Abstract
This study sought to determine if qualitative alterations in skeletal muscle mitochondrial respiration, associated with decreased mitochondrial efficiency, contribute to exercise intolerance in patients with chronic obstructive pulmonary disease (COPD). Using permeabilized muscle fibers from the vastus lateralis of 13 patients with COPD and 12 healthy controls, complex I (CI) and complex II (CII)-driven State 3 mitochondrial respiration were measured separately (State 3:CI and State 3:CII) and in combination (State 3:CI+CII). State 2 respiration was also measured. Exercise tolerance was assessed by knee extensor exercise (KE) time to fatigue. Per milligram of muscle, State 3:CI+CII and State 3:CI were reduced in COPD (P < 0.05), while State 3:CII and State 2 were not different between groups. To determine if this altered pattern of respiration represented qualitative changes in mitochondrial function, respiration states were examined as percentages of peak respiration (State 3:CI+CII), which revealed altered contributions from State 3:CI (Con 83.7 ± 3.4, COPD 72.1 ± 2.4%Peak, P < 0.05) and State 3:CII (Con 64.9 ± 3.2, COPD 79.5 ± 3.0%Peak, P < 0.05) respiration, but not State 2 respiration in COPD. Importantly, a diminished contribution of CI-driven respiration relative to the metabolically less-efficient CII-driven respiration (CI/CII) was also observed in COPD (Con 1.28 ± 0.09, COPD 0.81 ± 0.05, P < 0.05), which was related to exercise tolerance of the patients (r = 0.64, P < 0.05). Overall, this study indicates that COPD is associated with qualitative alterations in skeletal muscle mitochondria that affect the contribution of CI and CII-driven respiration, which potentially contributes to the exercise intolerance associated with this disease.
Keywords: COPD, exercise intolerance, mitochondrial dysfunction, mitochondrial respiration, muscle dysfunction
exercise intolerance and an increased oxygen (O2)-cost of physical activity are common features of chronic obstructive pulmonary disease (COPD) and are related to diminished quality of life and increased mortality in this population (4, 20, 31). While impaired lung function certainly plays a role in exercise intolerance, the fact that diminished physical function persists even after lung function is restored by transplantation or other therapeutic means (1, 5, 15) indicates that mechanisms peripheral to the pulmonary system are involved in this dysfunction. Impaired mechanical efficiency (i.e., increased ATP or O2 cost of contraction) has been documented in the exercising muscle of patients with COPD (3, 20, 31) and is thought to contribute to this debilitation. However, the underlying mechanisms involved in this decreased mechanical efficiency and their relationship to exercise intolerance in COPD remain uncertain.
During exercise, mechanical efficiency is influenced by both the efficiency of the mitochondria to resynthesize ATP (i.e., mitochondrial efficiency) and the efficiency of the muscle to translate ATP into mechanical work (i.e., ATP cost of contraction) (37). Our group recently reported that patients with COPD exhibit an increased ATP cost of muscle contraction which likely contributes to diminished mechanical efficiency (17, 18). However, mitochondrial efficiency, which can be influenced by either an alteration in the relative contributions of CI and CII-driven respiration in the electron transport chain (ETC) or an uncoupling of O2 consumption from ATP synthesis (i.e., nonphosphorylating respiration), was not investigated in this previous work (17, 18). Indeed, either or both of these scenarios of mitochondrial inefficiency could potentially play a role in the decreased mechanical efficiency and exercise intolerance observed in patients with COPD, but neither have been thoroughly investigated.
A shift in the contributions of CI and CII-driven respiration to peak respiration can affect mitochondrial efficiency, because electrons entering the ETC at CI, in the form of NADH, are at a higher energy state and subsequently result in the translocation of more protons than those entering at CII in the form of FADH2. Thus the FADH2-derived electrons ultimately yield fewer ATP per O2 consumed (i.e., a lower P/O ratio) than their NADH-derived counterparts (12). Therefore, a decreased contribution of CI-driven respiration in favor of a greater contribution of CII-driven respiration to overall respiration may result in a greater O2 cost for a given workload, and potentially result in exercise intolerance. Furthermore, uncoupled O2 consumption by the ETC occurs when hydrogen ions, pumped across the mitochondrial inner membrane in an O2-dependent manner, bypass ATP synthase and pass down the proton gradient by other means (e.g., uncoupling proteins), resulting in nonphosphorylating O2 consumption or proton leak (7). Currently, the extent to which uncoupled respiration is present in the skeletal muscle of patients with COPD is unclear. Indeed, the studies that have examined this facet of mitochondrial respiration in COPD have produced conflicting results reporting either exaggerated or unchanged uncoupling in COPD (22, 25, 29). Although these phenomena may contribute to the increased O2 cost of exercise recognized in patients with COPD (8, 22, 25) and ultimately negatively impact exercise tolerance, this hypothesis has not been extensively tested in this population.
Therefore, the purpose of this study was to determine if qualitative alterations in skeletal muscle mitochondrial respiration play a role in the exercise intolerance associated with COPD. Specifically, we hypothesized that, compared with healthy controls, patients with COPD would exhibit decreased mitochondrial efficiency with reduced utilization of CI-driven respiration relative to CII-driven respiration as well as increased uncoupling (State 2 respiration) and that this decreased mitochondrial efficiency may contribute to exercise intolerance during KE in the patients with COPD.
METHODS
Subjects.
Using newspaper advertisements, and word of mouth, 13 patients with moderate-to-severe COPD and 13 age-matched, healthy controls were recruited for this study based on spirometric evidence of airway obstruction (FEV1 < 80% predicted, FEV1/FVC < 0.70, where FEV1 is forced expiratory volume in 1 s and FVC is forced vital capacity), or the lack thereof (FEV1 > 80% predicted, FEV1/FVC > 0.70), respectively. These determinations were achieved by standard pulmonary function tests (9). Thigh volume and muscle mass were assessed by a series of circumference and skin fold thickness measurements of the upper leg, as previously described (19). Resting arterial O2 (SaO2) saturation was assessed with a pulse oximeter (Nellcor N-595, Pleasanton, CA) placed on the tip of the middle finger following 5 min of seated rest. All patients and controls completed the study with the exception of one control who withdrew for reasons unrelated to the study. The Institutional Review Boards at the University of Utah and the Salt Lake City Veterans Affairs Medical Center approved all protocols employed in this study. Accordingly, all subjects provided written informed consent before inclusion in this study.
Muscle biopsy.
Subjects reported to the laboratory for the muscle biopsy having refrained from vigorous exercise for 24 h. A muscle sample of the vastus lateralis muscle was obtained by a percutaneous needle biopsy 15 cm proximal to the knee at a depth of 3.5 cm under sterile conditions (31). Immediately after the muscle sample (∼150 mg) was taken from the leg, part of the sample (∼30 mg) was immersed in ice-cold biopsy preservation fluid (BIOPS) for respiratory analyses (23), while the remaining sample was immediately frozen and stored at −80°C for later histological and biochemical analysis.
Mitochondrial respiration and histochemical analyses.
Muscle samples were prepared and permeabilized for mitochondrial respiration analysis as described by Pesta et al. (23). Briefly, BIOPS-immersed fibers were carefully separated with fine-tip forceps and subsequently bathed in a BIOPS-based saponin solution (50 μg saponin/ml BIOPS) for 30 min. Following saponin treatment, muscle fibers were rinsed twice in ice-cold mitochondrial respiration fluid (MIR05) for 10 min each rinse. After rinsing for a total of 20 min, fibers were blotted with a paper towel to measure the weight of each sample (2-4 mg).
Muscle fibers were then placed in the temperature-controlled respiration chamber (Oxytherm, Hansatech Instruments. Norfolk, UK) in 2 ml MIR05 solution and warmed to 37°C. Note that the respiration chamber was calibrated daily, and that MIR05 was air saturated with O2 concentrations of ∼190 to ∼175 μM O2 from the start to finish of the experiment. After allowing the muscle fibers 10 min to equilibrate, mitochondrial respiratory function was assessed using the protocol described in Table 1 to determine the peak CI-driven respiration (State 3:CI), the peak CII-driven respiration (State 3:CII), and the peak CI+CII-driven respiration (State 3:CI+CII). Oxygen consumption not linked to phosphorylation (i.e., State 2) was also assessed (Table 1). Pilot studies indicated that the concentration of the substrates and inhibitors used were at saturating levels. Importantly, only samples that exhibited evidence of mitochondrial membrane integrity (less than a 10% increase in respiration in response to cytochrome c) were included in this study. Note that respiration data were acquired as the average respiration for the final minute of steady-state respiration for each step and that samples were run in duplicate and averaged across runs. Initially, respiration data were examined in terms of O2 flux per mg of tissue (wet weight) to obtain an indication of mitochondrial capacity per milligram of tissue. To further explore whether the mitochondria of patients with COPD exhibit qualitative changes in mitochondrial respiratory function, respiration was also examined as a percent of the peak respiration exhibited during State 3:CI+CII, as well as in terms of the ratio of State 3:CI to State 3:CII (i.e., CI/CII). As described by Pesta et al. (23), these internal ratios, which juxtapose one respiration state to another within an experimental run, provide insight into the qualitative function of the mitochondria in a mitochondrial content-independent manner, while introducing no extra variance. Immunofluorescence was used to determine muscle fiber type (Myosin Heavy Chain I: primary antibody BA-F8, secondary antibody Alexa Fluor 350; Myosin Heavy Chain IIa/IIx: primary antibody SC-71, secondary antibody Alexa Fluor 488) and capillarity (ab96884, Abcam, MA) with an Imager A2 microscope with Axiocam and accompanying software (Zeiss), as described in detail by Bloemberg and Quadrilatero (6). Chemicals used in the study were purchased from Sigma-Aldrich (St. Louis, MO), while primary antibodies for fiber typing were purchased from the Developmental Studies Hybridoma Bank (University of Iowa) and secondary antibodies from Invitrogen (Thermo Fisher Scientific).
Table 1.
Mitochondrial respiration protocol
| Step | Chemical Name (Concentration) | Major Site of Action | Respiration State |
|---|---|---|---|
| 1 | Malate (2 mM), glutamate (10 mM) | +Complex I (CI) | State 2 |
| 2 | ADP (5 mM) | +Complex V (CV) | State 3: CI |
| 3 | Succinate (10 mM) | +Complex II (CII) | State 3: CI+CII |
| 4 | Cytochrome c (10 μM) | Test of mitochondrial membrane integrity | |
| 5 | Rotenone (0.5 μM) | −CI | State 3: CII |
Description of the protocol used to assess mitochondrial respiratory function, the site of action of each chemical introduced to the preparation (+Substrate; −Inhibitor), and the respiration state associated with each step. Note that steady-state rates were achieved for each step, which took approximately 3 min, before proceeding to the next step.
Dynamic single leg knee extensor exercise.
Dynamic single leg KE exercise was performed on a custom-made KE ergometer (2, 33). After undergoing several familiarization visits, subjects performed exercise protocols to determine both maximum KE work rate (i.e., power) and endurance/exercise tolerance. Maximum KE work rate was determined with subject-specific incremental protocols (2–5 W/min) designed to reach a point of exhaustion within 8–12 min (30). KE endurance or exercise tolerance was determined by having subjects perform KE at 80% of their maximum work rate until exhaustion (34). In both KE tests, subjects were instructed to maintain a cadence of 60 rpm until exhaustion. Exhaustion or task failure was defined as the inability to maintain a cadence of greater than 50 rpm.
Assessment of physical activity.
Physical activity was assessed over the course of 7–10 days via accelerometry (Actigraph LLc, Pensacola Fl), which has recently been validated for use in COPD patients (28). These accelerometers, which assess physical activity in counts by summing the accelerations along three axes over the course of each minute, were worn by subjects during all waking hours, except when showering or swimming. Based on the recommendations of the manufacturer of the accelerometer, thresholds for sedentary, light, and moderate to vigorous activity were defined as <99, 100-1,959, and 1,952+ counts/min, respectively. Gait speed represents the usual walking speed (average of two trials) of the subjects over 10 m (35).
Statistical methods.
Differences in respiration states were analyzed with two-way repeated-measures ANOVA. Significant main effects or interactions were subsequently analyzed with Holm-Sidak post hoc test. Differences in subject characteristics between groups were made with independent samples t-tests. Correlations between variables were assessed with Pearson product-moment correlation. Data are represented as means ± SE and α = 0.05.
RESULTS
Subject characteristics.
As represented in Table 2, patients and controls were of similar age and BMI. However, by design, spirometric indices of lung function were decreased in the patients with COPD (P < 0.05). Nevertheless, resting O2 saturation was similar between groups. KE maximum work rate was not different between groups; however, KE endurance was greatly reduced in the patients with COPD (P < 0.05). Although all subjects underwent the biopsy procedure and completed all other testing, two of the patients with COPD failed to complete the KE protocols, for reasons unrelated to the study. Interestingly, there were no differences in fiber type or capillary density between the patients with COPD and the controls (P > 0.05). Gait speed and physical activity, assessed by both accelerometry and steps per day, were lower in the patients with COPD than the healthy controls (P < 0.05). Eleven of the thirteen patients with COPD were former smokers and reported an average time elapsed since quitting of 13 ± 3 yr (range 2–28 yr). Four of the controls were identified as former smokers and reported an average time elapsed since quitting of 39 ± 6 yr (range of 22–60 yr). One patient with COPD and none of the controls reported being current smokers. Aside from the medications listed in Table 2, subjects were free from any medications within the last 6 mo.
Table 2.
Subject characteristics
| Controls | COPD | P | |
|---|---|---|---|
| Subjects, n (female/male) | 12 (2f/10m) | 13 (3f/10m) | |
| Age, yr | 69 ± 2 | 66 ± 2 | 0.36 |
| BMI, kg/m2 | 26 ± 1 | 27 ± 2 | 0.73 |
| Lung function | |||
| FVC, liters | 4.8 ± 0.3 | 3.2 ± 0.2* | <0.001 |
| FEV1, liters | 3.5 ± 0.2 | 1.7 ± 0.2* | <0.001 |
| FEV1, %Predicted | 119 ± 6 | 55 ± 5* | <0.001 |
| FEV1/FVC, % | 79 ± 3 | 51 ± 4* | <0.001 |
| Resting SaO2, % | 95 ± 1 | 94 ± 1 | 0.57 |
| Physical activity and function | |||
| Knee extensor max, W | 29 ± 4 | 24 ± 4 | 0.38 |
| Knee extensor endurance, min | 14 ± 3 | 8 ± 1* | 0.04 |
| Gait speed, m/s | 1.38 ± 0.03 | 1.12 ± 0.08* | 0.007 |
| Steps per day | 5,387 ± 648 | 3,067 ± 250* | 0.004 |
| Sedentary physical activity, min/day | 1,245 ± 23 | 1,321 ± 15* | 0.01 |
| Light physical activity, min/day | 160 ± 16 | 111 ± 10 | 0.14 |
| Moderate-to-vigorous physical activity, min/day | 25 ± 4 | 8 ± 2* | 0.003 |
| Muscle characteristics | |||
| Quadriceps muscle mass, kg | 1.8 ± 0.1 | 1.4 ± 0.1 | 0.06 |
| Type 1 fibers, % | 40 ± 3 | 40 ± 5 | 0.61 |
| Type 2 fibers, % | 60 ± 3 | 60 ± 5 | 0.61 |
| NCAF | 2.7 ± 0.05 | 2.6 ± 0.05 | 0.78 |
| Medication history | |||
| Inhaled β-agonist/cholinergic antagonist, n | 0 | 7* | <0.001 |
| Corticosteroid, n | 0 | 0 | 1.00 |
| Statin-type drugs, n | 2 | 2 | 0.93 |
| Muscle relaxant, n | 0 | 2 | 0.14 |
Values are means ± SE.
COPD, chronic obstructive pulmonary disease; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; SaO2, arterial oxygen saturation; NCAF, number of capillaries around a fiber.
Medications listed are those taken over the past 6 mo.
Significant difference.
Mitochondrial respiration and muscle characteristics.
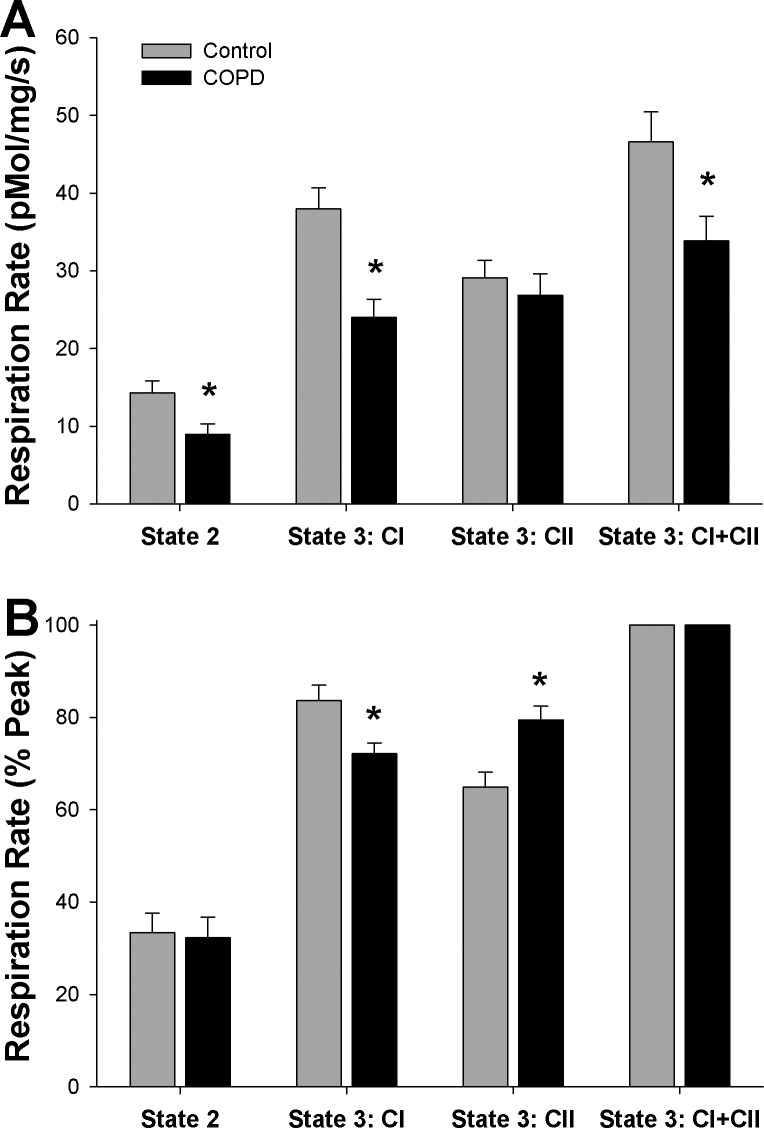
In terms of respiration per milligram of muscle, a significant interaction existed between subject group (i.e., control or COPD) and respiration states (P < 0.05), such that the impact of COPD on respiration depended on the respiration state under consideration. Notably, State 3:CI (37.9 ± 2.7 and 23.9 ± 2.3 pmol·mg−1·s−1, P < 0.05) and State 3:CI+CII (46.5 ± 3.8 and 33.8 ± 3.8 pmol·mg−1·s−1, P < 0.05) were both lower in patients with COPD (P < 0.05), while State 3:CII (29.0 ± 2.3 and 26.8 ± 2.8 pmol·mg−1·s−1, P > 0.05) was similar between groups. State 2 respiration, when expressed in terms of respiration per milligram of muscle, was not significantly different between controls and patients (14.2 ± 1.5 and 9.0 ± 1.3 pmol·mg−1·s−1, P = 0.18).
As wet weight muscle respiration indicated altered utilization of the CI and CII pathways in COPD, the relative contribution of each pathway in terms of percent of peak respiration during State 3:CI+CII (Fig. 1B) was explored. Consistent with the data represented in terms of wet weight respiration (Fig. 1A), a significant interaction between subject group and respiration state was observed (P < 0.05). Further analysis revealed a diminished contribution of the CI pathway in COPD (83.7 ± 3.4 and 72.1 ± 2.4% Peak, P < 0.05) and further revealed an exaggerated contribution of the CII pathway (64.9 ± 3.2 and 79.5 ± 3.0% Peak, P < 0.05). State 2 respiration represented as a percentage of peak respiration was not different between groups (33.5 ± 4.1 and 32.3 ± 4.5% Peak, P < 0.05). Note that cytochrome c had no effect on respiration in either subject group (P > 0.05).
Fig. 1.
Mitochondrial respiration of vastus lateralis muscle from patients with chronic obstructive pulmonary disease (COPD) and healthy controls. A: mitochondrial O2 flux per mg of muscle. B: mitochondrial respiration normalized to peak respiration observed during State 3 Respiration of Complex I and Complex II combined. *Significantly different from healthy control.
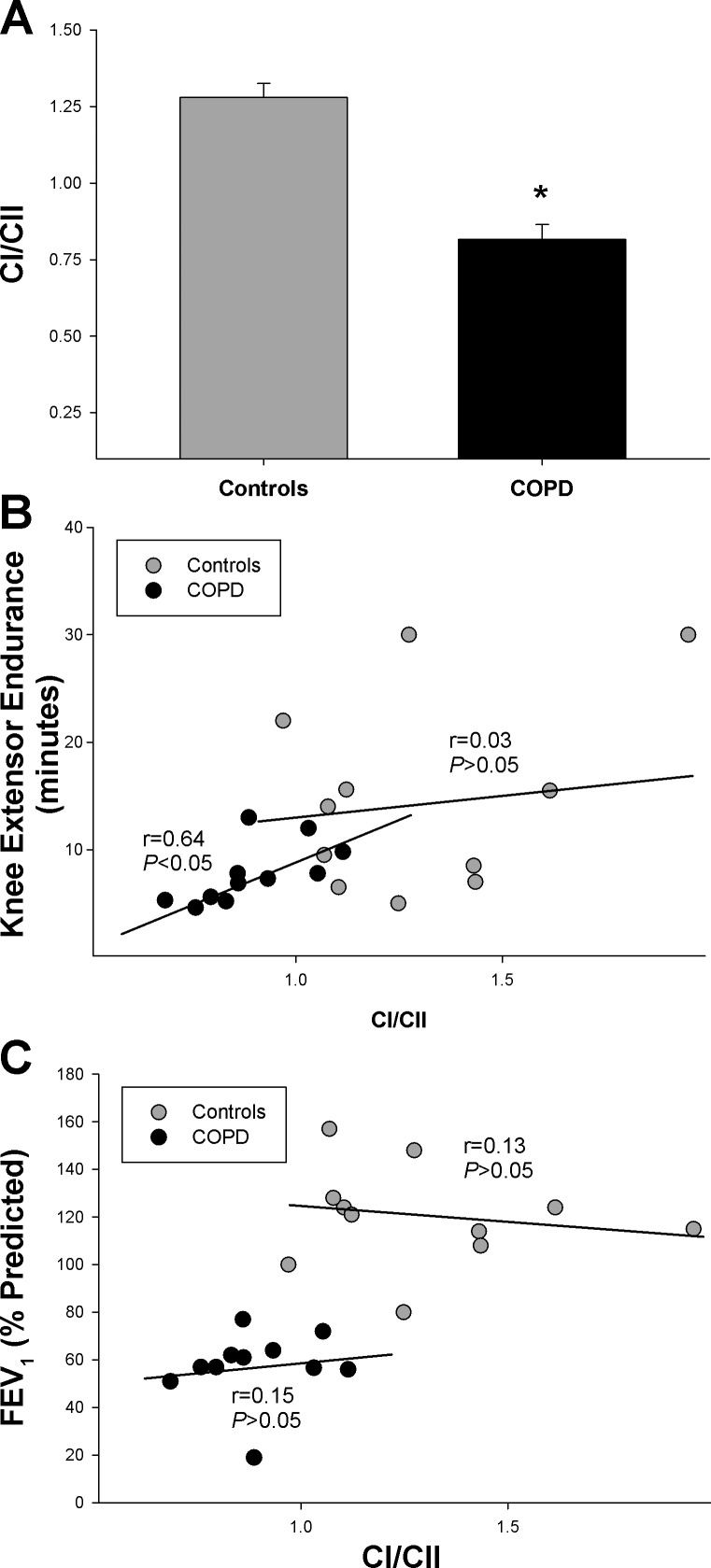
As it was hypothesized that patients would exhibit a greater reliance on CII-driven respiration than CI-driven respiration additional analysis of the relative contribution of the CI- and CII-driven pathways with the CI/CII was performed. As illustrated in Fig. 2A, CI/CII was significantly lower in patients with COPD compared with controls (0.82 ± 0.05 and 1.27 ± 0.05, respectively; P < 0.05).
Fig. 2.
Relative contributions of Complex I and Complex II-driven respiration and knee extensor endurance. A: ratio of State 3:CI to State 3:CII (i.e., CI/CII). B: relationship between CI/CII and knee extensor endurance. Note the significant relationship in the patients with COPD and lack of such a relationship in healthy controls. C: relationship between CI/CII and forced expiratory volume in 1 s (FEV1). *Significantly different from healthy controls.
Mitochondrial respiration, KE endurance, and lung function.
To explore the implications of this altered mitochondrial phenotype on exercise tolerance, the relationship between CI/CII and KE endurance (i.e., time to exhaustion) was examined (Fig. 2B). Overall, CI/CII exhibited a significant relationship with KE endurance (r = 0.43, P < 0.05) which appears to have been driven largely by the moderate correlation within the patients (r = 0.64, P < 0.05), as no relationship was found when only the healthy controls were included in the correlation analysis (r = 0.03; P = 0.94). As illustrated in Fig. 2C, lung function, as assessed by FEV1, also exhibited a significant relationship with CI/CII (r = 0.55, P < 0.05); however, this relationship may have been driven by group differences, as there was no relationship between FEV1 and CI/CII within each group (COPD: r = 0.15, P > 0.05; control: r = 0.13, P > 0.05). Additionally, across both groups, moderate-to-vigorous physical activity was significantly correlated with several qualitative parameters of mitochondrial function including State 3:CII %peak (r = 0.52, P < 0.05), and CI/CII (r = 0.49, P < 0.05). However, these relationships may have been partly driven by group differences, because when examined within each group, moderate-to-vigorous physical activity was unrelated to all indices of qualitative mitochondrial function (P > 0.05).
DISCUSSION
The purpose of this study was to determine if qualitative alterations in skeletal muscle mitochondrial respiration are associated with quadriceps exercise intolerance in patients with COPD. In terms of intrinsic mitochondrial function, patients with COPD exhibited a decreased reliance on CI-driven respiration and a greater reliance on the metabolically less-efficient CII-driven respiration, while uncoupled respiration was not different between groups. As detailed below, this study provides novel evidence that the quadriceps exercise intolerance exhibited by patients with COPD is associated with qualitative alterations in skeletal muscle mitochondrial respiratory function.
CI vs. CII-driven respiration in COPD.
As already recognized, the exercise intolerance exhibited by patients with COPD is thought to be partly related to changes within the muscle that result in an increased O2 cost of exercise (20, 31). Indeed, as has been reported previously (8, 22, 25), in the current study muscle fibers from patients with COPD exhibited significantly reduced rates of respiration per milligram of muscle (Fig. 1A). Certainly these reduced rates of O2 consumption may impair aerobic capacity, especially when coupled with the reduced muscle mass sometimes exhibited by patients with COPD (Table 2). However, such attenuated respiration rates do not necessarily confer a qualitative change in the mitochondrial function that could explain the increased O2-cost of exercise associated with COPD (25). Given that electrons entering the ETC at CII require more O2 to resynthesize a given amount of ATP than electrons entering at CI (12), we hypothesized that patients with COPD would also exhibit an increased reliance on the less-efficient CII-driven respiration compared with the more-efficient CI-driven respiration. Indeed, such a pattern of altered respiration was evident in patients with COPD as State 3:CI was significantly reduced in COPD compared with controls while State 3:CII was not (Fig. 1). Thus, in agreement with previous research (8), it appears that the reduction in State 3:CI+CII exhibited by patients with COPD in the current study was largely driven by diminished CI-driven respiration rather than CII-driven respiration. (Fig. 1A). Consequently, when considered in relative terms, the current data indicate that CI-driven respiration makes up a lesser proportion of peak respiration and provides a smaller metabolic contribution than CII-driven respiration in patients with COPD than in healthy controls (Figs. 1B and 2A).
In light of decreased CI-driven respiration, the maintenance of CII-driven respiration per milligram tissue in the patients with COPD likely prevented further loss of respiratory function in the muscle that would have occurred if CII-driven respiration was also decreased. However, it should be emphasized that in terms of ATP production, CI and CII-driven respiration are not equal. Even if CII-driven respiration was exaggerated to the point that it fully compensated for the diminished CI-driven respiration in terms of O2 consumption, given the lower P/O ratio, such augmented CII-driven respiration would not fully offset the decrement in terms of ATP production (12). Therefore, if patients with COPD do rely on this less efficient metabolic pathway more than controls, the mitochondria of the patients would be obligated to respire more than their healthy counterparts to produce the same amount of ATP. This appears to have been the case for the patients studied by Richardson et al. (31) and Medeiros et al. (20) who demanded greater amounts of O2 to perform the same amount of work as their healthy counterparts. To date the P/O ratio from combined CI + CII driven respiration has not been fully explored in COPD. Previously, Puente-Maestu et al. (27) reported that the P/O ratio of mitochondria from patients with COPD was similar to healthy controls, but in this case the P/O ratio was only measured during CII-driven respiration. Based upon our data, the P/O ratio is more likely to be depressed when both CI and CII are stimulated simultaneously as it may be the relative contribution of each pathway during physiological respiration and not necessarily the isolated function of each pathway that affects the P/O ratio in patients with COPD. While it is not clear if the qualitative alterations in mitochondrial respiration (i.e., reduced CI/CII) observed in the current study were associated with a decreased P/O ratio, it is clear that this altered respiratory profile was related to exercise intolerance among the patients, as those who exhibited low CI/CII (i.e., low contribution of CI with high contribution of CII) fatigued earlier than those patients who exhibited a greater CI/CII (Fig. 2).
Currently, it is not clear why the mitochondria of patients with COPD exhibit diminished CI-driven respiration compared with the mitochondria of healthy controls. Interestingly, Daussin et al. (10) reported a similar predominance of CII over CI-driven respiration in the mitochondria of sedentary individuals compared with those of endurance-trained individuals. In the current study, while both the patients and controls were relatively sedentary (36), the patients were less active than the controls (Table 2). Thus it is possible that physical inactivity played a role in the diminished role of CI-driven respiration in COPD; however, it has previously been reported that altered mitochondrial respiration persists in patients with COPD even when matched for physical activity (27). In this regard, it should be noted that the observed differences in respiration in the current study existed despite there being no significant difference in muscle fiber type (Table 2). Additionally, it has recently been suggested that, due to increased free radical production and decreased antioxidant defenses, the mitochondrial DNA of patients with COPD is prone to oxidative damage (14, 26). Interestingly, CII represents the only complex of the ETC that is not encoded, at least in part, by mitochondrial DNA. Thus the shift in ETC pathway to predominantly CII-driven respiration may potentially be the consequence of decreased CI respiration due to mitochondrial DNA damage. Certainly, further research is needed to determine the role of physical activity and mitochondrial DNA integrity in the diminished utilization of CI-driven respiration observed in patients with COPD.
Uncoupled respiration in COPD.
This study also tested the hypothesis that a component of the exercise intolerance typical of patients with COPD would be a consequence of an increase in uncoupled or nonphosphorylating O2 consumption. Contrary to our hypothesis, State 2 respiration per milligram of muscle was not significantly augmented among patients with COPD (Fig. 1A); in fact, it tended to be lower among patients with COPD. Additionally, there was no difference in State 2 respiration when expressed as a percentage of peak respiration (Fig. 1B). Thus this trend of reduced uncoupled respiration per milligram of tissue appears to be the consequence of reduced peak respiration rather than a qualitative change within the mitochondria. Thus, based on these data, it seems unlikely that exaggerated uncoupled respiration contributes to the exercise intolerance or the increased O2-cost of exercise common to patients with COPD. Nevertheless, as noted earlier, evidence regarding uncoupled respiration in COPD is conflicting and somewhat complex. For example, Picard et al. (25) reported no significant difference in State 2 respiration between patients with COPD and controls, while Naimi et al. (22) reported that patients with COPD had significantly greater State 2 respiration than controls. As it has been demonstrated that cachexic patients with COPD are more likely to exhibit exaggerated uncoupling (29), the disagreement between findings may to some extent reflect the heterogeneity of disease severity among patients.
Increased O2 cost of contraction in an O2-limited population.
Based upon the current data, patients with COPD exhibit decreased mitochondrial efficiency in terms of CI/CII, but not in terms of uncoupling. When considered in isolation this decrease in mitochondrial efficiency may not appear to be a severe limitation in COPD, but when viewed in combination with other physiological alterations present in this patient population it becomes clear that this qualitative change in mitochondrial function has the potential to severely exacerbate exercise intolerance in COPD. First, it is worth noting that in patients with COPD O2 delivery is often reduced (11, 13). For example, during exercise, particularly during large muscle mass exercise, patients with COPD exhibit varying degrees of pulmonary diffusion limitation that results in decreased blood O2 saturation and content, which may compromise muscle O2 delivery (13). Additionally, patients with COPD exhibit an increased work of breathing for a given level of exercise, which may limit locomotor muscle O2 delivery by redirecting a portion of a finite cardiac output, which may already exhibit a lower O2 content, away from the exercising locomotor muscles and toward the respiratory muscles (11). Thus, in patients with COPD, decreased mitochondrial efficiency may demand more O2 from an already reduced supply. Second, the consequences of decreased mitochondrial efficiency may be magnified by the increased ATP cost of contraction that our group recently observed in patients with COPD (17, 18). Specifically, it appears that not only do patients with COPD utilize a less efficient ETC pathway to synthesize ATP, but once synthesized, more ATP is required to elicit a contraction cycle, thereby exaggerating the O2 cost of contraction even more. Third, given the decreased respiratory capacity per milligram of muscle reported in this study (Fig. 1A) and other studies (8, 22, 25), the ability of patients with COPD to meet the increased demand for O2 consumption due to altered mitochondrial efficiency is potentially hindered.
Together the multiple alterations in O2 supply by the cardiopulmonary system and O2 demand by the exercising muscle in patients with COPD likely converge to create a supply-demand mismatch where the exercising muscle demands more O2 to perform a given amount work and this is compounded by a diminished O2 supply. While it is unclear whether all of these factors converged in the current patients with COPD, it is evident that the documented reduction in mitochondrial efficiency was related to exercise intolerance (Fig. 2B), thus implying that this phenomenon may play a role in the limited exercise capacity exhibited by these patients.
Experimental considerations.
While this study was designed to follow up on previous observations from our group (32) and others (20), which indicate that patients with COPD experience a greater O2 cost of exercise, it should be noted that not all studies have observed mechanical inefficiency in this heterogeneous patient population. Therefore, as mechanical efficiency of the quadriceps was not directly measured in the current study, it cannot, with complete certainty, be concluded that the patients studied here exhibited the mechanical inefficiency we have observed in other such patients. Nevertheless, given the inefficient mitochondrial profile observed in this study, and the increased ATP cost of contraction observed in another recent study comprised of many of the same subjects (18), it seems likely that the O2 cost of exercise is elevated in this group.
In this study we intentionally recruited sedentary, healthy, age-matched controls, but, due to the very low levels of physical activity typical of patients with COPD, the controls were still more physically active than the patients (Table 2). Therefore, as mitochondrial function is related to physical activity, it is possible that some of the differences in mitochondrial function are due to differences in physical activity rather than the disease itself. However, as physical activity did not explain the variation in CI/CII within each group, it seems unlikely that physical activity is the sole reason for the observed differences in mitochondrial function. Further research is needed to determine how much of the altered mitochondrial profile in COPD is due to the disease and how much is due to disease-associated disuse. Similarly, patients with COPD are often prescribed medications that may affect mitochondrial function (e.g., corticosteroids and statins) (16, 21), but it seems unlikely that drug therapy played a significant role in the current study as the patients and controls were mostly free of such medications for the past 6+ mo (Table 2).
Finally, what is referred to as peak respiration in this study represents the peak respiration observed during the described protocols, and is not necessarily the maximum respiration achievable by the mitochondria. Indeed, utilization of other, less-physiological protocols or other chemicals [e.g., tetramethyl phenylenediamine (TMPD)] (23) can result in respiration rates greater than those reported as peak respiration in this study.
Conclusions.
Qualitative alterations in skeletal muscle mitochondrial function that emphasize the relatively less efficient CII-driven respiration over the more efficient CI-driven respiration appear to contribute to decreased muscle function and exercise intolerance in patients with COPD. As patients with COPD often experience compromised O2 supply, such an increase in metabolic demand may yield deleterious effects even during the relatively modest physical demands of performing simple activities of daily living.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grant PO1-HL-09830.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.G., J.D.T., R.S.G., F.D., and R.S.R. conception and design of research; J.R.G., J.D.T., G.L., R.S.G., S.-Y.P., M.J.R., S.L., F.D., and R.S.R. performed experiments; J.R.G., J.D.T., G.L., R.S.G., S.-Y.P., M.J.R., and R.S.R. analyzed data; J.R.G., J.D.T., G.L., and R.S.R. interpreted results of experiments; J.R.G. prepared figures; J.R.G. drafted manuscript; J.R.G., J.D.T., G.L., R.S.G., S.-Y.P., M.J.R., S.L., F.D., and R.S.R. edited and revised manuscript; J.R.G., J.D.T., G.L., R.S.G., S.-Y.P., M.J.R., S.L., F.D., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for gracious participation.
REFERENCES
- 1.Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Dempsey JA. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol 299: R314–R324, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baarends EM, Schols AM, Akkermans MA, Wouters EF. Decreased mechanical efficiency in clinically stable patients with COPD. Thorax 52: 981–986, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 33: 1165–1185, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Bartels MN, Armstrong HF, Gerardo RE, Layton AM, Emmert-Aronson BO, Sonett JR, Arcasoy SM. Evaluation of pulmonary function and exercise performance by cardiopulmonary exercise testing before and after lung transplantation. Chest 140: 1604–1611, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2: 85–93, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Bronstad E, Tjonna AE, Dedichen HH, Kirkeby-Garstad I, Ha AK, Steinshamn S, Rognmo O, Haberg AK, Ingul CB, Wisloff U. High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur Respir J 40: 1130–1136, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, Calverley a PM, Chavannes N, Dillard T, Fahy B, Fein A, Heffner J, Lareau S, Meek P, Martinez F, McNicholas W, Muris J, Austegard E, Pauwels R, Rennard S, Rossi A, Siafakas N, Tiep B, Vestbo J, Wouters E, ZuWallack R. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23: 932–946, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Daussin FN, Zoll J, Ponsot E, Dufour SP, Doutreleau S, Lonsdorfer E, Ventura-Clapier R, Mettauer B, Piquard F, Geny B, Richard R. Training at high exercise intensity promotes qualitative adaptations of mitochondrial function in human skeletal muscle. J Appl Physiol 104: 1436–1441, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol 151: 242–250, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 1706: 1–11, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Knower MT, Dunagan DP, Adair NE, Chin R. Baseline oxygen saturation predicts exercise desaturation below prescription threshold in patients with chronic obstructive pulmonary disease. Arch Intern Med 161: 732–736, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Konokhova Y, Spendiff S, Jagoe T, Picard M, Kapchinsky S, Baril J, Bourbeau J, Hepple R, Taivassalo T. Mitochondrial DNA deletions correspond to high levels of oxidative DNA damage in COPD skeletal muscle. Am J Respir Crit Care Med 187: A3368, 2013. [Google Scholar]
- 15.Lands LC, Smountas AA, Mesiano G, Brosseau L, Shennib H, Charbonneau M, Gauthier R. Maximal exercise capacity and peripheral skeletal muscle function following lung transplantation. J Hear Lung Transplant 18: 113–120, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Larsen S, Ci MS, Stride N, Hey-mogensen M, Hansen CN, Bang LE, Bundgaard H, Dms C, Nielsen LB, Helge JW, Dela F. Simvastatin effects on skeletal muscle relation to decreased mitochondrial function and glucose intolerance. J Am Coll Cardiol 61: 44–53, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Layec G, Haseler LJ, Hoff J, Richardson RS. Evidence that a higher ATP cost of muscular contraction contributes to the lower mechanical efficiency associated with COPD: preliminary findings. Am J Physiol Regul Integr Comp Physiol 300: R1142–R1147, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layec G, Haseler LJ, Richardson RS. The effect of higher ATP cost of contraction on the metabolic response to graded exercise in patients with chronic obstructive pulmonary disease. J Appl Physiol 112: 1041–1048, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Layec G, Venturelli M, Jeong E, Richardson RS. The validity of anthropometric leg muscle volume estimation across a wide spectrum: From able-bodied adults to individuals with a spinal cord injury. J Appl Physiol 116: 1142–1147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medeiros WM, Fernandes MCT, Azevedo DP, de Freitas FFM, Amorim BC, Chiavegato LD, Hirai DM, O'Donnell DE, Neder JA. Oxygen delivery-utilization mismatch in contracting locomotor muscle in COPD: peripheral factors. Am J Physiol Regul Integr Comp Physiol 308: R105–R111, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Mitsui T, Azuma H, Nagasawa M, Iuchi T, Akaike M, Odomi M, Matsumoto T. Chronic corticosteroid administration causes mitochondrial dysfunction in skeletal muscle. J Neurol 249: 1004–1009, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Naimi AI, Bourbeau J, Perrault H, Baril J, Wright-Paradis C, Rossi A, Taivassalo T, Sheel AW, Rabol R, Dela F, Boushel R. Altered mitochondrial regulation in quadriceps muscles of patients with COPD. Clin Physiol Funct Imaging 31: 124–131, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810: 25–58, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Picard M, Godin R, Sinnreich M, Baril J, Bourbeau J, Perrault H, Taivassalo T, Burelle Y. The Mitochondrial phenotype of peripheral muscle in chronic obstructive pulmonary disease disuse or dysfunction? Am J Respir Crit Care Med 178: 1040–1047, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Puente-Maestu L, Lázaro A, Tejedor A, Camaño S, Fuentes M, Cuervo M, Navarro BO, Agustí A. Effects of exercise on mitochondrial DNA content in skeletal muscle of patients with COPD. Thorax 66: 121–127, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Puente-Maestu L, Perez-Parra J, Godoy R, Moreno N, Tejedor A, Gonzalez-Aragoneses F, Bravo JL, Villar Alvarez F, Camano S, Agusti A. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J 33: 1045–1052, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Rabinovich RA, Louvaris Z, Raste Y, Langer D, Van Remoortel H, Giavedoni S, Burtin C, Regueiro EMG, Vogiatzis I, Hopkinson NS, Polkey MI, Wilson FJ, Macnee W, Westerterp KR, Troosters T. Validity of physical activity monitors during daily life in patients with COPD. Eur Respir J 42: 1205–1215, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Rabinovich RA, Bastos R, Ardite E, Llinas L, Orozco-Levi M, Gea J, Vilaro J, Barbera JA, Rodriguez-Roisin R, Fernandez-Checa JC, Roca J. Mitochondrial dysfunction in COPD patients with low body mass index. Eur Respir J 29: 643–650, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B, Wagner PD. Determinants of maximal exercise V̇o2 during single leg knee-extensor exercise in humans. Am J Physiol Heart Circ Physiol 268: H1453–H1461, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SRD, Henry R, Mathieu-Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak V̇o2 with small muscle mass exercise. Am J Respir Crit Care Med 169: 89–96, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SRD, Henry R, Mathieu-Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak V̇o2 with small muscle mass exercise. Am J Respir Crit Care Med 169: 89–96, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75: 1911–1916, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Rossman MJ, Garten RS, Groot HJ, Reese V, Zhao J, Amann M, Richardson RS. Ascorbate infusion increases skeletal muscle fatigue resistance in patients with chronic obstructive pulmonary disease. Am J Physiol Regul Integr Comp Physiol 305: R1163–R1170, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S, Wu SS. Improvements in speed-based gait classifications are meaningful. Stroke 38: 2096–2100, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Tudor-Locke C, Bassett DR. How many steps/day are enough? Preliminary pedometer indices for public health. Sport Med 34: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Whipp BJ, Wasserman K. Efficiency of muscular work. J Appl Physiol 26: 644–648, 1969. [DOI] [PubMed] [Google Scholar]