Abstract
Adenomatous polyposis coli (APC) mutations are found in most colorectal tumours. These mutations are almost always protein-truncating, deleting both central domains that regulate Wnt signalling and C-terminal domains that interact with the cytoskeleton. The importance of Wnt dysregulation for colorectal tumourigenesis is well characterized. It is, however, unclear whether loss of C-terminal functions contributes to tumourigenesis, although this protein region has been implicated in cellular processes–including polarity, migration, mitosis, and chromosomal instability (CIN)–that have been postulated as critical for the development and progression of intestinal tumours. Since almost all APC mutations in human patients disrupt both central and C-terminal regions, we created a mouse model to test the role of the C-terminus of APC in intestinal tumourigenesis. This mouse (ApcΔSAMP) carries an internal deletion within Apc that dysregulates Wnt by removing the beta-catenin-binding and SAMP repeats, but leaves the C-terminus intact. We compared ApcΔSAMP mice with Apc1322T animals. The latter allele represented the most commonly found human APC mutation and was identical to ApcΔSAMP except for absence of the entire C-terminus. ApcΔSAMP mice developed numerous intestinal adenomas indistinguishable in number, location, and dysplasia from those seen in Apc1322T mice. No carcinomas were found in ApcΔSAMP or Apc1322T animals. While similar disruption of the Wnt signalling pathway was observed in tumours from both mice, no evidence of differential C-terminus functions (such as cell migration, CIN, or localization of APC and EB1) was seen. We conclude that the C-terminus of APC does not influence intestinal adenoma development or progression.
Keywords: APC, colorectal cancer, chromosomal instability, Wnt
Introduction
The adenomatous polyposis coli (APC) gene is mutated in patients with familial adenomatous polyposis (FAP) and in the majority of sporadic colorectal cancers. The APC gene product is a large, multi-domained protein containing binding sites for key Wnt pathway components, beta-catenin and axin [1], as well as cytoskeletal components including microtubule and end binding protein 1 (EB1) [2,3].
APC mutations occur early in the pathway of tumourigenesis, and almost all mutations found in adenomas and cancers are protein-truncating changes that lie within the beta-catenin-binding region of APC (Figure 1). It is now well established that such APC mutations disrupt the phosphorylation and subsequent degradation of beta-catenin, leading to an increase in Wnt signalling and activation of TCF/LEF transcription factors [4]. This occurs in two ways: first, through truncation of the protein before the SAMP repeats (codon 1564), which are required for the interaction of APC with axin and conductin; and second, through loss of some or all of the sets of 15- or 20-amino acid (aa) repeats that bind beta-catenin. The most common types of APC mutation result in a stable, but truncated protein that retains a small amount of beta-catenin binding and degradation activity [5,6]. Cells carrying these mutations have an increased, but sub-maximal, level of nuclear beta-catenin which is optimal for tumourigensis, possibly by increasing the number of crypt stem cells [7,8].
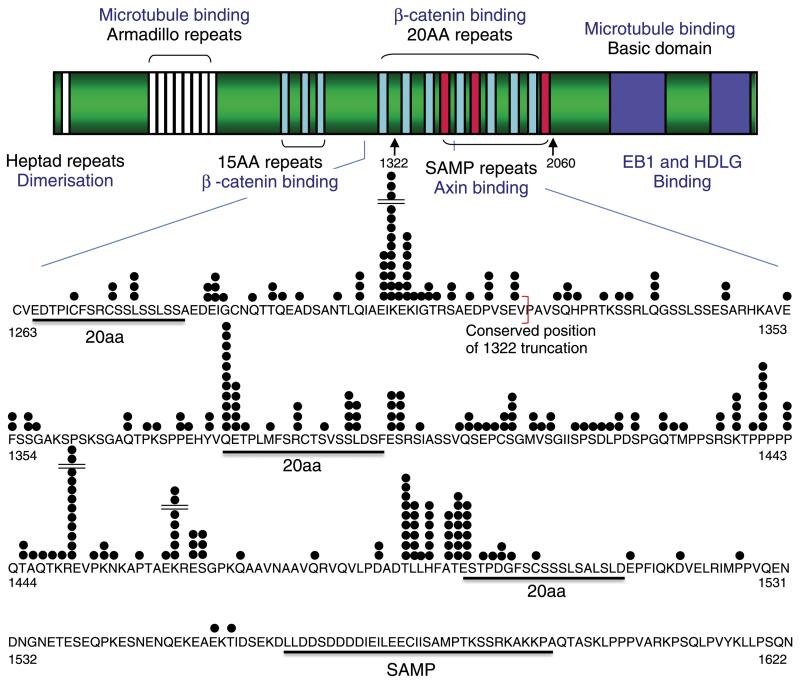
Figure 1.
Structure of the APC protein and position of mutations in human colorectal tumours. The diagram shows the major domains of the APC protein including regions that bind to beta-catenin, axin, and microtubules. The mutation cluster region is expanded to show the precise position of mutations, each represented by a black circle. The conserved position of the mouse codon 1322 truncation is marked by a red bracket. (Adapted from ref 48).
The beta-catenin-binding region of APC lies within the central part of the protein. Thus, almost all mutant APC proteins found in vivo also lack the molecule’s C-terminus. A central question is whether (i) absence of this distal part of APC is a bystander, secondary to disruption of beta-catenin binding; or (ii) loss of C-terminal function is required for tumourigenesis. The Wnt-signalling-associated role of APC in intestinal tumourigenesis has been intensely studied. However, the functions of the C-terminus—generally, interaction with cytoskeletal components—and their roles in tumourigenesis are much less well understood.
APC is now known to have a number of non-Wnt signalling, cytoskeleton-associated functions, including cell polarity and migration (reviewed in ref 9). APC localizes to and interacts directly with microtubules, at their plus ends [10,11] and also potentially via end-binding protein 1 (EB1) and the human homologue of discs large (DLG) [12]. These interactions stabilize the dynamic microtubule plus ends [13]. This microtubule-stabilizing role of APC influences cell polarity and by localizing APC at the tips of cell protrusions, which contributes to the cell migration of a number of different cell types in vitro and in vivo [14–17]. APC mutants that lack the C-terminus do not bind to microtubule ends as efficiently as their wild-type counterparts [18]. In all, there are three defined microtubule/cytoskeletal interacting domains in APC: the Armadillo repeats (aa 446–880); the basic domain (aa 2219–2580); and the EB1/DLG binding domain (aa 2733–2843). The last two of these domains lie within the C-terminus and are commonly deleted in the truncated APC proteins found in colorectal cancer.
APC may also have important functions in mitosis and in the prevention of chromosomal instability (CIN). In mouse embryonic stem (ES) cells and human colorectal cancer cell lines, APC associates with kinetochores in the mitotic spindle, although this is independent of the C-terminus microtubule- and EB1-interacting domains [19,20]. In colorectal cancer cell lines, there is a strong, but imperfect, correlation between a CIN+ phenotype and mutations in APC [21]. Moreover, overexpressing a truncated version of APC in CIN- cells has been shown to have a dominant-negative effect over the endogenous wild-type protein, resulting in mitotic defects typical of CIN+ cells, such as inefficient kinetochore attachments and misaligned metaphase chromosomes [20,22]. These studies have mainly taken place in cancer cell lines, although a few studies have also investigated mitotic defects in mouse tissue with a heterozygous Apc mutation and normal colon from FAP patients [21,23,24]. These show a disruption of the characteristic alignment of mitotic spindles in stem cell or transit amplifying cell compartments of precancerous tissue when compared with normal controls. It has therefore been proposed that cells with APC mutations are defective at sensing misaligned chromosomes and have loss or disruption of the mitotic spindle checkpoint [21]. Apparently contradictory evidence, however, shows that cells defective for APC still arrest after treatment with strongly depolymerizing agents such nocadozol, indicating that in some circumstances the checkpoint is still able to function despite the presence of mutant APC [22,25].
The importance of APC’s non-Wnt roles for tumourigenesis in vivo has not been clearly defined. While it is generally agreed that CIN (or at least aneuploidy and polyploidy) is a feature of most colorectal cancers, there is still much debate about when it occurs in the pathway of tumourigenesis. A number of studies argue strongly that it is necessary for the development of early adenomas, before or in conjunction with APC mutations, to account for the large number of mutations seen at this stage of tumour development [26,27]. Others, however, propose that mutations in adenomas accumulate at a rate which reflects the normal error rate of DNA replication and that CIN is a much later event in cancer development [28,29].
Separating the different cellular functions of APC to assess their roles in cancer has proved difficult in human cancers, since almost all tumours carry APC mutations that disrupt both beta-catenin-binding and microtubule-interacting domains [5,6,30–32]. A number of mouse models carrying comparable truncating mutations of APC have been well characterized. Those with mutations in or before the beta-catenin binding domain all develop adenomas in the small intestine with varying numbers and levels of dysplasia [8,17,33–39]. There is one model which carries a truncation of Apc at codon 1638, deleting all the C-terminal microtubule binding domains but only some of the beta-catenin binding sites and one of the axin-binding SAMP repeats [38]. However, these mice do not develop intestinal adenomas.
To date, no models exist of APC proteins that contain intact microtubule binding domains but have disruption of beta-catenin binding. In this study, we describe the construction and features of a mouse that carries a mutant Apc allele (ApcΔSAMP, ΔSAMP) with an internal, in-frame deletion from codon 1322 to 2006. This mutation removes six of the seven 20aa beta-catenin-binding repeats and all of the SAMP (axin-binding) repeats, but leaves the protein’s C-terminus intact. It allows us to test the two hypotheses that loss of C-terminal APC function is required for intestinal tumourigenesis and/or that absent C-terminal functions promote progression of tumours beyond early lesions. We have compared the phenotype of these mice with our Apc1322T (1322T) animals in which Apc is truncated at codon 1322, removing both beta-catenin and C-terminal domains.
Materials and methods
Generation of mice
The design of the ΔSAMP targeting vector is shown in Figure 2A. Mice were derived using standard methods. The vector was linearized and electroporated into 129Sv/J ES cells. Targeted ES cells were injected into C57Bl/6J blastocysts. The resultant chimeras were bred with C57Bl/6J mice for more than ten generations. Pgk-Cre mice were crossed with ΔSAMPs to delete the neomycin selectable marker. All ΔSAMP and 1322T animals were housed in the Barrier Unit of Cancer Research UK, Clare Hall Laboratories.
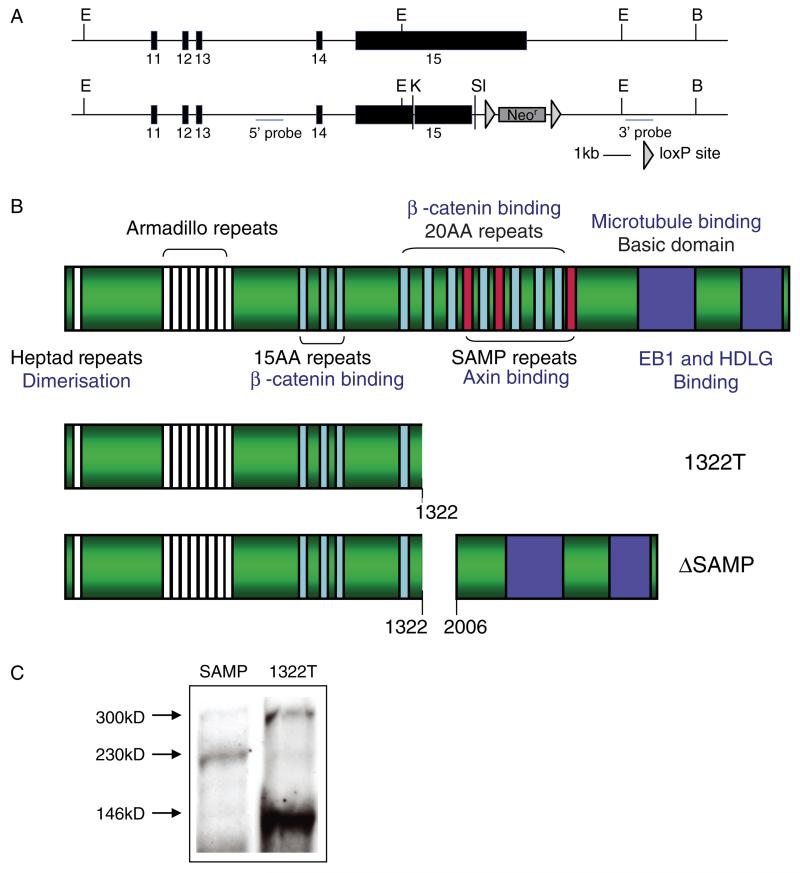
Figure 2.
Construction of ΔSAMP mice. (A) The top panel shows the wild-type allele and the lower panel shows the targeted ΔSAMP allele. Exons are numbered and restriction enzymes marked (B = BamHI; E = EcoR1; K = KpnI; Sl = SalI). (B) A cartoon showing the protein structure of APC and the deleted regions in the 1322T and ΔSAMP mutated proteins. (C) Western blot using anti-Apc antibody on tail fibroblasts derived from heterozygous 1322T and ΔSAMP mice showing the sizes of the mutated Apc protein relative to the full length 300 kD protein.
Western blotting to detect mutant Apc proteins
For analysis of full length and truncated Apc, tail-tip fibroblast cell pellets were lysed on ice for 15 min, denatured, loaded onto a 3% agarose gel, and run in SDS Tris–glycine buffer at 40 mA for 3–4 h. Samples were transferred to PVDF membrane by overnight capillary transfer in TBS buffer. After blocking for 1–2 h in a 5% milk–TBS solution, the membranes were incubated overnight at 4 °C with APC antibody (OP44; Calbiochem, Merck KGaA, Darmstadt, Germany) and incubated for 1–2 h at room temperature with HRP-conjugated rabbit anti-mouse secondary antibody (P0161; DakoCytomation, Dako, Glostrup, Denmark). ECL reagents were used for antibody detection (GE Healthcare, Chalfont St Giles, UK).
Phenotypic assessment
ΔSAMP or 1322T mice were sacrificed at a specified age or when symptomatic (anaemic secondary to polyps or suffering rectal prolapse). The intestinal tract was divided into four segments, three equal lengths of small intestine [proximal (SB1), middle (SB2), and distal (SB3)], and large intestine. Each segment was flushed with PBS and opened longitudinally onto filter paper. The samples were fixed overnight in 10% neutral buffered formalin (NBF) and stored in 70% ethanol. Preparations were stained with 0.2% methylene blue. Tumours were counted with a dissecting microscope at ×3 magnification and categorized according to size, with a cut-off of 0.1 mm diameter used for scoring. For histology, samples were processed using standard methods and analysed by histopathologists (MD, RP, and NAW).
Immunohistochemistry
Formalin-fixed, paraffin-embedded bowel sections from ΔSAMP and 1322T mice were de-waxed and rehydrated, and endogenous peroxidase was blocked using 1.6% H2O2 for 20 min. For antigen retrieval, sections were treated in a microwave oven in 10 mmol/l citrate buffer (pH 6.0) for 10 min. Sections were blocked with 10% normal serum for 30 min. Sigma polyclonal rabbit anti-beta catenin antibody (C2206) diluted to 1 : 1250 in 1% BSA or polyclonal rabbit anti-musashi (Msi1) antibody diluted to 1 : 150 (Chemicon; AB5977, Millipore, Billerica, MA, USA) was added for 1–2 h.
Sections analysed for beta-catenin were washed in PBS prior to applying biotinylated goat anti-rabbit secondary antibody (1 : 1250; DAKO, Dako, Glostrup, Denmark) for 1 h at room temperature and incubated in ABC (Vector Labs, Burlingham, CA, USA) for 30 min. DAB solution was applied for 2–5 min while being monitored microscopically. Slides were counterstained with haematoxylin, dehydrated, and mounted. Sections were assessed for nuclear beta-catenin staining, and the cell restricted distribution of nuclear beta-catenin was quantified in adenomas and background mucosa. Msi1-stained sections were incubated with secondary antibody (Molecular Probes goat anti-rabbit Alexa 488, Invitrogen, Carlsbad, CA, USA 1 : 300 dilution) for 1 h, immersed in Sudan black (0.1% in 70% IMS) to reduce autofluorescence, and mounted in hard set VECTAMOUNT (Vectors Labs) with DAPI. Positive cells were scored as present or absent in at least ten polyps from each type of mouse by MD, while blinded to polyp origin.
In situ hybridization
RNA in situ hybridization (ISH) was carried out using 4 μm serial sections from formalin-fixed, paraffin-embedded mouse intestines. The Lgr5 riboprobe has been described previously [7]. For hybridization control, a beta-actin probe was used. Riboprobes were generated by in vitro transcription using SP6 polymerase and labelled with 35S-UTP (GE Healthcare). Other methods were as described by Poulsom et al [40]. Signal intensity was scored in at least 12 polyps from each type of mouse (by SS and AL, blinded to polyp origin), as long as the beta-actin signal passed a quality control threshold. Total expression was calculated as the proportion of the epithelial component of a polyp that expressed the mRNA of interest (simultaneous bright-field and dark-field images for each adenoma were analysed using the ImageJ software package to measure the total area of the epithelial component of the adenoma and the area showing mRNA expression) multiplied by the intensity of the signal (0–3 determined by SS and AL.)
Expression microarray analysis
Adenomas were dissected from the SB1 portion of fresh intestinal tissue and snap-frozen in liquid N2). RNA was extracted and treated with RNase-free DNase I (Qiagen, Hilden, Germany). Quality was assessed on a Bioanalyzer (Agilent, Santa Clare, CA, USA). Three ΔSAMP and three 1322T tumour samples were prepared using One-Cycle Target Labeling and Control Reagents, hybridized to GeneChip® Mouse Genome 430A 2.0 Arrays, according to the manufacturer’s instructions (Affymetrix, Santa Clara, CA, USA). Output Cel files were analysed using the R statistical program with the robust multichip average (RMA) method, which is implemented in the BioConductor package ‘Affy’. Differentially expressed genes were then identified using the LIMMA (linear models of microarray analysis) package.
Cell migration
Five ΔSAMP, five 1322T, five Min, and one wild-type C57Bl/6J mice received an intra-peritoneal tritiated thymidine injection (0.5 mCi/kg) 17 h and a BrdU injection (50 mg/kg) 1 h prior to sacrifice. Intestines were then prepared, fixed, and processed for histological analysis as above. 5 μM sections were hydrolysed in 1 M HCl for 8 min and at 60 °C, and stained for BrdU with MAS 250 (Sera Lab, Loughborough, UK), peroxidase-conjugated anti-rat IgG, and incubated with a peroxidase substrate (diaminobenzidine and phosphate-buffered saline in addition to 0.3% hydrogen peroxide). Autoradiography was then carried out on these sections; slides were dipped in K2 emulsion (Ilford Ltd, Mobbersley, UK) dissolved in water warmed to 45 °C, left to dry on a cold plate, and exposed at 4 °C for 4 weeks. The slides were immersed in D-19 developer (Kodak, Paris, France) for 4 min, immersed in 1% acetic acid for 30 s, and finally fixed twice in 30% sodium thiosulphate for 4 min. The slides were then counterstained using Giemsa solution. Measurements were made of cell migration by recording the position of 3H-thymidine- and BrdU-labelled cells from individual crypts and villi. The rate of cell migration was determined by the difference between the highest 3H-thymidine and the BrdU-labelled cells. All tissues were analysed by MD blinded to the model under study.
Immunofluorescence of ES cells
ES cells were initially grown using feeder mouse embryonic fibroblasts in leukaemia inhibitory factor (LIF) supplemented medium. Differentiated cells were obtained by passaging without mouse embryonic fibroblasts (MEFs) and withdrawing LIF for 9–14 days. Cells were fixed in methanol at −20 °C for 30 min and permeabilized with 0.2% Triton X-100 for 10 min at room temperature. They were blocked with 10% goat serum for 30 min and incubated with anti-Apc Ali12-28 mouse monoclonal (1 : 80 dilution; Cancer Research UK, Cell Services) and anti-EB1 rabbit polycolonal (1 : 1000 dilution; Sigma) for 1 h. Secondary antibodies goat anti-mouse Fitc and goat anti-rabbit Cy5 (1 : 200 dilution; Abcam) were applied for 30 min, and cells were washed and stained with DAPI. Images were captured using a Zeiss 510 MetaHead confocal microscope.
Results
Construction of ApcΔSAMP (ΔSAMP) mice
We designed a targeting construct to create the ΔSAMP mice by in-frame deletion of codons 1322–2006 of Apc by homologous recombination in ES cells (Figure 2A). The resulting mutation was identical to that in the 1322T animals, but residues after codon 2006 were retained, including intact C-terminal microtubule- and EB1-interacting domains (Figure 2B). After deletion of the neomycinR cassette using a cross to Pgk-Cre animals, the mice were backcrossed onto the C57Bl/6J background for ten generations (and continuously for every generation thereafter, maintaining a stable heterozygous phenotype). Transcription across the deletion was confirmed by RT-PCR (data not shown) and correct production of the internally deleted Apc protein by western blot (Figure 2C).
ΔSAMP mice develop intestinal polyposis indistinguishable from 1322T mice
We tested the hypothesis that the presence of an intact Apc C-terminus in ΔSAMP mice would diminish the intestinal polyposis phenotype seen in 1322T mice. To this end, ΔSAMP heterozygous mice were aged, apparently developing normally, until they developed symptoms consistent with intestinal polyps (anaemia and hunching), at which time they were sacrificed and the number of polyps was counted. Typically, 190 adenomatous polyps developed between 70 and 90 days of age, with the majority of the polyps found in the duodenum and jejunum (SB1 and SB2) (Figures 3A and 3B). This polyp number and distribution were essentially the same as those seen previously in the 1322T mice (Wilcoxon test, p = 1.0). The size of these polyps was also indistinguishable from 1322T mice (Figure 3C). In addition, one to two colorectal and gastric adenomas were seen per mouse (Figure 3D) in both 1322T and ΔSAMP. No carcinomas were seen in either mutant.
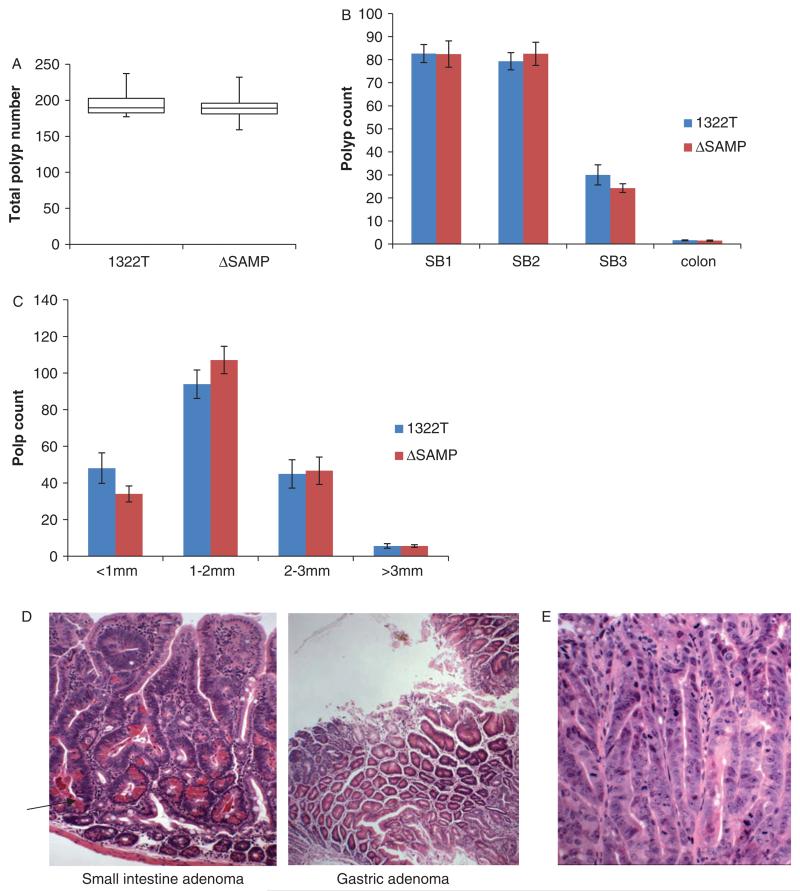
Figure 3.
The gross intestinal polyposis phenotype of ΔSAMP mice is indistinguishable from 1322T mice. (A) Box plot showing that the total polyp number found in 1322T (n = 12) and ΔSAMP (n = 10) mice is similar. (B) Graph showing the same distribution of polyps between the regions of the small intestine and colon in the two strains. (C) Bar chart showing the size of polyps in the small intestine in the two strains, (D) H&E staining showing a typical ΔSAMP small intestine adenoma with high numbers of Paneth cells (arrow) and a gastric adenoma. (E) Severely dysplastic areas in a ΔSAMP adenoma.
Next, we investigated the possibility that the Apc C-terminus modulated the severity of dysplasia in individual adenomas (Figure 3E). The proportions of severely dysplastic adenomas were very similar in 1322T and ΔSAMP animals [46% versus 45% (n = 50 for each strain), X21 = 0.50, p = 0.48], suggesting that the presence of the C-terminus has no effect on dysplasia at this stage of adenoma development. Similarly, the cellular composition of adenomas in the two strains was comparable, with high numbers of Paneth cells, particularly in early adenomas (Table 1). Molecular analysis showed loss of heterozygosity at Apc in all seven ΔSAMP polyps analysed, indicating homozygosity for the mutant Apc protein, as we had previously found in 1322T tumours [8]. We have never previously observed chromosomal aberrations indicative of CIN in 1322T tumours by array-comparative genomic hybridization; hence, as expected, none was found in ΔSAMP adenomas (details not shown).
Table 1.
Cell composition and beta-catenin protein expression in adenomas
| Normal crypt | Normal crypt | Early lesions <4 crypts) | Early lesions (<4 crypts) | Late lesions (>4 crypts) | Late lesions (>4 crypts) | |
|---|---|---|---|---|---|---|
| ΔSAMP N = 10 | 1322 N = 10 | ΔSAMP N = 10 | 1322 N = 13 | ΔSAMP N = 10 | 1322 N = 13 | |
| Mean cell number/crypt cross section (range in individual crypts) |
32.5 (27–41) | 29 (26–52) | 48 (32–81) | 43 (26–56) | 54 (28–71) | 46 (30–67) |
| Mean percentage enterocytes/cross section normal crypt or lesion |
69.8 (62–82) | 76.4 (72–81) | 50.7 (30–93.1) | 57.7 (28–95) | 85.6 (71–96) | 76.5 (52–95) |
| Mean percentage Paneth cells/cross section normal crypt or lesion |
22.1 (17–27) | 18.6 (15–25) | 48 (20–67) | 40.3 (17–63) | 15.8 (7.9–28) | 21.15 (5–45) |
| Mean percentage goblet cells/cross section normal crypt or lesion |
4.9 (4–11) | 6.9 (6–8) | 0.8 (0–2) | 1.97 (0–7) | 2.2 (0–4.7) | 1.23 (0–4.8) |
| Mean percentage neuroendocrine cells/cross section normal crypt or lesion |
1.75 (0–2) | 0.4 (0–2) | 0.5 (0–1) | 0.38 (0–2) | 0.6 (0–1) | 0 |
| Mean percentage enterocytes expressing nuclear beta catenin/cross section normal crypt or lesion |
1 (0–2) | 1 (0–2) | 4.5 (0–11) | 2.6 (0–13.3) | 11.7 (2–28) | 7.5 (0–32.9) |
| Mean percentage Paneth cells expressing nuclear beta-catenin/cross section normal crypt or lesion |
47.14 (20–80) | 42.3 (25–75) | 57 (32–87) | 66.9 (24–95) | 3.4 (0–10) | 1.3 (0–3.3) |
| Mean percentage goblet cells expressing nuclear beta-catenin/cross section normal crypt or lesion |
0 | 0 | 0 | 0 | 0 | 0 |
| Mean percentage neuroendocrine cells expressing nuclear beta-catenin/cross section normal crypt or lesion |
0 | 0 | 0 | 0 | 0 | 0 |
Molecular phenotype of ApcΔSAMP tumours is indistinguishable from that of Apc1322T mice
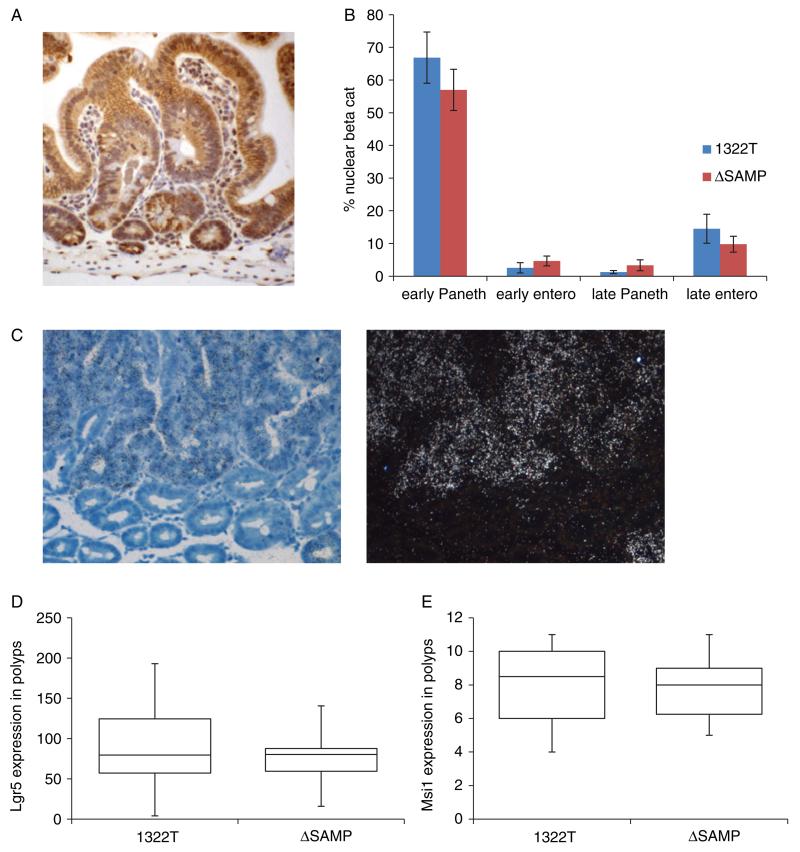
We had previously found that the 1322T mice had a more severe adenoma phenotype than the commonly used ApcR850X (Min) model, but that 1322T adenomas showed levels of nuclear beta-catenin expression and Wnt target gene expression intermediate between normal tissue and Min adenomas [7,8]. We found that ΔSAMP adenomas also showed this intermediate level of nuclear beta-catenin (Figures 4A and 4B; Wilcoxon p = 0.221). To further demonstrate the similarity in Wnt signalling levels, we also carried out Taqman QPCR of target genes in ES cells homozygous for the 1322T or ΔSAMP mutation. No significant differences were found between the two strains (Supporting information, Supplementary Figure 1). In addition, the high number of tumour cells expressing stem cell markers Lgr5 and Msi1 was seen in both 1322T adenomas and ΔSAMP tumours of similar size (Lgr5 Wilcoxon test p = 0.604, n = 59 1322, n = 14 ΔSAMP; Msi1 Wilcoxon test p = 0.819, n = 10) (Figures 4C–4E). Both strains also showed similarly high levels of crypt fission (data not shown).
Figure 4.
Beta-catenin and stem cell markers levels in ΔSAMP adenomas are the same as in 1322T adenomas. (A) IHC carried out with anti-beta-catenin antibody on ΔSAMP adenomas. (B) Cells with nuclear staining were counted in early and late lesions in both Paneth cells and enterocytes and compared with 1322T adenomas. Graph shows comparable numbers of cells with nuclear staining in each strain. (C) ISH carried out with a 35S-labelled Lgr5 riboprobe. Bright-field and dark-field images (20× objective magnification) of a typical tumour showing patches of intense Lgr5 expression (visualized by silver granules) and counterstained with Giemsa. (D) Box diagram showing similar Lgr5 expression scored in ΔSAMP and 1322T polyps. For each polyp, the area of staining was calculated as a proportion of the whole epithelial component and multiplied by the estimated mean intensity of the test mRNA. (E) Box diagram showing similar Msi1 expression scored in ΔSAMP and 1322T polyps.
We also investigated whether there were any more subtle differences detectable at the molecular level, due to the putative altered Eb1- and microtubule-binding capacity of the mutated Apc protein in 1322T compared with ΔSAMP animals. We carried out expression microarray analysis to look for differences in the mRNA expression between three 1322T and three ΔSAMP adenomas matched for size and location in SB1. After correction for multiple comparisons, no statistical differences were seen between adenomas from the two strains. After relaxing the statistical threshold to a nominal p = 0.05, we found that 66 genes showed some difference (Supporting information, Supplementary Table 1). However, the majority of these genes had low overall levels of expression and showed fold changes of less than 2. As expected, no known Wnt genes were contained in this list, but more surprisingly, no known cytoskeletal, mitotic checkpoint or cell cycle factors were found. Some differences were seen in ion transport or metal binding genes and possibly relate to differing levels of anaemia of the particular mice that were sacrificed.
Investigation of C-terminal Apc functions in ΔSAMP and 1322T mice
The C-terminus of APC is thought to be involved in cell migration. Although it was not possible to study this feature in adenomatous (homozygous mutant) tissue, we investigated it in the normal (heterozygous mutant) mucosa of 1322T and ΔSAMP mice. We did not observe any difference 24 h after labelling with tritiated thymidine or 1 h after labelling with BrdU between normal mucosa from 1322T or ΔSAMP animals; indeed, there was no difference between the mutant mice and wild-type littermates (Kruskal–Wallis test p = 0.111, n = 25 wt crypts, n = 37 1322, n = 21 ΔSAMP).
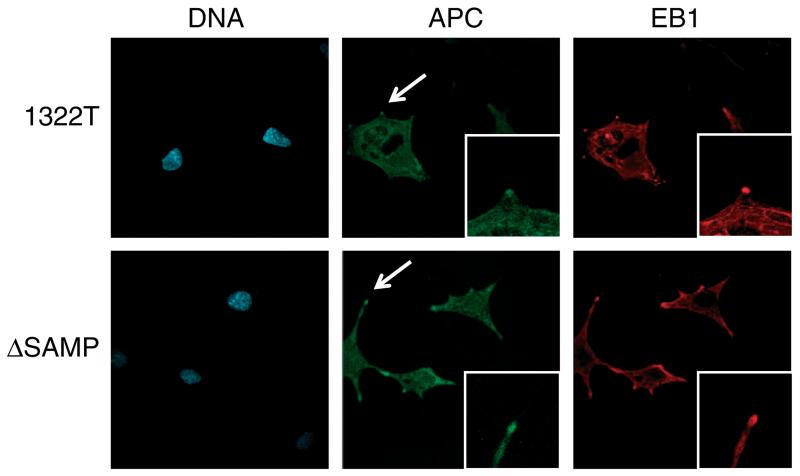
Since no major changes were seen in cell division and cytoskeletal components at the level of gene expression, we also investigated the effect of our ΔSAMP Apc mutation on Eb1. Since direct interaction between Apc and Eb1 is infrequent in interphase cells [13] and it is not possible to coimmunoprecipitate the endogenous proteins [3,41], we investigated co-localization of the proteins in dividing homozygous 1322T and ΔSAMP ES cells using immunofluorescence. (Since homozygous 1322T and ΔSAMP mice die in early gestation, it was not possible to derive homozygous embryonic fibroblasts.) Undifferentiated cells have mainly membranous and cytoplasmic Apc localization, with no difference in Apc or Eb1 localization between 1322T, ΔSAMP or wild-type cells (Supporting information, Supplementary Figure 2). However, these cells lack the protrusions where Apc C-terminal-dependent, cytoskeletal binding is usually visible. We therefore differentiated homozygous 1322T and ΔSAMP ES cells by withdrawing LIF. Apc localization to protrusions was seen in ΔSAMP cells, as expected, but was also present in 1322T cells (Figure 5). Similarly, Eb1 localization showed no difference between the cells. We concluded that in undifferentiated and differentiated ES cells, Apc can bind to microtubules independently of the C-terminus.
Figure 5.
Localization of Apc and EB1 in Apc-mutant differentiated ES cells. Immunofluorescence was carried out on differentiated homozygous ΔSAMP and 1322T ES cells with anti-Apc (green) and anti-EB1 (red) antibodies, and DNA was stained with DAPI (blue). The localization of both Apc and EB1 remained unchanged, both being present at the microtubules and cellular protrusions (arrowed and high-power view inset) in both Apc mutants.
Discussion
In order to investigate the role played by the Apc C-terminal microtubule binding regions in intestinal tumourigenesis, we have compared the phenotypes of two mouse strains: 1322T, carrying a truncated version of Apc without beta-catenin and microtubule binding domains; and ΔSAMP, carrying an internal deletion of the same 20 aa and SAMP-binding repeats as 1322T, but with an intact C-terminus. Both strains had an essentially identical intestinal polyposis phenotype, in terms of adenoma numbers, size, dysplasia, and location. In both cases, there were raised, but sub-maximal, levels of Wnt signalling in adenomas compared with Min mice, together with a high frequency of Lgr5-expressing stem-like cells and of crypt fission. Neither the ΔSAMP nor the 1322T adenomas showed evidence of chromosomal instability or a significant disruption of cell migration, and ES cells homozygous for either mutation showed no changes in Apc or Eb1 localization. We conclude that the functional effects of the two different mutations in Apc are indistinguishable at the gross phenotypic level and that the presence of the C-terminus has no measurable effect on the early tumourigenesis pathways which lead to the development of adenomas in the small intestine.
The data above suggest that the loss of C-terminus in Apc is not required for intestinal tumourigenesis, does not contribute to the severity of intestinal polyposis, and does not cause CIN. This is arguably somewhat surprising given the large body of data demonstrating the importance of the C-terminus microtubule interactions, but may be explained in several ways.
Firstly, we must note that the 1322T mouse, and virtually all other mouse strains carrying Apc mutations, only represents the early stages of tumourigenesis and the adenomas very rarely progress to invasive carcinomas. This is probably because the mice have to be sacrificed early, due to the effects of the large tumour burden, and the adenomas do not have sufficient time to accumulate additional mutations. It is interesting to note that we have not detected chromosomal-scale aberrations in any 1322T or ΔSAMP adenomas despite the fact that many show severe dysplasia, and indeed, CIN has not been found in Min or AOM/DSS induced adenomas, or even in mouse adenomas containing several further mutations in the human adenoma–carcinoma pathway [8] (Aleksic and Speicher, personal communication; Davis, unpublished). This supports the argument put forward by several groups that CIN is not necessary for adenoma formation [28,29]. We can conclude from this study that loss of the Apc C-terminus is not necessary to cause or promote adenoma development or progression. However, CIN can occur in severely dysplastic human colorectal adenomas [42] and is therefore still an important factor to consider. CIN in conjunction with loss of the Apc C-terminus might contribute to tumour progression after the loss of DNA damage checkpoints by mechanisms such as p53 mutation, and we note that we found no p53 mutations in 1322T or ΔSAMP adenomas (data not shown).
Secondly, much of the cell line work to investigate the role of APC in cell polarity and migration has been carried out in non-epithelial or non-intestinal cell types and there is evidence to suggest that APC localization and, more generally, its function are cell type- and context-dependent [43]. In the context of normal tissue, the epithelial cells of the intestine may show different effects of Apc mutations from MDCK cells, for example. Cultured ES cells and colorectal cancer cell lines are also likely to have constraints on genomic instability that are very different from those in an organized, hierarchical system such as the intestinal crypt. In addition, most of the dominant-negative constructs (and dominant CIN cell lines) contain truncated APC that is missing not only the C-terminus, but also some of the 20 aa and SAMP binding repeats [20,22,44]. They therefore do not distinguish between the Wnt and cytoskeleton-associated functions of APC. The longest mutant Apc protein tested in such experiments was the codon 1638 truncation in mouse ES cells. This contains three of the seven of the beta-catenin 20 aa binding sites, but in order to observe CIN in these ES cells without apoptosis occurring, it was necessary also to overexpress Bcl2 [19]. In short, it is possible that loss of the C-terminus of APC may increase the tendency to CIN, but this is generally insufficient to overcome intact mitotic checkpoints.
Lastly, many of the studies of the APC C-terminus studies have focused on the interactions with EB1. However, it is still not clear how important this interaction is in intestinal epithelial cells. In interphase cells, the interaction between the endogenous proteins is very infrequent and EB1 is not required for the localization of APC to microtubule plus ends or microtubule growth [13,45,46]. Similarly, EB1 localization is unaffected by Apc mutations [47], as we have found.
In conclusion, we have shown that there is no evidence that C-terminal associated functions of Apc play a role in the formation of intestinal adenomas or their progression to larger or more dysplastic lesions. It is the decreased beta-catenin binding capacity of mutated APC, and therefore increases in Wnt signalling, that is the most important factor at this early stage of the tumourigenesis pathway.
Supplementary Material
Table S1. List of genes showing differences in mRNA expression between 1322T and ΔSAMP mice in expression microarray analysis.
Figure S1. Taqman Q-PCR of Wnt targets in ES cells.
Figure S2. Localization of Apc and Eb1 in Apc-mutant ES cells.
Acknowledgment
We are grateful to colleagues from the Biological Resources Unit, and Histopathology Unit, London Research Institute, Cancer Research UK. We thank Chung-Yin Lee and Bill Otto for their help with cell migration assays. This work was funded by Cancer Research UK. Core infrastructure support to the Wellcome Trust Centre for Human Genetics, Oxford was provided by grant 090532/Z/09/Z.
Footnotes
No conflicts of interest were declared.
Supporting information may be found in the online version of this article.
References
- 1.Behrens J, Jerchow BA, Wurtele M, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 2.Munemitsu S, Souza B, Muller O, et al. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- 3.Su LK, Burrell M, Hill DE, et al. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- 4.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin–Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 5.Albuquerque C, Breukel C, van der Luijt R, et al. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet. 2002;11:1549–1560. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Lamlum H, Ilyas M, Rowan A, et al. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson’s ‘two-hit’ hypothesis. Nature Med. 1999;5:1071–1075. doi: 10.1038/12511. [DOI] [PubMed] [Google Scholar]
- 7.Lewis A, Segditsas S, Deheragoda M, et al. Severe polyposis in Apc(1322T) mice is associated with submaximal Wnt signalling and increased expression of the stem cell marker Lgr5. Gut. 2010;59:1680–1686. doi: 10.1136/gut.2009.193680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard P, Deheragoda M, Segditsas S, et al. The Apc 1322T mouse develops severe polyposis associated with submaximal nuclear beta-catenin expression. Gastroenterology. 2009;136:2204–2213. doi: 10.1053/j.gastro.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 9.Etienne-Manneville S. APC in cell migration. Adv Exp Med Biol. 2009;656:30–40. doi: 10.1007/978-1-4419-1145-2_3. [DOI] [PubMed] [Google Scholar]
- 10.Matsumine A, Ogai A, Senda T, et al. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science. 1996;272:1020–1023. doi: 10.1126/science.272.5264.1020. [DOI] [PubMed] [Google Scholar]
- 11.Mimori-Kiyosue Y, Shiina N, Tsukita S. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J Cell Biol. 2000;148:505–518. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10:865–868. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- 13.Kita K, Wittmann T, Nathke IS, et al. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol Biol Cell. 2006;17:2331–2345. doi: 10.1091/mbc.E05-06-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 15.Mogensen MM, Tucker JB, Mackie JB, et al. The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells. J Cell Biol. 2002;157:1041–1048. doi: 10.1083/jcb.200203001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathke IS, Adams CL, Polakis P, et al. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sansom OJ, Reed KR, Hayes AJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith KJ, Levy DB, Maupin P, et al. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994;54:3672–3675. [PubMed] [Google Scholar]
- 19.Fodde R, Kuipers J, Rosenberg C, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nature Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 20.Green RA, Kaplan KB. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J Cell Biol. 2003;163:949–961. doi: 10.1083/jcb.200307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldwell CM, Kaplan KB. The role of APC in mitosis and in chromosome instability. Adv Exp Med Biol. 2009;656:51–64. doi: 10.1007/978-1-4419-1145-2_5. [DOI] [PubMed] [Google Scholar]
- 22.Tighe A, Johnson VL, Taylor SS. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J Cell Sci. 2004;117:6339–6353. doi: 10.1242/jcs.01556. [DOI] [PubMed] [Google Scholar]
- 23.Fleming ES, Temchin M, Wu Q, et al. Spindle misorientation in tumors from APC(min/+) mice. Mol Carcinog. 2009;48:592–598. doi: 10.1002/mc.20506. [DOI] [PubMed] [Google Scholar]
- 24.Quyn AJ, Appleton PL, Carey FA, et al. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell. 2010;6:175–181. doi: 10.1016/j.stem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Green RA, Wollman R, Kaplan KB. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol Biol Cell. 2005;16:4609–4622. doi: 10.1091/mbc.E05-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loeb LA. Mutator phenotype in cancer: origin and consequences. Semin Cancer Biol. 2010;20:279–280. doi: 10.1016/j.semcancer.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michor F, Iwasa Y, Vogelstein B, et al. Can chromosomal instability initiate tumorigenesis? Semin Cancer Biol. 2005;15:43–49. doi: 10.1016/j.semcancer.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Shibata D, Lieber MR. Is there any genetic instability in human cancer? DNA Repair (Amst) 2010;9:858. doi: 10.1016/j.dnarep.2010.04.011. discussion 859–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomlinson I, Sasieni P, Bodmer W. How many mutations in a cancer? Am J Pathol. 2002;160:755–758. doi: 10.1016/S0002-9440(10)64896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurent-Puig P, Beroud C, Soussi T. APC gene: database of germline and somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1998;26:269–270. doi: 10.1093/nar/26.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi Y, Nagase H, Ando H, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 32.Sieber OM, Segditsas S, Knudsen AL, et al. Disease severity and genetic pathways in attenuated familial adenomatous polyposis vary greatly but depend on the site of the germline mutation. Gut. 2006;55:1440–1448. doi: 10.1136/gut.2005.087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colnot S, Niwa-Kawakita M, Hamard G, et al. Colorectal cancers in a new mouse model of familial adenomatous polyposis: influence of genetic and environmental modifiers. Lab Invest. 2004;84:1619–1630. doi: 10.1038/labinvest.3700180. [DOI] [PubMed] [Google Scholar]
- 34.Oshima M, Oshima H, Kitagawa K, et al. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci U S A. 1995;92:4482–4486. doi: 10.1073/pnas.92.10.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oshima M, Takahashi M, Oshima H, et al. Effects of docosahexaenoic acid (DHA) on intestinal polyp development in Apc delta 716 knockout mice. Carcinogenesis. 1995;16:2605–2607. doi: 10.1093/carcin/16.11.2605. [DOI] [PubMed] [Google Scholar]
- 36.Sasai H, Masaki M, Wakitani K. Suppression of polypogenesis in a new mouse strain with a truncated Apc(Delta474) by a novel COX-2 inhibitor, JTE-522. Carcinogenesis. 2000;21:953–958. doi: 10.1093/carcin/21.5.953. [DOI] [PubMed] [Google Scholar]
- 37.Shibata H, Takano H, Ito M, et al. Alpha-catenin is essential in intestinal adenoma formation. Proc Natl Acad Sci U S A. 2007;104:18199–18204. doi: 10.1073/pnas.0705730104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smits R, Kielman MF, Breukel C, et al. Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 1999;13:1309–1321. doi: 10.1101/gad.13.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 40.Poulsom R, Longcroft JM, Jeffery RE, et al. A robust method for isotopic riboprobe in situ hybridisation to localise mRNAs in routine pathology specimens. Eur J Histochem. 1998;42:121–132. [PubMed] [Google Scholar]
- 41.Berrueta L, Tirnauer JS, Schuyler SC, et al. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol. 1999;9:425–428. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- 42.Shih IM, Zhou W, Goodman SN, et al. Evidence that genetic instability occurs at an early stage of colorectal tumorigenesis. Cancer Res. 2001;61:818–822. [PubMed] [Google Scholar]
- 43.Langford KJ, Lee T, Askham JM, et al. Adenomatous polyposis coli localization is both cell type and cell context dependent. Cell Motil Cytoskeleton. 2006;63:483–492. doi: 10.1002/cm.20139. [DOI] [PubMed] [Google Scholar]
- 44.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 45.Barth AI, Siemers KA, Nelson WJ. Dissecting interactions between EB1, microtubules and APC in cortical clusters at the plasma membrane. J Cell Sci. 2002;115:1583–1590. doi: 10.1242/jcs.115.8.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma M, Leung L, Brocardo M, et al. Membrane localization of adenomatous polyposis coli protein at cellular protrusions: targeting sequences and regulation by beta-catenin. J Biol Chem. 2006;281:17140–17149. doi: 10.1074/jbc.M513027200. [DOI] [PubMed] [Google Scholar]
- 47.Morrison EE, Wardleworth BN, Askham JM, et al. EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–3477. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- 48.Rosin-Arbesfeld R, Townsley F, Bienz M. The APC tumour suppressor has a nuclear export function. Nature. 2000;406:1009–1012. doi: 10.1038/35023016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of genes showing differences in mRNA expression between 1322T and ΔSAMP mice in expression microarray analysis.
Figure S1. Taqman Q-PCR of Wnt targets in ES cells.
Figure S2. Localization of Apc and Eb1 in Apc-mutant ES cells.