Abstract
In the US, approximately 650 children are diagnosed with osteosarcoma and Ewing sarcoma (ES) each year. Five-year survival ranges from 65% to 75% for localized disease and <30% for patients with metastases. Recent findings include interval-compressed five drug chemotherapy improves survival with localized ES. In osteosarcoma a large international trial investigating the addition of ifosfamide/etoposide or interferon to standard therapy has completed accrual. For ES an ongoing trial explores the addition of cyclophosphamide/topotecan to interval-compressed chemotherapy. Trials planned by the Children’s Oncology Group will investigate new target(s) including IGF-1R and mTOR in ES, and RANKL and GD2 in osteosarcoma.
Keywords: blueprint, bone sarcoma, Ewing sarcoma, osteosarcoma
INTRODUCTION
The two most common malignant bone tumors in children and adolescents are Ewing sarcoma (ES) and osteosarcoma (OS). Progress continues to be made in improving the survival of patients with localized ES. Improving the outcome of patients with OS or metastatic or recurrent disease has been more challenging. Key findings from recent clinical trials include the recognition that interval-compressed five drug chemotherapy improves the survival of patients presenting with localized ES. In OS an international phase 3 trial investigating the addition of ifosfamide and etoposide or interferon to standard therapy has recently completed accrual but the results of that study are not yet available. For ES an ongoing Children’s Oncology Group (COG) phase 3 trial explores the addition of cyclophosphamide/ topotecan to now standard interval-compressed five drug chemotherapy. A major focus of clinical trials is to identify new agents with activity in ES and OS. Phase 2 trials planned for those with recurrent disease will investigate druggable target(s) of interest including IGF-1R and mTOR in ES, and RANKL, and GD2 in OS. A major focus of biology efforts is to identify preclinical therapeutic leads. All of these efforts will be described in more detail in this blueprint.
STATE OF THE DISEASE—CLINICAL
Ewing Sarcoma
Overview and incidence
ES is a solid tumor of uncertain origin made up of small round blue cells with minimal stroma and differentiation [1]. It is the second most common tumor of bone in children, but can also exist as a soft-tissue tumor [1]. The incidence is approximately 3 cases/million/year [2]. The tumor has a peak incidence in adolescent and young adults, exhibiting a slight male preponderance [3]. A striking, unexplained, epidemiologic finding is the significantly lower incidence of ES in individuals of African descent, compared to those of European ancestry [3].
Staging/stratification
The most significant prognostic factor for patients with ES is the presence or absence of overt metastatic disease [4]. Approximately 25% of patients have metastatic disease upon presentation. Metastatic disease is typically found in the lungs (60%), bone (43%), and/or bone marrow (19%) [5]. The COG recognizes two stages of ES: localized or metastatic. Other clinical prognostic factors have been found, such as location of tumor (pelvic tumors have a worse outcome), size of tumor (larger tumors do worse), age of patient (with older patients having worse outcome), and histologic response after induction chemotherapy (poorly responding tumors do worse) [6,7], but these factors have not entered into the routine stratification of treatment for ES in COG protocols. Likewise, potential biologic risk factors are not included in current risk-stratification and are an area of ongoing work by the committee.
Current outcome
While overt metastatic disease is prognostic, it is clear that the vast majority of patients (even those with localized disease) harbor micrometastatic deposits. Hence, the treatment of ES requires multidisciplinary approaches, including control of local disease (with surgery and/or radiation therapy), and control of micrometastatic disease (with chemotherapy). The outcome of patients with localized ES on the most recent COG completed trial (AEWS0031) is 73% EFS at 5 years (using interval-compressed VDC/IE), with a clear improvement over the 65% 5-year EFS observed with non-compressed VDC/IE (P = 0.048) [8]. In contrast, for patients with metastatic disease, 2- to 3-year EFS remains in the 20–30% range. There has been no significant improvement in decades [4]. Patients with recurrent disease have an EFS of less than 10% at 3 years [4].
Osteosarcoma
Overview and incidence
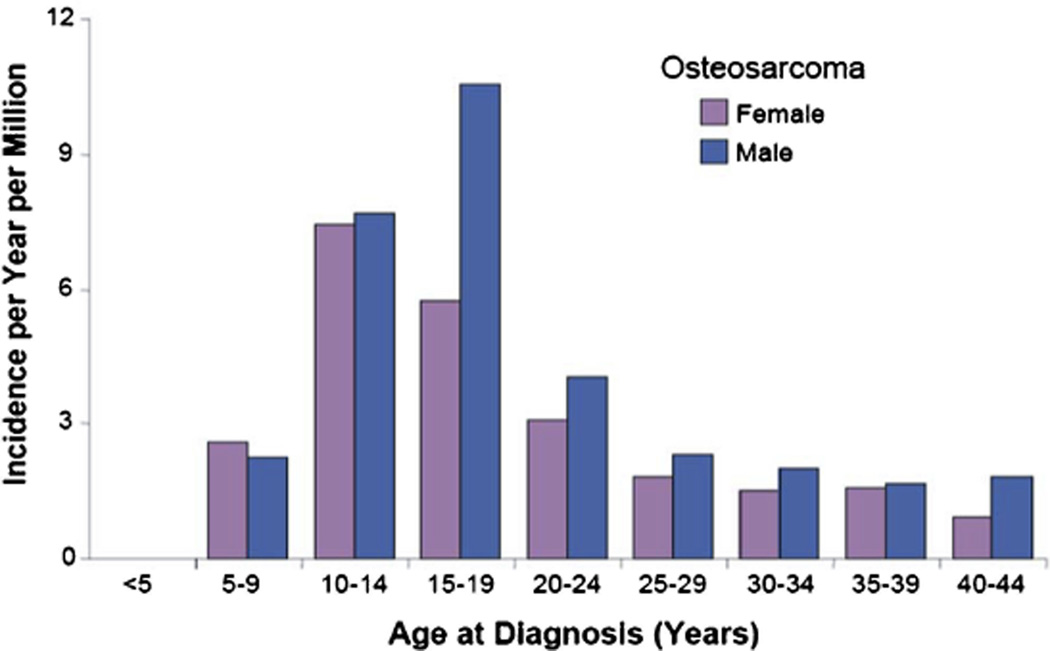
OS is the most common primary bone tumor. The incidence of OS is 4.8 per million per year [9,10]. The incidence peaks in adolescence (at age 16 for males and age 12 for females) [11,12]. While incidence rates are lower, OS also occurs in adults with at least an additional 200 cases diagnosed per year in people 20 and older (Fig. 1) [10].
Fig. 1.
Age distribution of osteosarcoma based on cases in the United States between 1975 and 1999 from SEER [2].
Staging/stratification
The principal prognostic factor in OS is stage with metastatic OS having a much worse prognosis than localized. Lung alone is the most common metastatic site (61%), followed by bone alone (15.8%), followed by lung and bone (13.9%), followed by other sites [13]. Ability to achieve a complete resection of all bulk disease is an important prognostic factor [14]. Consequently individuals with primary tumors in the axial skeleton have a worse prognosis than individuals with primary tumors in appendicular skeleton [14]. Additional poor prognostic factors include older age at diagnosis. The extent of tumor necrosis following neoadjuvant chemotherapy has been demonstrated, in many studies, to correlate with outcome [15].
For several reasons these prognostic factors are not utilized to select or modify treatment outside of a clinical trial. Extent of tumor necrosis and surgical remission status are not known at presentation but rather determined after neoadjuvant therapy. Intensifying treatment in response to poor prognostic variables has not yet been demonstrated to result in an improved outcome.
Current outcome
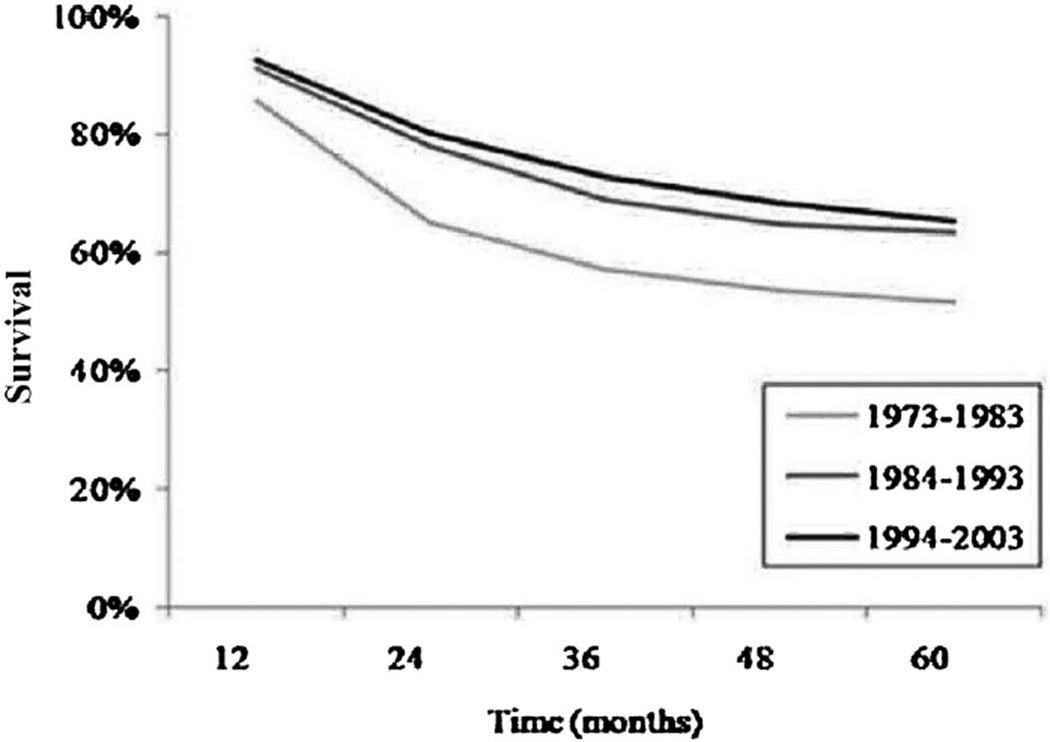
The 5- and 10-year overall survival for patients with localized OS is approximately 70% and 65% respectively [14,16,17]. Ten-year overall survival for patients with metastatic OS is 25% [13]. Overall survival following recurrence is less than 20% [18]. Outcomes have not changed for the past 2 decades (Fig. 2) [12]. This reflects the fact that medical therapy has remained essentially unchanged during this period with no new therapies identified to complement the activity of standard therapy with high-dose methotrexate, doxorubicin, and cisplatin.
Fig. 2.
Five-year overall survival in patients age 0–24 years over three decades 1973–2003 [12].
STATE OF THE DISEASE—BIOLOGICAL
Molecular Targets—Ewing Sarcoma and Osteosarcoma
Ewing sarcoma
ESs are characterized by the EWS/FLI transcription factor oncoprotein encoded by the t(11;22)(q24;q12) chromosomal translocation [19]. EWS/FLI isoforms are found in over 85% of ES cases and are required for the cancerous phenotype of ES cells. Early work suggested that the type 1 isoform of EWS/FLI conferred a survival advantage for patients with ES [20,21]. However, recent studies suggest that modern treatment protocols have eliminated the prognostic advantage of the Type 1 fusion [22,23]. In addition to EWS/FLI, other fusions have been identified in ES, raising important questions as to whether these are all truly ES, or some may represent a distinct biological subset [24,25]. This finding has important implications for diagnosis and therapy.
Given its critical role, EWS/FLI would appear to be the perfect molecular target. Ongoing work has focused on inhibiting the fusion by blocking its expression with cytarabine [26] or mithramycin [27] or its functions with YK-4–279 [28] or trabectadin [29]. Other investigators have focused on understanding the mechanistic basis of EWS/FLI function and its targets [30–44]. The overarching strategy is to identify new targets that can be functionally inhibited to block ES tumorigenesis. As an example, the importance of epigenetic dysregulation has recently come into focus and so the potential for epigenetic modifiers such as histone deacetylase inhibitors (e.g., vorinostat) and inhibitors of DNA methylation (e.g., decitabine) as novel agents requires investigation [45,46].
In addition to EWS/FLI, cooperative pathways are important for the transformed phenotype of ES (Table I). The IGF1 pathway has been shown to be of importance but a biologic understanding in terms of variable responses noted to IGF1R–blocking antibodies remains lacking [47–74]. Likewise, mTOR signaling appears to be upregulated in many of these tumors and provides another potential avenue for targeted therapeutics [75]. Subsets of ES have inactivating mutations in the p53 and RB pathways, and validation of the prognostic value of these alterations is being studied [48,49,76–85]. Stem-cell features also appear to be important for ES development, and genome-wide transcriptional and copy number analyses are striving to identify additional features relevant for the tumor’s behavior [86,87]. Finally, biologic features continue to be investigated, such as the significance of circulating tumor cells found in the blood or bone marrow of patients (by RT-PCR and flow cytometry) [88–93].
TABLE 1.
Pathways That Can Be Therapeutically Targeted in Ewing Sarcoma and Osteosarcoma
| Molecular target |
Example of inhibitory drug |
|
|---|---|---|
| Global metastases processes | ||
| Apoptosis/Anoikis | AKT | MK-2206 |
| mTOR | Temsirolimus | |
| Angiogenesis | VEGF | Bevacizumab |
| VEGFR | Sorafenib | |
| Preferential OS processes | ||
| Invasion | c-SRC | Saracatinib |
| Migration | HGF/c-MET | PF-02341066 |
| Proliferation/survival | IGF-1R | Cixutumumab |
Osteosarcoma
The search for OS-specific targets through the study of genetic aberrations or gene expression studies using OS tissues has not identified common and reproducible genetic lesions [94,95]. OS is usually characterized by extremely complex karyotypes without consistent recurring translocations, often seen in other sarcomas. In fact, the most consistent genetic finding, beyond dysregulation of p53 and RB, is significant aneuploidy [96]. This has suggested the possibility of an early defect in chromosomal stability or DNA repair/surveillance as a mechanism for the genesis of OS and the resultant bizarre aneuploidy that is devoid of consistent genetic aberrations across patients [97–99].
In the absence of consistently defined genetic aberrations, one approach towards therapeutic targeting may be based on common clinical features of the disease, such as its cellular environment or its propensity to metastasize early in its natural history (Table I). The integration of these common clinical features with biological studies that aim to uncover novel targets or can predict specific behaviors in patients are under active study. Translation of such data towards molecular targets should include therapeutic agents capable of disrupting various aspects the metastatic cascade. In addition, therapeutics that specifically or preferentially inhibits signaling pathways identified to participate in malignant osteo-blast/mesenchymal cell invasion, migration, proliferation, and survival should be actively evaluated. Importantly, the feasibility, tolerability, and activity of novel therapeutics, to be used in combination with conventional agents will be prioritized.
MAJOR RECENT FINDINGS
Ewing Sarcoma
In ES progress in improving the survival of patients presenting with localized disease has been steadily made dating back to the demonstration that the addition of ifosfamide and etoposide to the standard regimen of cyclophosphamide, doxorubicin and vincristine improves patient event free and overall survival [100]. In the past 5 years a randomized clinical trial (AEWS0031) of dose intensification via interval compression on 587 patients presenting with localized ES was completed. This trial demonstrated that intensive timing (cycles every 2 weeks) significantly improved the survival of these patients compared to standard timing (every 3 weeks). Event-free survival at a median of 5 years was 65% in the standard arm and 73% in the experimental, intensified arm, P = 0.048. The toxicity of the regimens was similar [101]. This study has changed the standard of care for the treatment of all patients with newly diagnosed localized disease.
Building upon the success of intensively timed, alternating, non-cross resistant chemotherapy regimens for the treatment of localized ES, a new randomized phase 3 trial was initiated and is ongoing. This study tests whether the addition of cyclophophosphamide, vincristine, and topotecan to the now standard intensively timed 5-drug chemotherapy regimen will further improve the survival of patients with localized ES. The feasibility of maintaining intensive timing with the addition of this third chemotherapy combination was proven in the AEWS07P1 study. The role of high dose chemotherapy with busulphan and melphalan and stem cell rescue is being investigated for the treatment of patients with newly diagnosed metastatic ES in the context of an international clinical trial (EuroEwing—AEWS0331).
A robust biology effort has been established in ES with 626 patients enrolled on the AEWS02B1 study and 275 patients enrolled on the AEWS07B1 study. Selected successes of this effort include an evaluation of the significance of translocation type on patient outcome [23], a molecular profiling study identifying ES that do not over-express BMI-1 as a distinct molecular subclass [102] as well as the development of tissue resources such as microarrays containing tumor samples that are linked to therapeutic studies and can be used for evaluation of prognostic factors.
The group is developing a series of studies evaluating new therapeutic approaches that potentially can be advanced to the experimental arm of studies in newly diagnosed localized patients. Prior studies include a study that successfully demonstrated the feasibility of administering vinblastine and celecoxib (as anti-angiogenic backbone) combined with a standard 5-drug regimen in newly diagnosed metastatic ES patients (AEWS02P1) [103]. A study was performed of cytarabine in patients with recurrent or refractory ES (AEWS0621) which unfortunately did not demonstrate major clinical activity [104]. Finally a study was performed testing bevacizumab combined with chemotherapy in patients with recurrent disease (AEWS0521) which demonstrated the feasibility of administering that therapy.
Osteosarcoma
A large collaborative international localized OS trial (Eura-mos—AOST0331) in conjunction with three other cooperative groups [Cooperative Osteosarcoma Study Group (COSS), European Osteosarcoma Intergroup (EOI), and Scandinavia Sarcoma Group (SSG)] has been completed. This study randomized the addition of interferon to standard chemotherapy in patients with a favorable histological response to neoadjuvant chemotherapy and randomized the addition of ifosfamide and etoposide to standard chemotherapy in the patients with an inferior histological response. This studies development included significant efforts addressing regulatory concerns in multiple countries [105]. As of June 2011, 2,260 patients were enrolled with the study at present having completed all planned accrual. The study includes correlative studies related to quality of life and tumor biology.
A biology effort has been established with an ongoing banking study AOST06B1. On the P9851 biology study, 1,105 eligible patients were enrolled and 490 patients were enrolled on the AOST06B1 study collecting/banking specimens on 1,595 patients. These specimens have been used as part of the NCI TARGET initiative and a NCI-led genome wide association study with early results published [106]. Analyses that are in process include gene expression studies that have been completed and are currently undergoing bioinformatic analyses, multi-platform analysis of tumor and germline nucleic acids, including CGH array, RNAseq, high-density sequencing, and a genome wide association study of germline DNA.
A series of feasibility studies have been performed evaluating new therapeutic approaches that potentially can be advanced to the experimental arm of studies in newly diagnosed localized patients. A phase 2 study has been performed evaluating the addition of zoledronate to standard chemotherapy for the treatment of patients with newly diagnosed metastatic OS (AOST06P1) demonstrating that this therapy has an acceptable toxicity profile. Similarly a phase 2 trial of trastuzumab in addition to standard chemotherapy for patients whose tumors express Her-2-neu (AOST0121) has demonstrated that this therapy is tolerable [107]. A phase 2 study of aerosolized GM-CSF for patients with lung metastases (AOST0221) similarly defined the feasibility of this treatment [108].
STRATEGIC APPROACH: TARGETED THERAPY
Ewing Sarcoma
Newly diagnosed population: More effective therapies applied to newly diagnosed patients with ES represent the best opportunity for improving survival. Approaches that are and can be tested include incorporating agents with a novel mechanism of action (either targeted or novel cytotoxic agents) to existing standard therapy. Based in large part on the improvement in survival achieved through intensive chemotherapy timing (AEWS0031), the current AEWS1031 study is testing in a randomized phase 3 trial the further intensification of therapy through the addition of cyclophosphamide, vincristine and topotecan to intensively timed 5-drug chemotherapy. If benefit is achieved through this addition, possibilities for further therapy intensification would include addition of a fourth regimen; temozolomide, vincristine, and irinotecan with or without novel agents, that will be initially assessed for safety and efficacy in patients with recurrent disease. The AEWS0331 study as well as the EURAMOS study have demonstrated the feasibility of performing international trials in rare tumors when necessary to achieve adequate patient accrual for a clinical trial addressing a meaningful question [105].
Metastatic ES
The poor prognosis of patients with newly diagnosed metastatic ES supports the utilization of novel agents in this population and these data can provide feasibility data for potential experimental arms of subsequent phase 3 randomized trials in the localized population. A study in development (AEWS1221) is assessing the feasibility of adding AMG479, an IGF-1R antibody, to intensively timed 5-drug chemotherapy in patients with newly diagnosed metastatic ES. Further investigations of IGF-1R antibody are supported by promising data obtained from several studies [50,52,53,74,109,110]. These clinical investigations in turn have been supported by promising pre-clinical data from the Pediatric Preclinical Testing Program (PPTP) among others [47,56,57]. This study is being planned as a randomized phase 2 trial to both obtain a preliminary estimate of the efficacy of the regimen as well as to obtain further data on the feasibility of intensively timed 5-drug chemotherapy in this patient population. This study will also define the feasibility of treating all bone metastases with stereotactic body radiotherapy (SBRT) as well as the feasibility of a 6-month maintenance phase with IGF-1R monoclonal antibody monotherapy. The trial also includes a series of critical biology studies to understand better heterogeneous response to this class of agents. At the completion of the ongoing phase 3 randomized trial (AEWS1031) in newly diagnosed ES patients, the subsequent study will be defined by the combined outcomes of the AEWS0331 which is being performed internationally, AEWS1221 and those performed in patients with recurrent disease.
Relapsed disease
Patients with relapsed disease have a poor prognosis and are in clear need of new treatments. Considerable data exist that temozolomide, vincristine, and irinotecan (VIT) has activity in the treatment of ES [111–113]. As this regimen is not utilized in the upfront treatment study VIT is a reasonable backbone on which to add novel agents both to treat these patients as well as to develop a chemotherapy combination that can be applied to the treatment of newly diagnosed patients. The concept that is being developed is a phase 2 randomized selection design comparing VIT and a rapamycin analogue with VIT and vorinostat and potentially VIT and a PARP inhibitor. The more active combination would undergo additional studies to define its feasibility with standard chemotherapy and assess its level of activity.
Trial design strategies
With localized disease in newly diagnosed patients the focus has been randomized phase 3 studies to improve and define standard front-line therapy (AEWS0331 previously and AEWS1031 currently). Other goals have been incorporated into each of these studies to address biological, quality of life, local control, and diagnostic radiology objectives. Studies in patients with relapsed disease have been performed to identify the initial feasibility and efficacy of novel agents and combination chemotherapy regimens (previously AEWS0521 testing bevacizumab, AEWS0621 testing cytarabine). These studies have generally been performed or will be performed as phase 2 or randomized phase 2 studies. Studies in patients with newly diagnosed meta-static ES have been performed as phase 3, phase 2 randomized or phase 2 studies defining the feasibility of combining novel agents with standard therapy and to a variable extent defining the activity of the regimen. A previous example was the AEWS02P1 study testing the addition of an anti-angiogenic regimen to standard chemotherapy. The AEWS1221 study is a randomized phase 2 study in newly diagnosed metastatic ES patients testing AMG479.
Osteosarcoma
Newly diagnosed disease. Localized osteosarcoma
While awaiting the AOST0331 results, studies will be performed to continue to investigate the impact of the addition of targeted agents to standard OS chemotherapy in patients with localized OS. The challenge is in identifying agents with preclinical and clinical data suggesting activity. The completed COG phase II studies including OS patients are ADVL0122 (imatinib), ADVL0421 (oxaliplatin), ADVL0524 (ixabepilone), and ADVL0525 (peme-trexed). In each study, there was no evidence of drug activity in OS. OS patients are also included in the phase II studies of IMC-A12 and MLN8237. If active novel agents are not identified in patients with relapsed OS, a randomized phase 3 trial in localized disease will likely not be performed until such agents are identified through a more robust phase 2 effort.
Metastatic osteosarcoma
In a prior phase 3 trial with a 2 × 2 factorial design (INT-0133), the BTC studied whether the addition of liposome encapsulated muramyl tripeptide phosphatidyletha-nolamine (L-MTP-PE, mifamurtide) to standard chemotherapy improved outcome in patients with newly diagnosed OS. Because of the study design, interpretation of the study results has been controversial [16,114]. The variability in interpretation of the study data and the non-uniformity of regulatory decisions has made the question regarding utility of L-MTP-PE in OS a high priority question. Patients with metastatic OS were included in INT-0133 but the study was not powered to evaluate the impact of L-MTP-PE on outcome in patients with metastatic disease. When the INT-0133 data are analyzed in patients with metastatic disease those who received L-MTP-PE have a higher EFS by approximately 8%, though this difference does not achieve statistical significance [115]. A consideration is performing another phase 3 trial in this patient population, powered sufficiently, to determine if this result can be replicated.
Relapsed population
Studies in the relapsed population will be directed at evaluating new agents for potential clinical activity. The two agents of greatest interest are RANKL antibody (Denosumab), and anti-GD2 antibody. RANKL and is receptor RANK, are members of the TNF family of with the physiologic function of regulating bone turnover. OS expresses both RANK and RANKL and RANK-RANKL interaction activates downstream signaling and modulates gene expression [116,117]. In several studies interfering with RANK-RANKL interaction in vivo decreases OS growth and metastatic tumor burden [118– 123]. GD2 is a cell surface sialic acid containing glycosphingolipid expressed normally in CNS, peripheral nerves and melanocytes. In neuroblastoma anti-GD2 antibody significantly improves event free and overall survival [124]. Over 90% of OS express GD2 [125]. In the phase I/Ib trial of the murine anti-GD2 antibody, 14.2G2a, plus IL-2, which predominantly focused on neuroblastoma one of two patients with OS treated on the study had a complete response persisting for 8 months [125,126]. Objective radiographic responses are rare in OS even with proven neoadjuvant chemotherapy in newly diagnosed patients. This is possibly due to the nature of the stromal tissue that contains calcified bone matrix. In these phase II trials to be initiated, end points more appropriate for OS such as progression free survival will be utilized rather than radiographic response rate. Patients with OS will be included in planned phase II trials of the pan-tyrosine kinase inhibitor, pazopanib and the combination of an IGF1R antibody, IMC-A12 and an mTOR inhibitor, Temsirolimus.
Trial design strategies
The trial designs that should be utilized should be tailored to the patient population and the extent of existing preclinical and clinical data. A randomized phase II trial design with EFS as an endpoint is a desirable design for the study of new agents in the recurrent patient population given the challenges in utilizing response rate as an endpoint. A randomized phase II trial with an EFS endpoint in the recurrent patient population will require approximately 120 patients. In order to justify a study of this size, it is necessary to have some evidence of clinical efficacy. This would suggest that for the evaluation of denosumab and GD2 antibody in the patient population with recurrent OS, a single arm phase II trial would be most appropriate as an initial study. A definitive randomized phase III trial design is being considered in patients with newly diagnosed metastatic OS, exploring the utility of L-MTP-PE. This choice of design is based both on the extent of pre-existing data and on the regulatory status of L-MTP-PE. In order for a phase III study of L-MTP-PE to be feasible, international collaboration will be required.
KEY TRIALS TO BE PURSUED
Ewing Sarcoma
Pivotal phase 3 trials
During much of the next 5 years the AEWS1031 study will be conducted, building upon prior successes in sarcomas. This study has the potential to improve survival of patients and thereby change the standard of care treatment of these patients.
Randomized phase 2 studies
AEWS1221 is a key trial intended to define the feasibility of adding AMG479 to intensively timed 5-drug chemotherapy and obtain a preliminary estimate of the efficacy of that regimen. This study builds on preclinical and clinical data that IGF-1R antibody has activity in the treatment of ES. This study will potentially provide the next experimental arm of a phase 3 trial in patients with newly diagnosed ES. The concept that is being developed for relapse is a phase 2 randomized selection design comparing VIT and a rapamycin analogue with VIT and vorinostat and potentially VIT and a PARP inhibitor. The more active combination would undergo additional studies to define its feasibility with standard chemotherapy and assess its level of activity.
Prioritization strategy
At present, clinical trials for patients with ES have been developed in a non-competing manner within the COG and indeed coordination has occurred internationally to minimize competition and redundancy. Our strategy for moving new agents forward has been to have safety defined by feasibility in phase 1 trials, efficacy in the patient population defined in phase 2 and randomized phase 2 trials in the ES patient population, feasibility in combination with chemotherapy defined in newly diagnosed patients with metastatic ES and ultimately its role in standard of care therapy defined by randomized phase 3 trials in patients with localized disease. If promising data are obtained from the AEWS1221 study it is likely that pursuing a randomized phase 3 trial with the experimental arm testing the efficacy of adding IGF-1R antibody to standard therapy in patients with localized ES would be of interest. If the data are not promising we would anticipate additional studies with the most promising VIT and new agent combination. Agents moved forward into clinical testing are largely based on the biology of the tumor particularly targets driven by the translocation and evidence of preclinical activity. It is critical that biological, radiographic, surgical, and quality of life questions are incorporated as secondary objectives when appropriate.
Osteosarcoma
Pivotal phase 3 trials
The feasibility of conducting a phase III trial evaluating MTP-PE in metastatic OS will be explored. In the trial design currently being considered for this study, the primary objective is to compare EFS in patients with newly diagnosed metastatic OS treated with standard chemotherapy with and without L-MTP-PE. To be feasible this trial will require international collaboration in order to enroll a sufficient number of patients, along with a commitment from the biopharmaceutical company sponsor.
Randomized phase 2 studies
The BTC aims to pursue randomized phase II trials in the recurrent OS patient population. However, a single arm phase II trial design is preferable for the agents currently of interest for study in this patient population, anti-GD2 antibody and RANKL antibody, denosumab. A number of unique factors should be taken into account with the single arm phase II trial design. Issues being addressed in the study design include: (i) recognition that many patients with recurrent OS will undergo complete resection of all measurable and evaluable disease prior to enrolling on a trial of systemic therapy; (ii) the possibility for differing extent of drug activity in microscopic versus gross residual disease states; and (iii) significant differences in EFS in patients with resectable versus unresectable disease. The primary endpoint for these single arm phase II trials will be EFS. If these single arm phase II trials demonstrate activity, a randomized phase II trial, combining the agent demonstrating activity with cytotoxic chemotherapy will be pursued.
Prioritization strategy
The primary focus in OS is on identifying new agents with activity in this disease via (i) supporting biologic investigation and (ii) phase II trials of new agents with novel approaches for detecting agent activity. The first priority for improving the treatment of patients with OS is identifying additional agents with clinical activity through performing single arm phase II studies in patients with recurrent OS. Concurrently, the feasibility of a phase III trial of L-MTP-PE in patients with metastatic OS will be pursued. There will be a continued focus on supporting biologic investigation through enrollments on the biology protocol and provision of outcome data for the TARGET initiative.
REFERENCES
- 1.Arndt CA, Rose PS, Folpe AL, et al. Common musculoskeletal tumors of childhood and adolescence. Mayo Clinic Proc. 2012;87:475–487. doi: 10.1016/j.mayocp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30:425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 3.Gurney JG, Swensen AR, Bulterys M. Malignant Bone Tumors. In: Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute SEER Program, NIH Pub. No. 99–4649; 1999. pp. 99–110. [Google Scholar]
- 4.Rodriguez-Galindo C, Spunt SL, Pappo AS. Treatment of Ewing sarcoma family of tumors: Current status and outlook for the future. Med Pediatr Oncol. 2003;40:276–287. doi: 10.1002/mpo.10240. [DOI] [PubMed] [Google Scholar]
- 5.Cangir A, Vietti TJ, Gehan EA, et al. Ewing’s sarcoma metastatic at diagnosis Results and comparisons of two intergroup Ewing’s sarcoma studies. Cancer. 1990;66:887–893. doi: 10.1002/1097-0142(19900901)66:5<887::aid-cncr2820660513>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: Analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 7.Bacci G, Ferrari S, Bertoni F, et al. Prognostic factors in nonmetastatic Ewing’s sarcoma of bone treated with adjuvant chemotherapy: Analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol. 2000;18:4–11. doi: 10.1200/JCO.2000.18.1.4. [DOI] [PubMed] [Google Scholar]
- 8.Womer RB, West DC, Krailo MD, et al. A randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J Clin Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute SEER Program; 1999. [Google Scholar]
- 10.Cancer epidemiology in older adolescents and young adults 15–29 years of age, including SEER incidence and survival: 1975–2000. Bethesda, MD: National Cancer Institute, NIH; 2006. [Google Scholar]
- 11.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 14.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 15.Goorin AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 16.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: A joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 18.Kempf-Bielack B, Bielack SS, Jurgens H, et al. Osteosarcoma relapse after combined modality therapy: An analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 19.Kovar H. Downstream EWS/FLI1—Upstream Ewing’s sarcoma. Genome Med. 2010;2:8. doi: 10.1186/gm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Alava E, Kawai A, Healey JH, et al. EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing’s sarcoma. J Clin Oncol. 1998;16:1248–1255. doi: 10.1200/JCO.1998.16.4.1248. [DOI] [PubMed] [Google Scholar]
- 21.Zoubek A, Dockhorn-Dworniczak B, Delattre O, et al. Does expression of different EWS chimeric transcripts define clinically distinct risk groups of Ewing tumor patients? J Clin Oncol. 1996;14:1245–1251. doi: 10.1200/JCO.1996.14.4.1245. [DOI] [PubMed] [Google Scholar]
- 22.Le Deley MC, Delattre O, Schaefer KL, et al. Impact of EWS-ETS fusion type on disease progression in Ewing’s sarcoma/peripheral primitive neuroectodermal tumor: Prospective results from the cooperative Euro-E.W.I.N.G 99 trial. J Clin Oncol. 2010;28:1982–1988. doi: 10.1200/JCO.2009.23.3585. [DOI] [PubMed] [Google Scholar]
- 23.van Doorninck JA, Ji L, Schaub B, et al. Current treatment protocols have eliminated the prognostic advantage of type 1 fusions in Ewing sarcoma: A report from the Children’s Oncology Group. J Clin Oncol. 2010;28:1989–1994. doi: 10.1200/JCO.2009.24.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierron G, Tirode F, Lucchesi C, et al. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet. 2012;44:461–466. doi: 10.1038/ng.1107. [DOI] [PubMed] [Google Scholar]
- 25.Sankar S, Lessnick SL. Promiscuous partnerships in Ewing’s sarcoma. Cancer Genetics. 2011;204:351–365. doi: 10.1016/j.cancergen.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stegmaier K, Wong JS, Ross KN, et al. Signature-based small molecule screening identifies cytosine arabinoside as an EWS/FLI modulator in Ewing sarcoma. PLoS Med. 2007;4:122. doi: 10.1371/journal.pmed.0040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grohar PJ, Woldemichael GM, Griffin LB, et al. Identification of an inhibitor of the EWS-FLI1 oncogenic transcription factor by high-throughput screening. J Natl Cancer Inst. 2011;103:962–978. doi: 10.1093/jnci/djr156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erkizan HV, Kong Y, Merchant M, et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma. Nat Med. 2009;15:750–756. doi: 10.1038/nm.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grohar PJ, Griffin LB, Yeung C, et al. Ecteinascidin 743 interferes with the activity of EWS-FLI1 in Ewing sarcoma cells. Neoplasia. 2011;13:145–153. doi: 10.1593/neo.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korner M, Waser B, Reubi JC. High expression of neuropeptide Y1 receptors in ewing sarcoma tumors. Clin Cancer Res. 2008;14:5043–5049. doi: 10.1158/1078-0432.CCR-07-4551. [DOI] [PubMed] [Google Scholar]
- 31.Lu C, Tilan JU, Everhart L, et al. Dipeptidyl peptidases as survival factors in Ewing sarcoma family of tumors: Implications for tumor biology and therapy. J Biol Chem. 2011;286:27494–27505. doi: 10.1074/jbc.M111.224089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zwerner JP, Joo J, Warner KL, et al. The EWS/FLI1 oncogenic transcription factor deregulates GLI1. Oncogene. 2008;27:3282–3291. doi: 10.1038/sj.onc.1210991. [DOI] [PubMed] [Google Scholar]
- 33.Beauchamp E, Bulut G, Abaan O, et al. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009;284:9074–9082. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel M, Simon JM, Iglesia MD, et al. Tumor-specific retargeting of an oncogenic transcription factor chimera results in dysregulation of chromatin and transcription. Genome Res. 2012;22:259–270. doi: 10.1101/gr.125666.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lessnick SL, Ladanyi M. Molecular pathogenesis of Ewing sarcoma: New therapeutic and transcriptional targets. Ann Rev Pathol. 2012;7:145–159. doi: 10.1146/annurev-pathol-011110-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toomey EC, Schiffman JD, Lessnick SL. Recent advances in the molecular pathogenesis of Ewing’s sarcoma. Oncogene. 2010;29:4504–4516. doi: 10.1038/onc.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gangwal K, Close D, Enriquez CA, et al. Emergent properties of EWS/FLI regulation via GGAA microsatellites in Ewing’s sarcoma. Genes Cancer. 2010;1:177–187. doi: 10.1177/1947601910361495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo W, Gangwal K, Sankar S, et al. GSTM4 is a microsatellite-containing EWS/FLI target involved in Ewing’s sarcoma oncogenesis and therapeutic resistance. Oncogene. 2009;28:4126–4132. doi: 10.1038/onc.2009.262. [DOI] [PubMed] [Google Scholar]
- 39.Kinsey M, Smith R, Iyer AK, et al. EWS/FLI and its downstream target NR0B1 interact directly to modulate transcription and oncogenesis in Ewing’s sarcoma. Cancer Res. 2009;69:9047–9055. doi: 10.1158/0008-5472.CAN-09-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen LA, Kowalewski AA, Lessnick SL. EWS/FLI mediates transcriptional repression via NKX2.2 during oncogenic transformation in Ewing’s sarcoma. PLoS ONE. 2008;3:1965. doi: 10.1371/journal.pone.0001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hancock JD, Lessnick SL. A transcriptional profiling meta-analysis reveals a core EWS-FLI gene expression signature. Cell Cycle. 2008;7:250–256. doi: 10.4161/cc.7.2.5229. [DOI] [PubMed] [Google Scholar]
- 42.Smith R, Owen LA, Trem DJ, et al. Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing’s sarcoma. Cancer Cell. 2006;9:405–416. doi: 10.1016/j.ccr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Kinsey M, Smith R, Lessnick SL. NR0B1 is required for the oncogenic phenotype mediated by EWS/ FLI in Ewing’s sarcoma. Mol Cancer Res. 2006;4:851–859. doi: 10.1158/1541-7786.MCR-06-0090. [DOI] [PubMed] [Google Scholar]
- 44.Gangwal K, Sankar S, Hollenhorst PC, et al. Microsatellites as EWS/FLI response elements in Ewing’s sarcoma. Proc Natl Acad Sci USA. 2008;105:10149–10154. doi: 10.1073/pnas.0801073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawlor ER, Thiele CJ. Epigenetic changes in pediatric solid tumors: Promising new therapeutic targets. Clin Cancer Res. 2012;18:2768–2779. doi: 10.1158/1078-0432.CCR-11-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel N, Black J, Chen X, et al. DNA methylation and gene expression profiling of ewing sarcoma primary tumors reveal genes that are potential targets of epigenetic inactivation. Sarcoma. 2012;2012:498472. doi: 10.1155/2012/498472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:1190–1197. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 48.Abudu A, Mangham DC, Reynolds GM, et al. Overexpression of p53 protein in primary Ewing’s sarcoma of bone: Relationship to tumor stage, response and prognosis. Br J Cancer. 1999;79:1185–1189. doi: 10.1038/sj.bjc.6690190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radig K, Schneider-Stock R, Rose I, et al. p53 and ras mutations in Ewing’s sarcoma. Pathology Res Practice. 1998;194:157–162. doi: 10.1016/S0344-0338(98)80016-2. [DOI] [PubMed] [Google Scholar]
- 50.Pappo AS, Patel SR, Crowley J, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor in patients with recurrent or refractory Ewing sarcoma family of tumors: Results of a phase II sarcoma alliance for research through Collaboration study. J Clin Oncol. 2011;29:4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juergens H, Daw NC, Geoerger B, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol. 2011;29:4534–4540. doi: 10.1200/JCO.2010.33.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang HJ, Angelo LS, Rodon J, et al. R1507, an anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, and EWS/FLI-1 siRNA in Ewing’s sarcoma: Convergence at the IGF/IGFR/Akt axis. PLoS ONE. 2011;6:26060. doi: 10.1371/journal.pone.0026060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garofalo C, Manara MC, Nicoletti G, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing’s sarcoma is dependent on insulin receptor signaling. Oncogene. 2011;30:2730–2740. doi: 10.1038/onc.2010.640. [DOI] [PubMed] [Google Scholar]
- 54.Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: A phase 1 expansion cohort study. Lancer Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurzrock R, Patnaik A, Aisner J, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 56.Kolb EA, Gorlick R, Lock R, et al. Initial testing (stage 1) of the IGF-1 receptor inhibitor BMS-754807 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2011;56:595–603. doi: 10.1002/pbc.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houghton PJ, Morton CL, Gorlick R, et al. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;54:921–926. doi: 10.1002/pbc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subbiah V, Anderson P, Lazar AJ, et al. Ewing’s sarcoma: Standard and experimental treatment options. Curr Treat Options Oncol. 2009;10:126–140. doi: 10.1007/s11864-009-0104-6. [DOI] [PubMed] [Google Scholar]
- 59.Kurmasheva RT, Dudkin L, Billups C, et al. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69:7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang F, Greer A, Hurlburt W, et al. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69:161–170. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herrero-Martin D, Osuna D, Ordonez JL, et al. Stable interference of EWS-FLI1 in an Ewing sarcoma cell line impairs IGF-1/IGF-1R signalling and reveals TOPK as a new target. Br J Cancer. 2009;101:80–90. doi: 10.1038/sj.bjc.6605104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cironi L, Riggi N, Provero P, et al. IGF1 is a common target gene of Ewing’s sarcoma fusion proteins in mesenchymal progenitor cells. PLoS ONE. 2008;3:2634. doi: 10.1371/journal.pone.0002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martins AS, Mackintosh C, Martin DH, et al. Insulin-like growth factor I receptor pathway inhibition by ADW742, alone or in combination with imatinib, doxorubicin, or vincristine, is a novel therapeutic approach in Ewing tumor. Clin Cancer Res. 2006;12:3532–3540. doi: 10.1158/1078-0432.CCR-05-1778. [DOI] [PubMed] [Google Scholar]
- 64.Benini S, Zuntini M, Manara MC, et al. Insulin-like growth factor binding protein 3 as an anticancer molecule in Ewing’s sarcoma. Int J Cancer. 2006;119:1039–1046. doi: 10.1002/ijc.21929. [DOI] [PubMed] [Google Scholar]
- 65.Prieur A, Tirode F, Cohen P, et al. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol. 2004;24:7275–7283. doi: 10.1128/MCB.24.16.7275-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strammiello R, Benini S, Manara MC, et al. Impact of IGF-I/IGF-IR circuit on the angiogenetic properties of Ewing’s sarcoma cells. Hormone Metabolic Res. 2003;35:675–684. doi: 10.1055/s-2004-814149. [DOI] [PubMed] [Google Scholar]
- 67.Toretsky JA, Steinberg SM, Thakar M, et al. Insulin-like growth factor type 1 (IGF-1) and IGF binding protein-3 in patients with Ewing sarcoma family of tumors. Cancer. 2001;92:2941–2947. doi: 10.1002/1097-0142(20011201)92:11<2941::aid-cncr10072>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 68.Toretsky JA, Kalebic T, Blakesley V, et al. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 69.Scotlandi K, Benini S, Sarti M, et al. Insulin-like growth factor I receptor-mediated circuit in Ewing’s sarcoma/peripheral neuroectodermal tumor: A possible therapeutic target. Cancer Res. 1996;56:4570–4574. [PubMed] [Google Scholar]
- 70.Yee D, Favoni RE, Lebovic GS, et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation A potential autocrine growth factor. J Clin Invest. 1990;86:1806–1814. doi: 10.1172/JCI114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borinstein SC, Barkauskas DA, Krailo M, et al. Investigation of the insulin-like growth factor-1 signaling pathway in localized Ewing sarcoma: A report from the Children’s Oncology Group. Cancer. 2011;117:4966–4976. doi: 10.1002/cncr.26112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Valen F, Winkelmann W, Jurgens H. Type I and type II insulin-like growth factor receptors and their function in human Ewing’s sarcoma cells. J Cancer Res Clin Oncol. 1992;118:269–275. doi: 10.1007/BF01208615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benini S, Manara MC, Baldini N, et al. Inhibition of insulin-like growth factor I receptor increases the antitumor activity of doxorubicin and vincristine against Ewing’s sarcoma cells. Clin Cancer Res. 2001;7:1790–1797. [PubMed] [Google Scholar]
- 74.Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 75.Zenali MJ, Zhang PL, Bendel AE, et al. Morphoproteomic confirmation of constitutively activated mTOR, ERK, and NF-kappaB pathways in Ewing family of tumors. Ann Clin Lab Sci. 2009;39:160–166. [PubMed] [Google Scholar]
- 76.de Alava E, Antonescu CR, Panizo A, et al. Prognostic impact of p53 status in Ewing sarcoma. Cancer. 2000;89:783–792. [PubMed] [Google Scholar]
- 77.Deneen B, Denny CT. Loss of p16 pathways stabilizes EWS/FLI1 expression and complements EWS/ FLI1 mediated transformation. Oncogene. 2001;20:6731–6741. doi: 10.1038/sj.onc.1204875. [DOI] [PubMed] [Google Scholar]
- 78.Lessnick SL, Dacwag CS, Golub TR. The Ewing’s sarcoma oncoprotein EWS/FLI induces a p53-dependent growth arrest in primary human fibroblasts. Cancer Cell. 2002;1:393–401. doi: 10.1016/s1535-6108(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 79.Huang HY, Illei PB, Zhao Z, et al. Ewing sarcomas with p53 mutation or p16/p14ARF homozygous deletion: A highly lethal subset associated with poor chemoresponse. J Clin Oncol. 2002;23:548–558. doi: 10.1200/JCO.2005.02.081. [DOI] [PubMed] [Google Scholar]
- 80.Ban J, Bennani-Baiti IM, Kauer M, et al. EWS-FLI1 suppresses NOTCH-activated p53 in Ewing’s sarcoma. Cancer Res. 2008;68:7100–7109. doi: 10.1158/0008-5472.CAN-07-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y, Tanaka K, Fan X, et al. Inhibition of the transcriptional function of p53 by EWS-FLI1 chimeric protein in Ewing Family Tumors. Cancer Lett. 2010;294:57–65. doi: 10.1016/j.canlet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 82.Kovar H, Jug G, Aryee DN, et al. Among genes involved in the RB dependent cell cycle regulatory cascade, the p16 tumor suppressor gene is frequently lost in the Ewing family of tumors. Oncogene. 1997;15:2225–2232. doi: 10.1038/sj.onc.1201397. [DOI] [PubMed] [Google Scholar]
- 83.Tsuchiya T, Sekine K, Hinohara S, et al. Analysis of the p16INK4, p14ARF, p15, TP53, and MDM2 genes and their prognostic implications in osteosarcoma and Ewing sarcoma. Cancer Genet Cytogenet. 2000;120:91–98. doi: 10.1016/s0165-4608(99)00255-1. [DOI] [PubMed] [Google Scholar]
- 84.Lopez-Guerrero JA, Pellin A, Noguera R, et al. Molecular analysis of the 9p21 locus and p53 genes in Ewing family tumors. Lab Invest. 2001;81:803–814. doi: 10.1038/labinvest.3780290. [DOI] [PubMed] [Google Scholar]
- 85.Obana K, Yang HW, Piao HY, et al. Aberrations of p16INK4A, p14ARF and p15INK4B genes in pediatric solid tumors. Int J Clin Oncol. 2003;23:1151–1157. [PubMed] [Google Scholar]
- 86.Cooper MJ, Hutchins GM, Cohen PS, et al. Human neuroblastoma tumor cell lines correspond to the arrested differentiation of chromaffin adrenal medullary neuroblasts. Cell Growth Differ. 1990;1:149–159. [PubMed] [Google Scholar]
- 87.Tirode F, Laud-Duval K, Prieur A, et al. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 88.West DC, Grier HE, Swallow MM, et al. Detection of circulating tumor cells in patients with Ewing’s sarcoma and peripheral primitive neuroectodermal tumor. J Clin Oncol. 1997;15:583–588. doi: 10.1200/JCO.1997.15.2.583. [DOI] [PubMed] [Google Scholar]
- 89.Zoubek A, Ladenstein R, Windhager R, et al. Predictive potential of testing for bone marrow involvement in Ewing tumor patients by RT-PCR: A preliminary evaluation. Int J Cancer. 1998;79:56–60. doi: 10.1002/(sici)1097-0215(19980220)79:1<56::aid-ijc11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 90.Peter M, Magdelenat H, Michon J, et al. Sensitive detection of occult Ewing’s cells by the reverse transcriptase-polymerase chain reaction. Br J Cancer. 1995;72:96–100. doi: 10.1038/bjc.1995.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dubois SG, Epling CL, Teague J, et al. Flow cytometric detection of Ewing sarcoma cells in peripheral blood and bone marrow. Pediatr Blood Cancer. 2010;54:13–18. doi: 10.1002/pbc.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pfleiderer C, Zoubek A, Gruber B, et al. Detection of tumour cells in peripheral blood and bone marrow from Ewing tumour patients by RT-PCR. Int J Cancer. 1995;64:135–139. doi: 10.1002/ijc.2910640211. [DOI] [PubMed] [Google Scholar]
- 93.Schleiermacher G, Peter M, Oberlin O, et al. Increased risk of systemic relapses associated with bone marrow micrometastasis and circulating tumor cells in localized ewing tumor. J Clin Oncol. 2003;21:85–91. doi: 10.1200/JCO.2003.03.006. [DOI] [PubMed] [Google Scholar]
- 94.Atiye J, Wolf M, Kaur S, et al. Gene amplifications in osteosarcoma-CGH microarray analysis. Genes Chromosomes Cancer. 2005;42:158–163. doi: 10.1002/gcc.20120. [DOI] [PubMed] [Google Scholar]
- 95.Khan J, Wei JS, Ringner M, et al. Classification and diagnostic prediction of cancers using gene expression profiling and artificial neural networks. Nat Med. 2001;7:673–679. doi: 10.1038/89044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ladanyi M, Gorlick R. Molecular pathology and molecular pharmacology of osteosarcoma. Pediatr Pathol Lab Med. 2000;19:391–413. [Google Scholar]
- 97.Al-Romaih K, Somers GR, Bayani J, et al. Modulation by decitabine of gene expression and growth of osteosarcoma U2OS cells in vitro and in xenografts: Identification of apoptotic genes as targets for demethylation. Cancer Cell Int. 2007;7:14. doi: 10.1186/1475-2867-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Selvarajah S, Yoshimoto M, Maire G, et al. Identification of cryptic microaberrations in osteosarcoma by high-definition oligonucleotide array comparative genomic hybridization. Cancer Genet Cytogenet. 2007;179:52–61. doi: 10.1016/j.cancergencyto.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 99.Gorlick R, Anderson P, Andrulis I, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: Meeting summary. Clin Cancer Res. 2003;9:5442–5453. [PubMed] [Google Scholar]
- 100.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 101.Womer RB, West DC, Krailo MD, et al. Randomized comarison of every-two-week v every-three-week chemotherapy in Ewing sarcoma family tumors (ESFT) [abstract] J Clin Oncol. 2008;26:10504. [Google Scholar]
- 102.Cooper A, van Doorninck J, Ji L, et al. Ewing tumors that do not overexpress BMI-1 are a distinct molecular subclass with variant biology: A report from the Children’s Oncology Group. Clin Cancer Res. 2011;17:56–66. doi: 10.1158/1078-0432.CCR-10-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Felgenhauer JL, Nieder ML, Krailo MD, et al. A pilot study of low-dose anti-angiogenic chemotherapy in combination with standard multiagent chemotherapy for patients with newly diagnosed metastatic ewing sarcoma family of tumors: A Children’s Oncology Group (COG) phase II study NCT00061893. Pediatr Blood Cancer. 2012 doi: 10.1002/pbc.24328. [Epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DuBois SG, Krailo MD, Lessnick SL, et al. Phase II study of intermediate-dose cytarabine in patients with relapsed or refractory Ewing sarcoma: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;52:324–327. doi: 10.1002/pbc.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marina N, Bielack S, Whelan J, et al. International collaboration is feasible in trials for rare conditions: The EURAMOS experience. Cancer Treat Res. 2009;152:339–353. doi: 10.1007/978-1-4419-0284-9_18. [DOI] [PubMed] [Google Scholar]
- 106.Jacobs KB, Yeager M, Zhou W, et al. Detectable clonal mosaicism and its relationship to aging and cancer. Nat Genet. 2012;44:651–658. doi: 10.1038/ng.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ebb D, Meyers P, Grier H, et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2545–2551. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arndt CA, Koshkina NV, Inwards CY, et al. Inhaled granulocyte-macrophage colony stimulating factor for first pulmonary recurrence of osteosarcoma: Effects on disease-free survival, immuno-modulation A report from the Children’s Oncology Group. Clin Cancer Res. 2010;16:4024–4030. doi: 10.1158/1078-0432.CCR-10-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim SY, Toretsky JA, Scher D, et al. The role of IGF-1R in pediatric malignancies. Oncologist. 2009;14:83–91. doi: 10.1634/theoncologist.2008-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: A phase 1 expansion cohort study. Lancer Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Casey DA, Wexler LH, Merchant MS, et al. Irinotecan and temozolomide for Ewing sarcoma: The Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53:1029–1034. doi: 10.1002/pbc.22206. [DOI] [PubMed] [Google Scholar]
- 112.Wagner LM, McAllister N, Goldsby RE, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007;48:132–139. doi: 10.1002/pbc.20697. [DOI] [PubMed] [Google Scholar]
- 113.Houghton PJ, Stewart CF, Cheshire PJ, et al. Antitumor activity of temozolomide combined with irinotecan is partly independent of O6-methylguanine-DNA methyltransferase and mismatch repair phenotypes in xenograft models. Clin Cancer Res. 2000;6:4110–4118. [PubMed] [Google Scholar]
- 114.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival—A report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 115.Chou AJ, Kleinerman ES, Krailo MD, et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: A report from the Children’s Oncology Group. Cancer. 2009;115:5339–5348. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mori K, Berreur M, Blanchard F, et al. Receptor activator of nuclear factor-kappaB ligand (RANKL) directly modulates the gene expression profile of RANK-positive Saos-2 human osteosarcoma cells. Oncol Rep. 2007;18:1365–1371. [PubMed] [Google Scholar]
- 117.Mori K, Le Goff B, Berreur M, et al. Human osteosarcoma cells express functional receptor activator of nuclear factor-kappa B. J Pathol. 2007;211:555–562. doi: 10.1002/path.2140. [DOI] [PubMed] [Google Scholar]
- 118.Lamoureux F, Richard P, Wittrant Y, et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: Blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007;67:7308–7318. doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 119.Lamoureux F, Picarda G, Rousseau J, et al. Therapeutic efficacy of soluble receptor activator of nuclear factor-kappa B-Fc delivered by nonviral gene transfer in a mouse model of osteolytic osteosarcoma. Mol Cancer Ther. 2008;7:3389–3398. doi: 10.1158/1535-7163.MCT-08-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Akiyama T, Choong PF, Dass CR. RANK-Fc inhibits malignancy via inhibiting ERK activation and evoking caspase-3-mediated anoikis in human osteosarcoma cells. Clin Exp Metastasis. 2010;27:207–215. doi: 10.1007/s10585-010-9319-y. [DOI] [PubMed] [Google Scholar]
- 121.Akiyama T, Dass CR, Shinoda Y, et al. Systemic RANK-Fc protein therapy is efficacious against primary osteosarcoma growth in a murine model via activity against osteoclasts. J Pharm Pharmacol. 2010;62:470–476. doi: 10.1211/jpp.62.04.0009. [DOI] [PubMed] [Google Scholar]
- 122.Beristain AG, Narala SR, Di Grappa MA, et al. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J Cell Sci. 2012;125:943–955. doi: 10.1242/jcs.094029. [DOI] [PubMed] [Google Scholar]
- 123.Molyneux SD, Di Grappa MA, Beristain AG, et al. PRKAR1a is an osteosarcoma tumor suppressor that defines a molecular subclass in mice. J Clin Invest. 2010;120:3310–3325. doi: 10.1172/JCI42391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heiner JP, Miraldi F, Kallick S, et al. Localization of GD2-specific monoclonal antibody 3F8 in human osteosarcoma. Cancer Res. 1987;47:5377–5381. [PubMed] [Google Scholar]
- 126.Frost JD, Hank JA, Reaman GH, et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: A report of the Children’s Cancer Group. Cancer. 1997;80:317–333. doi: 10.1002/(sici)1097-0142(19970715)80:2<317::aid-cncr21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]