Abstract
Perry syndrome is a rare form of autosomal dominant parkinsonism with respiratory failiure recently defined as being due to mutations in the DCTN1 gene. We describe a new family carrying a G71R mutation in the DCTN1 gene. The proband displayed a series of distinctive features not previously described in Perry syndrome: a disorder of vertical downward saccades accompanied by progressive midbrain atrophy, predominant non-motor symptoms responsive to L-DOPA, distinctive cranio-cervical L-DOPA induced dyskinesias, and a good response to high dose L-DOPA therapy and respiratory support. The family was initially thought to have autosomal dominant behavioural variant frontotemporal dementia with parkinsonism. This report expands the clinical definition of this distinctive syndrome.
Keywords: Perry Syndrome, Respiratory Failure, L-Dopa Responsive, Gaze Palsy, Dementia, Parkinsonism
Introduction
Perry syndrome is a rare autosomal dominant disorder (reported in 8 kindreds worldwide) with core features of hypoventilation, parkinsonism, weight loss and depression or psychiatric symptoms1,2. Cell loss in the substantia nigra is a uniform finding with some reports describing additional involvement of the locus coeruleus, nucleus tractus solitarius, dorsal nucleus of the vagus nerve, nucleus ambiguus and ventral medulla3. At a molecular level Perry syndrome is characterised by TAR DNA binding protein (TDP)-43 inclusions indicating a pathological overlap with amyotrophic lateral sclerosis and some forms of frontotemporal lobar degeneration (FTLD). Unlike in FTLD, pathological frontotemporal lobar atrophy is not a feature. Perry syndrome has recently been identified as being due to mutations in the DCTN1 gene, encoding the p150glued component of the dynactin complex, enabling further clinico-pathological delineation of this distinctive syndrome4. Penetrance is about 50%.
We report a new family with Perry syndrome presenting with features of behavioural variant frontotemporal dementia (bvFTD) later developing respiratory failure as well as autonomic disturbance, an abnormality of downgaze and distinctive L-DOPA responsive off-phenomena.
Case report
The proband was initially referred with suspected depression and was noted to have parkinsonism. His symptoms began at the age of 46. He had worked in computer-aided design. He spent the last 5 years of his working life as a warehouse operative (with decreasing productivity), retiring on ill health grounds at the age of 47. From the age of 46 his family described him becoming apathetic, less sociable and empathetic. Fluoxetine then lofepramine were prescribed with little benefit. At the age of 48 he began to walk more slowly, developed a staring expression and had falls. He had erectile dysfunction. When he was assessed at the age of 49 he had become reckless (especially with spending), mentally rigid and lacking initiative. His previously safe and reliable driving became erratic and risk-taking. He began to hoard. He would bolt food and there was mildly disinhibited behaviour, such as licking the bowl when dining. His wife remarked that he would frequently sigh, smack his lips or hum without apparent reason: these features were more prominent during sleep when they were interspersed with periods of rapid breathing and bruxism. There was no history of hallucinations. His father had died at the age of 56 with a post-mortem diagnosis of presenile dementia, and had originally been thought to have a psychiatric disorder; his paternal grandparents had both died at a young age. His mother died age 81 with late onset Parkinson’s disease.
On examination he was found to have generalized paucity of movement, axial rigidity with an en bloc posture on standing and turning, decreased arm swing and decreased blink rate, but no distal bradykinesia. There was no dyspraxia. His Mini-Mental State Examination score5 (MMSE) was 30/30, and Frontal Assessment Battery score6 was 16/18. Comprehensive neuropsychological assessment including attention span, verbal and visual memory, verbal reasoning, single word comprehension, and visuoperceptual and visuospatial construction skills revealed mildly reduced verbal fluency (total F-A-S letter fluency of 18 in 3 minutes) and mild slowing of information processing, but no other deficits. His recognition of emotions from facial expressions was within the control range7. His Neuropsychiatric Inventory Score (NPI) 8 was 20 (where a score of 0 signifies no behavioural abnormalities), with the most marked changes recorded in the domains of apathy and sleep disturbance.
He was started on L-Dopa and reported an improvement in movement and mood. At the age of 50 his family reported that he was becoming more short-tempered and he became very slow carrying out everyday activities. Later in the year there was evidence of distractibility (he left bags unattended at the airport). He had no insight into his balance problems. In the 26 months from his first hospital appointment his weight dropped from 75kg to 67kg.
At the age of 51, five years after the earliest symptoms, he developed diurnal episodes of tachypnoea lasting up to 20 minutes, on occasion associated with collapse. They were initially thought to be panic attacks. The breathing changes became more pronounced at the end of L-Dopa dose period. When reviewed eight months later he exhibited a series of autonomic and stereotypic wearing-off phenomena - hypersalivation, sweating, sniffing, repetitive facial grimacing with bruxism, worsening right leg tremor, as well as a feeling of inner tension, all responsive to L-DOPA. A year after commencement of treatment he was on 1000mg / day of L-DOPA (as co-careldopa), later building to more than 2000 mg/day of L-DOPA.
Disinhibited behaviour was becoming more marked. He required prompting for basic daily activities. Six years after onset, the patient had an episode of probable post-micturition syncope. A Holter tape revealed a persistent sinus tachycardia. Around this time his wife noticed choking and gagging noises during sleep. Two months later during the night he was found by his wife unconscious, making little respiratory effort. On admission to hospital his Glasgow Coma Score was 3, and his respiratory rate was 4/minute. He was in type II respiratory failure with a pO2 of 8.8kPa, a pCO2 of 10kPa and a blood pH of 7.2. Positive end expiratory pressure ventilation was initiated, he was transferred to the intensive care unit and was found to have an aspiration pneumonia. Subsequent sleep studies showed 2 desaturations (>4%) per hour on average. The pattern of desaturation was in keeping with central hypoventilation. Commencing regular non-invasive nocturnal ventilatory therapy (BIPAP) led to marked improvement in energy levels, alertness and concentration. He gained 7kg in weight during the first 3 months of non-invasive ventilatory therapy. Subsequently he also developed dysphagia as part of his wearing-off symptom complex. By the age of 53 he had marked slowing of downward saccades with preservation of horizontal saccades.
Neuropsychological assessment at this stage showed little evidence of cognitive deterioration: MMSE score remained 30/30 and performance in a series of focal cognitive domains was essentially unchanged, with only a mild increase in NPI score for abnormal behaviours (now 29). His dopaminergic therapy had been escalated to over 2g of cocareldopa/day as well as entacapone, with continued good response. With the provision of respiratory support and high dose L-DOPA treatment he and his family reported a good quality of life.
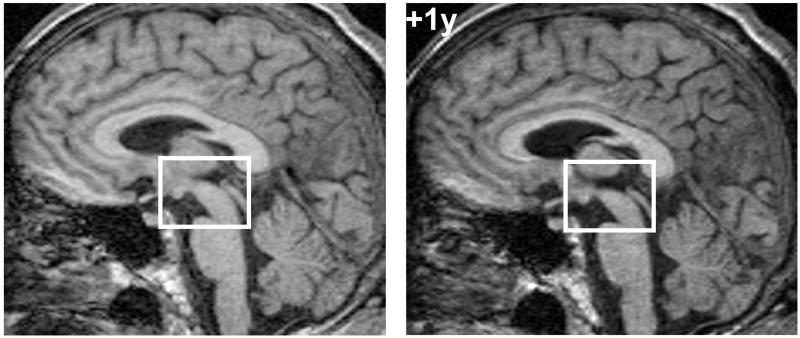
Serial brain MRIs were initially unremarkable but later showed mild diffuse cerebral atrophy and more pronounced focal midbrain atrophy (Figure 1). Video-EEG telemetry showed no evidence of epileptiform activity with frequent motor stereotypies.
Figure 1. MRI brain showing selective midbrain atrophy.
Sagittal T1-weighted volumetric MRI sections of the patient’s brain five years after symptom onset (left) and one year later (+1y), showing relatively selective midbrain atrophy. Images have been registered into a common space for comparison (the bounding box is provided to aid visualisation of midbrain change).
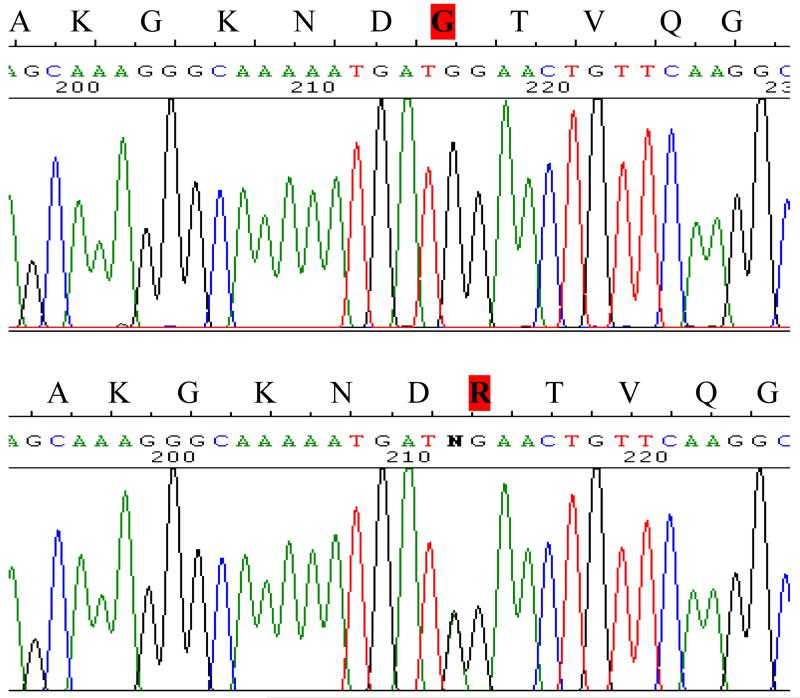
Sequence analysis of the tau (MAPT) and progranulin (GRN) genes was normal. Sequence analysis of the DCTN1 gene revealed a G71R mutation (Figure 2). The mutation was absent in a series of 355 Welsh control subjects.
Figure 2. Electropherogram showing mutation in DCTN1, compared to wild type.
DCTN1 G71R - Electropherogram showing the heterozygous mutation in DCTN1 exon 2 at nucleotide 212 G>A (lower panel) compared to the wild-type sequence (upper panel). Amino acid codes are given above the sequence, indicating that arginine (R) has been substituted for glycine (G) at position 71.
Discussion
This case fulfilled the proposed cardinal diagnostic features for probable Perry syndrome1. He had autosomal dominant inheritance with parkinsonism, respiratory failiure and apathy. Weight loss was present but not sustained, and with respiratory support his weight returned to pre-morbid levels. The diagnosis of Perry syndrome was confirmed by the identification of a pathogenic mutation in DCTN1. The patient was initially thought to have FTDP, however this was not supported by progressive cognitive decline or focal lobar atrophy. The diagnosis of Perry syndrome was not suspected until the development of respiratory failure.
Proposed supportive clinical diagnostic criteria for Perry syndrome include “No response to L-DOPA, or transient (<1 year) or erratic response” and “rapid progression”. This patient was strikingly L-DOPA responsive, with a variety of motor and non-motor off phenomena. Wearing off phenomena led to the escalation of L-DOPA to high levels. He did not have rapid progression, had an excellent response to respiratory support and remains largely independent nine years after the onset of symptoms. On L-DOPA treatment he displayed slow axial and cranio-cervical L-DOPA induced dyskinesia. As well as having early axial rigidity and falls this patient developed PSP-like features of slowing of vertical down-gaze and mid-brain atrophy, not previously described in Perry syndrome. Whilst dual pathology is conceivable in this case, we speculate that other patients with a familial FTDP and/or PSP like syndrome could have a mutation in DCTN-1.
This is the third family identified with the DCTN1 G71R mutation4. In Perry’s original description the earliest and most prominent symptom is treatment resistant mental depression and axial rigidity is also described9. In the other previously reported family with the G71R mutation from Turkey a long prodrome of decreased social-interest and apathy as well as a good response to L-DOPA are reported10. There is clinical heterogeneity in mutations of the DCTN-1 gene, with the nearby G59S mutation causing a lower motor neurone syndrome11 and the R1101K mutation causing ALS and FTD12. There is no consistent phenotypic variation associated with individual Perry syndrome causing mutations located in exon 2 of DCTN 1. Identification of further kindreds with pathogenic mutations of DCTN 1 and the longitudinal assessment of asymptomatic family members could broaden the phenotype further. The molecular pathology overlaps with the pathology of ALS, and the mechanism of neurodegeneration in patients with DCTN1 mutations will provide important insights into the mechanisms of TDP-43 related neurodegeneration13.
Acknowledgements
JDR is supported by a Brain Exit Scholarship and JDW by a Wellcome Trust Intermediate Clinical Fellowship.
This research is funded by the Medical Research Council (MRC) UK. Authors VM, EM, NW, and HRM are based at the MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff.
Grants
HRM: Parkinson’s Disease Society, Ipsen Fund, Medical Research Council, Wellcome Trust
NW: Parkinson’s Disease Society, Medical Research Council, Wellcome Trust, Action
Medical Research
JW: Wellcome Trust
JDR: Guarantors of Brain
Other Authors: none declared
Financial Disclosure (for previous 12 months)
Financial Disclosure (for previous 12 months)
Stock Ownership in medically related fields
All authors: none declared
Consultancies
All authors: none declared
Advisory Boards
HRM: Boerhinger-Ingelheim, Solvay. Other authors, none declared
Honoraria
HRM: Wellcome Trust. Other authors, none declared
Intellectual Property Rights
NW: Method for determining DNA copy number (GB0811500.8), Worldwide Patent Pending.
Other Authors: none declared
Expert Testimony
All authors: none declared
Employment
VN, EM, NW and HRM are employees of Cardiff University. JDR and JW are employed by University College London. MF is employed by Cardiff and Vale NHS trust. MH is employed by Gwent Healthcare NHS trust.
Contracts other than with main employer
All authors: none declared
Royalties
All authors: none declared
Footnotes
Financial disclosure related to this research
None of the authors have any conflicts of interest relating to the subject of this work.
References
- 1.Wider C, Wszolek ZK. Rapidly progressive familial parkinsonism with centralhypoventilation, depression and weight loss (Perry syndrome)-a literature review. Parkinsonism Relat Disord. 2008;14:1–7. doi: 10.1016/j.parkreldis.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Wider C, Dickson DW, Stoessl AJ, Tsuboi Y, Chapon F, Gutmann L, et al. Pallidonigral TDP-43 pathology in Perry syndrome. Parkinsonism Relat Disord. 2009;15:281–6. doi: 10.1016/j.parkreldis.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuboi Y, Dickson DW, Nabeshima K, Schmeichel AM, Wszolek ZK, Yamada T, et al. Neurodegeneration involving putative respiratory neurons in Perry syndrome. Acta Neuropathol. 2008;115:263–8. doi: 10.1007/s00401-007-0246-1. [DOI] [PubMed] [Google Scholar]
- 4.Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, et al. DCTN1 mutations in Perry syndrome. Nat Genet. 2009;41:163–5. doi: 10.1038/ng.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–6. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 7.Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto CA: 1976. [Google Scholar]
- 8.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 9.Perry TL, Bratty PJ, Hansen S, Kennedy J, Urquhart N, Dolman CL. Hereditary mental depression and Parkinsonism with taurine deficiency. Arch Neurol. 1975;32:108–13. doi: 10.1001/archneur.1975.00490440058009. [DOI] [PubMed] [Google Scholar]
- 10.Elibol B, Kobayashi T, Ataç FB, Hattori N, Şahin G, Gürer G, et al. Familial parkinsonism with apathy, depression and central hypoventilation (Perry’s syndrome) In: Mizuno Y, editor. Mapping the progress of Alzheimer’s and Parkinson’s disease. Kluwer Academic/Plenum Publishers; Boston, MA: 2002. pp. 285–90. [Google Scholar]
- 11.Puls I, Oh SJ, Sumner CJ, Wallace KE, Floeter MK, Mann EA, et al. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann Neurol. 2005;57:687–94. doi: 10.1002/ana.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munch C, Rosenbohm A, Sperfeld AD, Uttner I, Reske S, Krause BJ, et al. Heterozygous R1101K mutation of the DCTN1 gene in a family with ALS and FTD. Ann Neurol. 2005;58:777–80. doi: 10.1002/ana.20631. [DOI] [PubMed] [Google Scholar]
- 13.Wider C, Dachsel JC, Farrer MJ, Dickson DW, Tsuboi Y, Wszolek ZK. Elucidating the genetics and pathology of Perry syndrome. J Neurol Sci. 2009 doi: 10.1016/j.jns.2009.08.044. In press. www.elsevier.com/locate/jns. [DOI] [PMC free article] [PubMed] [Google Scholar]