Abstract
Background:
As an anesthetic adjuvant dexmedetomidine has been shown to provide good perioperative hemodynamic stability with minimum alveolar concentration sparing effect on inhalational anesthetic agents during laparoscopic surgeries performed under general anesthesia.
Aim:
The study was planned to investigate the effects of dexmedetomidine on attenuation of hemodynamic changes and requirements of intra-operative analgesic and inhalational anesthetic during laparoscopic surgeries and its postoperative side effects.
Materials and Methods:
A total of 70 patients scheduled for elective laparoscopic surgeries were randomized to receive bolus infusion of dexmedetomidine (group D) or saline (group S) 1 mcg/kg/h, followed by continuous infusion of the same, at the rate of 0.5 mcg/kg/h. Anesthesia was maintained with nitrous oxide in oxygen, muscle relaxant and isoflurane. Supplementation with end-tidal isoflurane was considered when heart rate (HR) and mean arterial blood pressure (BP) exceeded 20% of the baseline value. Hemodynamics, end-tidal isoflurane concentration and adverse events were recorded.
Results:
Intra-operative mean HR and mean BP in group D were lower than group S (P < 0.05) throughout the laparoscopy surgery. Requirement of intra-operative fentanyl, end-tidal isoflurane and postoperative tramadol were significantly more in group S compared to group D (P < 0.05) Statistically significant nausea and vomiting were noted in group S. Undue sedation and other adverse effects are comparable in both the groups.
Conclusion:
Dexmedetomidine as an adjuvant in general anesthesia for laparoscopic surgeries provided a stable hemodynamic profile in the perioperative period and effectively blunted pressor response to intubation and extubation, leading to minimal requirements for additional analgesics and potent inhalational agents. There were less adverse events.
Keywords: Dexmedetomidine, general anesthesia, hemodynamic effects, laparoscopic surgeries
INTRODUCTION
First laparoscopic cholecystectomy was successfully performed by Phillipe Mouret in 1987, since than laparoscopic surgeries have become the gold standard. The benefits of minimal access techniques include less pain, early mobilization, shorter hospital stay and better cosmetic results, which have further increased its applications.
During general anesthesia laryngoscopy, tracheal intubation and extubation are the critical events provoking transient, but marked sympathoadrenal response manifesting as hypertension and tachycardia. In addition, in laparoscopic surgery CO2 is routinely used to create pneumoperitoneum, which causes increased plasma level of catecholamine and vasopressin. Elevation of intra-abdominal pressure with raised diaphragm causes various adverse effects on the cardiovascular system such as decreased cardiac output, elevated arterial pressure and increased systemic and pulmonary vascular resistance leading to hypertension and tachycardia. Hence, a drug, which can blunt hemodynamic responses to laryngoscopy, intubation and pneumoperitoneum without having any adverse effects like respiratory depression and postoperative nausea and vomiting (PONV) was required for the purpose. Dexmedetomidine is an alpha-2-adrenergic agonist, which is the pharmacologically active dextro-isomer of medetomidine. It has properties of analgesia, sympatholysis and titrating sedation without major respiratory depression.[1] It reduces opioid requirements and stress response to surgery ensuring a stable hemodynamic state. It has distribution half-life of approximately 6 min, so can be used successfully for attenuating the stress response to laryngoscopy.[2]
We hypothesized that dexmedetomidine might be a useful adjuvant to general anesthesia to attenuate hemodynamic responses of pneumoperitoneum in laparoscopy surgeries.
The study was planned to investigate the effects of dexmedetomidine on attenuation of hemodynamic changes and there effects as adjuvant in anesthesia on analgesic and inhalation anesthetic requirements during laparoscopic surgeries and its postoperative adverse events.
MATERIALS AND METHODS
After Local Ethics Committee approval and written informed consent, a total of 70 patients of American Society of Anesthesiologists physical status I or II aged between 15 and 60 years of either sex were randomly selected for this study. They were scheduled for elective general laparoscopic surgeries of around 2 h under general anesthesia. Patients posted for emergency surgical procedures, patients with cardiovascular or respiratory, renal disorders, diabetes, hypertension, obesity, difficult airway, pregnant, currently breast feeding women, history of sleep apnea, psychiatric disorder were excluded from the study.
All the patients were double-blinded randomly divided into two groups. A closed enveloped technique was used to assign each patient to the saline group (group S, n = 35) or the dexmedetomidine group (group D, n = 35). An anesthesiologist (who was not one of the observers for the study) prepared injectable solutions containing either dexmedetomidine or 0.9% saline. The dexmedetomidine was supplied in 2 ml ampoules of 100 μg/ml concentration (Abbott, Chicago, IL, USA), and it was diluted with 48 ml of normal saline to yield a final concentration of 4 μg/ml. For each patient in group S, a 50 ml volume of 0.9% saline solution was prepared.
Routine monitoring consisted of noninvasive blood pressure (BP), electrocardiography, Peripheral oxygen saturation (SpO2), end-tidal carbon dioxide and isoflurane concentration measured continuously with a multigas analyzer (Philips).
An 18-gauge intravenous cannula was inserted, and the patient was preloaded with 5 ml/kg of crystalloids. Before induction of anesthesia, the group D patients were given dexmedetomidine 1 mcg/kg loading dose infusion over 10 min while in group S, patients received same dose of normal saline in the same rate infused over 10 min through injectomet Agilia (Fresenius Kabi, USA) infusion pump.
Balanced general anesthesia was administered for all the patients. The patients received premedication, intravenous doses of 0.2 mg glycopyrrolate, 2 mcg/kg fentanyl, 4 mg ondansetron before induction of anesthesia. Induction was achieved with 5 mg/kg intravenous thiopentone. Intubation was facilitated by 2 mg/kg intravenous succinylcholine, and muscle relaxation maintained with 0.5 mg/kg of atracurium intermittent bolus with using neuromuscular monitoring. The lungs were ventilated by maintaining a tidal volume of 7-10 ml/kg, a frequency of 12 breaths/min and an EtCO2 of 30-40 mmHg in 3 L/min of fresh gas flow with 66% nitrous oxide in oxygen in a closed circuit. Isoflurane inhalation was started with 0.6% in both the groups. Injection dexmedetomidine maintenance infusion of 0.5 mcg/kg/h started in group D, and saline infusion started in group S in the same dose. HR and noninvasive BP spo2 were measured at the following times: Baseline, after loading dose, after intubation, after 20 min of pneumoperitoneum, after 60 min of pneumoperitoneum, after infusion stopped, after extubation, at 2nd postoperative hour, at 4th postoperative hour.
Intra-operative bradycardia (pulse rate <50) was treated with atropine 0.6 mg. Intra-operative hypotension was treated with intravenous crystalloids and by reducing the isoflurane concentration and mephentermine. An increase in HR and/or mean arterial pressure (MAP) >20% from baseline values was treated by 1 mcg/kg of intravenous fentanyl. If there was no response within 5 min, the initial isoflurane concentration was increased by 0.2% increment every 4 min up to a maximum of 1% end-tidal concentration. Isoflurane inhalation was stopped 10 min before the end of surgery and dexmedetomidine was stopped at the end of skin incision closure. The total amount of fentanyl administered during each case was also recorded.
On completion of surgery, the neuromuscular blockade was reversed with 0.05 mg/kg neostigmine and 0.004 mg/kg glycopyrrolate. All patients were extubated, shifted to the recovery room and monitored for hemodynamics, analgesia, sedation and postoperative side effects up to 4 h after surgery.
Postoperative pain intensity was assessed using a 10 point visual analogue scale (VAS) on which 0 indicated no pain and 10 indicated the worst pain imaginable. Tramadol 100 mg intravenously was given if VAS >3 and ondansetron 4 mg intravenously was used to treat emesis. The degree of sedation was assessed using the 6 point Ramsay sedation scale:
1 = Anxious or agitated and restless or both.
2 = Cooperative, oriented and tranquil.
3 = Drowsy but responds to commands.
4 = Asleep, brisk response to light glabellar tap or loud auditory stimulus.
5 = Asleep, sluggish response to light glabellar tap or loud auditory stimulus.
6 = Asleep and unarousable.
Sedation score >3 is considered an undue sedation.
Statistical analysis
Statistical tests were performed using Statistical Package for the Social Sciences software, version 12.0 (SPSS Inc., Chicago, Illinois, USA). Independent t-test was used to compare the study group and the control group. Paired t-test was used to compare the variables before and after the intervention. Chi-square test was used to analyze the categorical data and for testing the association between the variables. For comparison of continuous data such as hemodynamic parameters ANOVA test was used. The results were expressed as mean ± 0 standard deviation (SD) 0 P < 0.05 was considered as significant.
OBSERVATION AND RESULTS
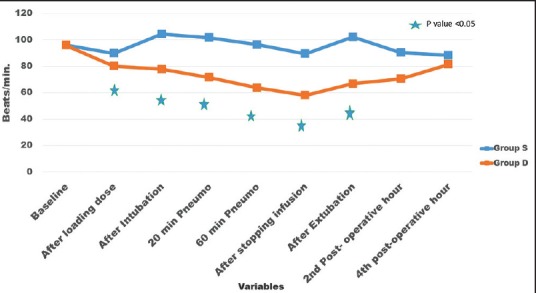
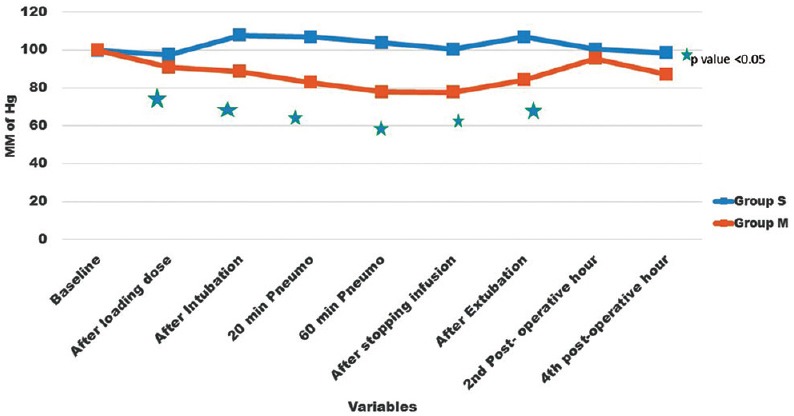
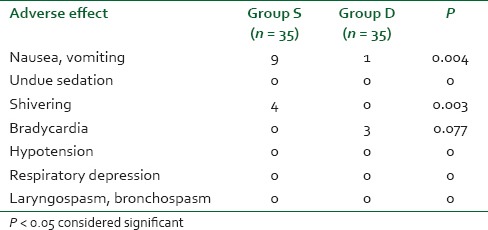
A total of 70 patients were randomly selected and compared using bolus injection, followed by infusion of dexmedetomidine (group D n = 35) or normal saline (group S n = 35) in laparoscopic surgeries for their efficacy in regard to pressor responses to intubation and extubation, hemodynamic stability, end-tidal isoflurane concentration and adverse events. As shown in Table 1 there was no significant difference in the age, sex, weight, height, body mass index, type and duration of surgery (P > 0.05). The mean duration of surgical procedure was around 60-120 min. Baseline mean HR was not significant between two groups (P > 0.05) as mean HR of group D was 95.94 ± 8.32 while in group S it was 96.05 ± 6.86 [Figure 1]. There was a significant reduction in HR following the loading dose of dexmedetomidine, after intubation, after 20 min of pneumoperitoneum, after 60 min of pneumoperitoneum, after infusion was stopped, after extubation, in group D (P < 0.05) as compared to saline group. Baseline MAP of group D was 99.94 ± 4.84 mmHg while in group S it was 99.71 ± 4.61 mmHg [Figure 2], which was not significant between two groups (P > 0.05). Decrease in MAP was found after loading dose, after intubation, after 20 min of pneumoperitoneum, after 60 min of pneumoperitoneum, after infusion stopped, after extubation, in group D compared to group S, which was significant (P < 0.05). Figure 3 shows, end-tidal isoflurane requirement was significantly less in group D when compared to group S (P < 0.05), especially it was increased after 20 min of pneumoperitoneum in group S. The intra-operative requirement of fentanyl was significantly higher in group S as compared in group D [Table 2]. The postoperative adverse effects like bradycardia were comparable between groups while shivering was statistically significant in group S [Table 3]. Four patients in group S experienced shivering and were treated with 25 mg tramadol. Bradycardia, noted in three patients in the dexmedetomidine group in the study, required treatment with 0.6 mg of atropine intravenously. Statistically significant nausea and vomiting were found in group S (9 patients [25.7%] as compared to 1 patient [2.8%] in group D). It was treated with ondansetron. Table 2 shows, 10 patients in group S (17.14%) required tramadol in the postoperative period for pain relief as compared to 2 patients in group D (5.71%), which was statistically significant (P < 0.05). Nine patient had sedation score slightly higher in group D, but none of the patient had an undue sedation, so the values are not statistically significant.
Table 1.
Demographic data and operative data
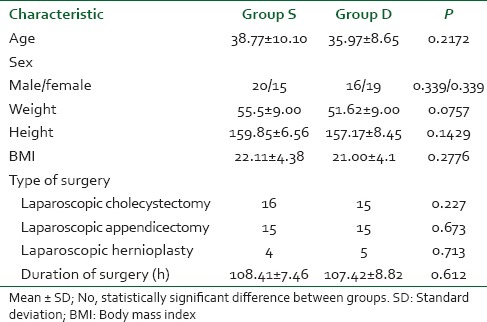
Figure 1.
Perioperative changes in heart rate (HR). Changes in HR over a period of time. Values are mean ± standard deviation significant difference (*P < 0.05) in group D compared to group S
Figure 2.
Perioperative changes of mean arterial blood pressure (BP). Changes in mean arterial BP over a period of time. Values are mean ± standard deviation significant difference (*P < 0.05) in group D compared to group S
Figure 3.
Intra-operative isoflurane requirement. End-tidal isoflurane concentration at different time interval. Values are mean ± standard deviation significant difference (*P < 0.05) in group D compared to group S at 20 min and 60 min after creation of pneumoperitoneum
Table 2.
Analgesic and antiemetic requirement
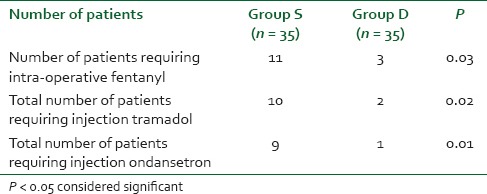
Table 3.
Adverse effect
DISCUSSION
In the present study, we demonstrated that bolus and intra-operative dexmedetomidine infusion decreased hemodynamic responses to various noxious stimuli perioperatively in laparoscopic surgeries. Thus, there was significant decrease in mean HR and MAP compared to saline infusion through-out the surgery (P < 0.05) which shows that infusion of dexmedetomidine (0.5 mcg/kg/h) was effective in reduction of HR and BP due to stress response of laparoscopic surgery.
Its hemodynamic effects are due to central sympatholytic and peripheral vasoconstrictive effects.[3,4,5] It causes a dose dependent decrease in arterial BP and HR associated with a decrease in serum norepinephrine concentrations. It activates receptors in the medullary vasomotor center, reducing norepinephrine turnover and decreasing central sympathetic outflow, resulting in alterations in sympathetic function, thereby suppressing the hemodynamic response to intubation, extubation without any side effects like respiratory depression and PONV. Additional effects result from the central stimulation of parasympathetic outflow and inhibition of sympathetic outflow from the locus coeruleus in the brainstem. These actions may have contributed to the findings in the hemodynamic profile in our patients who received dexmedetomidine. In patients undergoing general or gynecological surgery, numerous studies have shown that dexmedetomidine blunts the cardiovascular responses to intubation.[6,7,8]
Lawrence and De Lange found that a single dose of 2 μg/kg of dexmedetomidine before induction of anesthesia attenuated the hemodynamic response to intubation as well as that to extubation.[9] Bradycardia was observed at 1st and 5th min after administration. This might have been due to bolus administration. These drug related cardiovascular side effects were related to dosage and the speed of administration of the drug. In accordance to our study, other studies demonstrated that dexmedetomidine attenuates sympathoadrenal response to tracheal intubation, reduces perioperative anesthetic requirement[10] and hemodynamic stability.[11]
The end-tidal isoflurane concentration was significantly less in group D as compared to group S (P < 0.05) in this study. Numerous studies have shown that continuous intra-operative dexmedetomidine infusion can decrease anesthetic requirements of isoflurane by up to 47-90% in healthy patients[7,12] in the perioperative period. The alpha-2-adrenergic agonists inhibit central noradrenergic transmission, which is associated with a lowering of the minimum alveolar concentration (MAC) values of volatile anesthetic agents. Furthermore, other postsynaptic alpha-2 mechanisms may be involved. Use of dexmedetomidine produces intra-operative and postoperative opioid-sparing effect. Jaakola et al. evaluated analgesia after systemic administration of different doses of dexmedetomidine and fentanyl and found that dexmedetomidine had a moderate analgesic effect that was maximized at 0.5 mcg/kg.[8] Two patients required fentanyl in group D compared to eleven patients in group S in our study. Sedation and analgesia probably account for the MAC sparing effects of this class of compound. Central alpha-2-adrenoceptors in the locus ceruleus and receptors in the dorsal horn of the spinal cord are likely to be involved in these effects.[13] Dexmedetomidine infusion at a dose of 0.5 mcg/kg/h has specific analgesic effect and provides visceral pain relief.[14] In morbidly obese, dexmedetomidine produces a greater decrease in sympathovagal balance intra-operatively than fentanyl along with better postoperative analgesia.[15] Hemodynamic responses to the emergence from anesthesia and extubation are blunted with dexmedetomidine, and the centrally mediated sympatholytic effect has continued well into the postoperative period.[16,17]
Dexmedetomidine could result in cardiovascular depression, that is, bradycardia and hypotension. The activation of alpha-2-adrenoceptors, imidazoline-preferring receptors or both in the ventrolateral medulla and especially in the solitaries nucleus tract by dexmedetomidine causes bradycardia. We saw three patients had bradycardia in the postoperative period, which is statistically insignificant. This low incidence of bradycardia might be due to slow infusion of bolus. Our results are comparable to those of Bekker et al., who reported that dexmedetomidine, given at a similar dose, was effective in blunting the increase in systolic BP perioperatively, although it did not increase the incidence of hypotension or bradycardia.[18] Hogue et al. reported that dexmedetomidine preserves baroreflex sensitivity, and that patient had a normal HR response to BP. The noted slowing of the HR is mostly from sympathetic withdrawal and not due to enhanced vagal activity.[19] The incidence of postoperative bradycardia has been reported as high as 40% in healthy surgical patients who received dexmedetomidine, especially high doses. Usually, these temporary effects are successfully treated with atropine or epinephrine and volume infusions.
Turan et al. found that cough, difficulty in breathing, laryngeal edema and bronchospasm were not observed with a single dose of dexmedetomidine.[20] It may be that the analgesic and sedative characteristics of dexmedetomidine contribute to a lower level of sensitivity to laryngeal stimulation during extubation.
Dexmedetomidine provides sedation and analgesia with no accompanying respiratory depression in the postoperative period. Hall et al. found that small doses of dexmedetomidine provided significant sedation that could be easily reversed with verbal or physical stimuli, and it resolved 2 h after terminating the infusion.[1] In this study, we did not observe clinically significant undue sedation even though nine patient had sedation score ≤3 in group D. Similar to our study Gurbet et al. did not observe sedation in any patient who received intra-operative dexmedetomidine infused at a rate of 0.5 mcg/kg/h.[21] In the present study, VAS for pain was less in group D relative to group S and postoperative tramadol requirement was significantly less in group D relative to that in group S. The most likely site of the hypnotic/anesthetic action of dexmedetomidine is a postsynaptic alpha-2-adrenoceptor located in the locus coeruleus.[22] Animal studies indicate that systemic administration of alpha-2-adrenergic receptor agonists results in dose-dependent antinociception and sedation responses. Human data reveals a clear dose-response relationship for sedation, but not for analgesia, with systemic administration of these drugs. Gurbet et al. concluded in their study that continuous intravenous dexmedetomidine during abdominal surgery provides effective postoperative analgesia and reduces postoperative morphine requirements without increasing the incidence of side effects.[21] Dexmedetomidine when used as the sole substitute for remifentanil in ambulatory gynecologic laparoscopic surgery, provides better perioperative hemodynamic stability and postoperative analgesia.[23] Basar et al. concluded that a single dose of 0.5 mcg/kg of dexmedetomidine given preoperatively led to significant sedation with no change in recovery scores.[24] The analgesic, sedative/hypnotic and anxiolytic properties of dexmedetomidine makes this drug potentially useful for painful surgical procedures.
In this study, we noted statistically significant decreased incidence of PONV and requirement of rescue antiemetics in group D. The etiology of PONV after laparoscopic surgery is not fully understood, and still a major drawback of this type of surgery. Multiple factors are associated with an increased incidence of PONV, including patient, anesthetic and surgical factors. Islam Massad et al. concluded that combining dexmedetomidine to other anesthetic agents, results in more balanced anesthesia and a significant drop in the incidence of PONV, as well as reduces the requirements of postoperative rescue anti-emetic medications after laparoscopic gynecological surgeries.[25] There might be three possible explanations for this finding. The first of them is the significant drop in the requirement of each component of the balanced anesthetic mixture used. Secondly, dexmedetomidine is an alpha-2 adrenoreceptor agonist. This drug has an action on the locus coeruleus, which is a major noradrenergic cell group located in theopontine brain stem and appears to have regulatory effects on extracellular dopamine. The alpha-2 adrenoreceptor agonist of dexmedetomidine, which resembles that of clonidine, decreases the noradrenergic activity as a result of binding to alpha-2 presynaptic inhibitory adrenoreceptor in the locus coeruleus, an inhibition that possibly results in an anti-emetic effect. Thirdly, it is well-known that the gastrointestinal distension stimulates vagal visceral afferents; this in turn activates the vomiting center and induces nausea and vomiting. By increasing sympathetic outflow and decreasing parasympathetic outflow from the central nervous system, dexmedetomidine may exert its effect by increasing the gastric emptying and the gastrointestinal motility, which possibly has an important effect in decreasing nausea and vomiting. The incidence of nausea and vomiting after general anesthesia has been reported to be as high as 24% and after laparoscopy it is as high as 42% due to rapid peritoneal distension.
None of the patients in group D had postoperative shivering compared to four patients in group S. The mechanism of shivering in patients recovering from anesthesia though poorly understood, but volatile anesthetic agents are usually associated with altered temperature regulation. These might be due to decreased end-tidal concentration of inhaled isoflurane with dexmedetomidine as an adjuvant in laparoscopic surgeries. It suppresses shivering, possibly by their activity at alph-2B receptors in the hypothalamic thermoregulatory center of brain.
Limitations of the study
A limitation of this study was that there was a lack of BIS monitoring to monitor intra-operative awareness and the role of dexmedetomidine in ensuring hemodynamic stability.
CONCLUSION
Dexmedetomidine, as an adjuvant to general anesthesia for laparoscopic surgeries, provided a stable hemodynamic profile in the perioperative period and effectively blunted the presser response to intubation and extubation. Intra-operatively it decreased the requirements for additional analgesics and potent inhalational agents with less postoperative undesirable side effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 2.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 3.Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–42. doi: 10.1097/00000539-199711000-00033. [DOI] [PubMed] [Google Scholar]
- 4.Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003;99:65–70. doi: 10.1097/00000542-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Piascik MT, Soltis EE, Piascik MM, Macmillan LB. Alpha-adrenoceptors and vascular regulation: Molecular, pharmacologic and clinical correlates. Pharmacol Ther. 1996;72:215–41. doi: 10.1016/s0163-7258(96)00117-9. [DOI] [PubMed] [Google Scholar]
- 6.Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. Br J Anaesth. 1992;68:126–31. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 7.Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Dexmedetomidine infusion for maintenance of anesthesia in patients undergoing abdominal hysterectomy. Anesth Analg. 1992;75:940–6. [PubMed] [Google Scholar]
- 8.Jaakola ML, Ali-Melkkilä T, Kanto J, Kallio A, Scheinin H, Scheinin M. Dexmedetomidine reduces intraocular pressure, intubation responses and anaesthetic requirements in patients undergoing ophthalmic surgery. Br J Anaesth. 1992;68:570–5. doi: 10.1093/bja/68.6.570. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence CJ, De Lange S. Effects of a single pre-operative dexmedetomidine dose on isoflurane requirements and peri-operative haemodynamic stability. Anesthesia. 1997;52:736–44. doi: 10.1111/j.1365-2044.1997.169-az0303.x. [DOI] [PubMed] [Google Scholar]
- 10.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel CR, Engineer SR, Shah BJ, Madhu S. Effect of intravenous infusion of dexmedetomidine on perioperative haemodynamic changes and postoperative recovery: A study with entropy analysis. Indian J Anaesth. 2012;56:542–6. doi: 10.4103/0019-5049.104571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aantaa R, Jaakola ML, Kallio A, Kanto J. Reduction of the minimum alveolar concentration of isoflurane by dexmedetomidine. Anesthesiology. 1997;86:1055–60. doi: 10.1097/00000542-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 13.De Kock M, Crochet B, Morimont C, Scholtes JL. Intravenous or epidural clonidine for intra- and postoperative analgesia. Anesthesiology. 1993;79:525–31. doi: 10.1097/00000542-199309000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Cortinez LI, Hsu YW, Sum-Ping ST, Young C, Keifer JC, Macleod D, et al. Dexmedetomidine pharmacodynamics: Part II: Crossover comparison of the analgesic effect of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1077–83. doi: 10.1097/00000542-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Feld J, Hoffman WE, Paisansathan C, Park H, Ananda RC. Autonomic activity during dexmedetomidine or fentanyl infusion with desflurane anesthesia. J Clin Anesth. 2007;19:30–6. doi: 10.1016/j.jclinane.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Tanskanen PE, Kyttä JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97:658–65. doi: 10.1093/bja/ael220. [DOI] [PubMed] [Google Scholar]
- 17.Aksu R, Akin A, Biçer C, Esmaoglu A, Tosun Z, Boyaci A. Comparison of the effects of dexmedetomidine versus fentanyl on airway reflexes and hemodynamic responses to tracheal extubation during rhinoplasty: A double-blind, randomized, controlled study. Curr Ther Res Clin Exp. 2009;70:209–20. doi: 10.1016/j.curtheres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth Analg. 2008;107:1340–7. doi: 10.1213/ane.0b013e3181804298. [DOI] [PubMed] [Google Scholar]
- 19.Hogue CW, Jr, Talke P, Stein PK, Richardson C, Domitrovich PP, Sessler DI. Autonomic nervous system responses during sedative infusions of dexmedetomidine. Anesthesiology. 2002;97:592–8. doi: 10.1097/00000542-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Turan G, Ozgultekin A, Turan C, Dincer E, Yuksel G. Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816–20. doi: 10.1017/S0265021508004201. [DOI] [PubMed] [Google Scholar]
- 21.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53:646–52. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 22.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–52. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Salman N, Uzun S, Coskun F, Salman MA, Salman AE, Aypar U. Dexmedetomidine as a substitute for remifentanil in ambulatory gynecologic laparoscopic surgery. Saudi Med J. 2009;30:77–81. [PubMed] [Google Scholar]
- 24.Basar H, Akpinar S, Doganci N, Buyukkocak U, Kaymak C, Sert O, et al. The effects of preanesthetic, single-dose dexmedetomidine on induction, hemodynamic, and cardiovascular parameters. J Clin Anesth. 2008;20:431–6. doi: 10.1016/j.jclinane.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Massad IM, Mohsen WA, Basha AS, Al-Zaben KR, Al-Mustafa MM, Alghanem SM. A balanced anesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Med J. 2009;30:1537–41. [PubMed] [Google Scholar]


