (i.e., how close or distant was any improvement in the various parameters tested).
RESULTS
Subject demographics and clinical data.
Characteristics and body composition of MHD patients and control subjects are provided in Tables 1 and 2 of our companion paper (21). Features of the five subgroups (i.e., CTL, NT, ET, ST, and EST) are provided in Table 1 of Ref. 18. See also methods.
Muscle fiber proportions and morphology.
The muscle fibers of the vastus lateralis muscle were classified based on their immunoreactions to antibodies against specific MyHCs, and the proportions of type I, IIA, and IIX muscle fibers were determined for CTL subjects and MHD patients. Compared with baseline, analysis of the muscle biopsies at the end of study showed no significant changes in the proportions of type I, IIA, and IIX muscle fibers after either type of training (Fig. 1). Compared with baseline, after the specific type of exercise training, the mean CSA of both type I and IIA fibers decreased significantly by 10% in the EST group (Fig. 2), from 5,223 ± 440 to 4,683 ± 370 μm2 for type I fibers (P < 0.05) and from 4,749 ± 422 to 4,291 ± 387 μm2 for type IIA fibers (P < 0.05). No significant changes were observed after ET or ST alone.
Fig. 1.
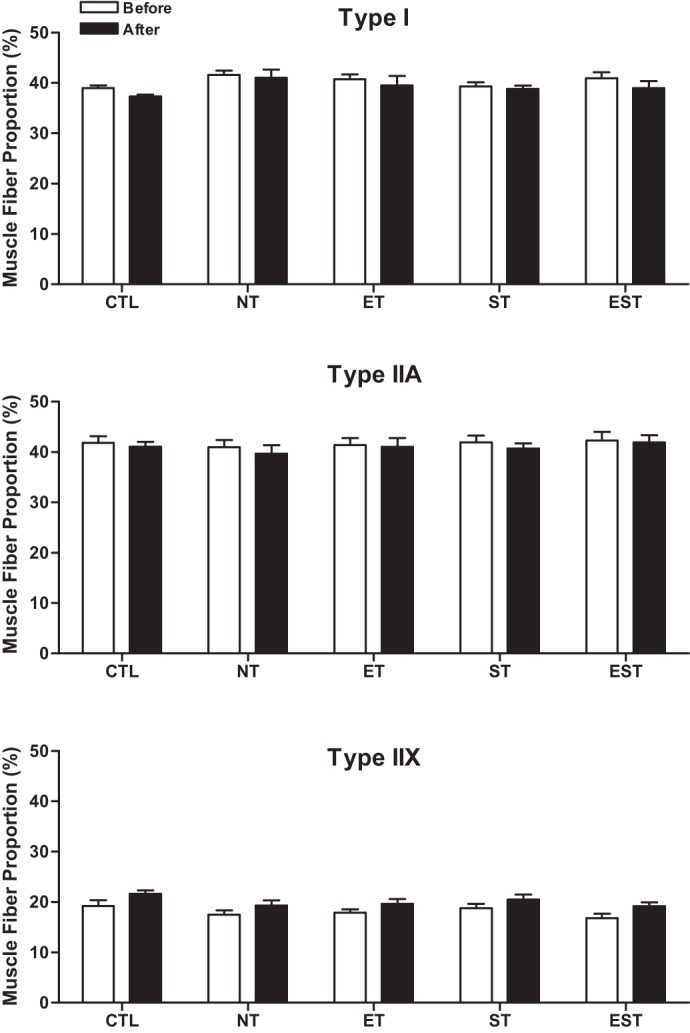
Mean proportions of type I, IIA, and IIX fibers in the vastus lateralis muscle before and after the training (T) period in healthy control (CTL) and maintenance hemodialysis (MHD) patients with no T (NT), endurance T (ET), strength T (ST), and their combination (EST). Values are means ± SE. Note: there were no significant changes in any muscle fiber type after either type of training.
Fig. 2.
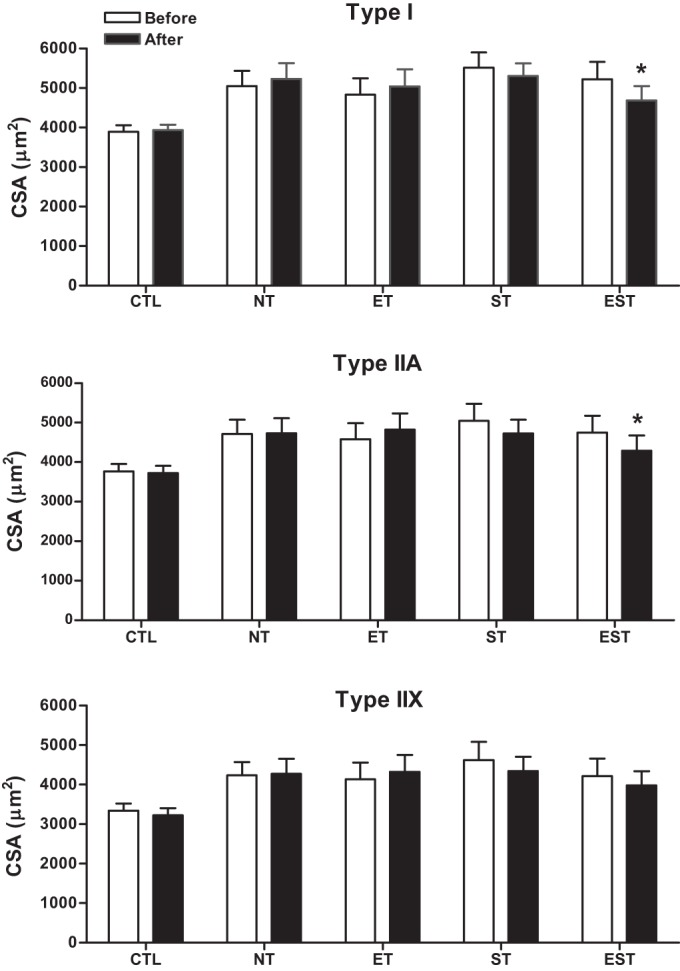
Mean fiber cross-sectional areas (CSA) from individual type I, IIA, and IIX fibers in the vastus lateralis muscle before and after the T period in CTL and MHD patients with NT, ET, ST, and EST. Values are means ± SE. Note: mean CSA of type I and IIA fibers decreased 10% with EST (*P < 0.05).
Mean SDH activity.
The mean SDH activities from individual type I, IIA, and IIX fibers of the vastus lateralis muscle were determined by a quantitative microdensitometric assay. Compared with baseline, after exercise training, there were no significant changes in the mean SDH activities of individual type I, IIA, and IIX fibers in any group. However, the mean SDH activity increased 16.3% in type IIA (P = 0.07) and 19.6% in type IIX (P = 0.06) fibers with ET and 8.9% in type IIA (P = 0.07) fibers with ST (Fig. 3).
Fig. 3.
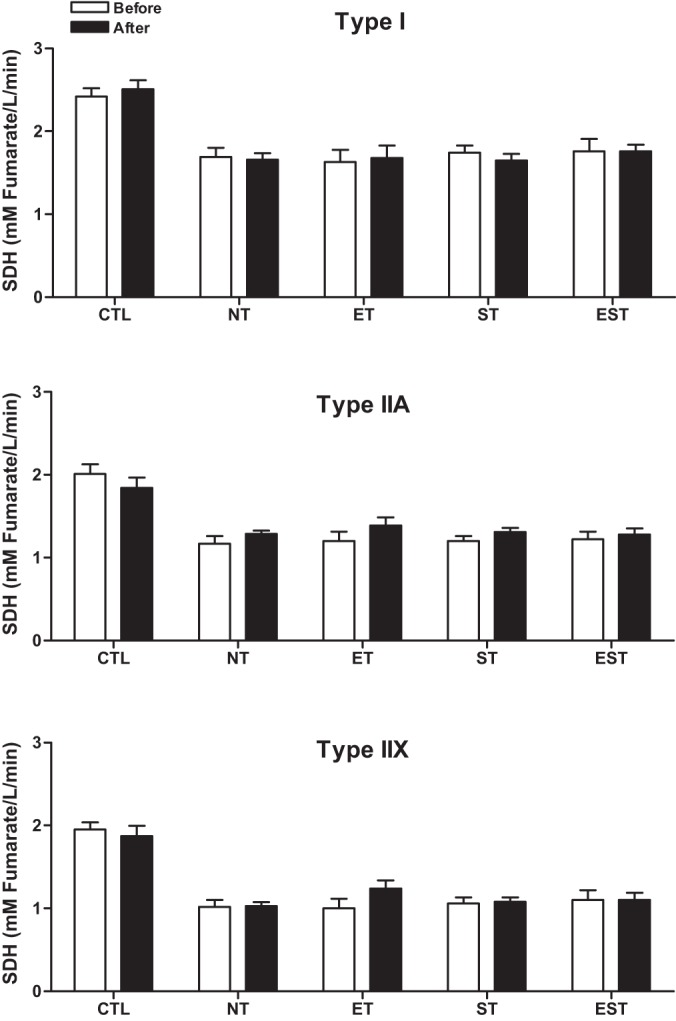
Mean succinate dehydrogenase (SDH) activity from individual type I, IIA, and IIX fibers in the vastus lateralis muscle before and after the T period in CTL and MHD patients with NT, ET, ST, and EST. Values are means ± SE. Note: mean SDH activity increased in type IIA (16.3%) and IIX (19.6%) fibers with ET and in type IIA (8.9%) fibers with ST but these changes were not significant.
Muscle fiber capillarity.
Compared with baseline, after training, the mean number of capillary contacts per individual fiber types increased significantly in type I (7%; P < 0.001), IIA (4%; P < 0.05), and IIX (3%; P < 0.05) fibers with EST and in type I (6%; P < 0.01) and IIA (4%; P < 0.05) fibers with ET (Fig. 4). No significant changes were observed with ST.
Fig. 4.
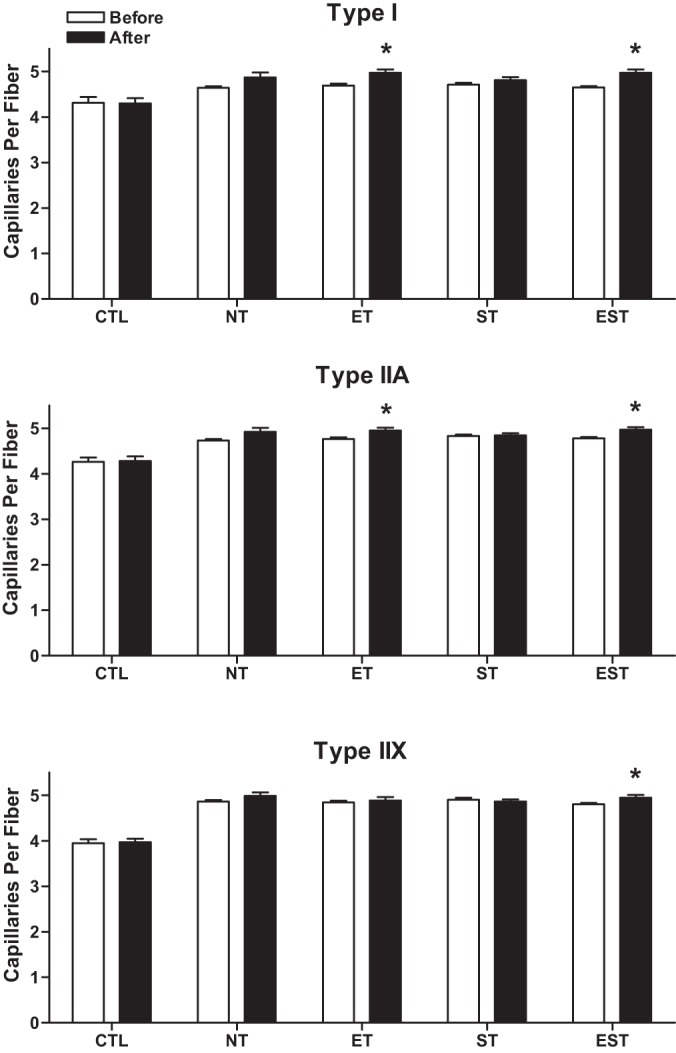
Mean number of capillaries per individual type I, IIA, and IIX fibers in the vastus lateralis muscle before and after the T period in CTL and MHD patients with NT, ET, ST, and EST. Values are means ± SE. Note: with EST the number of capillaries per individual fiber type increased in type I (7%, *P < 0.001), IIA (4%, *P < 0.05), and IIX (3%, *P < 0.05) fibers, whereas ET increased it in type I (6%, *P < 0.01) and type IIA (4%, *P < 0.05) fibers.
The capillary-to-fiber ratio, i.e., the total number of capillaries divided by the total number of fibers within a muscle section, increased significantly by 9.6% (P < 0.01) with ET and 11.0% (P < 0.001) with EST (Fig. 5). No significant changes were observed with ST.
Fig. 5.
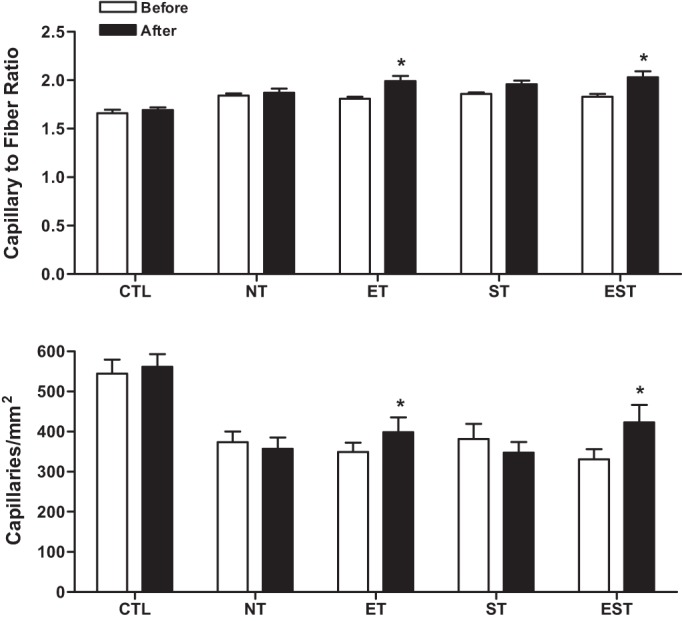
Mean capillary to fiber ratio (i.e., the total number of capillaries divided by the total number of fibers within a muscle section; upper panel) and mean capillary density (i.e., the number of capillaries per unit muscle fiber area; lower panel) in the vastus lateralis muscle before and after the training (T) period in healthy control (CTL) and MHD patients with no T (NT), endurance T (ET), strength T (ST) and their combination (EST).Values are means ± SEM. Note: The mean capillary to fiber ratio increased significantly by 9.6% (P < 0.01) with ET and 11.0% (P < 0.001) with EST. The mean capillary density significantly increased by 28% (P < 0.01) in EST patients and by 14.3% (P < 0.05) in the ET group.
Compared with baseline, after exercise training, the capillary density (i.e., the number of capillaries per square millimeter of muscle area) of the vastus lateralis muscle increased significantly in EST (28%; P < 0.01) and ET (14.3%; P < 0.05) patients (Fig. 5). There was no significant change in capillary density for the ST group.
DISCUSSION
In our companion paper (21), we postulated that impaired mitochondrial function and capillarity were important factors accounting for effort intolerance and decreased endurance in MHD patients. We also postulated that muscle fiber edema and likely reduced contractile tissue accounted for a reduction in force per unit CSA of muscle (i.e., reduced specific force/contractility), which would be an important factor accounting for impaired limb muscle strength in MHD patients. We also published previously improved endurance and strength in MHD patients subjected to exercise training (endurance, strength, or their combination) protocols (19, 34). Our current data indicate directions of change in several muscle characteristics that may contribute to the functional improvements reported (19).
Muscle fiber oxidative capacity.
At baseline, the mean SDH activities in type I, IIA, and IIX fibers of the vastus lateralis were reduced by 29, 40, and 47%, respectively, in our MHD cohort compared with healthy CTL subjects (21). These robust data are mirrored by muscle homogenate data for citrate synthase, cytochrome c oxidase, and β-hydroxyacyl-CoA dehydrogenase, other key oxidative mitochondrial enzymes (1, 7). Furthermore, a 27% reduction in the fractional synthesis rate of muscle mitochondrial proteins was reported with chronic renal failure (1), as well as distinct ultrastructural mitochondrial abnormalities in our MHD cohort (21).
In the present study, we did not observe a significant increase in SDH activity for any fiber type across groups. However, the fact that there was a 16.3 and 19.6% increment in SDH activity in type IIA and IIX fibers with ET (with a relatively small number of subjects), there is strong inference that these data might become significant with improved number of subjects and power, because combining all treatment groups yielded a significant increase in SDH activity (not reported). By contrast, the endurance training effects in both young and old (up to 70 years of age) subjects without comorbidities is robust in both groups for muscle citrate synthase activity in both the short and long terms (6, 22, 25, 35). The increment of oxidative capacity in our MHD subjects was much reduced compared with normal subjects, in whom an increment of 40% or more was observed with short-term endurance training (22, 26, 31). This suggests a much less robust response to exercise in our MHD patients. Our MHD patients were rather deconditioned (18, 19, 34), which likely decreased exercise intensity with concomitant reduced metabolic adaptation. In addition, there is disordered muscle protein turnover in patients with chronic renal failure (reduced mitochondrial protein synthesis and enhanced proteolysis). Many of the underlying factors (altered protein, amino acid, carbohydrate and lipid metabolism, inflammation, abnormal nutritional status, decreased daily physical activity, abnormal endocrine function, etc.) would be expected to persist during exercise training, thus blunting the adaptive response (18, 29). We speculate, because our ET cohort exhibited significant improvements in functional measures of endurance, that even small improvements in muscle oxidative capacity may be important contributors to the overall functional improvements noted.
Muscle fiber capillarity.
At baseline, we reported generally impaired capillarity in MHD patients (21), which would stress the diffusion capability for adequate nutrient and oxygen exchange, particularly under conditions of increased demand as in exercise (21, 30).
In the present study, significant increments in indices of capillarity were noted in those groups receiving endurance training. Of interest, studies in healthy subjects undergoing endurance training reported a similar increase in capillary density in young and old subjects, whereas other indices of capillarity increased by greater magnitudes [20–40% (10, 25)]. Apart from capillary density, other indices of capillarity were much more attenuated in the MHD patients compared with exercise training of healthy adult subjects. Again the intensity of training may have been a factor, as greater improvements in capillarity would be expected with enhanced intensity protocols, which our MHD subjects were unable to perform (25). The same argument has been applied to the frail elderly population in whom gains from rehabilitation programs may be attenuated because of limitations to muscle plasticity (5, 8, 33).
Muscle fiber morphometry.
At baseline in our MHD subjects, we reported increased CSA of all muscle fiber types (20), which we consider to be most likely due to fiber edema. This is consistent with the reported increased muscle intra- and extracellular total water in MHD patients (23). After exercise training in the present study, there were no changes in the CSA of any fiber type in any group except the EST patients, in whom a significant decline in CSA of 10% was observed in type I and type IIA fibers. Of note, in all the trained groups of our study, no differences were noted posttraining in fat free mass (FFM) of the limbs or of the whole body by DEXA scanning or by bioelectrical impedance measurements (19). Lack of hypertrophy, particularly with regard to the resistive training (ST and EST) in our study differs from the responses reported for healthy sedentary subjects, in which increments in quadriceps CSA of ∼6% were noted in elderly subjects with greater hypertrophy (14%) in young subjects with resistive training (20). There is also the concept of “interference effect” reported for concurrent endurance and strength training as demonstrated by Karavirta et al. (16), in which a 16% increment in type II fiber CSA of the vastus lateralis was observed with strength training, but no changes were observed in the combined strength and endurance training group. Thus our study patients differ in their morphometric profile response. This lack of morphological changes may reflect the decreased intensity of resistance training and the internal milieu of the MHD patients in which there are multiple stimuli promoting muscle proteolysis (28). Nevertheless marked increments in strength were observed in our MHD subjects. We postulate two possible mechanisms. First, strength training may have indeed induced anabolic effects in muscle fibers, as reflected by increased muscle IFG-1 (19) with a possible decrement in edema (as reflected by the decrease in fiber size in the EST group). This would provide more contractile muscle mass per fiber to improve strength and specific force. In addition, muscle levels of myostatin mRNA, a repressor of muscle anabolism and growth, was significantly reduced with training, and IL-6, which can promote muscle catabolism, was reduced by 40% in our MHD patients (19). Lastly, we previously reported a significant reduction in muscle atrogin-1 mRNA (a muscle-specific ubiquitin ligase, important in muscle proteolysis) after ST (compared with baseline) in our MHD subjects (38). Thus several pathway signals in our MHD subjects after training support increased muscle fiber protein synthesis and reduced muscle protein breakdown. Second, increased strength in the absence of hypertrophy could also be explained by neural adaptations to training, as reflected by significant increments of maximum integrated EMG in the vastus lateralis after either strength training or concurrent strength and endurance training (13). This reflects motor unit activation with training that results from either an increase in the number of active motor units and/or an increase in their firing frequencies (e.g., 12).
Two other studies of resistive training in MHD patients also showed significant improvements in leg muscle strength after restive training, but no significant change in lean body mass or muscle size (15, 17). Thus these studies would seem to support our findings described above.
Clinical implications and conclusions.
Our data have important clinical implications for MHD patients, because reduced aerobic capacity and muscle strength have been linked to poor survival (11, 32). Our data suggest that significant improvements in capillarity, and thus microvascular flow within the muscle for oxygen and nutrient transport and flux, account in part for improved endurance capacity with exercise training. This assumes no reduction in cardiac output and muscle blood flow as has been described in patients with chronic heart failure (27). It was also previously reported that patients with chronic renal failure retain the ability to increase the limb muscle mRNA abundance of VEGF, an angiogenic factor, with exercise (36). This, coupled with the strong “tendency” for increased SDH activity within individual muscle fiber types, suggest that MHD patients retain the ability to promote adaptations in the muscle “aerobic machinery,” although aerobic capacity is still markedly attenuated compared with healthy populations of untrained subjects (25). We also speculate that small biochemical and cellular adaptations may be sufficient to induce important physiological changes. Furthermore, our data provide strong inference for speculation that the improved strength observed in our subjects receiving resistive training may be explained in part by improved specific force and/or neuromuscular adaptations. Lastly, our study along with many in the literature, attests to the safety of exercise rehabilitation protocols that are geared to improve the strength and endurance of the exercising muscles in patients undergoing MHD.
GRANTS
This research was supported in part by funds from the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK054457 and National Heart, Lung, and Blood Institute Grant HL071227 and the resources of the General Clinical Research Center Grant M01-RR00425 and UCLA CTSI Grant UL1TR000124 of the National Center for Research Resources. R.C. occupies the Grancell/Burns Chair in the Rehabilitative Sciences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.I.L., M.F., T.W.S., R.C., and J.D.K. conception and design of research; M.I.L., M.F., T.W.S., R.C., and J.D.K. interpreted results of experiments; M.I.L. and M.F. drafted manuscript; M.I.L., M.F., H.W., T.W.S., R.C., and J.D.K. edited and revised manuscript; M.I.L. and J.D.K. approved final version of manuscript; M.F., H.W., T.W.S., R.C., and J.D.K. performed experiments; M.F. and H.W. analyzed data; M.F. prepared figures.
REFERENCES
- 1.Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab 278: E219–E225, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Blanco CE, Fournier M, Sieck GC. Metabolic variability within individual fibres of the cat tibialis posterior and diaphragm muscles. Histochem J 23: 366–374, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Blanco CE, Sieck GC, Edgerton VR. Quantitative histochemical determination of succinic dehydrogenase activity in skeletal muscle fibres. Histochem J 20: 230–243, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Boivin MA, Battah SI, Dominic EA, Kalantar-Zadeh K, Ferrando A, Tzamaloukas AH, Dwivedi R, Ma TA, Moseley P, Raj DSC. Activation of caspase-3 in the skeletal muscle during haemodialysis. Eur J Clin Invest 40: 903–910, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadore EL, Pinto RS, Bottaro M, Izquierdo M. Strength and endurance training prescription in healthy and frail elderly. Aging Dis 5: 183–195, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol 72: 1780–1786, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Conjard A, Ferrier B, Martin M, Callette A, Carrier H, Baverel G. Effects of chronic renal failure on enzymes of energy metabolism in individual human muscle fibers. J Am Soc Nephrol 6: 68–74, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Ehsani AA, Spina RJ, Peterson LR, Rinder MR, Glover KL, Villareal SDT, Binder EF, Holloszy JO. Attenuation of cardiovascular adaptations to exercise in frail octogenarians. J Appl Physiol 95: 1781–1788, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Fahal IH, Bell GM, Bone JM, Edwards RHT. Physiologic abnormalities of skeletal muscle in dialysis patients. Nephrol Dial Transplant 12: 119–127, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Gavin TP, Ruster RS, Carrithers JA, Zwetsloot KA, Kraus RM, Evans CA, Knapp DJ, Drew JL, McCartney JS, Garry JP, Hickner RC. No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol 55: 231–239, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Barany P, Heimberger O, Cederholm T, Stenvinkel P, Carrero JJ. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 9: 1720–1728, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häkkinen K. Neuromuscular adaptations during strength training, aging, detraining, and immobilization. Crit Rev Physiol Rehab Med 6: 161–198, 1994. [Google Scholar]
- 13.Häkkinen K, Alen M, Kraemer WJ, Gorostiaga E, Izquierdo M, Rusko H, Mikkola J, Häkkinen A, Valkeinen H, Kaarakainen E, Romu S, Erola V, Ahtiainen J, Paavolainen L. Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur J Appl Physiol 89: 42–52, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Johansen KL. Physical functioning and exercise capacity in patients on dialysis. Adv Ren Replace Ther 6: 141–148, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol 17: 2307–2314, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Karavirta L, Häkkinen A, Sillanpää E, Garcia-Lopez D, Kauhanen A, Haapasaari A, Alen M, Pakarinen A, Kraemer WJ, Izquierdo M, Gorostiaga EM, Häkkinen K. Effects of combined endurance and strength training on muscle strength, power and hypertrophy in 40–67-year-old men. Scand J Med Sci Sports 21: 402–411, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Kirkman DL, Mullins P, Junglee NA, Kumwenda M, Jibani MM, Macdonald JH. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle 5: 199–207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopple JD, Cohen AH, Wang H, Qing D, Tang Z, Fournier M, Lewis M, Casaburi R, Storer T. Effect of exercise on mRNA levels for growth factors in skeletal muscle of hemodialysis patients. J Ren Nutr 16: 312–324, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kopple JD, Wang H, Casaburi R, Fournier M, Lewis MI, Taylor W, Storer TW. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol 18: 2975–2986, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kraemer WJ, Hakkikinen K, Newton RU, Nindl BC, Volek JS, McCormick M, Gotshalk LA, Gordon SE, Fleck SJ, Campbell WW, Putukian M, Evans WJ. Effects of resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol 87: 982–992, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Lewis MI, Fournier M, Wang H, Storer TW, Casaburi R, Cohen AH, Kopple JD. Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol 112: 72–78, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meredith CN, Frontera WR, Fisher EC, Hughes VA, Herland JC, Edwards J, Evans WJ. Peripheral effects of endurance training in young and old subjects. J Appl Physiol 66: 2844–2849, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Montanari A, Graziani G, Borghi L, Cantaluppi A, Simoni I, Lorenzano E, Ponticelli C, Novarini A. Skeletal muscle water and electrolytes in chronic renal failure. Effects of long-term regular dialysis. Nephron 39: 316–320, 1985. [DOI] [PubMed] [Google Scholar]
- 24.Moore GE, Parsons DB, Stray-Gundersen J, Painter PL, Brinker KR, Mitchell JH. Uremic myopathy limits aerobic capacity in hemodialysis patients. Am J Kidney Dis 22: 277–287, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Murias JM, Kowalchuk JM, Richie D, Hepple RT, Doherty TJ, Patterson DH. Adaptations in capillarization and citrate synthase activity in response to endurance training in older and young men. J Gerontol A Biol Sci Med Sci 66: 957–964, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Orlander J, Aniansson A. Effect of physical training on skeletal muscle metabolism and ultrastructure in 70 to 75-year-old men. Acta Physiol Scand 109: 149–154, 1980. [DOI] [PubMed] [Google Scholar]
- 27.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302: H1050–H1063, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajan VR, Mitch WE. Muscle wasting in chronic kidney disease: the role of the ubiquitin proteasome system, and its clinical impact. Pediatr Nephrol 23: 527–535, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee CM, Kalantar-Zadeh K. Resistance exercise: an effective strategy to reverse muscle wasting in hemodialysis patients. J Cachexia Sarcopenia Muscle 5: 177–180, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sala E, Noyszewski EA, Campitol JM, Marrades RM, Dreha S, Torregrossa JV, Beers JS, Wagner PD, Roca J. Impaired muscle oxygen transfer in patients with chronic renal failure. Am J Physiol Regul Integr Comp Physiol 280: R1240–R1248, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52: 1888–1896, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 65: 719–724, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storer TW, Casaburi R, Sawelson S, Kopple JD. Endurance exercise training during hemodialysis improves strength, power, fatigability and physical performance in maintenance hemodialysis patients. Nephrol Dial Transplant 20: 1429–1437, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Suominen H, Heikkinen E, Liesen H, Michel D, Hollmann W. Effects of 8 weeks' endurance training on skeletal muscle metabolism in 56–70-year-old sedentary men. Eur J Appl Physiol Occup Physiol 37: 173–180, 1977. [DOI] [PubMed] [Google Scholar]
- 36.Wagner PD, Masanés F, Wagner H, Sala E, Miró O, Campistol JM, Marrades RM, Casademont J, Torregrosa V, Roca J. Muscle angiogenic growth factor gene responses to exercise in chronic renal failure. Am J Physiol Regul Integr Comp Physiol 281: R539–R546, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Casaburi R, Taylor WE, Aboellail H, Storer TW, Kopple JD. Skeletal muscle mRNA for IGF-IEa, IGF-II, and IGF-I receptor is decreased in sedentary chronic hemodialysis patients. Kidney Int 68: 352–361, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Storer TW, Casaburi R, Fournier M, Kopple JD. Skeletal muscle ubiquitin ligase atrogin-1 ligase mRNA is decreased with exercise training in maintenance hemodialysis patients. J Am Soc Nephrol 18: 484A, 2007. [DOI] [PubMed] [Google Scholar]


