Abstract
Objectives:
The purpose of this study was to determine analgesic efficacy of dexmedetomidine used as a continuous infusion without loading dose in postcardiac surgery patients.
Settings and Design:
A prospective, randomized, double-blind clinical study in a single tertiary care hospital on patients posted for elective cardiac surgery under cardiopulmonary bypass.
Interventions:
Sixty-four patients who underwent elective cardiac surgery under general anesthesia were shifted to intensive care unit (ICU) and randomly divided into two groups. Group A (n = 32) received a 12 h infusion of normal saline and group B (n = 32) received a 12 h infusion of dexmedetomidine 0.4 μg/kg/h. Postoperative pain was managed with bolus intravenous fentanyl. Total fentanyl consumption, hemodynamic monitoring, Visual Analogue Scale (VAS) pain ratings, Ramsay Sedation Scale were charted every 6th hourly for 24 h postoperatively and followed-up till recovery from ICU. Student's t-test, Chi-square/Fisher's exact test has been used to find the significance of study parameters between the groups.
Results:
Dexmedetomidine treated patients had significantly less VAS score at each level (P < 0.001). Total fentanyl consumption in dexmedetomidine group was 128.13 ± 35.78 μg versus 201.56 ± 36.99 μg in saline group (P < 0.001). A statistically significant but clinically unimportant sedation was noted at 6 and 12 h (P < 0.001, and P = 0.046 respectively). Incidence of delirium was less in dexmedetomidine group (P = 0.086+). Hemodynamic parameters were statistically insignificant.
Conclusions:
Dexmedetomidine infusion even without loading dose provides safe, effective adjunct analgesia, reduces narcotic consumption, and showed a reduced trend of delirium incidence without undesirable hemodynamic effects in the cardiac surgery patients.
Keywords: Cardiac surgery, dexmedetomidine, postoperative pain
INTRODUCTION
Dexmedetomidine has a relatively high ratio of α2/α1-activity (1620:1 as compared with 220:1 for clonidine) and therefore, is considered a full agonist of the α2 receptor.[1] This may result in more potent effects of sedation without unwanted cardiovascular effects from α1 receptor activation. In contrast to the gamma-amino butyric acid agonists and opiates, dexmedetomidine has a unique mechanism of action. It combines sedative, anxiolytic, sympatholytic, anti-delirious and analgesic sparing properties with minimal respiratory depression.[2,3] Whilst no single agent has all the desirable properties of an ideal agent, an ideal strategy would provide effective analgesia, anxiolysis, and reduce the risk of delirium and agitation with minimal cardio-respiratory depression.[4]
Recommended practice is to give a loading dose of 1 μg/kg over 10-20 min. In a study of 66 postsurgery patients receiving this loading dose, 11 out of 18 unwanted hemodynamic effects (mean arterial pressure <60 mmHg or heart rate <50 beat/min) occurred during loading.[5] A reduction in loading dose was suggested following a study in medical patients, especially if other sedative agents had been used prior to starting dexmedetomidine.[3]
We conducted this study to determine the analgesic efficacy of dexmedetomidine as a continuous infusion without loading dose in postcardiac surgery patients.
MATERIALS AND METHODS
The Hospital Ethics Committee approved this study and written informed consent was obtained from all the patients. Study was conducted between October 2012 and January 2013. Patient's inclusion criteria were: Age over 18 years, elective cardiac surgery using cardiopulmonary bypass (CPB) including coronary artery bypass graft, valve surgery, and atrial septal defect closure. Patients with elevated serum creatinine, ejection fraction <40%, arrhythmias, deranged hepatic function and known allergy to current medication were excluded. Apart from the study drug, all other management was according to unit protocols. All patients received a standard premedication oral gabapentin 600 mg 45 min before shifting to operating room and general anesthesia consisted of midazolam 0.1 mg/kg, fentanyl 10 μg/kg, propofol 100ug/kg/min and vecuronium 0.2 mg/kg. All patients were monitored with routine cardiac surgery hemodynamic monitoring. All patients were operated under normothermic, nonpulsatile CPB.
Study protocol
Sample size estimation showed that approximately 32 patients were needed to detect a clinically relevant reduction of the pain level by 25%, with a power of 0.80 and alpha of 5%. Sixty-four patients scheduled for elective cardiac surgery consented to participate in the study. After surgery, patients were transferred intubated and ventilated to a cardiothoracic intensive care unit (ICU) and then were prospectively randomized in a double-blind fashion into one of the two groups using computer. Group A (n = 32) received a 12 h infusion of normal saline and Group B (n = 32) received a 12 h infusion of dexmedetomidine 0.4 μg/kg/h without a loading dose. Postoperative pain was supplemented with intravenous fentanyl 25 μg intermittent bolus, whenever the Visual Analogue Scale (VAS) pain score was more than 5. Total fentanyl consumption, hemodynamic monitoring, VAS pain rating and Ramsay Sedation Scale (RSS) were charted every 6th hourly for 24 h postoperatively and followed-up till recovery from ICU. Richmond Agitation Sedation Scale (RASS) was used to categorize delirium.
Statistical analysis
Results on continuous measurements are presented as mean ± standard deviation and results on categorical measurements are presented in a number (%). Significance is assessed at 5% level of significance. Student's t-test (two-tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups and Chi-square/Fisher's exact test has been used to find the significance of study parameters on categorical scale between two or more groups. About 95% confidence interval has been computed to find the significant features.[6]
The statistical software namely SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0 and R environment version 2.11.1 were used for the analysis of the data and Microsoft word and Excel have been used to generate graphs, tables etc.
RESULTS
There were no statistically significant differences between patient demographics and types of surgery [Table 1] and intraoperative bypass time (CPB), aortic cross clamp time, duration of surgery and fentanyl consumption [Table 2].
Table 1.
Patient demographic data

Table 2.
Intra-operative data
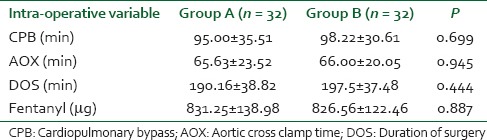
Dexmedetomidine treated patients had a statistically significant less VAS score of pain 3.31 ± 0.69, 4.34 ± 0.90, 4.13 ± 1.02, 4.11 ± 1.14and 4.75 ± 0.88 at 0, 6, 12, 18 and 24 h respectively [Table 3]. Fentanyl consumption in the first 24 h was less in dexmedetomidine group (128.13 ± 35.78 μg) than in saline group (201.56 ± 36.99 μg) with P < 0.001 [Table 4].
Table 3.
Comparison of VAS score in postoperative period
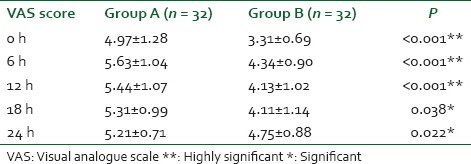
Table 4.
Comparison of total fentanyl use and incidence of delirium in the postoperative period

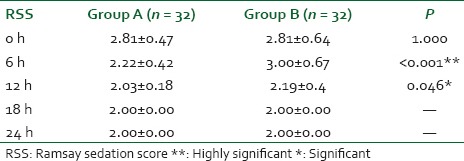
A statistically significant but clinically unimportant sedation was noted at 6 h (RSS of 3.00 ± 0.67, P < 0.001) and 12 h (RSS of 2.19 ± 0.4, P = 0.046) [Table 5].
Table 5.
Comparison of RSS in the postoperative period
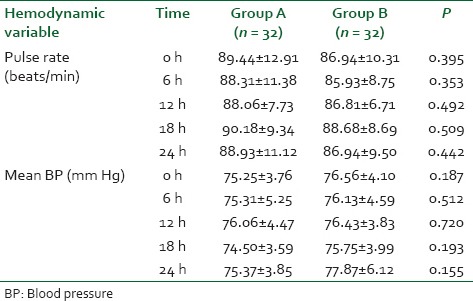
A reduced trend in delirium incidence was noted in dexmedetomidine group (3.1%) than in saline group (15.6%) with P = 0.086+ [Table 4]. Awakening time and hemodynamic parameters in postoperative period was statistically insignificant [Tables 6 and 7].
Table 6.
Awakening time in the postoperative period

Table 7.
Comparison of pulse and mean BP in the postoperative period
DISCUSSION
Dexmedetomidine is the dextro-stereoisomer and active ingredient of medetomidine, an agent used for many years in veterinary anesthesia. It is a highly selective α2 agonist with an affinity 8 times that of clonidine for the adrenoceptor.[7]
Dosage
The majority of adverse events (hypotension and bradycardia) occur as a result of the loading dose of dexmedetomidine. This was predictable from the known properties of α2 agonists and might have been avoided by omitting the loading dose and commencing the infusion while in the operating theatre. The protocol allowed neither boluses of study drug nor exceeding the maximum infusion rate (0.7 μg/kg/h dexmedetomidine). The hypotension and bradycardia occurring during the loading dose phase of the dexmedetomidine was only seen in cardiac patients. This is probably directly attributable to stimulation of the central postsynaptic α2 receptors causing inhibition of sympathetic activity or augmentation of parasympathetic activity.[5]
Hall et al. demonstrated in a randomized, double-blinded study involving small population of volunteers with healthy cardiovascular systems that small doses (0.2 and 0.6 μg/kg/h infusions, small and moderate doses respectively) of dexmedetomidine provided significant sedation and analgesia to the conventional pain therapy. Cardiovascular stability and respiratory function were both well maintained.[8]
In a study by Shehabi et al. on tertiary general ICU patients, unique feature was the omission of dexmedetomidine loading dose. Dexmedetomidine was started at 0.4 μg/kg/h in all patients then increased (maximum of 0.7 μg/kg/h) or decreased (minimum of 0.2 μg/kg/h) every 15 min as deemed clinically necessary.[9]
In a randomized controlled trial in 306 cardiac surgery patients showed that dexmedetomidine, at a median dose of 0.49 μg/kg/h, provided effective sedation and targeted analgesia without an increase in hypotension or vasopressor requirements. Furthermore, dexmedetomidine treatment significantly reduced the duration of delirium and promoted early extubation when compared with morphine based regimen.[10]
Dexmedetomidine can be used in patients expected to be ventilated for up to 48 h after complex surgery. The dose range is 0.2-0.7 μg/kg/h without the use of a loading dose. The infusion should be commenced in the operating room 30 min before skin closure or immediately after surgery.[4]
Lin et al. also have concluded that majority of the adverse events associated with dexmedetomidine administration occurred during or shortly after the loading dose and have recommended to use a lower loading infusion rate during the 1st h or eliminating the loading dose to reduce the incidence of hypotension.[11]
In the study by Shehabi et al., 20 critically ill patients received dexmedetomidine at a dose of 0.2-0.7 μg/kg/h, but a loading dose of dexmedetomidine was omitted because the patients had already received other sedative drugs, which were administered until 1 h after administration of dexmedetomidine was started.[9]
In our study, we used dexmedetomidine at 0.4 μg/kg/h without a loading dose in the immediate postoperative period after cardiac surgery, which had residual sedative and analgesic effects.
Hemodynamics
Venn et al. reported an incidence of 27% significant hemodynamic changes of which 61% occurred during the loading dose phase, with some patients being withdrawn from the study as a result.[5]
Ickeringill et al. showed a reduction of 8% in heart rate and 10% in systolic blood pressure from baseline after the commencement of dexmedetomidine without a loading dose. None of their patients had a clinically significant bradycardia, and only six patients (12%) required the introduction of a pressor for management of low blood pressure. However, the absence of a control group makes it difficult to identify a direct cause and effect in this group of patients where significant fluid shifts, blood loss and dynamic changes are likely to be occurring in the postoperative period. Postoperative warming in the cardiac surgery group and the concurrent use of inotropes and pressors may mask the true effect on blood pressure. They demonstrated that by avoiding a loading dose of dexmedetomidine, unwanted cardiovascular effects can be substantially minimized.[12]
In our study, there were no significant hemodynamic changes in the dexmedetomidine group when compared to the saline group. So by omitting a loading dose of dexmedetomidine, unwanted cardiovascular effects were avoided.
Analgesia and sedation
Dexmedetomidine has a strong synergistic effect with other sedatives and opioids, with a 50-70% reduction in propofol, midazolam and opioid requirements having been observed.[4] It is well established that dexmedetomidine has opioid-sparing effects.[8,13]
Ickeringill et al. studied in postsurgical patients without loading dose and found that 76% required no rescue sedation and 48% required no rescue analgesia. Of those requiring rescue analgesia, only 7 (14%) required significant additional analgesia. Cardiac group required the least additional sedation and or analgesia.[12]
Shahbaz et al. concluded that administration of dexmedetomidine before the completion of major surgical procedures associated with above-average postoperative pain significantly reduced by 66% the early postoperative need for morphine and 65% of the dexmedetomidine treated patients required no additional analgesic for the 1st h of recovery.[14]
Study by Venn et al. including 98 patients with complete data, 47 received dexmedetomidine, and 51 received placebo. Eighty-one patients (83%) underwent cardiac surgery requiring CPB. There were no overall differences in the distribution of Ramsay Sedation Scores between the dexmedetomidine and placebo groups while intubated.[5]
Shehabi et al. concluded that most patients had acceptable quality sedation as shown by 83% of observed RSS recorded between 2 and 5 over the study period. The overall effect on sedation and analgesia was similar in magnitude to studies on postoperative surgical patients with a consistent effect.[9]
Our study correlated well with the previous studies. We demonstrated that dexmedetomidine treated patients appreciated less pain and analgesic requirement throughout the postoperative period. There was statistically significant sedation in study group at 6 and 12 h when compared to the control group, although this was not significant clinically.
Delirium
We used RASS to define delirium. RASS has good reliability and validity and is increasingly recommended for use, though many others are also used.[15]
Although the exact mechanism by which dexmedetomidine counteracts agitation remains unclear, animal models show an increase in acetylcholine and reduction in noradrenaline levels in cerebrospinal fluid in response to dexmedetomidine, suggesting a central nervous system mediated effect.[16]
In a comparative study with lorazepam, dexmedetomidine infused for longer than 24 h up to 1.5 μg/kg/h in ventilated critically ill patients led to a significant reduction in delirium and number of days in coma.[17] Dexmedetomidine has also been successfully used in the management of emergence delirium after failure of conventional therapy in a cohort of critically ill mechanically ventilated patients.[16] In this study, the average dose used was 0.79 μg/kg/h and resulted in 86% of patients achieving a target Motor Activity Assessment Scale within 12 h. Similar results were shown in 111 patients treated for emergence delirium as assessed using the Ramsay Sedation Score.[18]
A recent randomized pilot study demonstrated the superiority of dexmedetomidine over haloperidol in the treatment of agitated delirious ventilated ICU patients.[19] A well-conducted multicenter randomized double-blind controlled trial has compared dexmedetomidine with midazolam in ventilated medical and surgical ICU patients for longer than 24 h. There was a 22.6% absolute reduction in the incidence and 48% reduction in the duration of delirium achieved with dexmedetomidine compared to midazolam in all patients, and in patients who were delirious at enrolment.[20]
Yapici et al. evaluated the use of dexmedetomidine to facilitate the weaning of delirious postoperative patients from mechanical ventilation. They concluded that dexmedetomidine may help to eliminate the emergence of agitation and can be a good treatment choice for the delirium state after cardiac surgery.[21]
In a meta-analysis of nine studies by Lin et al., the most striking finding was that sedation with dexmedetomidine is associated with lower risk of delirium following cardiac surgery. Dexmedetomidine could be a safe and efficacious sedative agent in cardiac surgery patients.[11]
Though our study was aimed to study delirium, dexmedetomidine treated patients had reduced the incidence of delirium (3.1%) while it was 15.6% in the control group.
Limitation
Though we have reported reduced incidence of delirium, the statistical significance was not determined because the sample size was estimated to calculate only analgesic efficacy without loading dose.
CONCLUSION
Dexmedetomidine infusion even without loading dose provides safe, effective adjunct analgesia, reduces narcotic consumption, and showed a reduced trend of delirium incidence without undesirable hemodynamic effects in the cardiac surgery patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 2.Gerlach AT, Dasta JF. Dexmedetomidine: An updated review. Ann Pharmacother. 2007;41:245–52. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- 3.Venn M, Newman J, Grounds M. A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensive Care Med. 2003;29:201–7. doi: 10.1007/s00134-002-1579-9. [DOI] [PubMed] [Google Scholar]
- 4.Shehabi Y, Botha JA, Ernest D. Clinical application, the use of dexmedetomidine in intensive care sedation. Crit Care Shock. 2010;13:40–50. [Google Scholar]
- 5.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 6.Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5:7–13. doi: 10.4103/0974-1208.97779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Hayashi Y, Maze M. Alpha 2 adrenoceptor agonists and Anesthesia. Br J Anaesth. 1993;71:108–18. doi: 10.1093/bja/71.1.108. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 9.Shehabi Y, Ruettimann U, Adamson H, Innes R, Ickeringill M. Dexmedetomidine infusion for more than 24 hours in critically ill patients: Sedative and cardiovascular effects. Intensive Care Med. 2004;30:2188–96. doi: 10.1007/s00134-004-2417-z. [DOI] [PubMed] [Google Scholar]
- 10.Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: A randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009;111:1075–84. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 11.Lin YY, He B, Chen J, Wang ZN. Can dexmedetomidine be a safe and efficacious sedative agent in post-cardiac surgery patients? A meta-analysis. Crit Care. 2012;16:R169. doi: 10.1186/cc11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ickeringill M, Shehabi Y, Adamson H, Ruettimann U. Dexmedetomidine infusion without loading dose in surgical patients requiring mechanical ventilation: Haemodynamic effects and efficacy. Anaesth Intensive Care. 2004;32:741–5. doi: 10.1177/0310057X0403200602. [DOI] [PubMed] [Google Scholar]
- 13.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 14.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 15.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 16.Shehabi Y, Nakae H, Hammond N, Bass F, Nicholson L, Chen J. The effect of dexmedetomidine on agitation during weaning of mechanical ventilation in critically ill patients. Anaesth Intensive Care. 2010;38:82–90. doi: 10.1177/0310057X1003800115. [DOI] [PubMed] [Google Scholar]
- 17.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007;298:2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi A, Okuda T, Kotani T, Oda Y. Efficacy of dexmedetomidine for controlling delirium in intensive care unit patients. Masui. 2007;56:1155–60. [PubMed] [Google Scholar]
- 19.Reade MC, O’sullivan K, Bates S, Goldsmith D, Ainslie WR, Bellomo R. Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: A randomised open-label trial. Crit Care. 2009;13:R75. doi: 10.1186/cc7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009;301:489–99. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 21.Yapici N, Coruh T, Kehlibar T, Yapici F, Tarhan A, Can Y, et al. Dexmedetomidine in cardiac surgery patients who fail extubation and present with a delirium state. Heart Surg Forum. 2011;14:E93–8. doi: 10.1532/HSF98.201011102. [DOI] [PubMed] [Google Scholar]


