INTRODUCTION
Since the first description of a surgical correction of craniosynostosis by Lane in 18921, various techniques have fallen in and out of favor. Initially, high rates of complications caused surgeons to abandon the procedure altogether2. But, gradually, surgical treatment again resurfaced3, mostly in the form of strip craniectomy and removal of the affected suture. Surgeons found, however, that this approach frequently resulted in re-stenosis, inadequate correction, and unacceptable aesthetic results despite a multitude of efforts to prevent re-fusion4. The advent of craniofacial surgery by Paul Tessier5, brought with it a more reliable and reproducible approach which was quickly popularized and has become the standard of care.
Over the past decade, craniofacial surgeons have witnessed a resurgence of strip craniectomies for the treatment of single and multi-suture craniosynostosis. The literature is now replete with descriptions of craniosynostosis treatment involving endoscopy7-18, springs19-26 and distraction devices30-35 in conjunction with strip craniectomies. The rationale frequently given for this reversion is shorter, safer operations with less blood loss. The studies which tout “minimally invasive approaches” often denegrate open cranial vault expansion.
The current approach of open cranial vault expansion still shares many similarities to Dr. Tessier's descriptions, but it too has evolved over the past decades. What was initially a lengthy, bloody surgery36, 37 with a long recovery and relatively high risk38, 39 has become a routine procedure at many craniofacial centers worldwide. But, previous research into the safety of these procedures has either focused mainly on blood loss, or has relied upon surgeon recall via surveys. Craniofacial teams and the treatment protocols they created have improved the outcomes, shortened the procedures and the recovery, and decreased the risk to the patient. At Seattle Children's Hospital, patients with craniosynostosis are evaluated by a multi-disciplinary team and are offered cranial vault expansion. The type of expansion is tailored to the age of the patient, type of synostotic suture and the shape of the resulting deformity. Those patients who elect to undergo cranial vault expansion are placed on a pathway that is meant to optimize their care from diagnosis through surgery to discharge from the hospital. Since 2002, this pathway has included the same team of pediatricians, surgeons and anesthesiologists who care for this condition. The aim of this study is to describe the multidisciplinary protocol for single suture craniosynostosis care at SCH and more rigorously analyze the safety of open cranial vault expansion in the setting of this paradigm.
PATIENTS AND METHODS
A retrospective chart review was performed for patients with craniosynostosis treated through the Craniofacial Center at Seattle Children's Hospital between the years of 2002 and 2006. Patients with single suture craniosynostosis diagnosed by physical exam and confirmed by CT scan who underwent cranial vault expansion were included. Patients with multi-suture involvement, syndromes, neurologic problems, premature patients (34 weeks gestation or earlier), and those who elected not to undergo surgery were excluded. We identified 123 eligible participants during the recruiting period and 96 were enrolled (10 families actively and 17 passively declined participation). Among the 96 enrolled patients, seven had surgery at institutions other than SCH (and were therefore ineligible for this study) and one had incomplete records for a total of 89 participants.
This cohort was identified as part of the ongoing infant learning project and therefore does not represent all patients treated during this timeframe. Patients selected for this study were approached for consent and then underwent a variety of neurodevelopmental assessments both before and after cranial vault expansion. It is for this reason we chose to exclude premature patients and those with pre-operative neurological problems to avoid bias within the neurodevelopmental testing. The outcome data from the infant learning project study will be presented elsewhere. The focus of this study is the safety of the operative intervention. The research was approved by the Seattle Children’s Hospital human subjects IRB #10998.
SURGICAL AND MEDICAL CARE
PRE-OPERATIVE CARE
The initial diagnosis was made by physical exam by multiple members of the craniofacial team including a craniofacial pediatrician, craniofacial plastic surgeon, and a pediatric neurosurgeon. A CT scan confirmed the diagnosis and aided in surgical planning. A pre-operative consultation with the anesthesia team was made to review medical and family history, physical exam, laboratory and other diagnostic test results and anesthetic risks. Additionally, patients are referred to the regional blood bank to discuss transfusion risks and the possibility of direct donation of blood.
Once the diagnosis was made, the planned surgical intervention and timing of the procedure was tailored to the suture affected. Isolated sagittal craniosynostosis diagnosed early (before 9 months of age) was treated with a modified pi procedure (fig.1). Late presenting sagittal craniosynostosis (after 9 months of age) was treated with a total cranial vault expansion (fig.2). Patients with lambdoid craniosynostosis were treated at 6 months of age by expanding and re-shaping the posterior cranial vault with a switch cranioplasty (fig.3). Both metopic and unicoronal craniosynostosis were treated with anterior vault expansion via frontal-orbital advancement between 9 and 12 months of age (fig.4 a&b). Operations were performed by one of two pediatric neurosurgeons in conjunction with one of two craniofacial plastic surgeons.
Figure 1.
Intra-operative view of markings for modified pi procedure for early treatment of sagittal craniosynostosis. The patient is positioned face down on a Mayfield headrest.
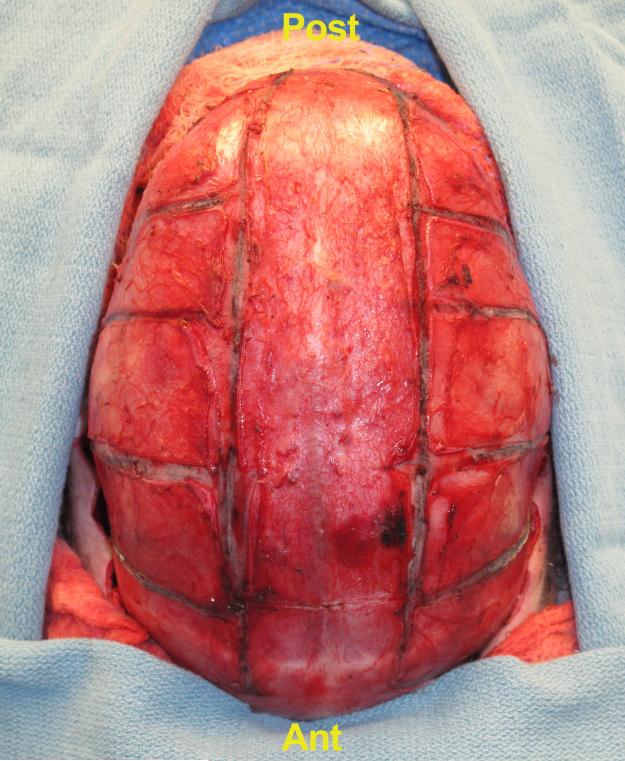
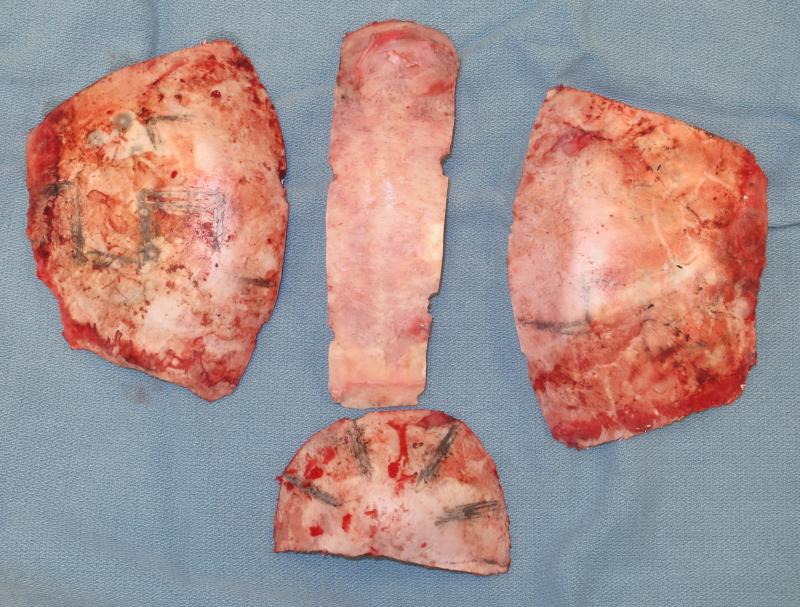
Figure 2.
Intra-operative view of bony segments removed during total cranial vault expansion for late treatment of sagittal craniosynostosis. These bones are re-shaped, expanded and replaced in situ to correct scaphocephaly.
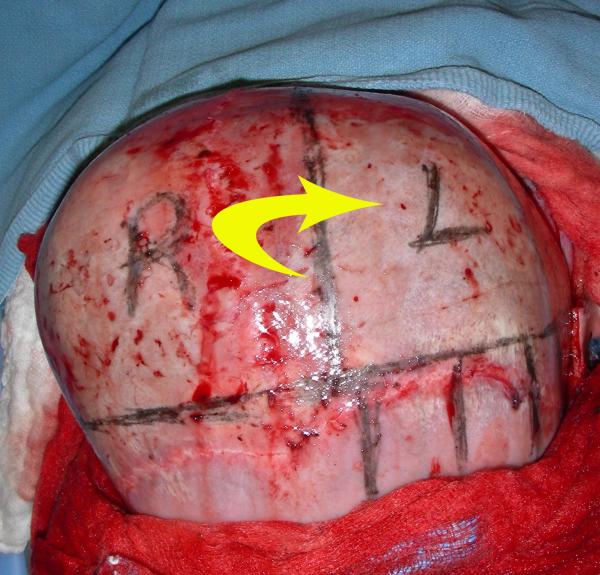
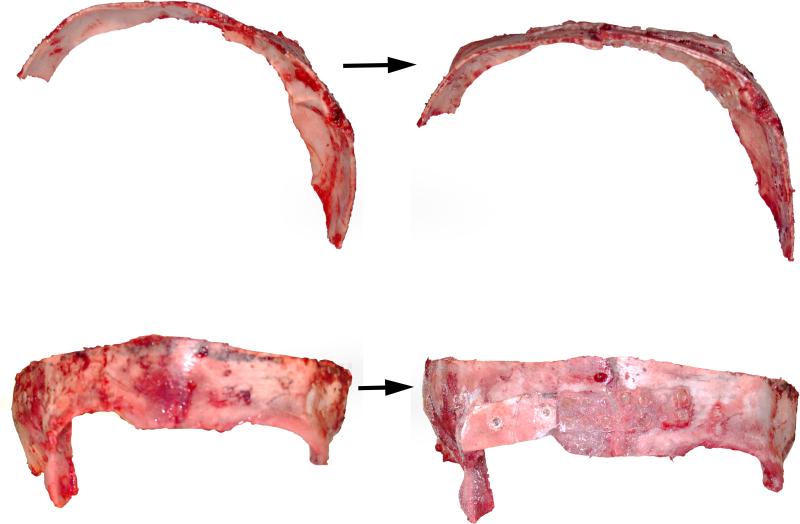
Figure 3.
Intra-operative markings for switch cranioplasty for treatment of left lambdoid craniosynostosis. After craniotomy, the two occipital halves are rotated and exchanged to correct the posterior plagiocephaly.
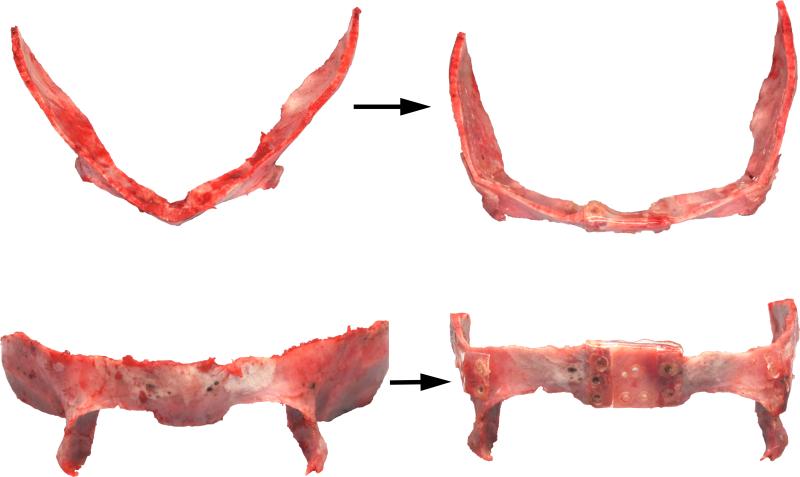
Figure 4a.
Intra-operative view of the orbital bandeau before (left) and after (right) reshaping for treatment of metopic craniosynostosis. The bandeau is widened with an interpositional calvarial bone graft and the lateral orbits are advanced with closing osteotomies to correct the trigonocephaly.
Figure 4b.
Intra-operative view of the orbital bandeau before (left) and after (right) reshaping for treatment of right unicoronal craniosynostosis. The bandeau is untwisted to advance the affected side and setback the “unaffected” side. The lateral orbit on the affected side is advanced with a closing osteotomy. The harlequin eye is corrected by shortening the lateral orbit and dropping the superior orbit with an onlay bone graft to correct the anterior plagiocephaly.
INTRA-OPERATIVE CARE
On the day of surgery, anesthesia was provided by a pediatric anesthesiologist who is a member of the craniofacial group. After induction of anesthesia, intravenous access was obtained (generally with two peripheral catheters), along with intra-arterial access. The endotracheal tube was placed and secured with a circum-mandibular wire; hyperventilation was initiated to reduce intracranial pressure until craniotomies were complete. Cross-matched packed red cells and fresh frozen plasma were available at the start of the case and generally, the first unit of blood was begun at the time of the first burr-hole craniotomy unless otherwise indicated. Administration of blood components was performed throughout the procedure based on vital signs, serial iSTAT® (Abbott Laboratories, Princeton, NJ) hematocrits, and other laboratory tests as well as observed blood loss.
Patients with sagittal craniosynostosis underwent a variation of the pi procedure described by Jane40, then modified by others41-49. The patient was placed prone, the scalp reflected, and parasagittal osteotomies were made to elevate the central strip of bone containing the fused suture. The dura was separated from the lambdoid and coronal sutures and multiple barrel stave osteotomies were made around these and within the parietal bones. Opening osteotomies were made along the occiput to flatten it and the barrel staves are outfractured just inferior to the squamosal suture. The central strip of bone was then shortened, rounded, and replaced in situ. The barrel staves were then shaped with a dissolvable suture, or fixed with resorbable plates. A sub-galeal Jackson-Pratt drain is placed in all patients and the scalp is closed in layers.
If a patient with sagittal craniosynostosis presented late (after 9 months), the surgeons expanded the cranial vault without utilizing the modified pi procedure. Depending on the location of the deformity, either the anterior and middle cranial vault were expanded or the posterior and middle cranial vaults using a comb expansion cranioplasty.
Patients with lambdoid craniosynostosis were also placed in prone position. After making the coronal incision and reflecting the scalp flaps, osteotomies are designed from the torcula posteriorly to the vertex anteriorly. The posterior calvarium is removed and halved. The two sides are then switched and fixated in an expanded, more rounded position using a resorbable plating system. The mastoid bulge is corrected with barrel stave osteotomies as is parietal asymmetry.
Patients with metopic or unicoronal craniosynostosis were positioned in the supine position for a frontal orbital advancement. The scalp was first reflected in the sub-periosteal plane, raising the temporalis muscles up with the scalp flap. The orbits were then freed superiorly from medial canthal tendon to the inferio-lateral orbital floor. C-shaped cuts were made from the inferior orbital rim up the lateral sidewall to the skull base. The bandeau was advanced at the affected supraorbital rim (unicoronal) or supraorbital rims (metopic) to correct brow flattening and better protect the globe. In metopic craniosynostosis, the bandeau was widened with bone grafts. The bandeau was then advanced and fixated with stainless steel wires at the lateral orbits and nasion and with resorbable plates at the temporal extensions. Further points of fixation were made by creating buttress grafts which extend from the sphenoid bone to the bandeau along the orbital roof. The frontal bones was then reshaped and fixated to the bandeau and parietal bones with wires and/or resorbable sutures.
POST-OPERATIVE CARE
Upon completion of the operation, the patient's airway was extubated and they were admitted to the ICU for close monitoring. Laboratory evaluations were obtained at the discretion of the intensivists based on hemodynamics, drain output, and the decision to transfuse blood products was made by the team based on the same parameters. Generally, a hematocrit of ≤20%, an INR of ≥1.4, a platelet count of ≤100,000/cm3, a fibrinogen of <100 mg% and/or active bleeding prompted transfusion of appropriate blood products. Once stable, patients were transferred from the ICU to the surgical in-patient ward to complete their recovery. Criteria for hospital discharge included adequate po intake, adequate po analgesia, discontinuance of the jp drain, declining edema such that at least one eye can be opened, and general medical stability.
DATA ANALYSIS
Patients within the cohort were grouped into the following categories based on diagnosis and operation performed: 1. Modified pi procedure 2. Total cranial vault expansion 3. Posterior vault switch cranioplasty, and 4. Frontal orbital advancement (FOA). Age and weight were determined at time of surgery as was the pre-operative hematocrit. Operative notes, anesthesia records and nurse's notes were reviewed to determine intra-operative complications and to estimate blood loss. Blood products transfused during and after surgery were recorded as were laboratory values. Postoperative events and laboratory results were extracted from the medical record into a standard data sheet (Table 1)
Table 1.
Data points collected by independent anesthesiologists, then compared for accuracy. Intra-operative events/PACU and complications
| Low O2 Saturation/O2 tension (< 80%) |
| Low HCT (25 or less) |
| Low PH/acidosis (7.20 or less) |
| Low Glucose (< 60) |
| Laboratory coagulopathy (INR > 1.5, platelets < 100, fibrinogen < 100) |
| Maintenance FiO2 (check one) |
| Air embolism |
| Hypothermia (T < 35 degrees) |
| Hypotension requiring pressor or resuscitation |
| Cardiac arrest requiring pressor or resuscitation |
| Complete extubation requiring reintubation and/or repositioning |
| Timing of planned |
| extubation: |
| Other: |
| Post-operative events (24 hour period after surgery) |
|---|
| Unplanned extubation requiring immediate reintubation |
| Respiratory failure requiring reintubation & ventilator support: |
| Hypotension requiring pressor resuscitation |
| Air embolism |
| Seizures |
| Abnormal CT scan |
| Notes on CT scan: |
| Sepsis |
| Laboratory coagulopathy |
| Other: |
| Post-operative events (during remainder of hospital stay) |
|---|
| Re-admit to ICU |
| Respiratory failure requiring reintubation & ventilator support |
| Seizures |
| Sepsis |
| Other: |
RESULTS
Of the eighty eight patients who fulfilled the inclusion criteria, 58% were male (n=51); 48% had sagittal (n=42), 26% metopic (n=23), 22% unicoronal (n=19), and 4% (n=4) had lambdoid craniosynostosis (Table 2). Forty two patients underwent frontal orbital advancement (FOA) at 10.5 months on average, 39 underwent modified pi procedure (average age 4.5months), 3 underwent total cranial vault(average age 9.75 months), and 4 underwent a posterior switch cranioplasty (average age 6 months).
Table 2.
Demographics
| cases in cohort = 88 | number | percentage |
|---|---|---|
| male | 51 | 58% |
| female | 37 | 42% |
| sagittal synostosis | 42 | 47% |
| metopic | 23 | 26% |
| unicoronal | 19 | 22% |
| lambdoid | 4 | 5% |
Length of procedure was recorded from time of induction until extubation and also varied between procedures (Table 3): FOA 5.2 hours; Modified pi 2.5 hours; Total Vault 4.9 hours; Switch Cranioplasty 4.6 hours. Blood loss was estimated by nursing records (suction canisters, sponge count) and anesthesia record (intra-operative hematocrit, hemodynamics and transfusion amounts) and the average differed between procedures as follows: FOA 385 mL; Modified pi 216 mL; Total Vault 600 mL; Switch Cranioplasty 207 mL. Intra-operative transfusion amounts of both packed cells (pRBC) and plasma (FFP) depended on procedure: FOA 385 mL pRBC and 110 mL FFP, Modified pi 200mL/33 mL, Total Vault 480 mL/165 mL, Switch Cranioplasty 385 mL/110 mL. No patients received platelets or cryoprecipitate intraoperatively. All but one patient received a transfusion of pRBC, FFP or both(99%). (Table 3)
Table 3.
Comparison of surgical timing, duration, blood loss and transfusion amounts between various open cranial vault procedures.
| Age at surgery (mos) | Duration (hours) | EBL (mL) | pRBC | FFP | platelets | |
|---|---|---|---|---|---|---|
| Modified Pi | 4.5 | 2.5 | 216 | 200 | 33 | 0 |
| Total Vault | 9.75 | 4.9 | 600 | 480 | 165 | 0 |
| FOA | 10.5 | 5.2 | 385 | 385 | 110 | 0 |
| Switch | 6 | 4.6 | 207 | 282 | 0 | 0 |
Fortunately, there were no deaths and no major intra-operative complications (Table 2). One patient unintentionally extubated; another desaturated (FiO2 < 80%) and became acidotic (pH < 7.2). Nine patients (10%) became hypothermic (T<35°C), usually during induction prior to draping. Two patients were coagulopathic by lab values and 55 (63%) had a hematocrit of 25 or less at some time during the operation. There were no transfusion reactions (Table 4).
Table 4.
Comparison of intra-operative events between procedures.
| Pi | Total Vault | FOA | Switch | |
|---|---|---|---|---|
| O2 sat < 80% | 0 | 0 | 1** | 0 |
| Hct < 25 | 25 | 1 | 25 | 4 |
| pH < 7.2 | 0 | 0 | 1** | 0 |
| glucose <60 | 0 | 0 | 0 | 0 |
| laboratory coagulopathy* | 1 | 0 | 1 | 0 |
| hypothermia (T<35 C) | 6 | 0 | 2 | 1 |
| extubation | 0 | 0 | 1** | 0 |
| hypotension (requiring pressors) | 0 | 0 | 0 | 0 |
| air embolism | 0 | 0 | 0 | 0 |
| cardiac arrest | 0 | 0 | 0 | 0 |
laboratory coagulopathy = INR >1.5, platelets < 100, fibrinogen < 100
represents a single patient who experienced an unplanned extubation, desaturated and became temporarily acidotic.
Post-operatively, patients stayed in the ICU for just over 1 day (1.2-1.5 days, mean = 1.4) and in the hospital for just over 3 days (3.2-3.6, mean = 3.4). There were no major post-operative complications (Table 5). One patient became apneic after a morphine bolus and required Narcan and readmission to the ICU and one patient had a seizure. One scalp hematoma was drained in the ICU, another was re-admitted on POD #4 with elevated temperature and was found to have transiently increased liver function tests, requiring no intervention. Post-operatively, 34% of the patients received a transfusion of blood, FFP, or both (Table 6). No patients returned to the operating room within the first 6 months after cranial vault expansion.
Table 5.
Comparison of post-operative events between procedures.
| Pi | Total Vault | FOA | Switch | |
|---|---|---|---|---|
| readmission to ICU | 1* | 0 | 0 | 0 |
| unplanned extubation | 0 | 0 | 0 | 0 |
| respiratory failure | 1* | 0 | 0 | 0 |
| hypotension | 0 | 0 | 0 | 0 |
| seizure | 0 | 0 | 1 | 0 |
| sepsis | 0 | 0 | 0 | 0 |
| laboratory coagulopathy | 11 | 2 | 11 | 0 |
this patient had respiratory failure due to morphine overdose and required readmission to the ICU
Table 6.
Number of patients receiving post-operative transfusions by surgery type.
| pRBC | FFP | Platelets | Cryoprecipitate | |
|---|---|---|---|---|
| Pi | 10 | 7 | 0 | 0 |
| Total Vault | 1 | 0 | 0 | 0 |
| FOA | 2 | 10 | 1 | 0 |
| Switch | 0 | 0 | 0 | 0 |
DISCUSSION
Open cranial vault expansion has been the standard of care for treatment of craniosynostosis for over 4 decades. It has proven effective in treating the functional and aesthetic deformities of the various single suture craniosynostoses51-56. The safety of the open procedure seems to have improved since its first description, but recent research into the safety of these procedures has relied upon surveys and surgeon recall rather than rigorously investigating the factors contributing to safety57.
In this study, every pre-op, intra-op and post op record was reviewed twice by unbiased anesthesiologists to record the data elements in Table 1 as well as all medications administered to the patients throughout their care. All variables which affect safety were reported here. In doing so, we aim to provide a rigorous analysis of the safety of open cranial vault surgery and the factors which lead to it.
In this cohort, there were no major intra-operative complications. In one patient, the endotracheal tube became dislodged and the patient required prompt re-intubation. The operation was then completed without further modification. More commonly, patients became hypothermic between induction and surgical draping. Although no patients found to be hypothermic went on to become coagulopathic, these may be causally related58 so the team has instituted use of heating lamps during this portion of the procedure to maintain euthermia.
Nearly all patients received transfusion of blood products. The anesthesia team has made a conscious effort to transfuse patients early, rather than after they have become hypovolemic, anemic or coagulopathic59, because previous studies regarding safety of cranial vault surgery have found under-resuscitation a primary factor leading to intra-operative complications60. Thus, since 2007, our anesthesia team has initiated blood transfusion at time of the craniotomy regardless of baseline hematocrit and has utilized plasma in addition to crystalloid to maintain intravascular volume toward the end of the case to minimize dilution coagulopathy. While this approach clearly increases our transfusion rate, it may contribute positive safety profile in this cohort.
Transfusion risks are fairly well known and often cited as an impetus to avoiding transfusions61, 62 and altering surgical techniques10, 30, 63 in craniofacial surgery. Current transfusion risks range from 1:5000 (TRALI) to 1:2,000,000 (HIV). While no transfusion complications occurred in this cohort, the sample size is admittedly small given the published incidence. That being said, no reported disease transmission has occurred at the Puget Sound Blood Center since testing began in 198564.
Much as surgical techniques for craniosynostosis have evolved over the decades, so too has the overall care provided to patients being treated with cranial vault expansion. In the past, solo practitioners may have been responsible for making the diagnosis, creating the surgical plan, implementing it, and then providing post-operative care. Increasingly, children with craniosynostosis are being treated through experienced, multi-disciplinary teams who create standardized protocols for pre-operative assessment, intra-operative care and post-operative recovery. Team care at SCH involves a standardized protocol of pre-operative imaging, blood work and consultation with anesthesiology and the blood bank in attempt to minimize complications. During surgery, the anesthesiologist and operating room staff are selected from a “craniofacial OR team” in order to maximize the experience level of all providers involved in the procedure. After surgery, ICU admission and specific discharge criteria are additional components instituted to maximize safety. Our team's approach to patients with craniosynostosis capitalizes upon the involvement of multiple experienced practitioners from the time of initial diagnosis until treatment is complete in order to maximize safety.
Since the creation of craniofacial surgery by Dr. Tessier, surgeons have continually modified and improved their technique in order to minimize risk to the patient. Equally important has been the development of a team approach. Our study's lack of a direct comparison cohort of patients cared for outside of the team setting prohibits the conclusion that the multidisciplinary approach is safer. Further studies directly comparing the outcomes of patients with single suture craniosynostosis treated within a team and those treated by solo practitioners are not only necessary, they may be mandatory in the future environment of managed or capitated health care. We believe that the team approach, with development of protocols and adherence to continual product improvement, contributed to the overall safety of cranial vault surgery in this cohort. Also not emphasized here, but likely as important is the overall number of cranial vault procedures performed per annum as high volume centers have shown improved outcomes in the treatment of other disorders65-71.
This study presents only a percentage of all the patients treated for craniosynostosis during this timeframe at our institution. Some may argue that the true risk of open cranial vault surgery is actually higher if syndromic and multi-suture craniosynostosis patients were included. We would agree as these cases tend to be more variable, more likely to display elevated intracranial pressure and often have other comorbidities most notably involving the airway. But, these patients are precisely the ones who would benefit most from multidisciplinary team care, peri-operative protocols and the experience provided by a high volume center. Surgical techniques will continue to evolve in order to improve patient safety. So, too must the craniofacial team evolve as they provide care before, during, and after cranial vault surgery.
REFERENCES
- 1.Lane L. Pioneer craniectomy for relief of mental imbecility due to premature sutural closure and microcephalus. JAMA. 1892;18:49–50. [Google Scholar]
- 2.Jacobi A. Non nocere. Med Red. 1894;45:609. [Google Scholar]
- 3.Faber H, Towne EB. Early operation in premature craniosynostosis for the prevention of blindness and other sequelae. Five case reports with follow up. Journal of Pediatrics. 1943;22:286–307. [Google Scholar]
- 4.Teng P. Premature synostosis of the sagittal suture, and its treatment. A modification of the linear craniectomy and the use of synthetic fabrics. J Neurosurg. 1962;19:1094–1097. doi: 10.3171/jns.1962.19.12.1094. [DOI] [PubMed] [Google Scholar]
- 5.Tessier P. [Total facial osteotomy. Crouzon's syndrome, Apert's syndrome: oxycephaly, scaphocephaly, turricephaly]. Ann Chir Plast. 1967;12:273–286. [PubMed] [Google Scholar]
- 6.Kohan E, Wexler A, Cahan L, et al. Sagittal synostotic twins: reverse pi procedure for scaphocephaly correction gives superior result compared to endoscopic repair followed by helmet therapy. J Craniofac Surg. 2008;19:1453–1458. doi: 10.1097/SCS.0b013e3181897390. [DOI] [PubMed] [Google Scholar]
- 7.Barone CM, Jimenez DF. Endoscopic craniectomy for early correction of craniosynostosis. Plast Reconstr Surg. 1999;104:1965–1973. doi: 10.1097/00006534-199912000-00003. discussion 1974-1965. [DOI] [PubMed] [Google Scholar]
- 8.Barone CM, Jimenez DF. Endoscopic approach to coronal craniosynostosis. Clin Plast Surg. 2004;31:415–422. vi. doi: 10.1016/j.cps.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Cartwright CC, Jimenez DF, Barone CM, et al. Endoscopic strip craniectomy: a minimally invasive treatment for early correction of craniosynostosis. J Neurosci Nurs. 2003;35:130–138. doi: 10.1097/01376517-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez DF, Barone CM. Endoscopy-assisted wide-vertex craniectomy, “barrel-stave” osteotomies, and postoperative helmet molding therapy in the early management of sagittal suture craniosynostosis. Neurosurg Focus. 2000;9:e2. doi: 10.3171/foc.2000.9.3.3. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez DF, Barone CM. Early treatment of anterior calvarial craniosynostosis using endoscopic-assisted minimally invasive techniques. Childs Nerv Syst. 2007;23:1411–1419. doi: 10.1007/s00381-007-0467-6. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez DF, Barone CM, Cartwright CC, et al. Early management of craniosynostosis using endoscopic-assisted strip craniectomies and cranial orthotic molding therapy. Pediatrics. 2002;110:97–104. doi: 10.1542/peds.110.1.97. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez DF, Barone CM, McGee ME, et al. Endoscopy-assisted wide-vertex craniectomy, barrel stave osteotomies, and postoperative helmet molding therapy in the management of sagittal suture craniosynostosis. J Neurosurg. 2004;100:407–417. doi: 10.3171/ped.2004.100.5.0407. [DOI] [PubMed] [Google Scholar]
- 14.Persing J. Endoscopy-assisted craniosynostosis. J Neurosurg. 2004;100:403–404. doi: 10.3171/ped.2004.100.5.0403. discussion 404-406. [DOI] [PubMed] [Google Scholar]
- 15.Stelnicki E, Heger I, Brooks CJ, et al. Endoscopic release of unicoronal craniosynostosis. J Craniofac Surg. 2009;20:93–97. doi: 10.1097/SCS.0b013e318190e2a6. [DOI] [PubMed] [Google Scholar]
- 16.Stelnicki EJ. Endoscopic treatment of craniosynostosis. Atlas Oral Maxillofac Surg Clin North Am. 2002;10:57–72. doi: 10.1016/s1061-3315(01)00007-5. [DOI] [PubMed] [Google Scholar]
- 17.MacKinnon S, Rogers GF, Gregas M, et al. Treatment of unilateral coronal synostosis by endoscopic strip craniectomy or fronto-orbital advancement: Ophthalmologic findings. J AAPOS. 2009;13:155–160. doi: 10.1016/j.jaapos.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Murad GJ, Clayman M, Seagle MB, et al. Endoscopic-assisted repair of craniosynostosis. Neurosurg Focus. 2005;19:E6. doi: 10.3171/foc.2005.19.6.7. [DOI] [PubMed] [Google Scholar]
- 19.Lauritzen CG, Davis C, Ivarsson A, et al. The evolving role of springs in craniofacial surgery: the first 100 clinical cases. Plast Reconstr Surg. 2008;121:545–554. doi: 10.1097/01.prs.0000297638.76602.de. [DOI] [PubMed] [Google Scholar]
- 20.Windh P, Davis C, Sanger C, et al. Spring-assisted cranioplasty vs pi-plasty for sagittal synostosis--a long term follow-up study. J Craniofac Surg. 2008;19:59–64. doi: 10.1097/scs.0b013e31815c94c8. [DOI] [PubMed] [Google Scholar]
- 21.Davis C, Lauritzen CG. Spring-assisted remodeling for ventricular shunt-induced cranial deformity. J Craniofac Surg. 2008;19:588–592. doi: 10.1097/SCS.0b013e31816aaa60. [DOI] [PubMed] [Google Scholar]
- 22.Davis C, Windh P, Lauritzen CG. Adaptation of the cranium to spring cranioplasty forces. Childs Nerv Syst. 2009 doi: 10.1007/s00381-009-1026-0. [DOI] [PubMed] [Google Scholar]
- 23.Davis C, Windh P, Lauritzen CG. Spring-assisted cranioplasty alters the growth vectors of adjacent cranial sutures. Plast Reconstr Surg. 2009;123:470–474. doi: 10.1097/PRS.0b013e3181954ce3. [DOI] [PubMed] [Google Scholar]
- 24.Maltese G, Tarnow P, Lauritzen CG. Spring-assisted correction of hypotelorism in metopic synostosis. Plast Reconstr Surg. 2007;119:977–984. doi: 10.1097/01.prs.0000252276.46113.ee. [DOI] [PubMed] [Google Scholar]
- 25.Guimaraes-Ferreira J, Gewalli F, David L, et al. Spring-mediated cranioplasty compared with the modified pi-plasty for sagittal synostosis. Scand J Plast Reconstr Surg Hand Surg. 2003;37:208–215. doi: 10.1080/02844310310001823. [DOI] [PubMed] [Google Scholar]
- 26.Lauritzen C, Sugawara Y, Kocabalkan O, et al. Spring mediated dynamic craniofacial reshaping. Case report. Scand J Plast Reconstr Surg Hand Surg. 1998;32:331–338. doi: 10.1080/02844319850158697. [DOI] [PubMed] [Google Scholar]
- 27.Lao WW, Denny AD. Internal distraction osteogenesis to correct symptomatic cephalocranial disproportion. Plast Reconstr Surg. 126:1677–1688. doi: 10.1097/PRS.0b013e3181ef8f65. [DOI] [PubMed] [Google Scholar]
- 28.Sugawara Y, Uda H, Sarukawa S, et al. Multidirectional cranial distraction osteogenesis for the treatment of craniosynostosis. Plast Reconstr Surg. 126:1691–1698. doi: 10.1097/PRS.0b013e3181ef8fc8. [DOI] [PubMed] [Google Scholar]
- 29.Choi JW, Ra YS, Hong SH, et al. Use of distraction osteogenesis to change endocranial morphology in unilateral coronal craniosynostosis patients. Plast Reconstr Surg. 126:995–1004. doi: 10.1097/PRS.0b013e3181e6c4b7. [DOI] [PubMed] [Google Scholar]
- 30.Akizuki T, Komuro Y, Ohmori K. Distraction osteogenesis for craniosynostosis. Neurosurg Focus. 2000;9:e1. doi: 10.3171/foc.2000.9.3.2. [DOI] [PubMed] [Google Scholar]
- 31.do Amaral CM, Di Domizio G, Tiziani V, et al. Gradual bone distraction in craniosynostosis. Preliminary results in seven cases. Scand J Plast Reconstr Surg Hand Surg. 1997;31:25–37. doi: 10.3109/02844319709010502. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi S, Honda T, Saitoh A, et al. Unilateral coronal synostosis treated by internal forehead distraction. J Craniofac Surg. 1999;10:467–471. doi: 10.1097/00001665-199911000-00002. discussion 472. [DOI] [PubMed] [Google Scholar]
- 33.Toth BA, Kim JW, Chin M, et al. Distraction osteogenesis and its application to the midface and bony orbit in craniosynostosis syndromes. J Craniofac Surg. 1998;9:100–113. doi: 10.1097/00001665-199803000-00003. discussion 119-122. [DOI] [PubMed] [Google Scholar]
- 34.Tellado MG, Lema A. Coronal suturectomy through minimal incisions and distraction osteogenesis are enough without other craniotomies for the treatment of plagiocephaly due to coronal synostosis. J Craniofac Surg. 2009;20:1975–1977. doi: 10.1097/SCS.0b013e3181bd2cd6. [DOI] [PubMed] [Google Scholar]
- 35.White N, Evans M, Dover MS, et al. Posterior calvarial vault expansion using distraction osteogenesis. Childs Nerv Syst. 2009;25:231–236. doi: 10.1007/s00381-008-0758-6. [DOI] [PubMed] [Google Scholar]
- 36.Kearney RA, Rosales JK, Howes WJ. Craniosynostosis: an assessment of blood loss and transfusion practices. Can J Anaesth. 1989;36:473–477. doi: 10.1007/BF03005352. [DOI] [PubMed] [Google Scholar]
- 37.Meyer P, Renier D, Arnaud E, et al. Blood loss during repair of craniosynostosis. Br J Anaesth. 1993;71:854–857. doi: 10.1093/bja/71.6.854. [DOI] [PubMed] [Google Scholar]
- 38.Converse JM, Wood-smith D, McCarthy JG. Report on a series of 50 craniofacial operations. Plast Reconstr Surg. 1975;55:283–293. [PubMed] [Google Scholar]
- 39.Whitaker LA, Munro IR, Salyer KE, et al. Combined report of problems and complications in 793 craniofacial operations. Plast Reconstr Surg. 1979;64:198–203. doi: 10.1097/00006534-197908000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Jane JA, Edgerton MT, Futrell JW, et al. Immediate correction of sagittal synostosis. J Neurosurg. 1978;49:705–710. doi: 10.3171/jns.1978.49.5.0705. [DOI] [PubMed] [Google Scholar]
- 41.Boulos PT, Lin KY, Jane JA, Jr., et al. Correction of sagittal synostosis using a modified Pi method. Clin Plast Surg. 2004;31:489–498. vii. doi: 10.1016/j.cps.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Guimaraes-Ferreira J, Gewalli F, David L, et al. Clinical outcome of the modified pi-plasty procedure for sagittal synostosis. J Craniofac Surg. 2001;12:218–224. doi: 10.1097/00001665-200105000-00003. discussion 225-216. [DOI] [PubMed] [Google Scholar]
- 43.Boop FA, Chadduck WM, Shewmake K, et al. Outcome analysis of 85 patients undergoing the pi procedure for correction of sagittal synostosis. J Neurosurg. 1996;85:50–55. doi: 10.3171/jns.1996.85.1.0050. [DOI] [PubMed] [Google Scholar]
- 44.Albright AL, Towbin RB, Shultz BL. Long-term outcome after sagittal synostosis operations. Pediatr Neurosurg. 1996;25:78–82. doi: 10.1159/000121101. [DOI] [PubMed] [Google Scholar]
- 45.Epstein N, Epstein F, Newman G. Total vertex craniectomy for the treatment of scaphocephaly. Childs Brain. 1982;9:309–316. doi: 10.1159/000120068. [DOI] [PubMed] [Google Scholar]
- 46.Fata JJ, Turner MS. The reversal exchange technique of total calvarial reconstruction for sagittal synostosis. Plast Reconstr Surg. 2001;107:1637–1646. doi: 10.1097/00006534-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Greene CS, Jr., Winston KR. Treatment of scaphocephaly with sagittal craniectomy and biparietal morcellation. Neurosurgery. 1988;23:196–202. doi: 10.1227/00006123-198808000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Pensler JM, Ciletti SJ, Tomita T. Late correction of sagittal synostosis in children. Plast Reconstr Surg. 1996;97:1362–1367. doi: 10.1097/00006534-199606000-00005. discussion 1368-1370. [DOI] [PubMed] [Google Scholar]
- 49.Weinzweig J, Baker SB, Whitaker LA, et al. Delayed cranial vault reconstruction for sagittal synostosis in older children: an algorithm for tailoring the reconstructive approach to the craniofacial deformity. Plast Reconstr Surg. 2002;110:397–408. doi: 10.1097/00006534-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Fearon JA, Ruotolo RA, Kolar JC. Single sutural craniosynostoses: surgical outcomes and long-term growth. Plast Reconstr Surg. 2009;123:635–642. doi: 10.1097/PRS.0b013e318195661a. [DOI] [PubMed] [Google Scholar]
- 52.Anderson PJ, David DJ. Late results after unicoronal craniosynostosis correction. J Craniofac Surg. 2005;16:37–44. doi: 10.1097/00001665-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Hansen M, Padwa BL, Scott RM, et al. Synostotic frontal plagiocephaly: anthropometric comparison of three techniques for surgical correction. Plast Reconstr Surg. 1997;100:1387–1395. doi: 10.1097/00006534-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Cohen SR, Maher H, Wagner JD, et al. Metopic synostosis: evaluation of aesthetic results. Plast Reconstr Surg. 1994;94:759–767. [PubMed] [Google Scholar]
- 55.Polley JW, Charbel FT, Kim D, et al. Nonsyndromal craniosynostosis: longitudinal outcome following cranio-orbital reconstruction in infancy. Plast Reconstr Surg. 1998;102:619–628. discussion 629-632. [PubMed] [Google Scholar]
- 56.Hilling DE, Mathijssen IM, Mulder PG, et al. Long-term aesthetic results of frontoorbital correction for frontal plagiocephaly. J Neurosurg. 2006;105:21–25. doi: 10.3171/ped.2006.105.1.21. [DOI] [PubMed] [Google Scholar]
- 57.Czerwinski M, Hopper RA, Gruss J, Fearon JA. Major morbidity and mortality rates in craniofacial surgery: an analysis of 8101 major procedures. Plast Reconstr Surg. 2010;126:181–186. doi: 10.1097/PRS.0b013e3181da87df. doi:10.1097/PRS.0b013e3181da87df. [DOI] [PubMed] [Google Scholar]
- 58.Lier H, Krep H, Schroeder S, et al. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65:951–960. doi: 10.1097/TA.0b013e318187e15b. [DOI] [PubMed] [Google Scholar]
- 59.Williams GD, Ellenbogen RG, Gruss JS. Abnormal coagulation during pediatric craniofacial surgery. Pediatr Neurosurg. 2001;35:5–12. doi: 10.1159/000050378. [DOI] [PubMed] [Google Scholar]
- 60.Munro IR, Sabatier RE. An analysis of 12 years of craniomaxillofacial surgery in Toronto. Plast Reconstr Surg. 1985;76:29–35. doi: 10.1097/00006534-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Fearon JA. Reducing allogenic blood transfusions during pediatric cranial vault surgical procedures: a prospective analysis of blood recycling. Plast Reconstr Surg. 2004;113:1126–1130. doi: 10.1097/01.prs.0000110324.31791.5c. [DOI] [PubMed] [Google Scholar]
- 62.Fearon JA, Weinthal J. The use of recombinant erythropoietin in the reduction of blood transfusion rates in craniosynostosis repair in infants and children. Plast Reconstr Surg. 2002;109:2190–2196. doi: 10.1097/00006534-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Hahn JF, Trusso R, Levy WJ. Craniosynostectomy with reduced blood loss. Neurosurgery. 1981;8:209–211. doi: 10.1227/00006123-198102000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Meghan Delaney D., MPH . personal communication with Craig Birgfeld. Seattle, WA: 2011. Acting Assistant Professor, University of Washington Assistant Medical Director, Puget Sound Blood Center. [Google Scholar]
- 65.Brattstrom V, Molsted K, Prahl-Andersen B, et al. The Eurocleft study: intercenter study of treatment outcome in patients with complete cleft lip and palate. Part 2: craniofacial form and nasolabial appearance. Cleft Palate Craniofac J. 2005;42:69–77. doi: 10.1597/02-119.2.1. [DOI] [PubMed] [Google Scholar]
- 66.Friede H, Lilja J. The Eurocleft Study: Intercenter study of treatment outcome in patients with complete cleft lip and palate. Cleft Palate Craniofac J. 2005;42:453–454. doi: 10.1597/05-038.1. author reply 454. [DOI] [PubMed] [Google Scholar]
- 67.Molsted K, Brattstrom V, Prahl-Andersen B, et al. The Eurocleft study: intercenter study of treatment outcome in patients with complete cleft lip and palate. Part 3: dental arch relationships. Cleft Palate Craniofac J. 2005;42:78–82. doi: 10.1597/02-119.3.1. [DOI] [PubMed] [Google Scholar]
- 68.Rosenstein SW, Grasseschi M, Dado D. The Eurocleft Study: Intercenter study of treatment outcome in patients with complete cleft lip and palate. Cleft Palate Craniofac J. 2005;42:453. doi: 10.1597/05-029.1. author reply 454. [DOI] [PubMed] [Google Scholar]
- 69.Semb G, Brattstrom V, Molsted K, et al. The Eurocleft study: intercenter study of treatment outcome in patients with complete cleft lip and palate. Part 1: introduction and treatment experience. Cleft Palate Craniofac J. 2005;42:64–68. doi: 10.1597/02-119.1.1. [DOI] [PubMed] [Google Scholar]
- 70.Semb G, Brattstrom V, Molsted K, et al. The Eurocleft study: intercenter study of treatment outcome in patients with complete cleft lip and palate. Part 4: relationship among treatment outcome, patient/parent satisfaction, and the burden of care. Cleft Palate Craniofac J. 2005;42:83–92. doi: 10.1597/02-119.4.1. [DOI] [PubMed] [Google Scholar]
- 71.Shaw WC, Brattstrom V, Molsted K, et al. The Eurocleft study: intercenter study of treatment outcome in patients with complete cleft lip and palate. Part 5: discussion and conclusions. Cleft Palate Craniofac J. 2005;42:93–98. doi: 10.1597/02-119.5.1. [DOI] [PubMed] [Google Scholar]