Abstract
Background:
We studied the effects of oral gabapentin and intravenous (I.V.) dexamethasone given together or separately 1 h before the start of surgery on intraoperative hemodynamics Postoperative analgesia and postoperative nausea vomiting (PONV) in patients undergoing gynaecological procedure.
Materials and Methods:
Patients were randomly divided into three groups: Group 1 (gabapentin, n = 46) received 400 mg gabapentin, Group 2 (dexamethasone, n = 46) received 8 mg dexamethasone and Group 3 (gabapentin plus dexamethasone, n = 46) received both 400 mg gabapentin and 8 mg dexamethasone I.V. 1 h before the start of surgery. Standard induction and maintenance of anesthesia were accomplished. Visual analog scale for pain was recorded for 12 h. Side effects were noted.
Results:
Hemodynamics at various time interval (0, 5, 10, 15, 20, 25 and 30 min) of laryngeal mask airway insertion and PONV were found significantly lower in Group 3 than in Group 1 and Group 2 (P < 0.05). The average time to first postoperative analgesic requirement at (visual analogue score >3) was significantly longer in Group 3 (510.00 ± 61.64 min) than in Group 1 (352.83 ± 80.61 min) and in Group 2 (294.78 ± 60.76 min), (P < 0.05).
Conclusion:
The present study concludes that the combination of oral Gabapentin and I.V. dexamethasone has significantly less hemodynamic changes, better postoperative analgesia and less incidence of PONV than individual administration of each drug.
Keywords: Analgesia, dexamethasone, gabapentin, postoperative nausea vomiting
INTRODUCTION
Pain is a dehumanizing experience that destroys the soul. Opioid derivatives are the most popular drugs used for this purpose, so current research in this field are focussed on finding new alternative drugs or drugs that can be combined with opioid to reduce the need for its use.[1]
Gabapentin and dexamethasone are two well tolerated and mechanistically diverse drugs that have each shown promise in the management of postoperative pain. Gabapentin, a structural analogue of Gamma-aminobutyric acid, is used as an anticonvulsant drug. Gabapentin has a selective effect on the nociceptive process involving central sensitization.[2,3,4]
Gabapentin's anti hyperalgesic effects result from an action at the alpha 2 delta units of voltage sensitive Ca channel.[2] Gabapentin is effective in treatment of postoperative nausea vomiting (PONV) by mitigation of tachykinin neurotransmitter activity.[5,6] Glucocorticoids are well-known for their analgesic, antiinflammatory, immune-modulating, and antiemetic effects, although the mechanisms by which glucocorticoids exert their action are far from clarified.[7]
The purpose of this study was to compare intraoperative hemodynamic changes, postoperative analgesia and PONV, following the use of gabapentin, dexamethasone together or separate 1 h before surgery.
MATERIALS AND METHODS
After approval from Institutional Ethics Committee and written consent of the patients, 138 normotensive female patients aged between 20 and 50 years (American Society of Anesthesiologists [ASA] physical status 1 and 2) undergoing gynaecological procedure were randomly assigned to three groups of 46 patients each. Exclusion criteria were cardiac disease, patients with compromised airway or morbid obesity, History of convulsion, allergy to the drug used, bleeding disorder, severe neurological deficit. Patient with history of respiratory, hepatic or renal disease (necessitating classification in ASA class III or above).
Study design was hospital based randomised analytical interventional type. Patients were randomly allocated into three groups. Group 1 (n = 46) received oral gabapentin 400 mg, Group 2 (n = 46) received intravenous (I.V.) 8 mg dexamethasone and Group 3 (n = 46) received 400 mg oral gabapentin + I.V. 8 mg dexamethasone 1 h before surgery. After the patients had been taken to the operating room, crystalloid infusion (ringer lactate) was started through 20-gauge cannula inserted in an appropriate vein, and mean arterial blood pressure (MAP), heart rate (HR) and peripheral oxygen saturation (SpO2) were monitored. Premedication with injection glycopyrolate (0.004 mg/kg) and injection midazolam (0.2 mg/kg) was given. After preoxygenation with 100% oxygen, anesthesia was induced with I.V. ketamine at a dose of 0.5 mg/kg and injection propofol 2.5 mg/kg. After induction, injection succinyl choline 2 mg/kg was given. Intermittent positive pressure ventilation was given with 100% oxygen for 1 min. Then laryngeal mask airway (LMA) was inserted. Thereafter MAP and HR were taken and followed by every 5 min, that means 0 min (at the time of LMA insertion), 5, 10, 15, 20, 25 and 30 min of LMA insertion. Anesthesia was maintained with 40% oxygen in 60% nitrous oxide with inhalational agent. After the end of surgery thorough oral suction was done. LMA removed and patient oxygenated with 100% oxygen for 5 min. Patients were transferred to the postanesthesia care unit. Assessment of postoperative pain was made with visual analogue scale score (VAS: 0 cm = no pain and 10 cm = worst pain imaginable). During the first 1 h in the postanesthesia care unit, then 2, 4, 6 and 12 h in the patient's room. Monitored the patient for 12 h or till the first dose of analgesic. When VAS values were >3, first dose of analgesic inj. Tramadol 100 mg I.V. was administered and noted.
The occurrence of any side effects, such as nausea vomiting were noted on patient request, or if nausea and vomiting occurred, ondansetrone 4 mg I.V. was given.
Statistical analysis
Categorical data, that is, ASA grade, incidence of adverse events (retching, nausea, vomiting) are presented as numbers (percent) and were compared among groups using Chi-square test. P < 0.05 are considered statistically significant. Groups were compared for demographic data (age, weight), duration of surgery, total duration of analgesia, hemodynamics by analysis of variance and t-test. Data is represented as mean and standard deviation (SD).
RESULTS
There were no significant differences among the three groups with respect to age, weight, duration of surgery [Table 1]. Cardiovascular responses are shown in Figures 1 and 2. The initial hemodynamic variables were similar in all groups. HR and MAP were significantly lower in Group 3 at 0, 5, 10, 15, 20, 25 and 30 min of LMA insertion than Group 1 and Group 2 (P < 0.05). Hemodynamics were similar in Group 1 and Group 2 [Tables 2 and 3]. VAS scores were found to be significantly lower in Group 3 than in Group 1 and Group 2. In Group 3 (oral gabapentin + I.V. dexamethasone) the mean time of total duration of analgesia with SD was 510.00 ± 61.64 min, which was significantly longer than Group 1 and Group 2. It was 352 ± 80.61 min in Group 1 (oral gabapentin). In Group 2 (I.V. dexamethasone) the mean time of total duration of analgesia with SD was 294.78 ± 60.76 min [Table 4 and Figure 3].
Table 1.
Demographic data and duration of surgery in the study groups (mean ± SD)
Figure 1.
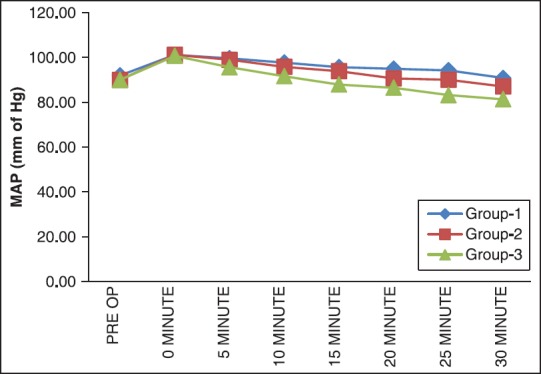
Distribution of mean arterial blood pressure at different time intervals
Figure 2.
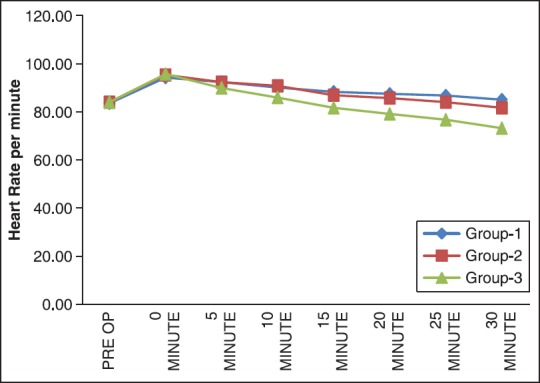
Distribution of mean heart rate at different time intervals
Table 2.
Comparison of mean±SD of MAP at various intervals in between the groups
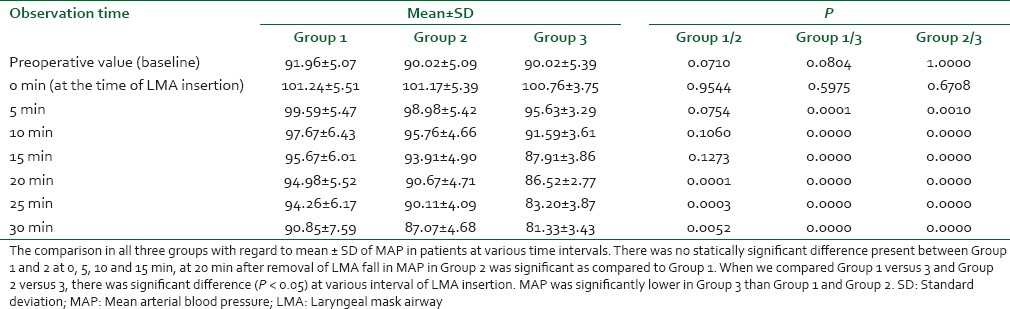
Table 3.
Comparison of mean ± SD of HR at various intervals in between the groups
Table 4.
Duration of analgesia; mean ± SD (min)
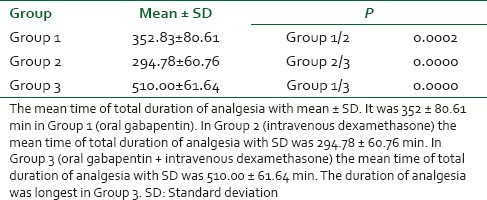
Figure 3.
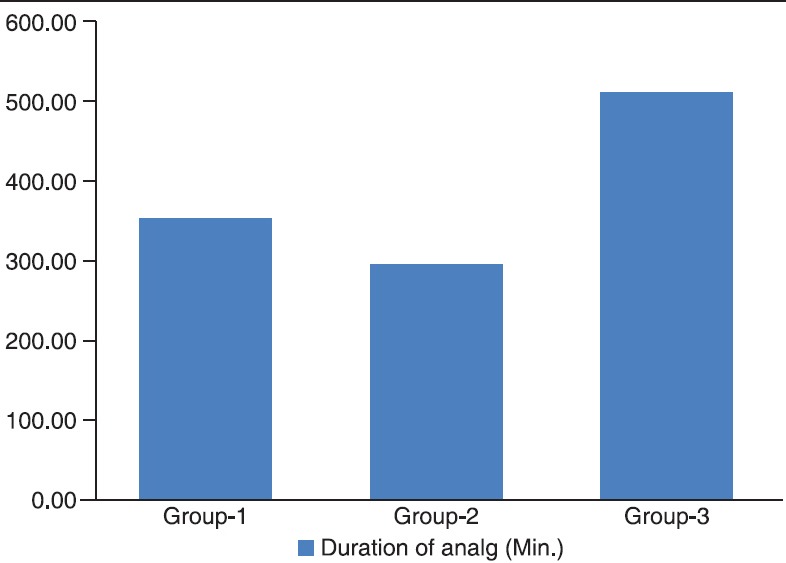
Mean duration of analgesia (min)
Postoperative nausea and vomiting occurred in 12 of 46 (26.09%) patients in Group 1, 4 of 46 (8.70%) patients in Group 2, and 2 of 46 (4.35%) patients in Group 3. The incidence of PONV was less frequent in Group 3 compared with all other groups (P < 0.001). Between Group 1 and Group 2, there was significantly reduced PONV in Group 2 than Group 1 [Table 5].
Table 5.
Incidence of PONV in between groups

DISCUSSION
The above mentioned results indicate that gabapentin and dexamethasone, administered together an hour before gynaecological procedure, result in better intraoperative hemodynamics, better postoperative analgesia and less PONV than individual administration of each drug.
Surgical procedures, endotracheal intubation, and anesthesia are stressful to the patient and may induce potentially harmful reactions, such as increases in HR and MAP.[8] on analysis there was statistically significant difference between Group 1 versus 3 and Group 2 versus 3. It is postulated that gabapentin and dexamethasone combination is more effective in attenuating hemodynamic response to LMA insertion. This finding is similar to finding of Koç et al. study,[7] they investigated the effects of gabapentin and dexamethasone given together or separately 1 h before the start of varicocele surgery on laryngoscopy, tracheal intubation, intraoperative hemodynamics. They found that Gabapentin and dexamethasone administered together an hour before varicocele surgery results in less laryngeal and tracheal intubation response, than individual administration of each drug.
Memis et al.[9] found that there was significant decrease in HR and MAP in group receiving 800 mg gabapentin 1, 3, 5 and 10 min after intubation compared to placebo and 400 mg gabapentin group. Our result is in favour of the study of Memis et al.
Our study is contrary to following studies. In our study gabapentin did not attenuate the HR and blood pressure due to lower single dose of gabapentin (400 mg) which we used. Fassoulaki et al.[10] have used gabapentin 1600 mg or placebo capsules at 6 hourly intervals starting the day (noon) before surgery. Our negative result may be explained by the finding of Kong and Irwin (2007) that the effects of gabapentin were dose dependent.
In animal models of nociception, gabapentin reduces hypersensitivity associated with nerve injury, inflammation, and pain after surgery.[11,12] Mechanical hyperalgesia surrounding the wound in postoperative patients and experimental, heat-induced secondary hyperalgesia share a common mechanism; namely, central neuronal sensitization that may contribute to some aspects of postoperative pain. Gabapentin's antihyperalgesic effects result from an action at alpha 2 delta subunits of voltage-dependent Ca2 channels which are up-regulated in the dorsal root ganglia and spinal cord after peripheral injury. Prevention of PONV by gabapentin due to mitigation of tachykinin neurotransmitter activity.
The analgesic effects of glucocorticoids are provided through inhibition of the phospholipase enzyme and subsequent blockage of both the cyclooxygenase and the lipoxygenase pathways in the inflammatory chain reaction, as well as suppression of tissue levels of bradykinin and release of neuropeptides from nerve endings. The mechanism by which glucocorticoids alleviate nausea and vomiting is not fully understood, but the effects are probably centrally mediated via inhibition of prostaglandin synthesis or inhibition of the release of endogenous opioids.
Our study is similar to Montazeri et al.[13] study, Koç et al. stud7 Mathiesen et al.,[14] Moore et al. 2011,[15] Pandey et al. 2004,[16] Baxendale et al. 1993.[17] Since we have used lower dose 400 mg oral gabapentin 1 h prior to surgery, so the results are not fully similar to above studies. We studied the effect of drug till the first dose of analgesic the various studies are done to analysis the effect of drug for 24 h.
Postoperative nausea and vomiting are a multifactorial problem, and several anesthetic and nonanesthetic factors must be controlled to obtain meaningful results.
Pandey et al. study, they studied[6] on prophylactic gabapentin for prevention of PONV in patients undergoing laparoscopic cholecystectomy. Iris Henzi et al. (2000) study, in a metaanalysis of 17 randomized controlled trials, a single dose of dexamethasone in combination with 5-HT3 receptor antagonists significantly reduced PONV when compared with placebo. Koç et al.[7] observed that in Group 3 (gabapentin + dexamethasone) when Gabapentin 800 mg and dexamethasone I.V. 8 mg administered together an hour before varicocele surgery prevents PONV better than individual administration of each drug.
Thus the finding of our study conclude that combination of oral gabapentin and I.V. dexamethasone is better for intraoperative hemodynamics changes, better postoperative analgesia and less incidence PONV.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–8. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 2.Mao J, Chen LL. Gabapentin in pain management. Anesth Analg. 2000;91:680–7. doi: 10.1097/00000539-200009000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Alden KJ, García J. Differential effect of gabapentin on neuronal and muscle calcium currents. J Pharmacol Exp Ther. 2001;297:727–35. [PubMed] [Google Scholar]
- 4.Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, et al. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology. 2002;42:353–66. doi: 10.1016/s0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- 5.Navari RM, Reinhardt RR, Gralla RJ, Kris MG, Hesketh PJ, Khojasteh A, et al. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754,030 Antiemetic Trials Group. N Engl J Med. 1999;340:190–5. doi: 10.1056/NEJM199901213400304. [DOI] [PubMed] [Google Scholar]
- 6.Pandey CK, Priye S, Ambesh SP, Singh S, Singh U, Singh PK. Prophylacti gabapentin for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: A randomized, double-blind, placebo-controlled study. J Postgrad Med. 2006;52:97–100. [PubMed] [Google Scholar]
- 7.Koç S, Memis D, Sut N. The preoperative use of gabapentin, dexamethasone, and their combination in varicocele surgery: A randomized controlled trial. Anesth Analg. 2007;105:1137–42. doi: 10.1213/01.ane.0000278869.00918.b7. [DOI] [PubMed] [Google Scholar]
- 8.Derbyshire DR, Smith G. Sympatoadrenal responses to anesthesia and surgery. Br J Anaesth. 1984;56:725–39. doi: 10.1093/bja/56.7.725. [DOI] [PubMed] [Google Scholar]
- 9.Memis D, Turan A, Karamanlioglu B, Seker S, Türe M. Gabapentin reduces cardiovascular responses to laryngoscopy and tracheal intubation. Eur J Anaesthesiol. 2006;23:686–90. doi: 10.1017/S0265021506000500. [DOI] [PubMed] [Google Scholar]
- 10.Fassoulaki A, Melemeni A, Paraskeva A, Petropoulos G. Gabapentin attenuates the pressor response to direct laryngoscopy and tracheal intubation. Br J Anaesth. 2006;96:769–73. doi: 10.1093/bja/ael076. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JK, Pan HL, Eisenach JC. Antiallodynic effect of intrathecal gabapentin and its interaction with clonidine in a rat model of postoperative pain. Anesthesiology. 2000;92:1126–31. doi: 10.1097/00000542-200004000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Field MJ, Holloman EF, McCleary S, Hughes J, Singh L. Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. J Pharmacol Exp Ther. 1997;282:1242–6. [PubMed] [Google Scholar]
- 13.Montazeri K, Kashefi P, Honarmand A. Pre-emptive gabapentin significantly reduces postoperative pain and morphine demand following lower extremity orthopaedic surgery. Singapore Med J. 2007;48:748–51. [PubMed] [Google Scholar]
- 14.Mathiesen O, Møiniche S, Dahl JB. Gabapentin and postoperative pain: A qualitative and quantitative systematic review, with focus on procedure. BMC Anesthesiol. 2007;7:6. doi: 10.1186/1471-2253-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore A, Costello J, Wieczorek P, Shah V, Taddio A, Carvalho JC. Gabapentin improves postcesarean delivery pain management: A randomized, placebo-controlled trial. Anesth Analg. 2011;112:167–73. doi: 10.1213/ANE.0b013e3181fdf5ee. [DOI] [PubMed] [Google Scholar]
- 16.Pandey CK, Sahay S, Gupta D, Ambesh SP, Singh RB, Raza M, et al. Preemptive gabapentin decreases postoperative pain after lumbar discoidectomy. Can J Anaesth. 2004;51:986–9. doi: 10.1007/BF03018484. [DOI] [PubMed] [Google Scholar]
- 17.Baxendale BR, Vater M, Lavery KM. Dexamethasone reduces pain and swelling following extraction of third molar teeth. Anaesthesia. 1993;48:961–4. doi: 10.1111/j.1365-2044.1993.tb07474.x. [DOI] [PubMed] [Google Scholar]


