Abstract
In mammals, a thermogenic mechanism exists that increases heat production and consumes energy. Recent work has shed light on the cellular and physiological mechanisms that control this thermogenic circuit. Thermogenically active adipocytes, namely brown and closely related beige adipocytes, differentiate from progenitor cells that commit to the thermogenic lineage but can arise from different cellular origins. Thermogenic differentiation shares some features with general adipogenesis, highlighting the critical role that common transcription factors may play in progenitors with divergent fates. However, thermogenic differentiation is also discrete from the common adipogenic program and, excitingly, cells with distinct origins possess thermogenic competency that allows them to differentiate into thermogenically active mature adipocytes. An understanding of this thermogenic differentiation program and the factors that can activate it has led to the development of assays that are able to measure thermogenic activity both indirectly and directly. By combining these assays with appropriate cell models, novel therapeutic approaches to combat obesity and its related metabolic disorders by enhancing the thermogenic circuit can be developed.
Keywords: Adipogenesis, Autologous transplants, Brown adipose tissue, Cell therapy, UCP1, Obesity, Preadipocyte, Thermogenesis
Background/significance
Obesity and metabolic syndrome has reached worldwide pandemic. According to the U.S. Center for Disease Control and Prevention, two-thirds of the US population is overweight or obese. More than one-third of US adults and approximately 17% of US children are obese. Worldwide, more than 1 billion adults (15% of the world population) are overweight and over 300 million people rank as truly obese. Obesity and insulin resistance are two hallmark features for a larger collection of metabolic syndromes. Indeed, in addition to the 20 million people with type 2 diabetes, it is estimated that over 40 million people in the U.S. have metabolic syndrome, and it is this collection of abnormalities which generates risk for many of our most common medical disorders, including type 2 diabetes, dyslipidemia, non-alcoholic fatty liver, cardiovascular disease, renal failure, and even some cancers.1, 2, 3, 4 The development of prevention and treatment strategies for obesity and its co-morbidities is of the utmost importance for the healthcare and research communities. One appealing approach that has garnered considerable interest over the last several years is the investigation of the therapeutic potential of brown adipose tissue (BAT).
BAT plays a pivotal role in thermogenesis, which is the process by which energy is released in the form of heat to maintain body temperature. In mice and humans, this process can be mediated by the function of uncoupling protein 1 (UCP1), which is expressed in the mitochondria of brown and brown-like (also known as beige or brite) adipocytes. Brown adipocytes are mostly located in the interscapular region in rodents and constitutively express UCP1 while beige adipocytes can be induced in white adipose tissue upon cold or β-adrenergic stimulations.5 In adult humans, UCP1-positive adipocytes are found in the neck, supraclavicular and spinal cord regions.6, 7, 8, 9
Simplifying the mechanism of UCP1 mediated thermogenesis
UCP1 is a proton leak channel in the inner mitochondrial membrane that uncouples the proton motive force generated by the electron transport chain (ETC) from ATP generation. UCP1 allows protons that are actively transported across the semi-permeable membrane by the ETC to pass back down their concentration gradient and bypass the V-ATPase, releasing energy as heat rather than to activate phosphorylation of ADP. One characteristic that distinguishes brown from beige adipocytes is that UCP1 expression is highly inducible by activators of thermogenic activity in beige adipocytes, while UCP1 is constitutively expressed at high levels in classical brown adipocytes.10, 11, 12, 13, 14
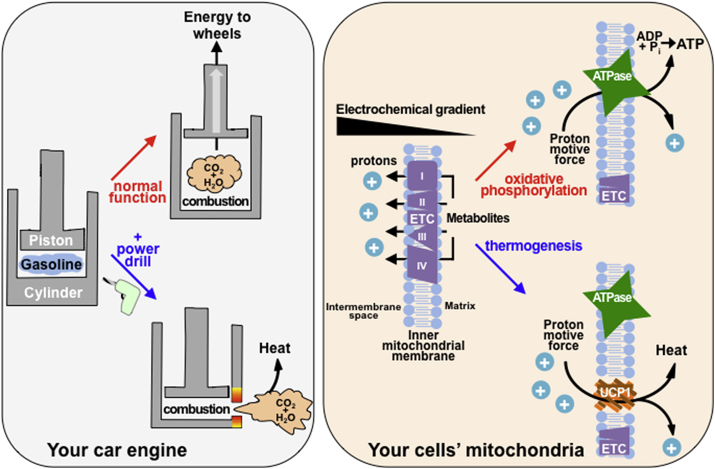
The molecular mechanism is easy to understand with the simple metaphor of a car engine (Fig. 1). During normal function, your car engine uses gasoline as an energy source to move the wheels forward, like cells use glucose and fatty acids as energy sources for their mitochondria to produce ATP. When the gas pedal is depressed and fuel is injected into the engine cylinder, an explosion is triggered that combusts the gasoline and produces carbon dioxide and water. This gas is contained in the cylinder, producing energy to push up the engine piston and propel the car forward and the cycle is then repeated. In cells, the electron transport chain uses fuel like glucose and fatty acids to pump protons into the inner mitochondrial membrane generating a proton motive force. During normal oxidative phosphorylation, the energy in these substrates would be used to “push” the ATPase enzyme that generates ATP akin to the way the gasoline combustion pushes the engine piston.15 The function of UCP1 can be thought of as having the same effect as a small hole drilled into the side of the engine cylinder in your car. Instead of pushing up the piston and doing work for your car, all of the gas and energy from combustion escapes through the hole without pushing the piston. In cells, thermogenesis occurs because the protons that might provide energy for the ATPase are simply released by UCP1 back into the mitochondrial matrix, like gas escaping an engine cylinder. It's easy to imagine sitting in your driveway, pressing the gas pedal and revving your car's engine, but going nowhere as you generate no force to push the engine pistons and move your car forward. Though you wouldn't go anywhere as you revved your engine and burned fuel, you would generate plenty of heat, the same way brown and beige fat are able to burn fuel and generate significant heat at the expense of ATP production using UCP1 mediated proton leak.
Figure 1.
The basic principle of UCP1 mediated thermogenesis compared to a car engine. Your car engine normally combusts gasoline to release CO2 and H2O inside of an enclosed engine cylinder. The gas released pushes up on a piston, sending its energy to the wheels so you can drive forward. Similarly, mitochondria shuttle metabolites through the ETC to store protons on one side of the inner mitochondrial membrane to generate an electrochemical gradient. The protons can pass down this gradient through an ATPase, which uses the proton motive force as energy to phosphorylate ADP, generating ATP. Just as drilling a hole in the engine cylinder would allow the gas produced by combustion to escape without pushing the piston, UCP1 is a channel that allows protons to pass down their electrochemical gradient without activating ATP production. This energy from the proton motive force is instead dissipated as heat, much like your car engine would heat up with holes drilled in the engine cylinders.
Activating a thermogenic mechanism
By activating the UCP1 mediated thermogenic mechanism, BAT has been shown to regulate fatty acid metabolism and glucose homeostasis.16, 17, 18 Given its influential role in the regulation of nutrient metabolism, it is not surprising that BAT mass/activity is inversely correlated to body mass index and percent body fat in humans.19, 20 Increasing the size and activation of brown and beige adipose tissue has the potential to alleviate the clinical sequelae of the metabolic syndrome making recent clinical efforts to demonstrate the feasibility of using cold regiments to activate BAT in humans an exciting frontier.21, 22, 23, 24
Therefore, brown and beige fat are the body's main tissues capable of activating a thermogenic circuit consisting of adipocytes that metabolize substrates in order to generate a proton motive force in mitochondria that is subsequently uncoupled from ATP production to generate heat. The steps of thermogenic differentiation can be co-opted by distinct progenitor cell types that produce mature cells with thermogenic capacity. Though they can share certain aspects of a common adipogenic and thermogenic differentiation program, cells at discrete stages of differentiation express surface proteins and transcriptional networks that are unique signatures of their specific cellular lineage. The factors that promote the thermogenic circuit at each of its stages are of great therapeutic interest in the field of obesity and diabetes and several comprehensive reviews have thoroughly listed these browning agents.14, 25 In this review, in addition to updating the list of browning agents, we will define the stages of cell differentiation and the inductive networks required to complete full differentiation and activation of the thermogenic circuit.
Adipose tissue development and physiology
Adipocytes are postmitotic and as a result hyperplasia of adipose tissue requires proliferation and differentiation of adipocyte precursor cells to form new mature adipocytes both during development and throughout life.26, 27 In mice, BAT arises developmentally from cells in the dermomyotome that express the transcription factors Pax728 and myogenic factor 5 (Myf5).29, 30 On the other hand, the majority of white adipocytes arise from cells lacking Myf5.31 During this process, multipotent progenitor cell populations are triggered to commit to specific lineages by induction cues in the stem cell niche. In adipose tissue, this niche is located in the stromal vascular fraction (SVF) and both human and mouse SVF contain cells that are capable of differentiating into several different lineages.32 Experiments carried out in mice have further revealed that SVF is a mixture of uncommitted and committed adipose progenitors.33, 34 During adult life, adipocyte turnover may be as frequent as 5% of mature cells per day requiring a large reservoir of these progenitor cells.35 To meet this need, committed preadipocytes are capable of significant expansion before they become fully differentiated.33
Following commitment to the adipocyte lineage, differentiation into mature adipocytes takes place and can be enhanced or inhibited by extrinsic cues. Some evidence suggests that the action of extrinsic signals to promote proliferation and differentiation of preadipocytes occurs in a perivascular niche.36, 37 In this model, cells that are located close to vasculature receive inductive cues that prompt their terminal differentiation and migration out of the niche. Once fully mature, brown and beige adipocytes are readily identifiable by their multilocular lipid droplets and by expression of UCP1 protein, which is essential to exert their thermogenic activity.
Factors determining adipogenic and thermogenic competency
During cell differentiation, certain stages of cell fate are irreversible and represent a commitment to a given lineage. Presumably, these stages correspond to distinct states of heterochromatin that allow cells access to genes that coordinately regulate cell identity. The primary stage is the canonical differentiation cascade triggering the commitment of mesenchymal progenitor cells to become the adipose lineage, i.e., the preadipocytes (Fig. 2). Using lineage tracing strategies, initial studies determined that only brown adipocytes arise from a Myf5-positive lineage while white and beige adipocyte precursors do not express Myf5.30 Subsequent analysis has indicated that although a small fraction of Myf5-positive cells are capable of undergoing white and beige adipogenesis, the majority of white and beige adipocytes are derived from a Myf5-negative lineage.31, 38 Though there are some disagreements regarding the exact contribution of the Myf5-positive and Myf5-negative lineage to each pool of mature adipocytes, the important concept is that Myf5 merely serves as a lineage marker, both adipogenic differentiation and thermogenic capacity are independent of expression of Myf5.
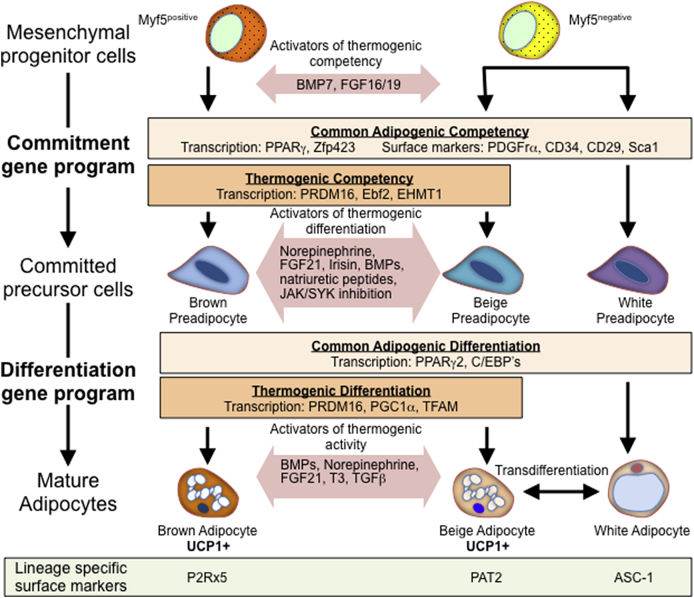
Figure 2.
Thermogenic differentiation. Thermogenic mature adipocytes arise from both Myf5-positive and Myf5-negative lineages that express a commitment gene program as they differentiate into committed precursor cells. This differentiation program has both aspects common to brown, beige and white preadipocytes as well as aspects distinct to thermogenically competent preadipocytes and can be enhanced by treatment with BMP7 or some members of the FGF family of proteins. Committed thermogenic precursor cells can further differentiate into mature adipocytes by activating a gene network that also shares common features with white adipogenesis, however, distinct thermogenic differentiation factors are also shared between brown and beige adipocytes. This process can also be enhanced by a large group of diverse activators of thermogenic differentiation. Finally, mature thermogenic adipocytes that express UCP1 can be further activated, yet distinct lineage specific markers that distinguish brown, beige and white adipocytes exist.
Mesenchymal progenitor cells differentiate into committed adipocyte progenitors in response to inductive signals such as bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs) and others that are expressed in the niche to activate brown or beige differentiation.12 Several members of these developmental factor superfamily have been implicated in the regulation of brown fat differentiation and functions as well as promoting browning of white adipose tissues, including BMP7, 4, 8 and FGF16, 19 and 21.39, 40, 41, 42, 43 Presumably, these signals act through cell surface receptors to activate signaling cascades that regulate expression of key competency genes. Consistent with an essential role of BMP7 in embryonic brown fat development,44 deletion of the BMP receptor BMPR1a in Myf5 expressing cells results in a paucity of interscapular brown adipose tissue.45 BMP7 signaling via Smad proteins suppresses expression of Pref-1, an EGF-repeat containing protein which inhibits adipogenic differentiation.46 Zinc-finger protein ZFP423 appears to be a determining factor for a preadipocyte commitment via interacting with the BMP-induced Smad protein and regulating peroxisome proliferator-activated receptor gamma (PPARγ) transcription.36 Expression of ZFP423 is suppressed by another zinc-finger protein ZFP521,47 highlighting that adipose commitment is under a rigorous transcriptional regulation. Interestingly, Pref-1 marks mesenchymal precursors at as early as embryonic day 10.5, which is prior to the expression of ZFP423 and PPARγ.48
Cell sorting strategies have also led to a significant body of knowledge describing the cell surface antigens that mark cells committed to the adipose lineage. Notably, all progenitor cells that are committed to the adipocyte lineage express platelet derived growth factor receptor (PDGFR) α/β, CD34, CD29 and stem cell antigen 1 (Sca1).33, 34, 49 However, it remains unknown whether these proteins are required to prime a cell for adipogenic differentiation or they simply provide tools for isolation of committed adipose progenitors.
Transcriptional control of thermogenic fate
As surface markers that define progenitor cells that give rise to brown, beige or white adipocytes remain to be identified, efforts have been undertaken to identify transcriptional regulators that mark committed preadipocytes. The transcriptional regulator PRD1-BF1-RIZ1 homologous domain containing 16 (PRDM16) controls a regulatory switch in preadipocytes that is required for brown adipocyte differentiation.30 Overexpression of PRDM16 in myogenic precursors induces their differentiation into brown adipocytes.30 Surprisingly, deletion of this factor in Myf5-expressing progenitors has minimal effects on embryonic brown fat development.50 Adipocyte-specific ablation of PRDM16 has little impact on differentiation and function of classical brown adipocytes, but results in impairment of beige adipocyte formation in response to cold or β3-adrenergic stimulation.51 Interestingly, activation of PRDM16 is independent of the DNA binding domain of PRDM16, implicating other potential transcription factors that may act in concert with PRDM16 to promote or repress transcription.52 Recently, the transcription factor early B cell factor 2 (Ebf2) was shown to mark cells from both white and brown adipose tissue that were committed to the thermogenic fate.53, 54 Ebf2 expression marks cells that arise from both the Myf5-positive and Myf5-negative lineage that give rise to brown and beige adipocytes, suggesting that Ebf2 and its targets poise these cells to become thermogenic competent after differentiation. Ebf2 is thought to act as a co-regulator of gene expression with PRDM16.
As previously mentioned, thermogenic competency may also be a function of chromatin state maintained by a large repertoire of histone modifying enzymes. In human embryonic stem cells, PRDM16 has specific histone 3 lysine 9 (H3K9) methyltransferase activity. The H3K9 methylation state is a critical determinant of gene activity and preadipocytes that do not express PRDM16 may fail to properly modify chromatin associated with the thermogenic differentiation program.55, 56 In addition to its own histone modifying activity, PRDM16 associates other chromatin modifying enzymes, including the proteins C-terminal-binding-protein-1 (Ctbp1) and Ctbp2. These two proteins are known to further recruit histone deacetylase enzymes to modify chromatin.57, 58 Moreover, the PRDM16 complex also contains the protein euchromatic histone-lysine N-methyltransferase 1 (EHMT1) which is required to suppress myogenic differentiation of precursor cells by methylating histones associated with muscle specific gene promoters to suppress them.59
The mechanisms that link chromatin state, transcriptional regulation and surface marker patterns and ultimately determine adipose cell fate and thermogenic potential are still being teased apart. Based on the current understanding, cells from different lineages share common programs that allow them to fully access the adipogenic and thermogenic gene networks required to differentiate into white, beige or brown adipocytes.
Factors regulating thermogenic differentiation and activity
After commitment of precursor cells to an adipocyte lineage, a second key step in the formation of mature adipocytes relies on gene expression networks that complete differentiation from competent cells. The transcriptional regulators able to activate programs of terminal differentiation have been extensively characterized and reviewed elsewhere.33, 60, 61, 62, 63, 64, 65 Critically, terminal differentiation of white preadipocytes shares a common fundamental transcriptional program with brown and beige adipogenesis that is dependent on expression of PPARγ and the CCAAT/enhancer-binding proteins (specifically C/EBPα/β/δ).66, 67 Downstream targets of these transacting regulatory factors are required for the formation of mature adipose tissue in committed brown, beige and white adipocytes.
Similar to the commitment stage of differentiation, a thermogenic differentiation program exists that is discrete from the transcriptional machinery that promotes adipogenesis. It is known that the thermogenic circuit in brown and beige fat can be activated by the sympathetic nervous system in response to cold; however, other pathways and circulating factors could also induce thermogenic activity either by modulating the sympathetic input or through independent pathways. The canonical pathway regulating BAT metabolic activity in response to cold relies on sympathetic input resulting in the release of the catecholamine neurotransmitter norepinephrine (NE) to activate G-protein coupled adrenergic receptors and increase intracellular cAMP. This process could be mimicked by pharmacological compounds such as the β3-adrenergic receptor agonist CL 316,243. These stimuli enhance BAT thermogenic activity and induce beige adipocyte formation in white adipose tissue.68 The latter is mediated by increased differentiation of precursor cells into thermogenically competent cells as well as direct transdifferentiation.69 In addition to the canonical catecholamine pathway, several alternate pathways capable of increasing BAT thermogenic activity and/or brown fat differentiation have been described.12, 70 These include classical hormones, such as thyroid hormone and insulin, and newly identified endocrine factors, such as bile acid, natriuretic peptides, FGF21, irisin and BMPs. Recently, cellular tyrosine kinases in the Jak and Syk family that negatively regulate thermogenic differentiation have been identified.71 Thermogenic gene expression is driven by PRDM16 and the transcriptional co-activator proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), which activates mitochondrial biogenesis with mitochondrial transcription factor A (TFAM).72, 73 In many respects, this thermogenic program is again shared between brown and beige adipocytes, however, it is clear that some differences exist as these two cell types have distinct gene expression profiles.6 During the later stages of differentiation, the thermogenic program could be further enhanced by both sympathetic dependent and independent pathways.14, 70
Screening for thermogenic factors
Until recently, a lack of human brown adipocyte cell models has prevented drug screening using human relevant cell types. To meet this need, several groups have reported the generation of cell lines that can be induced to become brown/beige adipocytes.74, 75 Perhaps the best cellular system would be progenitors derived from bona fide human brown adipose tissue as differentiated cells from these progenitors serve as the standard for genuine human brown adipocytes. These models present exciting opportunities to screen for drugs that can enhance thermogenic competency and activity, however, careful assay design is required in order to properly assess which effect any given drugs has. Critical to this experimental design is the treatment timeframe (Fig. 3A). As discussed above, lineage commitment precedes differentiation. This means that treatment paradigms that test compounds during the late stages of differentiation require cells that have been committed to the adipogenic lineage to gauge the full effect, whereas identification of reagents that promote the commitment of progenitor cells to a thermogenic fate requires treatment of uncommitted cells prior to adipogenic induction.
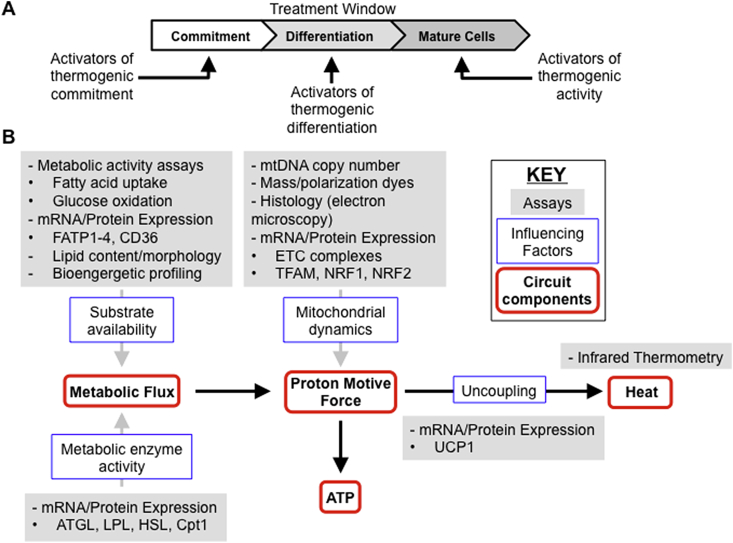
Figure 3.
Assaying the thermogenic circuit. (A) Adipogenic differentiation in vitro can be approximately divided into three phases: commitment, differentiation and maturity. Treatment with reagents that enhance each phase must be carried out in the appropriate treatment window. (B) The thermogenic circuit at the cellular level is composed of the metabolic flux through the cell that generates a proton motive force, which can either be used to generate ATP or uncoupled to generate heat. To measure the activity of the cellular thermogenic circuit, influencing factors such as substrate availability, metabolic enzyme activity, mitochondrial dynamics and UCP1 expression are often measured using a broad range of different assays.
Another opportunity for development lies in the assays used to characterize the thermogenic circuit. In order to increase activity of the thermogenic circuit, many influencing factors that can be measured act on circuit components. These factors are commonly measured by mRNA or protein expression, notably in the case of UCP1, the factor directly upstream of heat production (Fig. 3B).
Measuring thermogenesis
As UCP1 is located in mitochondria, many assays also determine metrics of mitochondrial dynamics. To determine mitochondrial mass, mitochondrial DNA copy number is often measured using quantitative PCR. It is also possible to assess mitochondrial mass histologically by electron micrograph and several dyes are commercially available that specifically stain mitochondria. Care should be taken that staining intensity is representative of mitochondrial mass as some dyes, specifically Mitotracker Red, are sensitive to the mitochondrial membrane potential state. Potential indicative of increased proton motive force may not be associated with a browning effect, however, as UCP1 mediated uncoupling would actually decrease the membrane potential even if metabolic flux were high. Transcriptional regulators of mitochondrial biogenesis such as TFAM and nuclear respirator factors 1 and 2 (NRF1 and NRF2) can also be assessed. Finally components of the ETC are often quantified by immunoblotting to measure the capacity of mitochondria to generate the proton motive force.
In order for mitochondria to generate the proton motive force, fuel must be broken through many pathways that serve to provide substrates to the ETC. Metabolic flux is usually measured in a steady state or implied by the concentrations or activities of the enzymes that control rate limiting steps in the breakdown of fuel such as the lipases adipose triglyceride lipase (ATGL), lipoprotein lipase (LPL), hormone sensitive lipase (HSL) and carnitine palmitoyltransferase 1(Cpt1), which controls entry of fatty acids into mitochondria. As thermogenic cells commonly use glucose or fatty acids as fuel, uptake of these metabolites can be measured. Along these lines, expression of key fatty acid uptake proteins such as fatty acid transport proteins 1–4 (FATP1-FATP4) and CD36 can be measured. Recently, high throughput image analysis of lipid droplet morphology has also been used as a metric of substrate availability based on the assumption that an increasing number of smaller lipid droplets increases the surface area to volume ratio and allows increased access to lipid molecules by water soluble lipases in the cytoplasm to facilitate metabolism.71
Currently, one limitation is that most of the assays used to characterize thermogenesis, especially in vitro, do not directly measure heat production. Thermal imaging at a sub-cellular scale is possible, however, it requires nanoparticle injection into fixed cells and has yet to be adapted to a high throughput methodology.76 It could be useful to develop high throughput thermometry techniques assay the thermogenic circuit by directly measuring heat production.
Another similar technological limitation exists when determining the efficacy of potential thermogenic factors in vivo. Most efforts to detect brown adipose tissue activity and mass in humans rely on metabolite uptake to discriminate thermogenically active tissue, however the need for these techniques to be standardized has been recognized.77
Therapeutic applications of the thermogenic circuit
Gaining a better understanding of the thermogenic circuit must ultimately be translated into therapeutic applications, primarily to treat the metabolic syndrome. The individual components of the thermogenic circuit make a range of diverse therapeutic strategies possible (Fig. 4). There are two main categories of therapies to activate thermogenesis in humans. The first strategy relies on treatments given directly to humans while the second strategy is based on cell-based therapies.
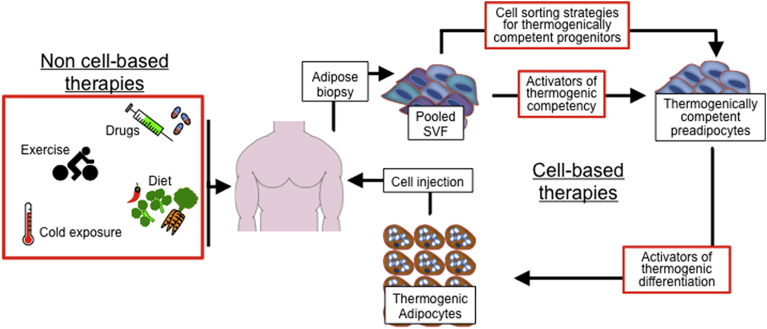
Figure 4.
Therapeutic interventions to enhance the thermogenic circuit. Two different basic types of therapy for enhancing the thermogenic circuit in man are possible. First, non cell-based therapies such as drugs, exercise, diet and therapeutic cold exposure are possible. Second, cell-based therapies rely on adipose tissue biopsy to provide SVF cells, which can be used to produce thermogenically competent preadipocytes ex vivo using either drug treatment of cell sorting strategies. These cells can be expanded and differentiated to produce large amounts of thermogenic adipocytes for autologous transplantation back into the donor. Treatment of cells with activators of thermogenic competency and differentiation ex vivo limits potential side effects of treatments on the rest of the body.
Currently, treatments that directly activate the thermogenic circuit in humans are quite diverse. Although cold exposure is an effective way of activating brown adipose tissue and several studies have demonstrated the clinical feasibility of this approach,21, 22, 24 therapeutic cold exposure is uncomfortable for humans. Diet and exercise based interventions are also attractive given the pleiotropic benefits of these strategies. In particular, foods that contain capsaicin have been reported to activate brown adipose tissue activity.78 Several drugs already clinically available have been shown to activate brown adipose tissue, such as the β-3 adrenergic agonist mirabegron.79 These treatments are promising but side effects, particularly to the cardiovascular system, may pose potential problems for treatment of obese subjects.
Cell based therapies using autologous cell transfer to activate the thermogenic circuit may provide another way of potential treatment.80 After biopsy, cells can be cultured and treated to enhance thermogenic competency or specific cell surface markers can be used for FACS sorting strategies to enrich for thermogenically competent progenitors. Once in culture, these cells can be expanded and differentiated into thermogenically active adipocytes before implantation back into the donor. One significant advantage of this strategy is that cells can be treated with compounds that enhance thermogenic competency and differentiation in vitro, avoiding effects on other cell types that might occur if humans were treated in vivo. Additionally, adipose stromal cells are highly proliferative and could potentially serve as a large source of thermogenically active adipocytes to combat obesity.
Understanding the thermogenic circuit is a key to developing strategies to combat obesity and its sequelae. Crucial to this is knowledge describing how thermogenically active cells differentiate. By studying the process by which cells commit to a thermogenically active fate experiments can be designed that identify regulators at key steps of thermogenic differentiation. The hope is that these regulators can be translated into human therapy either by activating the thermogenic circuit in vivo or by generating cells ex vivo that can augment thermogenesis.
Acknowledgments
We thank M. Leung for expert editorial assistance. We apologize to those whom we were unable to reference due to space limitations. This work was supported in part by National Institutes of Health (NIH) Grant (R01DK077097), Joslin Diabetes Center's Diabetes Research Center (P30DK03836), a research grant from the American Diabetes Foundation (ADA 7-12-BS-191), and by funding from the Harvard Stem Cell Institute (to Y-H.T). M.D.L was supported by NIH fellowships (T32DK007260 and F32DK102320).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Kopelman P.G. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Hsu I.R., Kim S.P., Kabir M., Bergman R.N. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr. 2007;86:s867–s871. doi: 10.1093/ajcn/86.3.867S. [DOI] [PubMed] [Google Scholar]
- 3.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 4.Cornier M.A., Dabelea D., Hernandez T.L. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrovic N., Walden T.B., Shabalina I.G., Timmons J.A., Cannon B., Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J., Bostrom P., Sparks L.M. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cypess A.M., White A.P., Vernochet C. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lidell M.E., Betz M.J., Leinhard O.D. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;9:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 9.Jespersen N.Z., Larsen T.J., Peijs L. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 12.Schulz T.J., Tseng Y.H. Brown adipose tissue: development, metabolism and beyond. Biochem J. 2013;453:167–178. doi: 10.1042/BJ20130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend K.L., Tseng Y.H. Brown adipose tissue: recent insights into development, metabolic function, and therapeutic potential. Adipocyte. 2012;1:13–24. doi: 10.4161/adip.18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J., Cohen P., Spiegelman B.M. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabe M., Wang H., Oster G. The mechanochemistry of V-ATPase proton pumps. Biophys J. 2000;78:2798–2813. doi: 10.1016/S0006-3495(00)76823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanford K.I., Middelbeek R.J., Townsend K.L. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartelt A., Bruns O.T., Reimer R. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 18.Townsend K.L., Tseng Y.H. Brown fat fuel utilization and thermogenesis. Trends Endocrinol Metab. 2014;25:168–177. doi: 10.1016/j.tem.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cypess A.M., Lehman S., Williams G. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 21.Blondin D.P., Labbe S.M., Phoenix S. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2014;593:701–714. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijgen G.H., Sparks L.M., Bouvy N.D. Increased oxygen consumption in human adipose tissue from the “brown adipose tissue” region. J Clin Endocrinol Metab. 2013;98:E1230–E1234. doi: 10.1210/jc.2013-1348. [DOI] [PubMed] [Google Scholar]
- 23.van der Lans A.A., Hoeks J., Brans B. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vosselman M.J., Vijgen G.H., Kingma B.R., Brans B., van Marken Lichtenbelt W.D. Frequent extreme cold exposure and brown fat and cold-induced thermogenesis: a study in a monozygotic twin. PLoS One. 2014;9:e101653. doi: 10.1371/journal.pone.0101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonet M.L., Oliver P., Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta. 2013;1831:969–985. doi: 10.1016/j.bbalip.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Joe A.W., Yi L., Even Y., Vogl A.W., Rossi F.M. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q.A., Tao C., Gupta R.K., Scherer P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepper C., Fan C.M. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atit R., Sgaier S.K., Mohamed O.A. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 30.Seale P., Bjork B., Yang W. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Gurmaches J., Guertin D.A. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuk P.A., Zhu M., Ashjian P. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry R., Rodeheffer M.S. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz T.J., Huang T.L., Tran T.T. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigamonti A., Brennand K., Lau F., Cowan C.A. Rapid cellular turnover in adipose tissue. PLoS One. 2011;6:e17637. doi: 10.1371/journal.pone.0017637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta R.K., Mepani R.J., Kleiner S. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang W., Zeve D., Suh J. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Gurmaches J., Guertin D.A. Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta. 2014;1842:340–351. doi: 10.1016/j.bbadis.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian S.W., Tang Y., Li X. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci USA. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittle A.J., Carobbio S., Martins L. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konishi M., Mikami T., Yamasaki M., Miyake A., Itoh N. Fibroblast growth factor-16 is a growth factor for embryonic brown adipocytes. J Biol Chem. 2000;275:12119–12122. doi: 10.1074/jbc.275.16.12119. [DOI] [PubMed] [Google Scholar]
- 42.Tomlinson E., Fu L., John L. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 43.Fisher F.M., Kleiner S., Douris N. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng Y.H., Kokkotou E., Schulz T.J. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulz T.J., Huang P., Huang T.L. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H., Schulz T.J., Espinoza D.O. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010;30:4224–4233. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang S., Akerblad P., Kiviranta R. Regulation of early adipose commitment by Zfp521. PLoS Biol. 2012;10:e1001433. doi: 10.1371/journal.pbio.1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hudak C.S., Gulyaeva O., Wang Y. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014;8:678–687. doi: 10.1016/j.celrep.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodeheffer M.S., Birsoy K., Friedman J.M. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 50.Harms M.J., Ishibashi J., Wang W. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014;19:593–604. doi: 10.1016/j.cmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen P., Levy J.D., Zhang Y. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kajimura S., Seale P., Kubota K. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajakumari S., Wu J., Ishibashi J. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang W., Kissig M., Rajakumari S. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci USA. 2014;111:14466–14471. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenfeld J.A., Wang Z., Schones D.E., Zhao K., DeSalle R., Zhang M.Q. Determination of enriched histone modifications in non-genic portions of the human genome. BMC Genomics. 2009;10:143. doi: 10.1186/1471-2164-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barski A., Cuddapah S., Cui K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Kajimura S., Seale P., Tomaru T. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008;22:1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chinnadurai G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol Cell. 2002;9:213–224. doi: 10.1016/s1097-2765(02)00443-4. [DOI] [PubMed] [Google Scholar]
- 59.Ohno H., Shinoda K., Ohyama K., Sharp L.Z., Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504:163–167. doi: 10.1038/nature12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farmer S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farmer S.R. Molecular determinants of brown adipocyte formation and function. Genes Dev. 2008;22:1269–1275. doi: 10.1101/gad.1681308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kajimura S., Seale P., Spiegelman B.M. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–262. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosen E.D., MacDougald O.A. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 64.Seale P., Kajimura S., Spiegelman B.M. Transcriptional control of brown adipocyte development and physiological function–of mice and men. Genes Dev. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vernochet C., McDonald M.E., Farmer S.R. Brown adipose tissue: a promising target to combat obesity. Drug News Perspect. 2010;23:409–417. doi: 10.1358/dnp.2010.23.7.1487083. [DOI] [PubMed] [Google Scholar]
- 66.Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid- activated transcription factor [published erratum appears in Cell 1995 Mar 24;80(6): following 957] Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 67.Wu Z., Puigserver P., Spiegelman B.M. Transcriptional activation of adipogenesis. Curr Opin Cell Biol. 1999;11:689–694. doi: 10.1016/s0955-0674(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 68.Ghorbani M., Claus T.H., Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 69.Smorlesi A., Frontini A., Giordano A., Cinti S. The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes Rev. 2012;13:83–96. doi: 10.1111/j.1467-789X.2012.01039.x. [DOI] [PubMed] [Google Scholar]
- 70.Villarroya F., Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 71.Moisan A., Lee Y.K., Zhang J.D. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nat Cell Biol. 2015;17:57–67. doi: 10.1038/ncb3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Z., Puigserver P., Andersson U. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 73.Vernochet C., Mourier A., Bezy O. Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell Metab. 2012;16:765–776. doi: 10.1016/j.cmet.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elabd C., Chiellini C., Carmona M. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells. 2009;11:2753–2760. doi: 10.1002/stem.200. [DOI] [PubMed] [Google Scholar]
- 75.Silva F.J., Holt D.J., Vargas V. Metabolically active human brown adipose tissue derived stem cells. Stem Cells. 2014;32:572–581. doi: 10.1002/stem.1595. [DOI] [PubMed] [Google Scholar]
- 76.Kucsko G., Maurer P.C., Yao N.Y. Nanometre-scale thermometry in a living cell. Nature. 2013;500:54–58. doi: 10.1038/nature12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cypess A.M., Haft C.R., Laughlin M.R., Hu H.H. Brown fat in humans: consensus points and experimental guidelines. Cell Metab. 2014;20:408–415. doi: 10.1016/j.cmet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toneshiro T., Aita S., Kawai Y., Iwanaga T., Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr. 2012;95:845–850. doi: 10.3945/ajcn.111.018606. [DOI] [PubMed] [Google Scholar]
- 79.Cypess A.M., Weiner L.S., Roberts-Toler C. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim E.H., Heo C.Y. Current applications of adipose-derived stem cells and their future perspectives. World J Stem Cells. 2014;6:65–68. doi: 10.4252/wjsc.v6.i1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]