Abstract
Objective
To compare the effectiveness of two home-based behavioral interventions to promote wheelchair users exercise adoption and maintenance over 12 months.
Design
Randomized controlled trial, with participants stratified into groups based on disability type (stable, episodic, progressive) and support partner availability.
Setting
Exercise occurred in participant preferred locations (e.g., home, recreation center), with physiological data collected at the university-based exercise lab.
Participants
One hundred twenty-eight inactive wheelchair users (64 women) with sufficient upper arm mobility for arm-based exercise enrolled. Participants on average were 45 years old, lived with their impairment for 22 years, with spinal cord injury (46.1%) most commonly reported as causing mobility impairment.
Interventions
Both groups received home-based exercise interventions. The staff-supported group (n= 69) received intensive exercise support, while the self-guided group (n= 59) received minimal support. Both received exercise information, resistance bands, instructions to self-monitor exercise, regularly-scheduled phone calls, and handwritten cards.
Main Outcome Measures
The primary outcome derived from weekly self-reported exercise. Secondary outcomes included physical fitness (aerobic/muscular) and predictors of exercise participation.
Results
The staff-supported group reported significantly greater exercise (~ 16 minutes/week) than the self-guided group over the year (t=10.6, p=0.00), with no significant between group difference in aerobic capacity (t=0.76, p=0.45) and strength (t=1.5, p=0.14).
Conclusions
Although the staff-supported group reported only moderately more exercise, the difference is potentially clinically significant as they also exercised more frequently. The staff-supported approach holds promise for encouraging exercise among wheelchair users, yet additional support may be necessary to achieve more exercise to meet national recommendations.
Keywords: exercise, people with disabilities, wheelchair, intervention, randomized controlled trial
Nearly 1 in 5 Americans live with a disability,1 and this is expected to increase.2 Persons with disabilities are significantly more likely to be sedentary and less likely to be active3–5–a troubling health disparity given the substantial health benefits of an active lifestyle6, 7 which may be greater for those with disabilities than the general population7, 8 as they experience a higher incidence9 and prevalence10 of numerous chronic health conditions. Barriers to being physically active are compounded for those with mobility impairments. In addition to general exercise barriers (e.g., motivation, time), they face numerous unique disability-related barriers (e.g., lack of affordable/accessible transportation, knowledgeable health professionals, and accessible equipment and facilities).11–14 Those with severe mobility impairments (e.g., spinal cord injury, SCI) also face equipment, resource, and environmental barriers to being active. While strong evidence shows exercise has specific health and functional benefits for people with disabilities,8, 15 data regarding effective strategies for adopting and maintaining an active lifestyle despite disabilities is limited and conflicting. For example, studies have reported both minimal or no effects16, 17 and significant increases in physical activity.18–23
Successful interventions have used different behavioral approaches, including phone calls to support activity efforts.18, 20–23 While the frequency and content of calls has varied,18, 20, 21 they focus on developing specific goals,19, 22, 23 providing support,18, 20, 21 and addressing barriers.19, 20, 22 Based on this evidence and our pilot study results,19 which found individual counseling/education increased physical activity in mobility-impaired women, we designed a theory-based, multi-component 52-week intervention to compare the effectiveness of two home-based interventions designed to increase the adoption and maintenance of home and community-based exercise among manual wheelchair users. Secondarily, we examined factors associated with exercise adherence.
We hypothesized that: (1) the staff-supported experimental group would engage in significantly more aerobic and strength exercise at 12 and 52 weeks compared with the self-guided comparison group; (2) the experimental group would demonstrate significantly greater increases in aerobic capacity and muscular strength than the comparison group; and (3) exercise adherence would be greatest for those reporting fewer barriers, higher exercise self-efficacy, fewer health problems, less pain, and less fatigue.
Methods
Theoretical framework
The multi-component intervention was based on Social Cognitive Theory24, 25 and the Relapse Prevention model.26, 27 Intervention components included building self-efficacy and behavioral skills using strategies successfully applied in the general population and listed by the U.S. Task Force on Community Preventive Services (the Community Guide)28 as effective for promoting exercise: goal setting, self-monitoring, building social support, and preventing relapse. The 12 month study had a six month intervention period and six month follow-up.
Recruitment and Eligibility
We previously published details of the recruitment methods.29 Strategies included posting fliers, advertising, and presenting at community events. Participants were offered a $10 giftcard for referring others.
Telephone screening assessed the following inclusion criteria: (1) an impairment for ≥ six months necessitating manual or powered wheelchair use for mobility outside the home; (2) age between 18–65; (3) not currently physically active (< 150 minutes/week of moderate or vigorous exercise); (4) sufficient upper arm mobility for aerobic exercise; and (5) physician approval; and exclusion criteria: (1) body mass index ≥ 50; (2) MD-identified contraindications for unsupervised exercise; (3) cognitive impairment precluding self-directing daily activities; and (4) current or planned pregnancy.
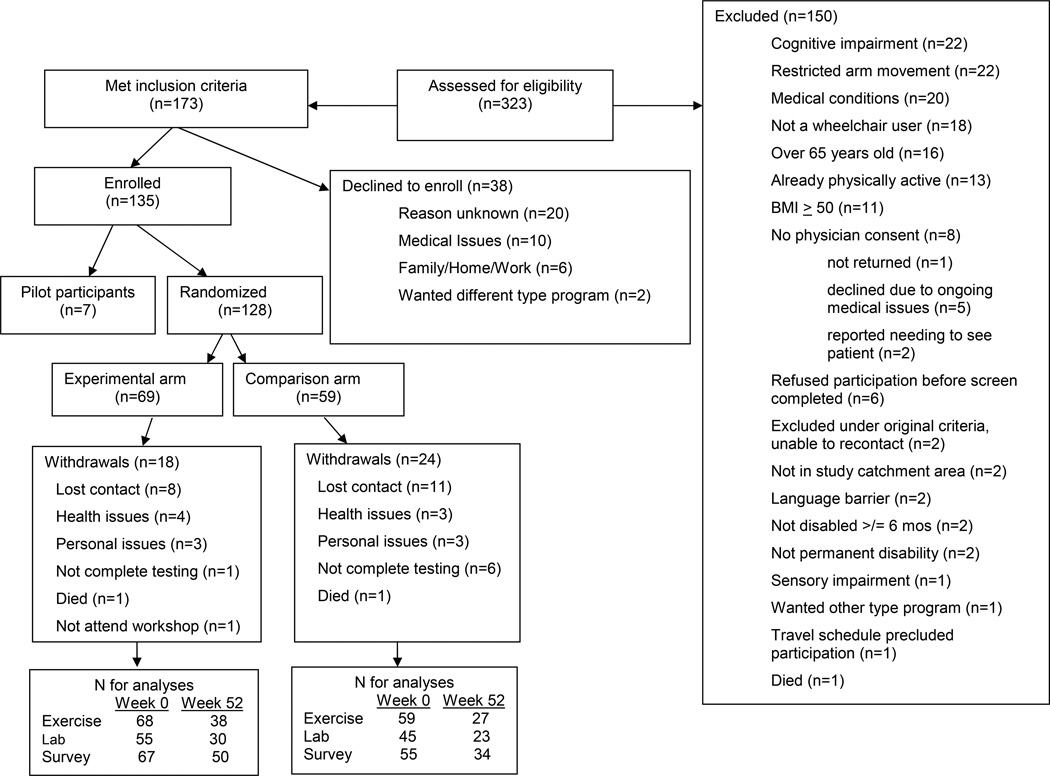
Recruitment/enrollment into 10 cohorts occurred over 3 years. Of 355 screened individuals, 173 were eligible (see CONSORT, Figure 1). Thirty-eight declined participation and 135 enrolled. The initial 7 participants served to pilot the intervention and methods and were excluded from subsequent analyses. All had physician clearance and provided written informed consent, approved by the Human Subject’s Committee (HSC #10053).
Fig 1.
The CONSORT flow chart
Randomization
Eligible participants were randomized to either the staff-supported intervention group or the self-guided comparison group following stratification on availability of a partner to support their effort to increase exercise (yes/no) and their disability type (stable, episodic, or progressive).
Statistical Power
A-priori power calculation on the primary outcome (self-reported aerobic exercise minutes/week) indicated a sample size of 104 (52/group) was required for 80% power, with a conservatively assumed high correlation of .60 among repeated measures, a moderate group difference (Cohen’s d= 0.50), and an anticipated attrition rate of 16%. This is considerably smaller than differences (median d= 0.69) observed in a previous pilot study19 and similar published studies,30–32 suggesting adequate power for the final sample of 128 participants.
Intervention
Elements common to both groups
Protocol and intervention details were previously published.33 Briefly, both groups received (1) disability-specific educational information (health benefits of exercise, aerobic and strength training distinctions, accessible exercises and locations); (2) resistance bands; (3) instruction/encouragement for self-monitoring; (4) 15 regularly-scheduled calls; and (5) handwritten cards for birthdays, holidays, and major events. The goal was to increase exercise either at home or at another self-selected location while maximizing options to deal with individual limitations, enjoyment, and schedule. Support intensity, described below, was the primary difference between groups.
All participants were provided an individual target heart rate (THR) for adopting moderate-intensity aerobic exercise. THR was prescribed at 55–75% heart rate reserve (HRR)34 plus resting HR. Peak HR was the highest HR observed during the peak aerobic capacity test described below. For participants without medical clearance to perform this test, peak HR for prescribing THR was estimated as 220 – age in years.
Staff-supported experimental group
Intervention components designed to increase exercise knowledge, self-efficacy, and self-management skills included: (1) attending a one day educational workshop, during which individualized exercise plans were developed for: (2) setting specific exercise goals, (3) establishing plans to prevent relapses, and (4) identifying people to support exercise efforts. (5) Study staff phone calls, also providing exercise support (phone calls were completed weekly for two months, biweekly the next two months, and monthly for months 5 and 6). Callers addressed 11 topics over 15 scheduled calls, with flexibly designed scripts to maximize meeting individual experiences and needs (specific topics are described elsewhere33). (6) Participants received a monthly two-page newsletter for the year. Participants unable to attend the workshop received a DVD containing workshop content and developed their exercise plan during the initial support call.
Self-guided comparison group
This group received a minimal-contact intervention that included similarly scheduled 15 phone calls. Educational materials mailed to participants were reviewed during the first phone call. Subsequent calls were limited to thanking or requesting return of logs and inquiring about exercise-related injuries.
Outcomes Measures
The primary study outcome was self-reported weekly minutes of aerobic exercise, reported weekly for 52 weeks. Secondary outcomes included peak aerobic capacity and maximal strength. All staff completing fitness evaluations were blinded to group assignment.
Demographic data collected at baseline included birthdate, sex, race and ethnicity, mobility impairment etiology, impairment onset date, marital status, education, employment, healthcare coverage, and household income.
Self-reported weekly exercise
Participants reported type, duration, and frequency of aerobic exercise, including HR during aerobic exercise, whether they performed strength exercise, and occurrence of disability-related health problems on standardized, preprinted logs, which had space for open-ended comments. We previously used this approach with mobility-impaired women. We provided reminders for late or missing logs and $5 giftcards on 5 occasions for returning logs in postage-paid envelopes. On three occasions (months 3, 6, 9), participants wore ActiGraph® (Pensacola, FL, model GT1Ma) on their wrist during all waking hours for 5 consecutive days to assess validity of self-reported exercise.
Anthropometric data were collected at baseline and months 3, 6, and 12 in a medical center exercise laboratory. Participants were compensated for time and transportation. Protocol details, summarized below, were previously published.33
Body weight was measured using an accessible Seca scale (#664b). Maximal strength was assessed with a one-repetition maximum free weight bench press. Participants performed up to five lifts with increasing weight until they could not use proper technique or reported inability to continue. Peak aerobic capacity was assessed during a graded, discontinuous arm crank test (two-minute stages, work load increased 5–15 watts/stage depending on conditioning level) using a SciFit Pro I ergometerc (50 rpm). Oxygen consumption was assessed continuously using a ParvoMedics’ True One® 2400 metabolic cartd. Peak aerobic capacity was the highest oxygen observed. The highest HR observed was used to calculate the THR. Blood pressure and heart rate (EKG) were monitored during all tests. A physician was present for participants at risk for cardiovascular events.
Self-reported perceptions of exercise and health were collected at baseline and months 3, 6 and 12. Surveys were mailed before every fitness appointment and returned at the appointment.
The PARTS35 evaluates mobility-impaired individuals’ participation in five daily life domains and was used for a disability severity index based on reporting assistance needed for seven personal care activities (grooming, dressing, bathing, meal preparation, eating, bladder care, bowel care) from someone else and with assistive equipment. Responses (0=no help, 1= some help, 2=a lot of help) were summed over the 14 items; higher scores indicated greater severity. We observed high internal consistency (alpha=0.81 at baseline).
The 18-item Lee Fatigue Scale (LFS)36 measures perceived fatigue and has strong internal consistency (alphas=0.91–0.96) across different populations36, 37 and in our study sample (alphas=0.87–0.88 across baseline, months 3, 6, 12) Two Short Form-3638 items asking how much bodily pain in the past four weeks and how much pain interfered with normal work comprised the Bodily Pain subscale. Internal consistency on this subscale was high to strong (alphas=0.80–0.91). The 16-item Barriers to Health Activities among Disabled Persons (BHADP)39 assessed perceived exercise barriers with two subscales: Motivation (7 items) and External Barriers (9 items). It has high internal consistency (alpha=0.82) and good discriminant validity between individuals with and without disabilities,39 which was also confirmed in this study (alphas=0.76–0.84). The 7-item Exercise subscale of the Self-Rated Abilities for Health Practices Scale (SRAHP)40 measured exercise self-efficacy. Internal consistency for the SRAHP in our study was high (alphas=0.79–0.82).
Treatment Fidelity
Intervention delivery fidelity was assessed for: workshop attendance, log return, phone call delivery, and appropriate provision (or not) of exercise support. Call tracking included number of calls attempted/completed, percent completed, and number and percent of scripted topics delivered (staff-supported group only).
Data Analyses
Primary analyses were longitudinal comparisons of outcomes between the two groups over exercise adoption (baseline-12 weeks) and maintenance (13–52 weeks). To address hypotheses 1 and 2, we used mixed modeling to account for interdependency among observations collected at multiple time points. A proper error covariance structure was determined based on the model fit indicated by log-likelihood, Akaike Information Criterion (AIC), Bayesian Information Criterion. Mixed modeling estimated the time-related (linear, quadratic, cubic, etc.) changes and group differences in each outcome over the 12-month period.
Factors influencing intervention effectiveness were also assessed (Hypothesis 3). Best subset mixed modeling analyses identified the best model for predicting weekly aerobic exercise minutes. We identified the best model separately for each group because initial analyses indicated significant group differences predicting aerobic exercise minutes. Potential predictors included demographic, peak aerobic capacity, disability-related and psychosocial health variables, and health problems. Time-variant (linear) change was a priori selected as a covariate. A total of 2,047 models (2m − 1, where m= number of potential predictors) could be constructed from the set of variables. Corrected AIC (AICC) and Minimal Description Length (MDL) were compared across these 2,047 models using the SAS macro ALLMIXED2.33, 41
The Actigraph® (model GT1M) provided an objective measure of activity to validate our self-report exercise measure. We examined correlations between accelerometer derived minutes of moderate/vigorous activity42 and minutes of moderate/vigorous activity in bouts 10 minutes/day with self-reported log data.
All analyses were based on an intention-to-treat approach. Partial sets of outcome measurements were not lost but analyzed via restricted maximum likelihood estimation in the mixed models. Statistical significance was set at α=0.05; all analyses used SAS 9.3.43
Results
The groups did not differ on socio-demographic characteristics, disability profiles, or health events (Table 1). On average, participants were 45 years old, lived with their disabling condition for 22 years, and needed little ADL assistance (disability severity=7.7 on a 0–28 scale). Most at least attended some college (78%); only 36.4% were employed. S CI (46%) was the most common cause of impairment.
Table 1.
Participant demographics
| Full sample | Staff-supported intervention group |
Self-guided comparison group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N | M | SD | N | M | SD | N | M | SD |
| Age (year) | 128 | 44.5 | 12.5 | 69 | 46.0 | 12.1 | 59 | 42.9 | 13 |
| Years live with disability | 124 | 22.3 | 15.9 | 66 | 23.3 | 16 | 58 | 21.0 | 15.7 |
| Age at disability onset | 124 | 22.4 | 18.1 | 66 | 22.9 | 17 | 58 | 21.7 | 19.5 |
| Disability severity | 117 | 7.7 | 5.1 | 64 | 7.1 | 4.9 | 53 | 8.4 | 5.3 |
| Exercise at week 0 | |||||||||
| Aerobic exercise (min/wk) | 127 | 23.7 | 5.7 | 68 | 25.0 | 8.5 | 59 | 22.1 | 7.4 |
| Aerobic exercise (days/wk) | 127 | 0.8 | 0.1 | 68 | 0.7 | 0.2 | 59 | 0.9 | 0.2 |
| Resistance exercise (days/wk) | 127 | 0.9 | 0.2 | 68 | 0.8 | 0.2 | 59 | 1.1 | 0.2 |
| Fitness | |||||||||
| Peak VO2 | 100 | 13.2 | 0.04 | 55 | 12.7 | 0.6 | 45 | 13.8 | 0.6 |
| Strength | 97 | 152.7 | 6.4 | 55 | 143.5 | 8.3 | 43 | 164.9 | 9.9 |
| Body weight (in kg) | 127 | 86.7 | 2.3 | 68 | 83.0 | 2.7 | 59 | 90.9 | 3.9 |
| Fatigue1 | 122 | 72.7 | 2.2 | 67 | 72.0 | 2.8 | 55 | 73.6 | 3.8 |
| Bodily pain2 | 122 | 56.7 | 2.3 | 67 | 57.7 | 3.3 | 55 | 55.4 | 3.1 |
| Total exercise barriers3 | 122 | 29.6 | 0.6 | 67 | 29.4 | 0.9 | 55 | 29.9 | 0.7 |
| Exercise self-efficacy4 | 122 | 22.1 | 0.4 | 67 | 22.6 | 0.5 | 55 | 21.5 | 0.6 |
| N | % | N | % | N | % | ||||
| Gender | 128 | ||||||||
| Male | 64 | 50 | 38 | 55.1 | 26 | 44.1 | |||
| Female | 64 | 50 | 31 | 45.9 | 33 | 55.9 | |||
| Race/Ethnicity | 128 | ||||||||
| White | 102 | 79.7 | 52 | 75.4 | 50 | 84.7 | |||
| Black | 15 | 11.7 | 10 | 14.5 | 5 | 8.5 | |||
| Mutti-Racial | 8 | 6.3 | 5 | 7.2 | 3 | 5.1 | |||
| Other | 3 | 2.3 | 2 | 2.9 | 1 | 1.7 | |||
| Education1 | 122 | ||||||||
| >= High school | 27 | 22.1 | 15 | 22.7 | 12 | 21.4 | |||
| Some college/Bachelors | 63 | 51.6 | 32 | 48.5 | 31 | 55.4 | |||
| Some graduate/Graduate degree | 32 | 26.2 | 19 | 28.8 | 13 | 23.2 | |||
| Employment2 | |||||||||
| Employed (full/part) | 121 | 44 | 36.4 | 26 | 40.0 | 18 | 32.1 | ||
| Unemployed | 47 | 38.8 | 25 | 38.8 | 22 | 39.3 | |||
| Student/Retired/Volunteer | 30 | 24.8 | 14 | 21.5 | 16 | 28.6 | |||
| Primary disability | |||||||||
| SCI | 59 | 46.1 | 35 | 50.7 | 24 | 40.7 | |||
| CP/SB | 26 | 20.3 | 8 | 11.6 | 18 | 30.5 | |||
| MS | 10 | 7.8 | 4 | 5.8 | 6 | 10.2 | |||
| Other | 10 | 7.8 | 6 | 8.7 | 4 | 6.8 | |||
| Amputation | 8 | 6.3 | 4 | 5.8 | 4 | 6.8 | |||
| Orthopedic | 5 | 3.9 | 5 | 7.2 | 0 | 0 | |||
| Post Polio | 4 | 3.1 | 2 | 2.9 | 2 | 3.4 | |||
| Fibromylagia/Lupus | 3 | 2.3 | 2 | 2.9 | 1 | 1.7 | |||
| Stroke/TBI | 3 | 2.3 | 3 | 4.3 | 0 | 0 | |||
| Health events reported (over 52 wks) | 8.5 | 10.4 | 69 | 8.7 | 10.4 | 59 | 8.2 | 10.4 | |
1, 2, 3, 4LFS, SF-36, BHADP, and SRAHP scale scores, respectively.
Retention
One-third (n=42) of participants withdrew or were lost to follow up. While more withdrew from the self-guided (40.7%) than the staff-supported group (26.1%), this difference was not significant (χ2=3.07, p=0.08). Dropouts (40.5±11.4 years) were significantly younger than completers (46.5±12.7 years) (t= −2.6, p<0.05), but did not differ by sex, time with disability, height, weight, strength, or self-reported outcomes (all p’s>0.05).
Accelerometer Data
Self-report exercise minutes were moderately correlated with accelerometer data: moderate activity minutes/day (r=0.43, p< 0.01), vigorous activity minutes/day (r=0.31, p< 0.01), and moderate or vigorous activity minutes/day in 10 minute bouts (r=0.48, p< 0.01).
Self-reported exercise
Mixed modeling revealed significant group differences in exercise adoption and maintenance. The staff-supported group spent significantly more time (17 more minutes/week) and 0.5 more days/week in aerobic exercise over the first 12 weeks (adoption) and ~15 more minutes/week and 0.6 more days/week during weeks 13–52 (maintenance) compared to the self-guided group. Table 2 depicts average time (minutes/week) and average number of days/week for aerobic and resistance training.
Table 2.
Estimated means and standard errors
| Staff-supported intervention group | Self-guided comparison group | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 1 | Week 12 | Week 26 | Week 52 | Week 0 | Week 1 | Week 12 | Week 26 | Week 52 | |||||||||||||
| Variable | M | SD | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | Group* | Time** |
| Exercise | ||||||||||||||||||||||
| Aerobic exercise (min/week) | 25.0 | 8.5 | 52.7 | 4.9 | 51.7 | 3.9 | 50.3 | 3.4 | 47.8 | 5.4 | 22.1 | 7.4 | 36.4 | 5.1 | 35.3 | 4.2 | 34.0 | 3.9 | 31.5 | 5.9 | 0.00 | 0.52 |
| Aerobic exercise (days/week) | 0.7 | 0.2 | 2.1 | 0.1 | 2.0 | 0.1 | 1.9 | 0.1 | 1.7 | 0.2 | 0.9 | .2 | 1.5 | 0.2 | 1.4 | 0.1 | 1.3 | 0.1 | 1.2 | 0.2 | 0.00 | 0.15 |
| Resistance exercise (days/wk) | 0.8 | 0.2 | 2.0 | 0.1 | 1.9 | 0.1 | 1.8 | 0.1 | 1.6 | 0.2 | 1.1 | 0.2 | 1.7 | 0.1 | 1.6 | 0.1 | 1.5 | 0.1 | 1.3 | 0.2 | 0.02 | 0.09 |
| Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 months | |||||||||||||||
| Variable | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | Group* | Time** | ||||
| Fitness | ||||||||||||||||||||||
| Peak VO2 (ml/kg/min) | 12.7 | 0.6 | 13.6 | 0.6 | 13.3 | 0.6 | 13.3 | 0.6 | 13.3 | 0.6 | 14.3 | 0.7 | 13.9 | 0.7 | 13.9 | 0.6 | 0.45 | 0.02 | ||||
| Maximal bench press strength (kg) | 143.6 | 8.0 | 152.6 | 7.8 | 158.8 | 8.0 | 162.8 | 8.4 | 161.4 | 9.1 | 170.5 | 9.0 | 176.7 | 9.1 | 180.7 | 9.5 | 0.14 | 0.01 | ||||
| Body weight (kg) | 83.5 | 3.1 | 83.5 | 3.1 | 83.5 | 3.1 | 83.5 | 3.1 | 91.4 | 3.3 | 91.4 | 3.3 | 91.3 | 3.3 | 91.3 | 3.4 | 0.09 | 0.95 | ||||
Note. Raw means are reported for week 0.
p value for group difference.
p value for weekly/monthly linear change (i.e., increase or decrease), except for maximum VO2 (cubic change; i.e., increase from baseline to 3 months, decrease from 3 to 6 months, and maintenance afterward) and strength (quadratic change; i.e., rapid then slow increase).
Physiological outcomes
There were significant within, but not between group differences for change in peak aerobic capacity and maximal strength over 12 months (Table 2). There were no significant between or within group differences for change in body weight.
Predictors of exercise adherence and maintenance
Although the best subset mixed modeling results suggested time with disability, health problems, fatigue, bodily pain, exercise self-efficacy, and exercise barriers predicted exercise adoption and maintenance, the best fit for each group contained different variables (Table 3). After accounting for other predictors only exercise barriers (for staff-supported group) and exercise self-efficacy (for self-guided group) significantly predicted weekly minutes of aerobic exercise over 12-months.
Table 3.
Best subset mixed modeling results for weekly aerobic exercise minutes
| Staff-supported intervention group |
Self-guided comparison group |
|||||
|---|---|---|---|---|---|---|
| Effect | b | SE | p | b | SE | p |
| Intercept | 90.34 | 29.86 | 0.004 | −43.81 | 25.59 | 0.093 |
| Years lived with disability | 0.15 | 0.45 | 0.740 | −0.04 | 0.39 | 0.917 |
| Month (linear change) | 1.08 | 0.97 | 0.264 | 1.35 | 0.88 | 0.132 |
| Health problems (count) | −0.27 | 1.15 | 0.814 | −1.28 | 0.86 | 0.137 |
| Fatigue1 | −0.17 | 0.17 | 0.321 | 0.31 | 0.16 | 0.055 |
| Bodily pain2 | 0.04 | 0.19 | 0.842 | |||
| Total exercise barriers3 | −1.74 | 0.77 | 0.025 | |||
| Exercise self-efficacy4 | 2.27 | 0.90 | 0.014 | |||
1, 2, 3, 4LFS, SF-36, BHADP, and SRAHP scale scores, respectively.
Treatment Fidelity
There were no between group differences for number of calls attempted/completed or exercise logs returned (68.8% staff-supported vs 60.7% self-guided). Both groups completed two-thirds of planned calls (66.9% staff-supported vs 63.5% self-guided).
Discussion
The limited evidence regarding effective strategies to promote exercise for people with disabilities report varied success.16, 18, 20, 21 Our study compared behavioral and physiological outcomes over 12 months between wheelchair users in a staff-supported or self-guided intervention group, where intensity of exercise support was the major distinguishing feature. Our intervention included empirically supported components for increasing exercise (i.e., individualized counseling,19–23 exercise goals,19, 22, 23 self-monitoring, ongoing phone-based support,22,23,33,34 and barrier problem solving20, 22). However, the magnitude of increase in exercise that we observed was lower than expected.
As hypothesized, both groups adopted exercise and significantly improved aerobic capacity and strength. Providing additional staff-support resulted in increased aerobic exercise of only 16 minutes/week compared to the self-guided group (51 vs. 34 minutes/week). While modest, the difference is potentially clinically significant as the staff-supported group also exercised more frequently, both each week (see aerobic/strength days, Table 2) and over the year. Weekly exercise data reveals that more than half (61%) of staff-supported participants on average over the year reported some aerobic exercise each week compared to less than half (48%) of the self-guided group (data not shown). Although additional/different support may be necessary for wheelchair users to meet the ACSM’s activity guidelines44 (a minimum of 30 minutes of moderate intensity five days/week or 20 minutes of vigorous intensity activity three times/week, or equivalent combination), recent evidence-based clinical guidelines indicate that individuals with SCI need only 40 minutes/week of moderate to vigorous exercise for fitness increases.45 Notably, the staff-supported experimental participants achieved and maintained this level (average= 51 minutes/week), while the self-guided group fell short (average = 34 minute/week).
To place these results in context, our staff-supported intervention increased exercise more than some other interventions for wheelchair users,16, 17, 23, 46, 47 although less than others.18–20 Differences between our study and previous studies include a primary outcome of contemporaneous self-reports, combined with objective assessment (accelerometry). Other studies have used only retrospective self-reports,20–23 which have been shown to overestimate activity levels due to faulty recollection, erroneous perceptions, social desirability bias.48–51 For example, retrospective survey data from the National Health and Nutrition Examination Survey shows 60% report meeting physical activity guidelines, whereas less than 5% met activity guidelines when assessed by accelerometer.52 Our study also found retrospective estimates of physical activity using the Behavioral Risk Factor Surveillance System physical activity module yielded vastly higher values (over 300 minutes/week by 3 and 6 months) than the contemporaneous exercise logs (~51 minutes/week). Similar methodological issues may have occurred in other studies,18, 20, 21 where physical activity was based upon self-reports of time generally spent in physical activity20, 21 or performed over one week18 but used to represent a three to six month timeframe, in the absence of objective activity monitoring. Thus, while the levels of exercise in this study were less than some similar studies,18–20 we argue that our exercise log data more likely reflect reality and future studies investigating physical activity measurement validity and reliability for individuals with disabilities are warranted.
We encountered other methodologic issues worth noting. First, participants experienced substantial delays (mean of 76.7 days ± 52.9 days) between eligibility determination, when their motivation was likely highest, and program initiation. These delays primarily occurred to recruit sufficient participants for a cohort. Allowing participants to enter an ongoing program upon eligibility determination could better capitalize on initial motivation. Second, many participants did not have access to or familiarity with accessible exercise equipment. Obtaining exercise equipment or locating a suitable exercise facility took time and connecting individuals with options may facilitate better adoption.18 Third, workshop attendees expressed interest in reconnecting with one another and others have found benefits from social interaction during structured exercise interventions.53 Thus, including mechanisms that facilitate participant interaction may enhance individuals’ adherence. Fourth, health problems hindered many from being as active as they hoped. Our 110 participants experienced 418 health events, including allergies, colds, flus, and serious events requiring hospitalizations, surgeries, or extensive treatment. Strategies for such “down periods” might enhance exercise interventions for individuals with disabilities.
Limitations
Despite adequately powered a-priori, our study was underpowered with a sample size lower than expected. Although attrition was similar to other studies, it was higher than estimated (~33%). However, dropouts were not significantly different than completers, as the latter included participants who maintained exercise and those who did not.
Conclusions
Exercise behavior is challenging to change. This staff-supported intervention demonstrated increased exercise adoption and maintenance that was significantly, but only moderately greater than the self-guided approach. Significant increases in peak aerobic capacity and muscular strength were shown in both groups. Although the average minutes/week of aerobic exercise fell short of the ACSM’s guidelines for the general population44 and those with SCI,54 the staff-supported group met the new evidence-based guidelines for individuals with SCI.45 This study contributes to the evidence base of approaches for promoting activity among those with disabilities. The staff-supported approach, which attempted to eliminate transportation and environmental barriers, holds promise for promoting aerobic exercise among wheelchair users. Yet, additional support may be necessary to achieve more weekly exercise. We observed participants making connections and sharing knowledge during workshops and most expressed interest to reconnect. Incorporating strategies that facilitate peer support may further increase weekly exercise. The innovative use of technology such as computers/tablets/smartphones may help increase adoption through facilitating virtual social and professional support while avoiding transportation barriers.55 Others have successfully used peer-support56, 57 within internet-based interventions promoting behavior change.
Acknowledgements
The study was funded by NICHD/NIH grant #R01 HD048628 and the work was supported in part by Frontiers: The Heartland Institute for Clinical and Translational Research, (University of Kansas Medical Center’s CTSA; UL1RR033179). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, NICHD or NCMRR. This study is registered at www.clinicaltrials.gov (No. NCT00866112).
List of Abbreviations
- SCI
spinal cord injury
- ACSM
American College of Sports Medicine
- U.S.
United States
- HSC
Human Subjects Committee
- MD
medical doctor
- THR
target heart rate
- HRR
heart rate reserve
- HR
heart rate
- DVD
digital video disc
- FL
Florida
- LFS
Lee Fatigue Scale
- BHADP
Barriers to health Activities among Disabled Persons
- MDL
Minimal Description Length
- ADL
activities of daily living
- EKG
electrocardiogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was conducted at the Gerontology Center of the LifeSpan Institute at the University of Kansas and General Clinical Research Center at the University of Kansas Medical Center.
Findings from this study were presented at the 139th Annual meeting of the American Public Health Association to the Disability Section, in Washington, DC.
Accelerometer, model GT1M, ActiGraph, 49 East Chase Street, Pensacola, FL 32502
Accessible scale, Seca model #664, Seca North American West, Medical Scales and Measuring Systems, Seca Corp., 13601 Benson Avenue, Chino, CA 91710, USA
Arm Ergometer, SciFit Pro I, SciFit Corporate Headquarters, 5151 S 110th E. Ave, Tulsa, OK 74146
Metabolic cart, ParvoMedics’ True One® 2400, ParvoMedics, 8152 South 1715 East, Sandy, UT 84093
References
- 1.Brault M. Americans with disabilities: 2005. Curr Popul Rep. 2008:70–117. [Google Scholar]
- 2.Institute of Medicine. The future of disability in America. Washington, DC: National Academy Press; 2007. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Physical activity among adults with a disability-United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56(39):1021–1024. [PubMed] [Google Scholar]
- 4.Healthy People 2020. Leisure time physical activity-none (percent) [2010 February 24];2012 Available from: URL: http://www.healthindicators.gov/Indicators/Noleisure-timephysicalactivity_1313/Profile/Data.
- 5.Healthy People 2020. Aerobic physical activity: >150 min/week moderate or >75 minutes/week vigorous physical activity (percent) [cited 2012 2–20];2010 Available from: URL: http://www.healthindicators.gov/Indicators/Aerobic-physical-activity-150-minweek-moderate-or-75-minutesweek-vigorous-physical-activity-percent_1319/National_0/Profile/Data.
- 6.Seaman J. Physical activity and fitness for persons with disabilities. Research Digest. 1999;3(5):1–6. [Google Scholar]
- 7.Heath GW, Fentem PH. Physical activity among persons with disabilities - A public health perspective. In: Holloszy JO, editor. Exercise and sport sciences. Williams & Wilkins; 1997. pp. 195–234. [PubMed] [Google Scholar]
- 8.Rimmer JH, Chen MD, McCubbin JA, Drum C, Peterson J. Exercise intervention research on persons with disabilities: what we know and where we need to go. Am J Phys Med Rehabil. 2010;89(3):249–263. doi: 10.1097/PHM.0b013e3181c9fa9d. [DOI] [PubMed] [Google Scholar]
- 9.Rasch EK, Magder L, Hochberg MC, Magaziner J, Altman BM. Health of community-dwelling adults with mobility limitations in the United States: Incidence of secondary health conditions. Part II. Arch Phys Med Rehabil. 2008;89:219–230. doi: 10.1016/j.apmr.2007.08.159. [DOI] [PubMed] [Google Scholar]
- 10.Rasch EK, Hochberg MC, Magder L, Magaziner J, Altman BM. Health of community-dwelling adults with mobility limitations in the United States: Prevalent health conditions. Part I. Arch Phys Med Rehabil. 2008;89:210–218. doi: 10.1016/j.apmr.2007.08.146. [DOI] [PubMed] [Google Scholar]
- 11.Froehlich AK, Nary DE, White GW. Identifying barriers to participation in physical activity for women with disabilities. SCI Psychosocial Processes. 2002;15:21–28. [Google Scholar]
- 12.Rimmer JH, Rubin SS, Braddock D. Barriers to exercise in African American women with physical disabilities. Archives of Physcial Medicine and Rehabilitation. 2000;81(2):182–188. doi: 10.1016/s0003-9993(00)90138-2. [DOI] [PubMed] [Google Scholar]
- 13.Rimmer JH, Riley B, Wang E, Rauworth A, Jurkowski J. Physical activity participation among persons with disabilities: Barriers and facilitators. Am J Prev Med. 2004;26(5):419–425. doi: 10.1016/j.amepre.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Driver S, Ede A, Dodd Z, Stevens L, Warren AM. What barriers to physical activity do individuals with a recent brain injury face? Disabil Health J. 2012 doi: 10.1016/j.dhjo.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Martin Ginis KA, Jorgenson S, Stapleton J. Exercise and sport for persons with spinal cord injury. Physical Medicine and Rehabilitation. 2012;4(11):894–900. doi: 10.1016/j.pmrj.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Zemper ED, Tate DG, Roller S, Forchheimer M, Chiodo A, Nelson VS, et al. Assessment of a holistic wellness program for persons with spinal cord injury. Am J Phys Med Rehabil. 2003;82(12):957–968. doi: 10.1097/01.PHM.0000098504.78524.E2. [DOI] [PubMed] [Google Scholar]
- 17.Kosma M, Cardinal BJ, McCubbin JA. A pilot study of a web-based physical activity motivational program for adults with physical disabilities. Disabil Rehabil. 2005;27(23):1435–1442. doi: 10.1080/09638280500242713. [DOI] [PubMed] [Google Scholar]
- 18.Wise HH, Thomas J, Nietert PJ, Brown DD, Sword DO, Diehl N. Home physical activity programs for the promotion of health and wellness in individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. 2011;14(4):122–132. [Google Scholar]
- 19.Froehlich-Grobe K, White GW. Promoting physical activity among women with mobility impairments: a randomized controlled trial to assess a home- and community-based intervention. Arch Phys Med Rehabil. 2004;85(4):640–648. doi: 10.1016/j.apmr.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Rimmer JH, Hsieh K, Graham BC, Gerber BS, Gray-Stanley JA. Barrier removal in increasing physical activity levels in obese African American women with disabilities. J Womens Health. 2010;19(10):1869–1876. doi: 10.1089/jwh.2010.1941. [DOI] [PubMed] [Google Scholar]
- 21.Rimmer JH, Rauworth A, Wang E, Heckerling PS, Gerber BS. A randomized controlled trial to increase physical activity and reduce obesity in a predominantly African American group of women with mobility disabilities and severe obesity. Prev Med. 2009;48(5):473–479. doi: 10.1016/j.ypmed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Arbour-Nicitopoulos KP, Ginis KAM, Latimer AE. Planning, leisure-time physical activity, and coping self-efficacy in persons with spinal cord injury: A randomized controlled trial. Archives of Physical and Medical Rehabilitation. 2009;90(12):2003–2011. doi: 10.1016/j.apmr.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Latimer AE, Ginis KAM, Arbour KP. The efficacy of an implementation intention intervention for promoting physical activity among individuals with spinal cord injury: A randomized controlled trial. Rehabil Psychol. 2006;51(4):273–280. [Google Scholar]
- 24.Bandura A. Social foundations of thought and action: Social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 25.Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 26.Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavioral medicine: Changing health lifestyles. New York: Brunner/Mazel; 1980. pp. 410–452. [Google Scholar]
- 27.Marlatt GA, Gordon JR. Relapse prevention. Warm Beach, Washington: The Guilford Press; 1985. [Google Scholar]
- 28.Kahn EB, Ramsey LT, Brownson RC, Heath GW, Howze EH, Powell KE, et al. The effectiveness of interventions to increase physical activity. Am J Prev Med. 2002;22(4S):73–107. doi: 10.1016/s0749-3797(02)00434-8. [DOI] [PubMed] [Google Scholar]
- 29.Nary DE, Froehlich-Grobe K, Aaronson L. Recruitment issues in a randomized controlled exercise trial targeting wheelchair users. Contemp Clin Trials. 2011;32(2):188–195. doi: 10.1016/j.cct.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King AC, Taylor B, Haskell WL, DeBusk RF. Strategies for increasing early adherence to and long-term maintenance of home-based exercise training in healthy middle-aged men and women. Am J Cardiol. 1988;61:628–632. doi: 10.1016/0002-9149(88)90778-3. [DOI] [PubMed] [Google Scholar]
- 31.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, Blair SN. Reduction in cardiovascular disease risk factors: 6-month results from Project Active. Prev Med. 1997;26:883–892. doi: 10.1006/pmed.1997.0218. [DOI] [PubMed] [Google Scholar]
- 32.King AC, Haskell WL, Taylor B, Kraemer HC, DeBusk RF. Group- vs home-based exercise training in healthy older men and women. JAMA. 1991;266(11):1535–1542. [PubMed] [Google Scholar]
- 33.Froehlich-Grobe K, Aaronson LS, Washburn RA, Little TD, Lee J, VanSciver A, et al. An exercise trial for wheelchair users: Project workout on wheels. Contemp Clin Trials. 2012;3(2):351–363. doi: 10.1016/j.cct.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate: a longitudinal study. Annales of Medicinae Experimentalis et Biologiae Fenniae. 1957;35(3):307–315. [PubMed] [Google Scholar]
- 35.Gray DB, Hollingsworth HH, Stark SL, Morgan KA. Participantion survey/mobility: Psychometric properties of a measure of participation for people with mobility impairments and limitations. Arch Phys Med Rehabil. 2006;87:189–197. doi: 10.1016/j.apmr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Lee KA, Hicks G, Nino-Mucia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 37.Meek P, Nail L, Barsevick A, Schwartz A, Stephen S, Whimer K, et al. Psychometric testing of fatigue instrument for use with cancer patients. Nurs Res. 2000;49:181–190. doi: 10.1097/00006199-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceputal framework and item selection. Med Care. 1992;30(6):473–481. [PubMed] [Google Scholar]
- 39.Becker H, Stuifbergen AK, Sands D. Development of a scale to measure barriers to health promotion activities among persons with disabilities. Am J Health Promot. 1991;5(6):449–454. doi: 10.4278/0890-1171-5.6.449. [DOI] [PubMed] [Google Scholar]
- 40.Becker H, Stuifenbergen A, Oh HS, Hall S. Self-rated abilities for health practices: A health self-efficacy measure. Health Values. 1993;17(5):42–50. [Google Scholar]
- 41.Fernandez G. Model selection in PROC MIXED - A user-friendly SAS® macro application. 2007 [Google Scholar]
- 42.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 43.SAS Institute. SAS/STAT 9.3 user's guide. Cary, NC: SAS; 2002–2010. [Google Scholar]
- 44.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 45.Martin Ginis KA, Hicks AL, Latimer AE, Warburton DE, Bourne C, Ditor DS, et al. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord. 2011;49(11):1088–1096. doi: 10.1038/sc.2011.63. [DOI] [PubMed] [Google Scholar]
- 46.Block P, Vanner EA, Keys CB, Rimmer JH, Skeels SE. Project Shake-It-Up: Using health promotion, capacity building and a disability studies framework to increase self efficacy. Disabil Rehabil. 2010;32(9):741–754. doi: 10.3109/09638280903295466. [DOI] [PubMed] [Google Scholar]
- 47.Warms CA, Belza BL, Whitney JD, Mitchell PH, Stiens SA. Lifestyle physicial activity for individuals with spinal cord injury: a pilot study. Am J Health Promot. 2004;18(4):288–291. doi: 10.4278/0890-1171-18.4.288. [DOI] [PubMed] [Google Scholar]
- 48.Matthews CE. Use of self-report instruments to assess physical activity. In: Welk G, editor. Physical activity assessments for health-related research. Champaign, IL: Human Kinetics Publishers; 2002. pp. 107–121. [Google Scholar]
- 49.Sallis JF, Saelens BE. Assesment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2007;71(2):1–14. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- 50.Washburn RA, Montoye HJ. The assessment of physical activity by questionnaire. American Journal of Epidemiology. 1986;123:563–576. doi: 10.1093/oxfordjournals.aje.a114277. [DOI] [PubMed] [Google Scholar]
- 51.Matthews CE, Moore SC, George SM, Sampson J, Bowles HR. Improving self-reports of active and sedentary behaviors in large epidemiologic studies. Exercise and Sport Sciences Review. 2012;40(3):118–126. doi: 10.1097/JES.0b013e31825b34a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S. adults: Compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011;40(4):454–461. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Neuberger GB, Aaronson LS, Gajewski B, Embretson SE, Cagle PE, Loudon JK, et al. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness and disease outcomes of rheumatoid arthritis. Arthritis Care Res. 2007;57:943–952. doi: 10.1002/art.22903. [DOI] [PubMed] [Google Scholar]
- 54.Figoni SF. Spinal cord disabilities: Paraplegia and tetraplegia. In: Durstine JL, Moore GE, editors. ACSM's exercise management for persons with chronic diseases and disabilities. 2nd ed. Champaign, IL: Human Kinetics; 2003. pp. 247–253. [Google Scholar]
- 55.Gerber BS. The chronic disease self-management program: extending reach through the itnernet. Med Care. 2006;44(11):961–963. doi: 10.1097/01.mlr.0000244923.80923.1e. [DOI] [PubMed] [Google Scholar]
- 56.Lorig K, Ritter PL, Laurent DD, Plant K. Internet-based chronic disease self-management: A randomized trial. Med Care. 2006;44(11):964–971. doi: 10.1097/01.mlr.0000233678.80203.c1. [DOI] [PubMed] [Google Scholar]
- 57.VanVoorhees BW, Gollan J, Fogel J. Pilot study of internet-based early intervention for combat-related mental distress. Journal of Rehabilitation Research and Development. 2012;49(8):1175–1190. doi: 10.1682/jrrd.2011.05.0095. [DOI] [PubMed] [Google Scholar]