Abstract
Background:
Satisfactory analgesia is of great importance in the labor. The clinical efficacy and side effects of remifentanil in the management of labor pain had been evaluated. Dexmedetomidine (DMET) demonstrates an antinociceptive effect in visceral pain conditions. Aims of the study were to assess whether the combination of DMET with remifentanil would produce a synergistic effect that results in lower analgesic requirements. Furthermore, whether this combination would have less maternal and neonatal adverse effects.
Patients and Methods:
Sixty American Society of Anesthesiologists physical status I-II pregnant women had been enrolled into this study. All were full term (37-40 weeks’ gestation), singleton fetus with cephalic presentation in the first stage of spontaneous labor. They were divided into two groups group (I) Patient-controlled IV remifentanil analgesia (bolus dose 0.25 μg/kg, lockout interval 2 min) increased by 0.25 μg/kg to a maximum bolus dose 1 μg/kg in addition to a loading dose of DMET 1 μg/kg over 20 min, followed by infusion at 0.5 μg/kg/h group (II) Patient-controlled IV remifentanil analgesia (PCA) (bolus dose 0.25 μg/kg, lockout interval 2 min) increased by 0.25 μg/kg to a maximum bolus dose 1 μg/kg in addition to a the same volume of normal saline as a loading dose, followed by a continuous saline infusion. Visual analog scale score, maternal, and fetal complications and patients’ satisfaction were recorded.
Results:
Patients receiving a combination of PCA remifentanil and DMET had a lower pain score compared with remifentanil alone in the second stage of labor (P = 0.001). The Total consumption of remifentanil was reduced by 53.3% in group I. There was an increased incidence of maternal complications and a lower patient satisfaction score in group II.
Conclusion:
DMET has an opioid sparing effect; a combination of DMET and remifentanil produces a synergistic effect that results in lower analgesic requirements and less maternal and neonatal adverse events.
Keywords: Dexmedetomidine, labor pain, patient-controlled analgesia, remifentanil
INTRODUCTION
Satisfactory analgesia is of paramount importance in the labor. Epidural analgesia is considered the gold standard in the treatment of labor associated pain.[1] However, its use is restricted in patients with contraindications and in those who refuse to receive it because of its invasive nature and the potential complications.[2]
Remifentanil is a novel agent that has been used in the past decade for surgical anesthesia, sedation for mechanically ventilated patients and postoperative analgesia.[3]
Remifentanil is a synthetic mu receptor agonist characterized by rapid onset and offset of action.[4] This would make remifentanil suitable for administration via patient-controlled analgesia (PCA), which can be used for analgesia during labor.[5] Remifentanil peak effect is reached at 2 min, the duration of action is 20 min, and the context-sensitive half-life is for 3 min (independent of the duration of infusion). Remifentanil is rapidly metabolized to an inactive metabolite (remifentanil acid) by plasma and tissue estrases, and it is eliminated completely by the tissue esterase in 9-10 min. After intravenous (IV) administration, the plasma concentration in a pregnant woman is half that of the nonpregnant due to the larger volume of distribution and higher clearance. It is eliminated quickly in neonate by rapid metabolism and redistribution.[6] The use of opioid in labor is frequently limited by maternal side effect including sedation, oxygen desaturation, nausea, and vomiting. Furthermore, there is a concern about fetal heart rate (FHR) abnormality and neonatal depression.
Clonidine, an alpha 2-adrenergic receptor (α2-AR) agonist, has been widely used and investigated as an analgesic adjuvant for anesthesia and pain therapy. Dexmedetomidine (DMET) belongs to the same family but presents with a different and more favorable pharmacokinetic profile.
Dexmedetomidine was first introduced into clinical practice as a short-term IV sedative in the intensive care unit. Because the drug also demonstrates analgesic properties related to α2-AR binding (DMET is 8-10-fold more selective for α2-AR than clonidine), several studies have investigated its use as a systemic analgesic adjuvant, mostly in the acute perioperative setting.[7]
In the recent use of DMET in obstetric analgesia, because of its high lipophilicity, DMET is retained in placental tissue and passes less readily than clonidine into the fetal circulation (0.77 maternal/fetal index; DS 0.06)[8] and thereby is less likely to cause harmful fetal bradycardia. It also increases the frequency and amplitude of uterine contractions directly and in a dose-dependent fashion[9] suggesting further advantages for its use as an analgesic adjunct during labor.
Dexmedetomidine also demonstrates an antinociceptive effect in visceral pain conditions.[10] Furthermore, the drug also possesses attractive properties such as maternal hemodynamic stability and anxiolysis.
Dexmedetomidine sedation, analgesia, and sympatholysis are due to its effects on α2-agonist receptors on the locus caeruleus and the spinal cord.[11]
We assume that the combination of DMET with remifentanil produces a synergistic effect that results in lower analgesic requirements and less maternal and neonatal adverse events such as nausea and vomiting, without increasing the incidence of respiratory depression.
PATIENTS AND METHODS
We present a prospective, double-blind, randomized controlled trial. Ethical approval was granted by Ain Shams University Hospital Ethics Committee 60 patients who had American Society of Anesthesiologists (ASA) physical status I-II were recruited. All women were full-term ≥37 weeks’ gestation, singleton fetus with cephalic presentation in the first stage of spontaneous labor.
Informed written consents were obtained from all parturient. Those who had a known relevant drug allergy, significant respiratory depression from previous exposure to opioid or obstetric complications were excluded from the study.
Upon arrival in the labor room, an IV line was placed. The protocol for pain relief started when the interval between contractions was <5 min, cervical dilatation was ≥3 cm and the pain level using a visual analog scale (VAS) during contractions was ≥50 mm. They were given the option to opt out of the study at any time if they wished to choose an alternative pain management.
The recruited women were randomized into two equal groups each contains 30 patients: Randomization of patients was performed using sealed envelope design. All parturient were provided with a PCA device. The machine was loaded with a 50-ml syringe containing remifentanil 20 μg/ml.
Group I: (Remifentanil-DMET group) patient-controlled IV remifentanil analgesia (PCA) (bolus dose 0.25 μg/kg, lockout interval 2 min) increased by 0.25 μg/kg to a maximum bolus dose 1 μg/kg in addition to a loading dose of DMET 1 μg/kg over 20 min, followed by infusion at 0.5 μg/kg/h was carried out by a technician who was not involved in data collection, who made up identical syringes and infusions of DMET and normal saline under sterile conditions.
Group II: (Remifentanil group) patient-controlled IV remifentanil analgesia (PCA) (bolus dose 0.25 μg/kg, lockout interval 2 min) increased by 0.25 μg/kg to a maximum bolus dose 1 μg/kg in addition to a the same volume of normal saline as a loading dose, followed by a continuous saline infusion.
Monitoring of vital data was performed by a one to one assigned nurse. Maternal heart rate (HR) and oxygen saturation (SaO2) were monitored continuously throughout the study period. Noninvasive blood pressure was measured by a cuff in the contralateral limb to PCA and was registered along with respiratory rate (RR) every 15 min. FHR was continuously monitored by cardiotocography. The FHR-tracings were analyzed by an obstetrician according to the department's clinical guidelines, and remifentanil was stopped if pathological changes occurred such as; absence of accelerations, decreased beat-beat variability, bradycardia, tachycardia, or late decelerations.
An oxygen source and Naloxone were available in labor and birth room.
When maternal SaO2 was <95% then oxygen was administrated by nasal prong with a rate of 2-3 L/m.
When the SaO2 remained <92% or the RR was <9 or patient drowsy (sleepy but respond to verbal stimulation) the anesthetist was notified and remifentanil analgesia was temporarily stopped. The pain therapy was restarted on one step lower dose when physiological parameters were normalized.
Pain scores were recorded and measured hourly using a VAS scale (a scale of 0-100 mm, where 0 = No pain and 100 = Worst imaginable pain). Total remifentanil consumption was also recorded.
The degree of sedation was monitored using a five-point scale (1 = Awake, 2 = Drowsy, 3 = Arousable to voice, 4 = Arousable to touch, 5 = Unarousable). The observations of nausea, vomiting and itching were also registered.
Patients who developed severe nausea and vomiting were initially given an IV bolus of metoclopramide 10 mg, followed by ondansetron 4 mg if metoclopramide was unsuccessful.
An assessment of patients’ satisfaction using a four-point scale (0 = Totally dissatisfied, 1 = Moderately dissatisfied, 2 = Reasonably satisfied, 3 = Totally satisfied with pain relief).
The baby's Apgar scores at 1 and 5 min after delivery were recorded.
Statistical methods
Before the study, a power analysis was performed to determine the minimal acceptable number of patients in each group based on remifentanil consumption. The minimal sample size was 28 patients per group with type I error (alpha) = 0.05 and type II error (beta) = 0.1 with power of the test 90%. IBM SPSS statistics (version 18, IBM® Corp., USA, 2012) was used for data analysis. Data are expressed as mean ± standard deviation or frequency and percentages as appropriate. Unpaired Student's t-tests were used to see statistical significance difference for interval variables and Chi-square tests were performed for categorical variables between the groups. P value < 0.05 was considered as statistically significant [Flow Chart 1].
Flow Chart 1.
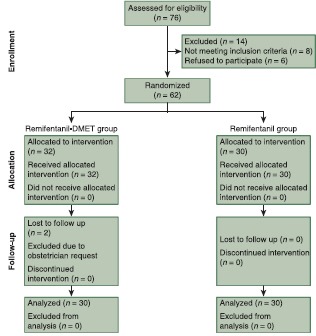
The CONSORT E-flow chart
RESULTS
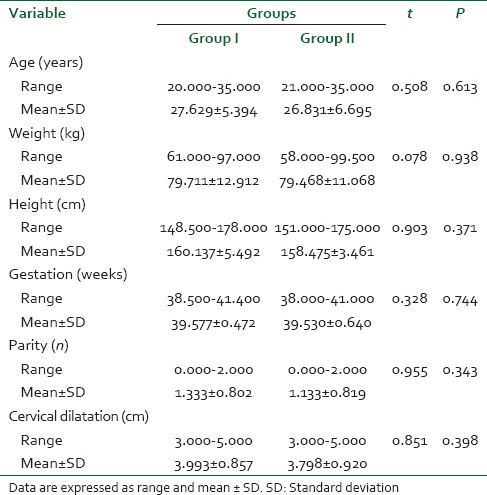
The analgesic procedures were carried out in 60 women, both primiparas and multiparas fulfilling the ASA I-II criteria. Both groups were comparable with regard to age, weight, height, gestational age and baseline cervical dilatation (P < 0.05). The characteristics of the patients are presented in Table 1.
Table 1.
Patient characteristics and obstetric data
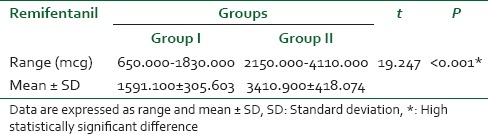
Changes in pain score during the entire study duration are shown in Figure 1. Following an initial reduction, pain score gradually increased with the progress of labor. Patients of the (remifentanil-DMET group) had a lower pain score compared with the (remifentanil group) in late stage of labor (P < 0.001). Total remifentanil consumption was reduced by 53.3% in the first group [Table 2].
Figure 1.
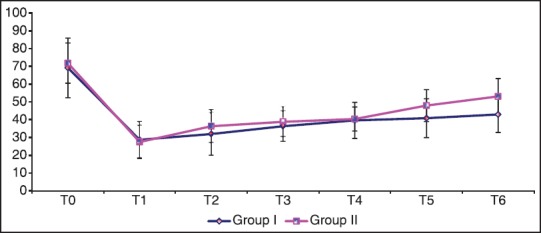
Visual analog scale pain score; data are expressed as mean ± standard deviation
Table 2.
Total remifentanil consumption (in mcg)
Respiratory and cardiovascular complications [Table 3] showed significantly higher incidence of desaturation in the remifentanil group with no statistically significant differences as regard RR, hypotension or bradycardia among both groups.
Table 3.
Respiratory and cardiovascular complications
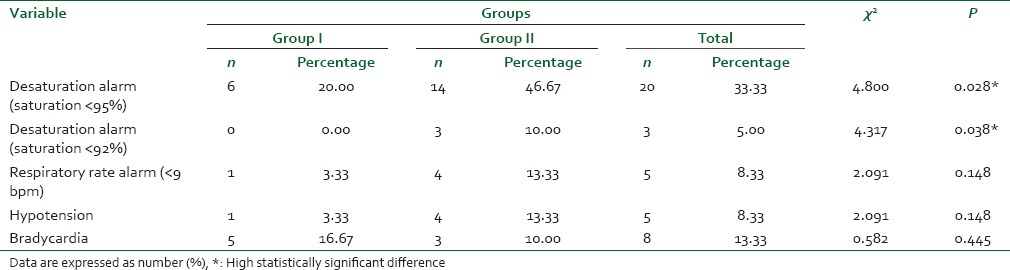
Incidence of nausea and vomiting was significantly higher in the remifentanil group compared with the remifentanil-DMET group (P = 0.010) and (P = 0.038), respectively. Incidence of itching in the remifentanil group was higher than that in the remifentanil-DMET group with no statistical significance (P = 0.121) [Table 4]. The sedation scores did not differ significantly between the two groups.
Table 4.
Side effects and complications
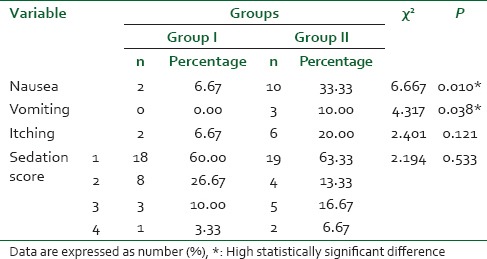
Overall, parturient in remifentanil-DMET group were more satisfied compared to those in the remifentanil group (P = 0.0013) [Table 5].
Table 5.
Patient's satisfaction
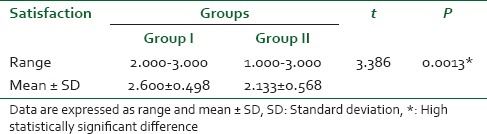
Two women in the remifentanil-DMET group and five in the remifentanil group had nonreassuring FHR in the form of fetal bradycardia which was transient (<1 min).
On average, the Apgar scores were similar between groups at 1 and 5 min. There was no report of neonatal complication [Table 6].
Table 6.
Fetal monitoring and outcome
DISCUSSION
Epidural analgesia is the most effective method for pain relief during labor. However, it may not be the best option for women with a contraindication. Alternative strategies for pain relief may be necessary. Systemic opioids have been used as an alternative with widespread but accompanied with maternal and neonatal side effects.
In the current study adding DMET to remifentanil lead to decrease total remifentanil consumption by 53.3%, better analgesia especially in the second stage of labor and less maternal and neonatal side effects.
Palanisamy et al.[12] reported the successful use of continuous-infusion DMET as an analgesic adjunct in IV PCA with fentanyl for labor in a patient with occult spina bifida.
Abu-Halaweh et al.[13] reported a case of an obese diabetic patient with severe eclampsia who rejected spinal analgesia for labor, and received only DMET, achieving mild pain scores and superficial sedation during the infusion, with no other side effects. The patient eventually underwent C-section under general anesthesia due to late persistent decelerations.
Mendoza[14] described two patients who received IV analgesia with remifentanil. Both required rapidly increasing titrations in order to achieve pain scores under 4/10, and they developed severe pain during the advanced active phase of labor despite high infusion doses of remifentanil. Ultimately, the quality of analgesia improved in both patients following continuous infusion of DMET leading to sympatholysis and superficial sedation that enabled an adequate interaction with their environment, with no evident clinical side effects that might have impaired their hemodynamic condition or the fetal status.
Hanoura et al.[15] recorded successful adding of DMET to regular mixture of epidural anesthetics in women undergoing elective cesarean section; they recorded improvement of intraoperative conditions and the quality of postoperative analgesia without maternal or neonatal significant side effects.
Other authors described the use of DMET in pregnant women for nonobstetric surgery[16] and for C-section.[17]
Gurbet et al.[18] investigated the efficacy of DMET versus placebo for postoperative analgesia after total abdominal hysterectomy. The two groups had similar pain scores, but the patients who received DMET required a lower cumulative amount of morphine during the first 48 h after surgery.
Arain et al.[19] examined 34 patients scheduled for elective inpatient surgery and randomized them equally to receive either DMET (initial loading dose of 1 μg/kg over 10 min, followed by 0.4 mg/kg/h, discontinued at the end of surgery) or morphine sulfate (0.08 mg/kg) 30 min before the end of surgery. The groups had similar pain scores, but the morphine group required 66% more morphine to achieve the same analgesic effect.
Several studies had evaluated the clinical efficacy and side effects of remifentanil in the management of labor pain.
Tveit et al.[20] compared the analgesic efficacy and side effects of remifentanil with standard epidural analgesia during labor, dose of remifentanil was 0.15 μg/kg with 0.15 mcg/kg increments until relief of pain, parturient receiving epidural analgesia reported some better pain scores compared to remifentanil PCA, but all differences were nonsignificant. Remifentanil produced more sedation, desaturation (SaO2 <92%) and need for supplemental oxygen. Fetal and neonatal outcome was reassuring, and they concluded that there is a higher risk for sedation and desaturation with remifentanil necessitating close monitoring.
Volmanen et al.[21] used remifentanil PCA bolus doses from 0.2 to 0.8 μg/kg reported desaturation in 54% (13/24), sedation in 29% (7/24) of patients and 54% FHR changes.
Nausea and vomiting are a recognized effect of opioid analgesia. The incidence reported with remifentanil has ranged from 0%[22] to as high as 60%.[23]
CONCLUSION
While neuraxial analgesia is clearly superior to opioids in providing pain relief during labor, there is a need for opioids when patients have contraindications or exhibit a lack of preference for neuraxial analgesia. DMET has an opioid sparing effect; a combination of DMET and remifentanil produces a synergistic effect that results in lower analgesic requirements and less adverse events such as nausea and vomiting, without increasing the incidence of respiratory depression.
Footnotes
Source of Support: Nil
Conflict of Interest: No potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
REFERENCES
- 1.Halpern SH, Muir H, Breen TW, Campbell DC, Barrett J, Liston R, et al. A multicenter randomized controlled trial comparing patient-controlled epidural with intravenous analgesia for pain relief in labor. Anesth Analg. 2004;99:1532–8. doi: 10.1213/01.ANE.0000136850.08972.07. [DOI] [PubMed] [Google Scholar]
- 2.Mordani K, Macarthur A. Anesthesia considerations of preeclamptic patients. In: Baker PN, Kingdom JC, editors. Preeclampsia: Current Perspectives on Managements. New York: Parthenon Publishing; 2004. pp. 196–9. [Google Scholar]
- 3.Egan TD. Pharmacokinetics and pharmacodynamics of remifentanil: An update in the year 2000. Curr Opin Anaesthesiol. 2000;13:449–55. doi: 10.1097/00001503-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Volmanen P, Akural E, Raudaskoski T, Ohtonen P, Alahuhta S. Comparison of remifentanil and nitrous oxide in labour analgesia. Acta Anaesthesiol Scand. 2005;49:453–8. doi: 10.1111/j.1399-6576.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 5.Hill D. The use of remifentanil in obstetrics. Anesthesiol Clin. 2008;26:169–82, viii. doi: 10.1016/j.anclin.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Davis PJ, Stiller RL, Wilson AS, McGowan FX, Egan TD, Muir KT. In vitro remifentanil metabolism: The effects of whole blood constituents and plasma butyrylcholinesterase. Anesth Analg. 2002;95:1305–7. doi: 10.1097/00000539-200211000-00038. [DOI] [PubMed] [Google Scholar]
- 7.Wagner DS, Brummett CM. Dexmedetomidine: As safe as safe can be. Semin Anesth Perioper Med Pain. 2006;25:77–83. [Google Scholar]
- 8.Ala-Kokko TI, Pienimäki P, Lampela E, Hollmén AI, Pelkonen O, Vähäkangas K. Transfer of clonidine and dexmedetomidine across the isolated perfused human placenta. Acta Anaesthesiol Scand. 1997;41:313–9. doi: 10.1111/j.1399-6576.1997.tb04685.x. [DOI] [PubMed] [Google Scholar]
- 9.Karaman S, Evren V, Firat V, Cankayali I. The effects of dexmedetomidine on spontaneous contractions of isolated gravid rat myometrium. Adv Ther. 2006;23:238–43. doi: 10.1007/BF02850129. [DOI] [PubMed] [Google Scholar]
- 10.Ulger F, Bozkurt A, Bilge SS, Ilkaya F, Dilek A, Bostanci MO, et al. The antinociceptive effects of intravenous dexmedetomidine in colorectal distension-induced visceral pain in rats: The role of opioid receptors. Anesth Analg. 2009;109:616–22. doi: 10.1213/ane.0b013e3181a9fae2. [DOI] [PubMed] [Google Scholar]
- 11.Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001;7:221–6. doi: 10.1097/00075198-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Halaweh SA, Al Oweidi AK, Abu-Malooh H, Zabalawi M, Alkazaleh F, Abu-Ali H, et al. Intravenous dexmedetomidine infusion for labour analgesia in patient with preeclampsia. Eur J Anaesthesiol. 2009;26:86–7. doi: 10.1097/EJA.0b000e000000f3fb. [DOI] [PubMed] [Google Scholar]
- 13.Palanisamy A, Klickovich RJ, Ramsay M, Ouyang DW, Tsen LC. Intravenous dexmedetomidine as an adjunct for labor analgesia and cesarean delivery anesthesia in a parturient with a tethered spinal cord. Int J Obstet Anesth. 2009;18:258–61. doi: 10.1016/j.ijoa.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Mendoza JM. Dexmedetomidine as adjunct for analgesia in labor: A report of two cases. Rev Colomb Anestesiol. 2012;40:79–81. [Google Scholar]
- 15.Hanoura SE, Hassanin R, Singh R. Intraoperative conditions and quality of postoperative analgesia after adding dexmedetomidine to epidural bupivacaine and fentanyl in elective cesarean section using combined spinal-epidural anesthesia. Anesth Essays Res. 2013;7:168–72. doi: 10.4103/0259-1162.118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souza KM, Anzoategui LC, Pedroso WC, Gemperli WA. Dexmedetomidine in general anesthesia for surgical treatment of cerebral aneurysm in pregnant patient with specific hypertensive disease of pregnancy: Case report. Rev Bras Anestesiol. 2005;55:212–6. doi: 10.1590/s0034-70942005000200008. [DOI] [PubMed] [Google Scholar]
- 17.Toyama H, Wagatsuma T, Ejima Y, Matsubara M, Kurosawa S. Cesarean section and primary pulmonary hypertension: The role of intravenous dexmedetomidine. Int J Obstet Anesth. 2009;18:262–7. doi: 10.1016/j.ijoa.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53:646–52. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 19.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 20.Tveit TO, Seiler S, Halvorsen A, Rosland JH. Labour analgesia: A randomised, controlled trial comparing intravenous remifentanil and epidural analgesia with ropivacaine and fentanyl. Eur J Anaesthesiol. 2012;29:129–36. doi: 10.1097/EJA.0b013e32834dfa98. [DOI] [PubMed] [Google Scholar]
- 21.Volmanen P, Sarvela J, Akural EI, Raudaskoski T, Korttila K, Alahuhta S. Intravenous remifentanil vs. epidural levobupivacaine with fentanyl for pain relief in early labour: A randomised, controlled, double-blinded study. Acta Anaesthesiol Scand. 2008;52:249–55. doi: 10.1111/j.1399-6576.2007.01509.x. [DOI] [PubMed] [Google Scholar]
- 22.Balki M, Kasodekar S, Dhumne S, Bernstein P, Carvalho JC. Remifentanil patient-controlled analgesia for labour: Optimizing drug delivery regimens. Can J Anaesth. 2007;54:626–33. [PubMed] [Google Scholar]
- 23.Evron S, Glezerman M, Sadan O, Boaz M, Ezri T. Remifentanil: A novel systemic analgesic for labor pain. Anesth Analg. 2005;100:233–8. doi: 10.1213/01.ANE.0000143351.64538.BC. [DOI] [PubMed] [Google Scholar]


