Abstract
This study tested a behavioral intervention to increase dental attendance among rural Oregonian low-income women and their children. It utilized a multi-site, single-blind, randomized trial design. Four hundred women were randomized into one of four conditions to receive prenatal or postpartum motivational interviewing/counseling (MI) or prenatal or postpartum health education (HE). Counselors also functioned as patient navigators. Primary outcomes were dental attendance during pregnancy for the mother and for the child by age 18 months. Attendance was obtained from the Oregon Division of Medical Assistance Programs and participant self-report. Statewide self-reported utilization data were obtained from the Oregon Pregnancy Risk Assessment Monitoring System (PRAMS). Maternal attendance was 92% in the prenatal MI group and 94% in the prenatal HE group (RR = 0.98; 95% CI = 0.93–1.04). Children’s attendance was 54% in postpartum MI group and 52% in the postpartum HE group (RR = 1.03; 95% CI = 0.82–1.28). Compared to statewide PRAMS, attendance was higher during pregnancy for study mothers (45% statewide; 95% CI = 40–50%) and for their children by 24 months (36% statewide; 95% CI = 27–44%). MI did not lead to greater attendance when compared to HE alone and cost more to implement. High attendance may be attributable to the counselors’ patient navigator function.
Trial Registration: ClinicalTrials.gov Identifier NCT01120041
Keywords: motivational interviewing, oral health, RCT, community, pregnancy, PRAMS
Introduction
The perinatal period is an important time to focus on improving oral health. Maternal care has been shown to disrupt oral transmission of tooth decay pathogens from mother to child and delaying infection of the child by the mother results in improved oral health (Köhler & Andréen, 1994). Prevention programs in pregnant women can be effective (Gomez et al., 2001). Nevertheless, many fail to see the importance of care during pregnancy, while others experience barriers (Singhal et al., 2014).
Klamath County, Oregon conducted a demonstration to promote attendance (Milgrom et al., 2008). Women received counseling by a dental hygienist through the health department, who also functioned as a patient navigator. Almost 56% visited the dentist compared to less than 9% statewide. Interviews confirmed the centrality of the navigator function (Le et al., 2009). There was a reduction in tooth decay among the children (Milgrom et al., 2010). Grembowski and colleagues (2008), in Washington, showed child utilization is higher when mothers have a regular source of care and mothers reported their children were healthier.
Barriers also exist to care for children (Garg et al., 2013) and programs have been developed to increase attendance and impact the knowledge and attitudes of dentists (Grembowski & Milgrom, 2000). Investigators have attempted counseling interventions to encourage parents to begin care for their children by age one (Lee et al., 2004), establish a dental home (Brickhouse et al., 2013), and reduce childhood dental disease (Ramos-Gomez et al., 2012). One such counseling approach, motivational interviewing (MI), is a client-centered but directive counseling strategy. A brief MI approach (BMI), designed for use in primary care settings, has shown promise in prenatal care (Bogaerts et al., 2013) and within dental settings (Weinstein et al., 2006; Harrison et al., 2012; Broughton et al., 2013). Weinstein and others (2006) randomized 240 parents of six to18 month infants to a BMI intervention or HE. BMI took 45 minutes, included six follow-up calls and two postcards over a year, and focused on home oral hygiene and diet. After two years there was a 46% reduction in tooth decay in the BMI group. Although BMI resulted in reduced tooth decay, there were limitations; number and length of contacts differed between conditions, and MI delivery fidelity was not monitored. The active part of the intervention may have been the increased contact with the interventionist, whose role was similar to a patient navigator, rather than the intervention’s content. This is consistent with the observed difference in the number of fluoride varnish applications between the groups and no difference among any of the measures of oral health behaviors. In another dental MI study, caregivers of African-American preschool children were randomized to one of two groups – MI plus oral health education DVD vs. DVD alone (Ismail et al., 2011). In contrast to the previous study, fidelity of MI interventionists was assessed and found to be weak, and the intervention had limited effectiveness on controlling dental disease.
To build on the successful community intervention in Oregon (Milgrom et al., 2008; Milgrom et al., 2010), address the aforementioned studies’ limitations (e.g., behavioral intervention and counselor fidelity), and examine a maternal child health approach to reducing tooth decay, “Baby Smiles” tested a two-phase brief motivational counseling intervention to increase dental attendance among rural low-income women and their children. Additionally, a cost minimization analysis was conducted to compare the cost-effectiveness of the brief motivational intervention to health education to understand the cost implications of implementing this approach.
Study Hypotheses
The study had three primary hypotheses: (1) women in prenatal MI would have a greater frequency of utilization than women in prenatal HE, (2) children of women in postpartum MI would have a greater frequency of preventive care by 18 months than children of women in postpartum HE, and (3) children of women in prenatal-postpartum MI would have greater preventive utilization than children of women in prenatal HE-postpartum MI. In this last comparison, we examined if providing mothers with MI for utilizing care for themselves would have a carryover effect and strengthen motivation to utilize care for their children. Eighteen months was chosen as the cut-off to evaluate if mothers met the recommendation to establish care for their child.
The study had secondary hypotheses; (1) as baseline readiness to change increases, the effects of MI on prenatal attendance and children’s attendance for preventive care during their first 18 months of life will increase [Note: readiness baseline for prenatal attendance assessed prior to prenatal intervention and readiness baseline for children’s attendance assessed prior to postpartum intervention], and (2) number of preventive home practices taken by mothers to prevent tooth decay in their children at 18 months postpartum will be greater among women in postpartum MI compared to HE. Exploration of the effects of other moderators (e.g., maternal depression, dental anxiety, oral health impact, and perceived stress) was assessed without a priori hypotheses.
Methods
Four hundred pregnant women living in four rural Oregon counties and eligible for Medicaid (Oregon Health Plan; OHP) were randomly allocated to one of four groups: (1) prenatal MI followed by postnatal MI, (2) prenatal MI followed by postnatal HE, (3) prenatal HE followed by postnatal MI, and (4) prenatal HE followed by postnatal HE. Descriptions of the design and intervention’s fidelity monitoring have been published (Milgrom et al., 2013; Weinstein et al., 2014).
Participants and Setting
Participation was limited to English-speaking women ≥15 years old in their first or second trimester who were eligible for Medicaid, and who planned to reside with their infants for the entire study. Study sites were four rural counties in Oregon (Douglas, Jefferson, Josephine, and Lincoln). Participants were clients at public health departments and were recruited by department staff and enrolled by Baby Smiles counselors. There was one counselor per county with the exception of Lincoln, which had a counselor at each of two sites.
University of Washington and Oregon State Public Health Division/Multnomah County Public Health IRBs approved the study. Participants gave informed consent.
Efforts were made to increase awareness and knowledge of community dentists and physicians. Materials were distributed and visits were made to local dental and medical groups. Managed dental care programs centralized appointments and trained personnel to prioritize pregnant women and young children. Community involvement resulted in incorporation of a patient navigator function in the counselors’ roles. As part of the navigator role, counselors, after getting an affirmative response from participants about their desire to visit the dentist, assisted the women with making appointments. Navigator assistance was available for all study conditions. Oregon provided insurance coverage for pregnant women with family incomes below 185% of the Federal Poverty Level. The period of coverage was the duration of pregnancy plus two months. Coverage was available for children from birth.
Interventions
Intervention protocols were scripted to ensure consistency. At the end of the intervention session, counselors assisted both MI and HE participants who wanted a dental appointment.
MI Interventions
Both the prenatal and postpartum MI interventions included written protocols and video-recorded “real life” examples to guide discussion and assure fidelity. MI interventions also included a fillable written plan for participants to take home after the session. As part of the intervention, follow-up phone calls were made to check-in on participants’ plans, report unanticipated problems, and inquire about readiness to make a dental appointment if not previously made.
Prenatal phase
The intervention was delivered in-person by the counselor immediately after enrollment and baseline measures assessment. Counselors focused on barriers to care during pregnancy and identified participants’ dental needs, dental risks, reinforced needs, and navigated barriers to care. Written prenatal educational materials from the National Maternal Child Oral Health Resource Center (NMCOHRC) included Two Healthy Smiles: Tips to Keep You and Your Baby Healthy. Women also received information about dental coverage, guidelines to being a successful patient, and a “prescription” for oral health. Counselors made follow-up phone calls four and six weeks following the intervention.
Postpartum phase
The postpartum intervention was delivered in-person, approximately nine months after the birth except for 33 cases (10.6%) where the postpartum intervention was by phone. Counselors identified participants’ needs for their child’s dental health, the dental risks for their children, reinforced their needs, and navigated barriers to professional care and personal oral hygiene. They presented a menu of preventive strategies such as oral hygiene and dietary practices and the age 1 dental visit from which the mother could choose and showed a brief video on parenting behaviors and professional services for preventing tooth decay. Written postpartum health education materials included NMCOHRC materials (Two Healthy Smiles: Tips to Keep You and Your Baby Healthy; A Healthy Smile for Your Child: Tips to Keep Your Child Healthy; Topical Fluoride Recommendations for High Risk Children Under Age 6 Years), “prescription” for oral health, and a ‘first dental visit’ card. Counselors made a follow-up call six weeks after intervention delivery.
HE Interventions
Educational materials provided in the prenatal and postpartum HE conditions were the same as the MI interventions. Intervention delivery mode and timing were the same for HE groups as MI groups. In contrast to MI, HE protocols were short with simple instructions to provide automated educational information followed by asking participants about their readiness to make a dental appointment. In the prenatal HE group, a computerized slide show presenting the NMCOHRC messages was played. In the postpartum HE group, participants were shown the same video as the postpartum MI group, but without any discussion of the information. In contrast to MI interventions, HE interventions’ follow-up phone calls only asked about participants’ reports of unanticipated problems and their readiness to make a dental appointment if not previously made.
Schedule and Study Measures
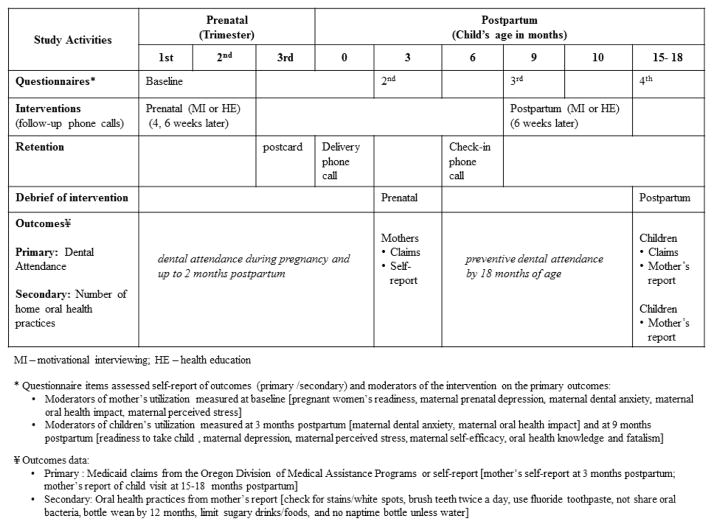
Figure 1 depicts study events and measures. Baseline measures were collected prior to delivery of the intervention. The prenatal and postpartum interventions’ follow-up assessments, three months post-baseline and approximately 15–18 month postpartum also included a debriefing survey about the MI and HE conditions. Participants received incentives throughout the study in the form of gift cards (up to $80), a baby gift, and toothpaste/toothbrushes for mothers/children. Study measures included mother’s readiness to visit the dentist (scoring range 0–10, where higher scores indicate greater readiness; Coolidge et al., 2011), prenatal depression (Center for Epidemiologic Studies Depression Scale [CES-D]; scoring range 0–60, where higher scores indicate more depressive symptoms; Radloff, 1977), maternal perceived stress (Perceived Stress Scale [PSS]: scoring range 0–40, where higher scores indicate greater stress; Cohen & Williamson, 1988), maternal oral health impact (Oral Health Impact Profile short form [OHIP]: scoring range 0–56, where higher scores indicate lower oral health quality of life; de Oliveira & Nadanovsky, 2006), and maternal dental anxiety questions [M-DAS]: scoring range 5–25, where higher scores indicate greater dental anxiety; Humphris et al., 1995). Post-partum: readiness to take child to the dentist, maternal dental anxiety, maternal depression, maternal perceived stress, maternal self-efficacy, maternal oral health knowledge, and fatalism (Adair et al., 2004; Finlayson et al., 2005).
Figure 1.
Overview of the Baby Smiles study design and measures.
Primary Outcome Measure – Dental Attendance
Primary outcome measures were dental attendance during pregnancy and up to two months postpartum, and preventive dental attendance by 18 months of age for the child. These outcomes were ascertained using Medicaid claims obtained from the Oregon Division of Medical Assistance Programs (DMAP). Data included all claims from May 1, 2010 to October 31, 2013 for the primary outcome measures (any dental service by pregnant/postpartum women and preventive dental services by children) defined by Current Dental Terminology codes (CDT codes - 100s, 1000s; routine and problem-based dental exams, cleanings, and fluoride treatments). This range allowed us to have a record of utilization for most children by 18 months of age. DMAP merged the list of participants and their ID numbers with their Medicaid Claims. Data were de-identified. Because some women had coverage in addition to Medicaid or could have paid out of pocket, we included self-reports in the study questionnaires. Based on the responses, we added 12 visits made during pregnancy (by women in MI). We added 30 child visits (18 - postpartum MI; 12 - postpartum HE). Visits were confirmed by counselors. Moderators for the intervention effect on the primary outcomes were also assessed by questionnaire. Potential moderators of maternal utilization were measured at baseline: readiness to visit the dentist, prenatal depression, perceived stress, oral health impact, and dental anxiety. Moderators of children’s utilization were measured at three months (dental anxiety and oral health impact) and nine months (readiness to take child to the dentist, postnatal depression, perceived stress, self-efficacy, oral health knowledge, and fatalism).
Secondary Outcome Measures – Oral Health Practices
The secondary outcome measures, number of home oral health practices taken by mothers to prevent tooth decay in their children, were assessed at study end (15–18 months after child’s birth) by questionnaire. These practices included checking teeth for stains/white spots, twice daily tooth brushing, using fluoride toothpaste, not sharing oral bacteria (cleaning pacifiers with mouth or sharing utensils), bottle weaning by 12 months, limiting sweetened drinks and sugary foods to less than twice a day, and not using a bottle during nap time unless filled with water.
Sample Size Determination
Sample sizes were determined to provide adequate power to test three a priori hypotheses. Sample sizes were calculated based on chi-square test using a two-sided significance level of 0.016 to account for the three primary comparisons. Given the primary outcome, dental utilization, would be obtained from Medicaid on all subjects except those that explicitly refused participation, we assumed a 10% rate of attrition by study end. Unequal group sizes were used because a greater number of subjects were required for the two intervention groups receiving postpartum MI to test the third hypothesis. Milgrom et al (2008) found only 8.8% of pregnant women served by Medicaid in Oregon received dental care. To account for a potential effect of HE, we assumed a higher rate of dental utilization during pregnancy, 15 to 25%, in the HE prenatal groups. Based on a sample size of 180 women in the prenatal MI and HE conditions, power was ≥85% to demonstrate a twofold or greater increase in utilization for the prenatal MI (Hypothesis 1). In a National Survey of Children’s Health (Hale, 2003) the use of preventive dental care by children during the first 18 months was 10%. In a study of the Washington’s State Access to Baby and Child Dentistry program only 12% of Medicaid-enrolled children had any utilization during 24 and 48 months of age and only 2.4% of children had utilization during 12 to 24 months of age (Milgrom et al., 1997; Grembowski & Milgrom, 2000). To account for a potential effect of HE, we assumed a higher rate of preventive dental utilization, 10 to 20%, during the first 18 months of life for children of mothers in the postpartum HE condition. Based on sample size of 266 in the postpartum MI condition and 94 in the HE condition, power was determined be ≥95% to demonstrate a 2.5 to three-fold increase in preventive utilization for children of mothers in postpartum MI (Hypothesis 2). For the third comparison, based on a sample size of 133 in each of the postpartum groups (HE-MI and MI-MI), power was ≥80% to demonstrate a 1.5–2 fold increase in preventive utilization for children of mothers who receive the prenatal and postpartum MI as compared to children of mothers who only received postpartum MI, assuming rates of 20% to 40% (Hypothesis 3).
Randomization
Participants were randomly assigned at the prenatal baseline visit to one of four intervention groups. Randomization was stratified on counselor/county health department to ensure the study groups would be balanced within each counselor. Blocking, with block sizes of four or eight were used to ensure the groups would be balanced across the study period. To prevent determination of the intervention assignment, which could be possible with fixed block sizes, computer-generated permuted blocks of size four or eight, were randomly chosen with ¾ and ¼ probability (Efird, 2011). The randomization list was prepared using the “sample” function of the R statistical software (R Foundation for Statistical Computing, Vienna, Austria, V. 2.5.1).
Allocation and Blinding
After consent, the counselor opened the sealed and sequentially numbered study packet containing the assigned prenatal intervention, and then prior to the nine month postpartum intervention the study data manager emailed the counselor the intervention. Participants were not informed of intervention assignments.
Statistical Methods
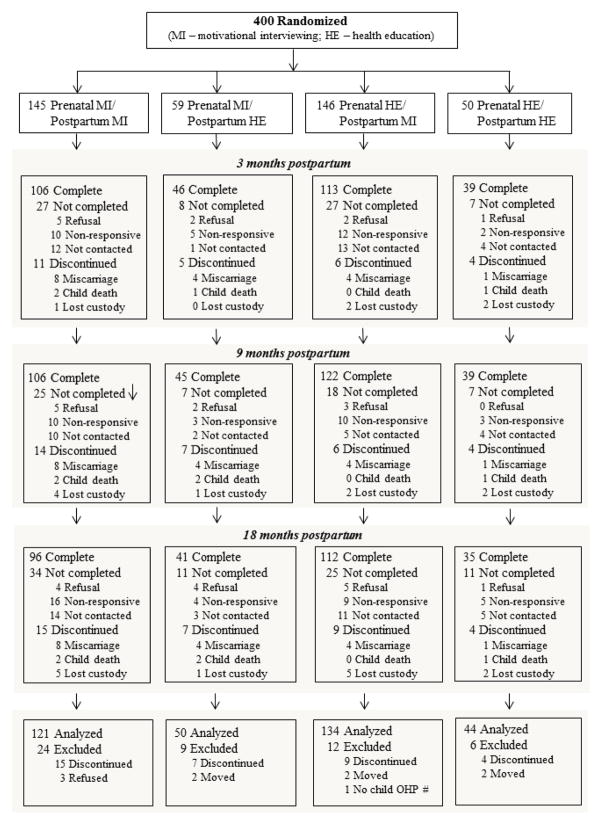
For the analysis of the primary outcomes all women and children were included in their assigned group regardless of how many of the MI or HE activities completed. Exceptions were that children’s receipt of preventive care was limited to only live-born and resident children (Figure 2). Sixteen subjects were excluded because of withdrawal, moving, or missing Medicaid ID (Figure 2). Primary outcomes were compared according to the three a priori hypotheses (prenatal MI versus HE for mother utilization, and for child preventive utilization postpartum MI versus HE, and prenatal-postpartum MI-MI versus HE-MI). In addition, child preventive utilization was compared for prenatal-postpartum MI-MI, HE-MI, and MI-HE versus HE-HE. Log-linear regression analyses were used for these group comparisons (Spiegelman & Hertzmark, 2005), and all regression models adjusted for potential counselor/county health department differences by inclusion of indicator variables for counselor in the regression model. Log-linear regression analysis was used to assess for the a priori (readiness) and other exploratory moderation effects on mother utilization and child preventive utilization by assessing for an interaction between the hypothesized moderators and intervention group. The same group comparisons used for the primary outcomes were used to compare the intervention group on the secondary outcomes at 15 to 18 months postpartum. Analyses were based only on mothers with questionnaire data at 15 to 18 months postpartum (280/349).
Figure 2.
Flow diagram of the Baby Smiles participant recruitment, randomization, study design and final disposition (e.g., completion of study questionnaires and/or debrief at specific study events) of participating mothers.
Comparison of Study Outcomes with Statewide Data
Statewide self-report data were obtained from PRAMS surveys (PRAMS for 2010 births; PRAMS for 2007 births; and PRAMS-2 for 2007 births) to compare dental attendance trends to Baby Smiles participants and their children to pregnant women and their children in the four counties. These data were also sought to compare dental attendance of our study participants with that of low-income Oregon mothers generally. To create this comparison, we included any PRAMS respondent who reported “Oregon Health Plan or Medicaid” as their prenatal care insurance in response to the question “Did any of these insurance plans help you pay for prenatal care? (Check all that apply).” Mother’s use of dental services was coded as “yes” if she answered “Yes, I went to a dentist or dental clinic” in response to the PRAMS question, “This question is about the care of your teeth during your most recent pregnancy.” Children’s utilization was coded as “yes” if the mother answered yes to the PRAMS-2 question: “Has your two-year old ever been to a dentist or dental clinic?” Rates of dental utilization for pregnant women and their children were computed along with 95% confidence intervals (CI). No hypothesis tests were performed given PRAMS subjects were recruited using different methods (i.e., randomly sampled subjects rather than study volunteers) and the outcomes were assessed differently. Rates and confidence intervals were computed using methods that accounted for the complex sampling design of the PRAMS data [Proc Surveyfreq and Surveylogistic; SAS software (Version 9.3; SAS Institute, Cary, NC)].
Cost Minimization Analysis
Costs were estimated using the local public health agency and societal perspectives because this research is relevant to public health agencies and practitioners wishing to understand the cost implications of an MI vs. HE intervention to increase dental attendance at the county level (Weinstein et al., 1996; Van Keulen et al., 2010). The societal perspective, a standard in cost effectiveness analysis, includes all the resources used in the intervention whereas the public health agency perspective includes only the costs attributed to the public health agency. Resources were grouped into set-up (fixed) costs and variable costs (Ruger et al., 2009). Research-driven costs, such as gathering data as part of the clinical trial, were excluded. Data sources were clinical trial and contract records for the intervention counties.
Total set-up costs include training costs and community outreach. The trainer was assumed to be a local health education specialist and the community involvement champion would be a local dentist. The salary and benefits for these personnel were estimated using Bureau of Labor Occupational Employment Statistics and previous research (Bureau of Labor Statistics. Occupational Employment Statistics. 2014. Available at: http://www.bls.gov/oes/home.htm; Ruger et al., 2009). Training-related travel costs for the trainer and counselors were estimated using the federal per diem and reimbursement rates published by the US General Services Administration for that year.
Variable costs, from an agency perspective, included counselor and supervisor time costs and intervention resources. From a societal perspective, variable costs also included counselor, supervisor, and participant time costs and intervention resources. Participant salaries and benefits were estimated using Oregon hourly minimum wage rates for each year (US Department of Labor: Wage and Hour Divison. Minimum Wage Laws in the States. 2014. Available at: http://www.dol.gov/whd/minwage/america.htm). Amount of time spent on the intervention was collected prospectively in participants’ sessions, which recorded start and end times for each participant across intervention conditions. The loaded hourly pay of counselors and supervisors was obtained as noted earlier. Because of participants’ random assignment to the conditions, we assumed there were no differences in average patient costs (i.e., lost wage and leisure time, travel time, appointment waiting time) between groups.
Discounting was applied such that costs were represented in 2012 dollars because 2012 was the final project year. Discounted costs were estimated by multiplying the total costs each year by the percent inflation between that year and 2012. Inflation percentages were based on the current US inflation rates established by the Consumer Price Index.
Results
Recruitment and enrollment occurred between May 6, 2010 and August 2, 2011. The first prenatal follow-up began June 15, 2010. The first study end follow-up call was January 10, 2012 and the last study end follow-up occurred May 20, 2013. Figure 2 shows the flow and final disposition of participants. Fifty-one (12.7%) participants were excluded from the data analysis; most of the exclusions were due to discontinuations due to loss of the fetus/child either through miscarriage (n = 17), child death (n = 5) and loss of custody (n = 13), and other exclusions were due to requesting to be withdrawn from the study (n = 3), moving out of state (n = 12) and missing the child’s Medicaid ID (n = 1). Baseline data provided by mothers excluded from the analysis of study outcomes, compared to mothers included in the analysis, reported higher levels of perceived stress (mean ± SD = 17 ± 7 vs. 14 ± 7; p=0.01), more symptoms of depression (18 ± 11 vs. 14 ± 9; p=0.04), higher oral health impact scores (13 ± 14 vs. 10 ± 10; p=0.11), and were more likely to have two or more children (30% vs. 19%; p=0.11).
Table 1 shows baseline characteristics for Baby Smiles participants. The mean age was 24 years of age (range = 15–41). Most participants were White (79%), not Hispanic (91%), and married or living with a partner (60%). Almost half of the participants (49%) were first-time mothers, and nearly 40% had some college or vocational training beyond high school. With regard to previous dental attendance, at baseline, one-third had not visited a dentist within the last two years and 23% had done so for a dental emergency. Over 90% of the women were either in the preparation (36%) or action (56%) phase of the readiness ladder for obtaining dental care. Baby Smiles participants appeared to have low dental anxiety (only 14% with an anxiety score ≥19), low perceived stress (mean score = 14), and relatively high oral health quality of life (mean score = 10). For perceived stress, participants had a lower mean score compared to a US national sample of female respondents (mean score = 20.2; Cohen & Williamson, 1988). Furthermore, Baby Smiles participants had few symptoms of prenatal depression: One-third (35%) scored 16 points or higher on the CES-D, where scores of 16 to 26 indicate mild depression (Zich et al., 1990). Groups were comparable across baseline characteristics, except prenatal HE groups had a greater proportion of mothers with a CES-D score >16 (40 to 42% vs. 28 to 30%; p=0.04) and prenatal MI groups had on average higher scores on the M-DAS (12.6 to 13.2 vs. 11.6 to 11.9; p=0.05).
Table 1.
Baseline characteristics of Baby Smiles participants: Based on the entire sample and by prenatal and postpartum motivational interviewing and/or health education intervention groups.
| Characteristics a | All (N = 349) | Intervention Groups
|
|||
|---|---|---|---|---|---|
| MI-MI (n = 121) | HE-MI (n = 134) | MI-HE (n = 50) | HE-HE (n = 44) | ||
| Age (years) at enrollment | 24.2 ± 4.9 | 24.1 ± 4.8 | 24.3 ± 5.1 | 24.3 ± 5.1 | 24.3 ± 4.5 |
| Race (White) | 79.0 (275) | 78.5 (95) | 76.7 (102) | 82.0 (41) | 84.1 (37) |
| Ethnicity (Hispanic) | 8.9 (31) | 10.7 (13) | 8.3 (11) | 8.0 (4) | 6.8 (3) |
| Number of children | |||||
| 0 | 48.7 (170) | 47.1 (57) | 48.5 (65) | 50.0 (25) | 52.3 (23) |
| 1 | 32.7 (114) | 30.6 (37) | 35.1 (47) | 36.0 (18) | 27.3 (12) |
| ≥2 | 18.6 (65) | 22.3 (27) | 16.4 (22) | 14.0 (7) | 20.5 (9) |
| Education level (highest) | |||||
| High school diploma or less | 59.6 (208) | 59.5 (72) | 59.7 (80) | 76. 0 (38) | 40.9 (18) |
| Trade/vocational training, some college, 2-year degree | 37.5 (131) | 38. 0 (46) | 37.3 (50) | 22.0 (11) | 54.5 (24) |
| 4-year degree or higher | 2.9 (10) | 2.5 (3) | 3.0 (4) | 2.0 (1) | 4.5 (2) |
| Marital status | |||||
| Married or living with partner | 60.2 (210) | 55.4 (67) | 64.2 (86) | 54. 0 (27) | 68.2 (30) |
| Single/never married | 30.4 (106) | 29.8 (36) | 28.4 (38) | 42.0 (21) | 25. 0 (11) |
| Separated, divorced, or other | 9.5 (33) | 14.9 (18) | 7.5 (10) | 4.0 (2) | 6.8 (3) |
| Last dentist visit | |||||
| <6 months ago | 24.1 (84) | 23.1 (28) | 24.6 (33) | 32.0 (16) | 15.9 (7) |
| 6–12 months ago | 15.2 (53) | 14.9 (18) | 24.6 (33) | 12.0 (6) | 18.2 (8) |
| 1–2 years ago | 26.9 (94) | 28.9 (35) | 15.7 (21) | 14.0 (7) | 27.3 (12) |
| >2 years ago | 33.8 (118) | 33.1 (40) | 29.9 (40) | 42.0 (21) | 38.6 (17) |
| Reason for last dental visit | |||||
| Emergency | 22.5 (78) | 25.0 (30) | 15.7 (21) | 26.5 (13) | 32.6 (14) |
| Check-up and cleaning | 46.5 (161) | 48.3 (58) | 48.5 (65) | 42.9 (21) | 39.5 (17) |
| Scheduled treatment | 30.9 (107) | 26.7 (32) | 35.8 (48) | 30.6 (15) | 27.9 (12) |
| Study Measures b | |||||
| Maternal dental anxiety c (0–56) | 12.2 ± 5.1 | 12.6 ± 5.5 | 11.6 ± 4.6 | 13.2 ± 5.6 | 11.9 ± 5.1 |
| M-DAS score ≥19 | 14.3 (50) | 14.9 (18) | 9.7 (13) | 24.0 (12) | 15.9 (7) |
| Readiness to see dentist d (0–10) | 9.1 ± 1.3 | 9.0 ± 1.4 | 9.3 ± 1.1 | 8.9 ± 1.8 | 9.1 ± 1.3 |
| Precontemplative (0–1) | 0.0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Contemplative (2–7) | 8.3 (29) | 10.7 (13) | 5.2 (7) | 10.0 (5) | 9.1 (4) |
| Preparation (8–9) | 35.5 (124) | 35.5 (43) | 35.8 (48) | 36.0 (18) | 34.1 (15) |
| Action (10) | 56.2 (196) | 53.7 (65) | 59.0 (79) | 54.0 (27) | 56.8 (25) |
| Maternal OHIP-14 e (0–56) | 10.3 ± 10.4 | 10.7 ±11.1 | 10.0 ± 9.8 | 10.0 ± 9.7 | 10.3 ±11.1 |
| Maternal PSS f (0–40) | 14.4 ± 6.8 | 14.9 ± 7.6 | 14.4 ± 6.7 | 13.4 ± 5.8 | 14.4 ± 6.1 |
| Prenatal depression g (0–60) | 14.2 ± 8.9 | 14.1 ± 9.2 | 15.0 ± 8.7 | 11.9 ± 7.1 | 15.2 ± 9.9 |
| CES-D score ≥16 | 35.1 (120) | 30.3 (36) | 40.0 (52) | 28.0 (14) | 41.9 (52) |
Note. Baseline characteristics are summarized as Mean ± standard deviation, or the percentage and number of participants in the intervention groups, % (n).
Abbr. CES-D=Center for epidemiologic studies depression scale. HE=Health education. HE-HE=Prenatal and postpartum HE. HE-MI=Prenatal HE and postpartum MI. M-DAS=Maternal dental anxiety scale. MI=Motivational interviewing. MI-HE=Prenatal MI and postpartum HE. MI-MI=Prenatal and postpartum MI. PSS=Perceived stress scale. OHIP-14=Oral health impact profile-14, short form.
Variables with missing data: Race (n=1); Ethnicity (n=1); Reason for last dental visit (n=3); OHIP-14 (n=2); Perceived stress scale (n=3); and CES-D scale (n=7).
For select study measures, the range in total possible scores is provided in parentheses.
Maternal dental anxiety: assessed using M-DAS; higher scores indicate greater dental anxiety; ≥19 indicate the lower threshold for mild anxiety.
Readiness to see dentist: Scoring values correspond to different stages of readiness; higher score indicates a greater readiness to see dentist.
Maternal OHIP-14: higher scores indicate lower oral health quality of life.
Maternal PPS: higher scores indicate greater perceived stress.
Prenatal depression: assessed using CES-D; higher scores indicate greater depressive symptoms; ≥16 indicate the lower threshold for mild depression.
Maternal and Child Dental Attendance – Primary Outcomes
Table 2 shows the results for the Baby Smiles’ study primary outcome measures on dental attendance from both DMAP and questionnaire self-report data for three primary comparisons; (1) maternal dental attendance between the prenatal MI and HE groups, and (2) children’s dental attendance between the postpartum MI and HE groups and (3) children’s dental attendance between the MI-MI and the HE-MI groups. There were no significant differences between the MI and HE groups for the mothers’ prenatal attendance (RR=0.98; 95% CI = 0.93–1.04) nor for children’s attendance in the remaining two comparisons: postpartum MI vs. HE (RR=1.03; 95% CI = 0.82–1.28) or MI-MI vs. HE-MI groups (RR=1.05; 95% CI = 0.80–1.36). Mothers’ dental attendance in the prenatal period was very high (prenatal MI vs. HE: 92% vs. 94%) while children’s dental attendance was almost half that of maternal attendance for both comparisons (postpartum MI vs. HE: 54% vs. 52%; MI-MI vs. HE-MI: 52% vs. 55%). Neither maternal prenatal attendance nor children’s attendance between the MI and HE varied by counselor (p>0.20). Adjusting for baseline differences did not change results (e.g., MI vs. HE for maternal prenatal attendance [adjusted RR = 0.98; 95% CI = 0.92–1.03] and children’s attendance [adjusted RR = 0.97; 95% CI = 0.80–1.17]).
Table 2.
Maternal and child dental attendance based on Medicaid claims using the Oregon Division of Medical Assistance Program and self-report by the mother.
| Total N | DMAP a
|
DMAP + Self report b
|
|||||
|---|---|---|---|---|---|---|---|
| % (n) | RR | 95% CIs | % (n) | RR | 95% CIs | ||
|
|
|
||||||
| Maternal Attendance c | |||||||
| Prenatal Intervention | |||||||
| MI Group | 171 | 85.4 (146) | 0.91 | 0.83, 0.98 | 92.4 (158) | 0.98 | 0.93, 1.04 |
| HE Group | 178 | 94.4 (168) | 1.00 | Referent | 94.4 (168) | 1.00 | Referent |
| Child Attendance d | |||||||
| Postpartum Intervention | |||||||
| MI Group | 255 | 46.7 (119) | 1.19 | 0.90, 1.58 | 53.7 (137) | 1.03 | 0.82, 1.28 |
| HE Group | 94 | 39.4 (37) | 1.00 | Referent | 52.1 (49) | 1.00 | Referent |
| Prenatal-Postpartum Intervention Groups e | |||||||
| MI-MI | 94 | 47.1 (57) | 1.30 | 0.84, 2.00 | 52.1 (63) | 1.10 | 0.77, 1.56 |
| HE-MI | 134 | 46.3 (62) | 1.24 | 0.80, 1.89 | 55.2 (74) | 1.16 | 0.78, 1.72 |
| MI-HE | 50 | 42.0 (21) | 1.12 | 0.67, 1.86 | 56.0 (28) | 1.13 | 0.80, 1.58 |
| HE-HE | 44 | 36.4 (16) | 1.00 | Referent | 47.7 (21) | 1.00 | Referent |
Note. Attendance outcomes are summarized as Relative risk ratios (RR), adjusted for counselor, accompanied by their 95% confidence intervals (CIs).
Abbr. HE=Health education. HE-HE=Prenatal and postpartum HE. HE-MI=Prenatal HE and postpartum MI. MI=Motivational interviewing. MI-HE=Prenatal MI and postpartum HE. MI-MI=Prenatal and postpartum MI. N=Total number of participants in each group. n=number of participants.
Maternal and child dental attendance:
DMAP: based only on Medicaid claims;
DMAP + Self-report: based on Medicaid claims and mother self-report.
Maternal attendance: Includes any dental care utilization that occurred during pregnancy and includes 2 months postpartum.
Child attendance: Includes any non-restorative or preventive (i.e., “non-emergent”) dental utilization care.
Other comparison between MI-MI vs. HE-MI for DMAP: RR = 1.05, 95% CIs: 0.80, 1.36; DMAP + Self report: RR = 0.97, 95% CIs: 0.77, 1.22.
Additional results (median [interquartile range, IQR]) based on DMAP data (excluding self-report information) included a similar number of maternal dental visits for the prenatal MI and HE groups (number of visits = 2 [2–4] vs. 2 [2–3]; Mann-Whitney, p=0.08), dental attendance by children at 12 months of age (28% vs. 23%, p=0.36), and the age of the child at their first dental visit between 6 to 18 months (12.6 [12–14]). Moreover, for the women who had a maternal dental visit, 86.6% received diagnostic, 83.1% imaging, and 80.6% preventive services, while 52.5% had routine restoration, 24.5% extractions, 11.1% complex restoration, 7.6% periodontal procedures, or 6.7% endodontic procedures.
Preventive Oral Health Practices – Secondary Outcomes
Preventive practices for children were assessed at the study end (15–18 month postpartum, N=280) and included: checking for white spot lesions by lifting the lip, tooth brushing frequency, use of fluoridated toothpaste, transmission of cavity-causing bacteria through sharing utensils, pacifiers and pre-chewing food, limiting sweet snacks and foods to less than two times per day, not using a bottle at naptime, and weaning from the bottle at 12 months. Log-linear regression analyses revealed that there were no differences in preventive oral health practices of mothers in the MI and HE groups and no difference between the MI-MI and HE-MI groups. The majority (>85%) of mothers checked their children’s teeth for stains or white spot lesions regardless of study group. Overall, 62% of mothers reported brushing their children’s teeth two or more times per day and 25% reported brushing once per day. In addition, more than half engaged in preventive activities for their children, with the exception of bottle weaning by 12 months, and for most groups, less than 50% of mothers weaned by one year.
Moderators of Utilization
Moderation analyses were conducted for readiness at baseline for mothers’ utilization and at nine months for children’s utilization. Exploratory moderation analyses were conducted for depression, dental anxiety, oral health impact and perceived stress measured at baseline for mothers’ utilization. For children’s utilization, exploratory moderation analyses were conducted for depression, perceived stress, oral health fatalism, oral health self-efficacy, bottle use knowledge and child oral hygiene (measured at nine months postpartum), and dental anxiety and oral health impact (measured three months postpartum). There were no significant effects of the a priori or exploratory moderating variables tested.
Cost Minimization Analysis
Table 3 shows total costs and costs per participant for each condition and for Baby Smiles overall. Variable costs comprised a higher proportion of total project costs. The MI-MI group had the highest costs, and HE-HE had the lowest costs. The difference in costs to a public health agency to implement a similar brief MI intervention versus a HE intervention was $170.58 per participant ($2012 dollars). Table 3 also shows the total costs and participant costs using a societal perspective. The difference in costs between MI-MI and HE-HE using the societal perspective was $172.29.
Table 3.
Project-related costs for Baby Smiles (in 2012): Overall costs and costs per participant from the public health agency and societal perspectives grouped by prenatal and postpartum interventions.
| Project-related costs (in dollars) | Prenatal-Postpartum Intervention Groups
|
Total for Project | |||
|---|---|---|---|---|---|
| MI-MI (N = 145) | MI-HE (N = 59) | HE-MI (N = 146) | HE-HE (N = 50) | ||
|
|
|
|
|||
| Public Health Agency Perspective | |||||
| Total set-up cost for project | $13,700.00 | $3,797.00 | $5,794.00 | $476.00 | $23,757.00 |
| Set-up cost per participant | $94.48 | $64.36 | $39.68 | $9.52 | $59.39 |
| Total variable cost for project | $37,767.15 | $13,021.42 | $28,951.63 | $8,867.11 | $88,607.31 |
| Variable cost per participant | $266.92 | $232.70 | $213.09 | $181.30 | $221.52 |
| Total project costs per participant | $361.40 | $297.05 | $252.78 | $190.82 | $280.91 |
| Societal Perspective | |||||
| Total set-up cost for project | $13,700.00 | $3,797.00 | $5,794.00 | $476.00 | $23,757.00 |
| Set-up cost per participant | $94.48 | $64.36 | $39.68 | $9.52 | $59.39 |
| Total variable cost for project | $39,621.94 | $13,706.79 | $30,610.29 | $9,408.36 | $93,347.37 |
| Variable cost per participant | $279.42 | $244.11 | $224.52 | $192.09 | $233.37 |
| Total project costs per participant | $373.90 | $308.46 | $264.21 | $201.61 | $292.76 |
Note. HE=Health education. HE-HE=Prenatal and postpartum HE. HE-MI=Prenatal HE and postpartum MI. MI=Motivational interviewing. MI-HE=Prenatal MI and postpartum HE. MI-MI=Prenatal and postpartum MI. N=Total number of participants in each group. n=number of participants.
Visiting the dentist– Comparison with PRAMS
Attendance during pregnancy and early childhood was assessed within PRAMS to determine if Baby Smiles participants had greater attendance. Attendance data revealed 45.1% (95% CI = 40.2–50.0) of pregnant women on OHP visited the dentist statewide and 60.5% (95% CI = 42.6–78.3) for the four study counties. Attendance data for children on Medicaid showed 35.6% (95% CI = 27.3–43.9%) visited the dentist statewide. Medicaid children’s utilization among the four study counties was much lower (8.1%; 95% CI = 1.5–14.8%), but the number of survey respondents from the four counties was small.
Discussion
This study was conducted in a setting with community workers as interventionists and standardized protocols. Outcomes were drawn from publicly available claims data. Thus, confidence is increased that the results are valid and potentially generalizable to other communities in which low-income women and children have insurance and access to care.
“Baby Smiles” asked whether women who received a brief MI intervention during the prenatal and/or postpartum periods would be more likely to seek services for themselves or their children compared to women who received health education without interpersonal support. We found no difference between the intervention conditions in terms of mothers’ or children’s dental attendance. However, baseline assessments showed that the majority of participants were ready to visit the dentist. Additionally, explanation of the findings may lay in the patient navigator function in all conditions. Mostly, Baby Smiles participants were an educated and highly functioning group of women motivated to seek care. Characteristics expected in a low-income population–dental anxiety, perceived stress, poor oral health quality of life–were low. Thus with guidance in making and keeping dental appointments rates of attendance were high. This is consistent with findings of Le and colleagues (2009).
Given lack of group differences in prenatal attendance, we examined whether study participation itself led to increased attendance by comparing the state-specific PRAMS variables related to visiting a dentist during pregnancy and childhood. PRAMS results for 2010 births revealed almost half of low-income women in Oregon and over 60% of low-income women within the four study counties visited the dentist during pregnancy or soon after. While these percentages are higher than reported from a previous study, they are less than those of the Baby Smiles groups. Since the earlier work, there have been efforts (Oral Health Care During Pregnancy Expert Workgroup, 2012) focused on oral health care during pregnancy. Also, as part of the community involvement aspects of this study, extensive efforts were made to reduce barriers to care among providers. It is likely these efforts resulted in more attention to pregnant women’s oral health needs and created “ready” dentists. Furthermore, it is possible that with most of the participants being so close to the top of the ‘readiness ladder’, the effect of brief MI was indistinguishable from HE.
Overall, the percentage of children who visited the dentist was lower than that for mothers. While the first year dental visit is promoted by national groups, there is still work to do to ensure that low-income children are seen.
Similar to the results of the analysis of the primary outcome measures, we found no differences between groups in secondary outcomes. More than half of the mothers, regardless of group, performed most preventive activities. Over 85% reported checking for white spot lesions or staining. Over 60% reported using fluoridated toothpaste when brushing their children’s teeth. Given the cautious “directions for use” of fluoride toothpaste on the toothpaste tubes, it was interesting that mothers complied with recommendations within the health education materials and MI sessions to use fluoridated toothpaste. In addition, mothers appeared to be open to or complied with information on reducing the transmission of cavity-causing bacteria. In examining the questions that made up the transmission of germs construct (pacifier use, sharing utensils, pre-chewing foods), less than 10% reported cleaning the pacifier with their mouths, approximately 80% did not share utensils, and only 1% reported pre-chewing children’s food.
Costs reflect anticipated costs to public health agencies to administer this intervention to increase use of care among rural, low-income women and young children. Ongoing project costs (i.e., variable costs), including personnel, contributed to a greater share of total costs than setup. This suggests that agencies may need to plan for ongoing funding. The MI-MI intervention was most costly; the second most costly was prenatal MI followed by postnatal HE. The cost difference between prenatal MI/postnatal HE and prenatal HE/postnatal MI stems from differences in training. The prenatal MI training was longer than the training provided to administer the post-natal MI although this time was inflated because of the need to document intervention fidelity and hiring counselors with no previous MI experience. Despite need to allocate limited resources, agencies may see the benefit in hiring a counselor to provide HE not solely for providing education but to assist in patient navigation. Participant time valued at minimum hourly wage was the only addition to societal costs making the difference in costs between the agency and societal perspectives very small.
Limitations
This study found no differences between MI and HE for promoting attendance in low-income mothers and their children. MI, although promising in oral health has had varied success. In the current RCT, participants benefited from counselors functioning as patient navigators. Inclusion of the navigator role likely had an impact on participants’ behavior and lessened differences between treatments. Nevertheless, a decision was made that not providing the navigation was neither ethical nor practical in a community trial. Moreover, selection bias is likely present. Women enrolled in this study were volunteers. Study volunteers tend to have various reasons for engaging in research including obtaining ancillary health services. This was evident in the number of women who scored high in readiness to visit the dentist across the groups, likely obfuscating the outcome. And finally, although we believe that our results are generalizable to rural-dwelling, low-income women who have access to dental insurance and services, it is possible that the comparative effects of MI and HE differ in low readiness communities and/or communities where health care providers and dentists have little integration.
In summary, in this community RCT an MI approach did not lead to greater dental attendance among low-income, pregnant women and their children when compared to HE alone. High levels of attendance are likely attributable to their high level of readiness and/or the counselor’s patient navigator function. Compared to the statewide PRAMS, attendance was higher for Baby Smiles study mothers and their children in both MI and HE groups.
Research Highlights.
Dental attendance was similar between the motivational interviewing counseling and health education groups.
Baby Smiles participants’ dental attendance was higher than other Oregon women.
Counselors’ patient navigator role may have impacted dental attendance.
Higher costs were incurred for counseling compared to health education only.
Acknowledgments
Research reported in this publication was supported National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number U54DE019346. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge and thank our community-based research team and community partners who contributed to this effort: Baby Smiles research team; Ruth Converse, Lari Peterson, Linda Stohlman, Bev Cole, Marilyn Carter, Amanda Claxton, Bethany Bickel, Shelley Paeth, Alisha Schade, Silvia Navarro, Patty Barker; the Oregon Dental Care Organizations – Advantage Dental Plans, Capitol Dental Care, and Willamette Dental Group; and our four community health partner organizations. We appreciate the cooperation of the Division of Medical Assistance Programs of the State of Oregon, Oregon Public Health Division and CDC’s Division of Reproductive Health (Pregnancy Risk Assessment Monitoring System-PRAMS). We also acknowledge and thank Dr. Ken Rosenberg for his review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christine A Riedy, Email: Christine_Riedy@hsdm.harvard.edu, Northwest Center to Reduce Oral Health Disparities, Department of Oral Health Sciences, Box 357475, University of Washington, Seattle, WA USA 98195-7475. Harvard School of Dental Medicine, 188 Longwood Ave, Boston, MA 02115, (Present Address).
Philip Weinstein, Email: philw@uw.edu, Northwest Center to Reduce Oral Health Disparities, Department of Oral Health Sciences, Box 357475, University of Washington, Seattle, WA USA 98195-7475.
Lloyd Mancl, Email: llman@uw.edu, Northwest Center to Reduce Oral Health Disparities, Department of Oral Health Sciences, Box 357475, University of Washington, Seattle, WA USA 98195-7475.
Gayle Garson, Email: garson@uw.edu, Northwest Center to Reduce Oral Health Disparities, Department of Oral Health Sciences, Box 357475, University of Washington, Seattle, WA USA 98195-7475.
Colleen E Huebner, Email: colleenh@uw.edu, Northwest Center to Reduce Oral Health Disparities, Department of Health Services, Box 357230, University of Washington, Seattle, WA USA 98195-7230.
Peter Milgrom, Email: dfrc@uw.edu, Northwest Center to Reduce Oral Health Disparities, Department of Oral Health Sciences, Box 357475, University of Washington, Seattle, WA USA 98195-7475.
David Grembowski, Email: grem@uw.edu, University of Washington School of Public Health, Box 357660, 1959 NE Pacific St, Seattle, WA 98195-7660.
Megan Shepherd-Banigan, Email: msb23@uw.edu, University of Washington School of Public Health, Box 357660, 1959 NE Pacific St, Seattle, WA 98195-7660.
Darlene Smolen, Email: darlenesmolen@gmail.com, Northwest Center to Reduce Oral Health Disparities, Department of Oral Health Sciences, Box 357475, University of Washington, Seattle, WA USA 98195-7475.
Marilynn Sutherland, Email: msutherland@co.klamath.or.us, Klamath County Department of Public Health, 305 Main Street, Klamath Falls, OR 97601.
References
- 1.Adair PM, Pine CM, Burnside G, et al. Familial and cultural perceptions and beliefs of oral hygiene and dietary practices among ethnically and socio-economically diverse groups. Community Dental Health. 2004;21(1 Suppl):102–111. [PubMed] [Google Scholar]
- 2.Bogaerts AF, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van Den Bergh BR. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. International Journal of Obesity. 2013;37(6):814–821. doi: 10.1038/ijo.2012.162. [DOI] [PubMed] [Google Scholar]
- 3.Brickhouse TH, Haldiman RR, Evani B. The impact of a home visiting program on children’s utilization of dental services. Pediatrics. 2013;132(Suppl 2):S147–S152. doi: 10.1542/peds.2013-1021N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broughton JR, Maipi JT, Person M, Thomson WM, Morgaine KC, Tiakiwai SJ, Kilgour J, Berryman K, Lawrence HP, Jamieson LM. Reducing disease burden and health inequalities arising from chronic disease among indigenous children: an early childhood caries intervention in Aotearoa/New Zealand. BMC Public Health. 2013;13:1177. doi: 10.1186/1471-2458-13-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park: Sage; 1988. [Google Scholar]
- 6.Coolidge T, Skaret E, Heima M, Johnson EK, Hillstead MB, Farjo N, Asmyhr O, Weinstein P. Thinking about going to the dentist: a contemplation ladder to assess dentally-avoidant individuals’ readiness to go to a dentist. BMC Oral Health. 2011;11:4. doi: 10.1186/1472-6831-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Oliveira BH, Nadanovsky P. The impact of oral pain on quality of life during pregnancy in low-income Brazilian women. Journal of Orofacial Pain. 2006;20(4):297–305. [PubMed] [Google Scholar]
- 8.Efird J. Block randomization with randomly selected block sizes. International Journal of Environmental Research and Public Health. 2011;8(1):15–20. doi: 10.3390/ijerph8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlayson TL, Siefert K, Ismail AI, Delva J, Sohn W. Reliability and validity of brief measures of oral health-related knowledge, fatalism, and self-efficacy in mothers of African American children. Pediatric Dentistry. 2005;27(5):422–428. [PMC free article] [PubMed] [Google Scholar]
- 10.Garg S, Rubin T, Jasek J, Weinstein J, Helburn L, Kaye K. How willing are dentists to treat young children?: a survey of dentists affiliated with Medicaid managed care in New York City, 2010. Journal of the American Dental Association. 2013;144(4):416–425. doi: 10.14219/jada.archive.2013.0135. [DOI] [PubMed] [Google Scholar]
- 11.Gomez SS, Weber AA, Emilson C. A prospective study of a caries preventive program in pregnant women and their children five and six years of age. Journal of Dentistry for Children. 2001;68:191–195. [PubMed] [Google Scholar]
- 12.Grembowski D, Milgrom PM. Increasing access to dental care for Medicaid preschool children: the Access to Baby and Child Dentistry (ABCD) program. Public Health Reports. 2000;115(5):448–459. doi: 10.1093/phr/115.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grembowski D, Spiekerman C, Milgrom P. Linking mother and child access to dental care. Pediatrics. 2008;122(4):e805–e814. doi: 10.1542/peds.2008-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale KJ. American Academy of Pediatrics Section on Pediatric Dentistry. Oral health risk assessment timing and establishment of the dental home. Pediatrics. 2003;111:1113–1116. doi: 10.1542/peds.111.5.1113. [DOI] [PubMed] [Google Scholar]
- 15.Harrison RL, Veronneau J, Leroux B. Effectiveness of maternal counseling in reducing caries in Cree children. Journal of Dental Research. 2012;91(11):1032–1037. doi: 10.1177/0022034512459758. [DOI] [PubMed] [Google Scholar]
- 16.Humphris GM, Morrison T, Lindsay SJ. The Modified Dental Anxiety Scale: validation and United Kingdom norms. Community Dental Health. 1995;12(3):143–150. [PubMed] [Google Scholar]
- 17.Ismail AI, Ondersma S, Jedele JM, Little RJ, Lepkowski JM. Evaluation of a brief tailored motivational intervention to prevent early childhood caries. Community Dentistry and Oral Epidemiology. 2011;39(5):433–448. doi: 10.1111/j.1600-0528.2011.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köhler B, Andréen I. Influence of caries-preventive measures in mothers on cariogenic bacteria and caries experience in their children. Archives of Oral Biology. 1994;39(10):907–911. doi: 10.1016/0003-9969(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 19.Le M, Riedy CA, Weinstein P, Milgrom P. Barriers to utilization of dental services during pregnancy: A qualitative analysis. ASDC Journal of Dentistry for Children. 2009;76:46–52. [PMC free article] [PubMed] [Google Scholar]
- 20.Lee RS, Milgrom P, Huebner CE, Conrad DA. Dentists’ perceptions of barriers to providing dental care to pregnant women. Womens Health Issues. 2010;20(5):359–365. doi: 10.1016/j.whi.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JY, Rozier RG, Norton EC, Kotch JB, Vann WF., Jr Effects of WIC participation on children’s use of oral health services. American Journal of Public Health. 2004;94(5):772–777. doi: 10.2105/ajph.94.5.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milgrom P, Hujoel P, Grembowski D, Ward JM. Making Medicaid child dental services work: a partnership in Washington state. Journal of the American Dental Association. 1997;128(10):1440–1446. doi: 10.14219/jada.archive.1997.0066. [DOI] [PubMed] [Google Scholar]
- 23.Milgrom P, Lee RSY, Huebner CE, Conrad DA. Medicaid reforms in Oregon and suboptimal utilization of dental care of women of childbearing age. Journal of the American Dental Association. 2010;141(6):688–695. doi: 10.14219/jada.archive.2010.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milgrom P, Ludwig S, Shirtcliff RM, Smolen D, Sutherland M, Gates PA, Weinstein P. Providing a dental home for pregnant women: A community access program to address dental care access. Journal of Public Health Dentistry. 2008;68(3):170–173. doi: 10.1111/j.1752-7325.2007.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milgrom P, Riedy CA, Weinstein P, Mancl LA, Garson G, Huebner CE, Smolen D, Sutherland M. Design of a community-based intergenerational oral health study: “Baby Smiles”. BMC Oral Health. 2013;13(1):38. doi: 10.1186/1472-6831-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milgrom P, Sutherland M, Shirtcliff M, Ludwig S, Smolen D. Children’s tooth decay in a public health program to encourage low-income pregnant women to utilize dental care. BMC Public Health. 2010;10:76. doi: 10.1186/1471-2458-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oral Health Care During Pregnancy Expert Workgroup. Oral Health Care During Pregnancy: A Summary of a Consensus Development Expert Workgroup Meeting. Washington, DC: National Maternal and Child Oral Health Resource Center, Georgetown University; 2012. [Accessed January 5, 2015]. http://www.mchoralhealth.org/materials/consensus_statement.html. [Google Scholar]
- 28.Phipps KR, Rosenberg KD, Sandoval AP. Dental care during pregnancy Oregon 2000. Presented at the Annual Meeting of the American Public Health Association; November 19, 2003; San Francisco. 2003. [Accessed January 8, 2015]. https://public.health.oregon.gov/HealthyPeopleFamilies/DataReports/prams/Pages/prepub.aspx. [Google Scholar]
- 29.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 30.Ramos-Gomez FJ, Gansky SA, Featherstone JD, Jue B, Gonzalez-Beristain R, Santo W, Martinez E, Weintraub JA. Mother and youth access (MAYA) maternal chlorhexidine, counselling and paediatric fluoride varnish randomized clinical trial to prevent early childhood caries. International Journal of Paediatr Dentistry. 2012;22(3):169–179. doi: 10.1111/j.1365-263X.2011.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruger JP, Emmons KM, Kearney MH, Weinstein MC. Measuring the costs of outreach motivational interviewing for smoking cessation and relapse prevention among low-income pregnant women. BMC Pregnancy and Childbirth. 2009;9:46. doi: 10.1186/1471-2393-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singhal A, Chattopadhyay A, Garcia AI, Adams AB, Cheng D. Disparities in unmet dental need and dental care received by pregnant women in Maryland. Maternal and Child Health Journal. 2014;18(7):1658–1666. doi: 10.1007/s10995-013-1406-7. [DOI] [PubMed] [Google Scholar]
- 33.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and difference. American Journal of Epidemiology. 2005;163(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 34.Van Keulen HM, Bosmans JE, van Tulder MW, Severens JL, de Vries H, Brug J, Mesters I. Cost-effectiveness of tailored print communication, telephone motivational interviewing, and a combination of the two: results of an economic evaluation alongside the Vitalum randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity. 2010;7:64. doi: 10.1186/1479-5868-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinstein P, Harrison R, Benton T. Motivating mothers to prevent caries: confirming the beneficial effect of counseling. Journal of the American Dental Association. 2006;137(6):789–793. doi: 10.14219/jada.archive.2006.0291. [DOI] [PubMed] [Google Scholar]
- 36.Weinstein P, Milgrom P, Riedy CA, Mancl LA, Garson G, Huebner CE, Smolen D, Sutherland M. Treatment fidelity of brief motivational interviewing and health education in a randomized clinical trial to promote dental attendance of low-income mothers and children: community-based intergenerational oral health study: “Baby Smiles”. BMC Oral Health. 2014;14(1):15. doi: 10.1186/1472-6831-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. Journal of the American Medical Association. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 38.Zich JM, Attkisson CC, Greenfield TK. Screening for depression in primary clinics: the CES-D and the BDI. International Journal in Psychiatry and Medicine. 1990;20:259–277. doi: 10.2190/LYKR-7VHP-YJEM-MKM2. [DOI] [PubMed] [Google Scholar]