Abstract
Mechanical ventilation (MV) is an important aspect in the intraoperative and early postoperative management of lung transplant (LTx)-recipients. There are no randomized-controlled trials of LTx-recipient MV strategies; however there are LTx center experiences and international survey studies reported. The main early complication of LTx is primary graft dysfunction (PGD), which is similar to the adult respiratory distress syndrome (ARDS). We aim to summarize information pertinent to LTx-MV, as well as PGD, ARDS, and intraoperative MV and to synthesize these available data into recommendations. Based on the available evidence, we recommend lung-protective MV with low-tidal-volumes (≤6 mL/kg predicted body weight [PBW]) and positive end-expiratory pressure for the LTx-recipient. In our opinion, the MV strategy should be based on donor characteristics (donor PBW as a parameter of actual allograft size), rather than based on recipient characteristics; however this donor-characteristics-based protective MV is based on indirect evidence and requires validation in prospective clinical studies.
Keywords: Lung transplantation, primary graft dysfunction, acute respiratory distress syndrome, mechanical ventilation, tidal volume, lung protective ventilation, ventilator induced lung injury
INTRODUCTION
Lung transplantation (LTx) is an important treatment option for select patients with end-stage pulmonary disease. Remarkable progress has been made since the modern LTx era began in 19831. The field of LTx has grown rapidly over the last thirty years with improved surgical techniques and medical management strategies2,3. However there is little information on mechanical ventilation (MV) strategies after LTx, and no guidelines specific to this setting exist4,5.
Primary graft dysfunction (PGD) represents one of the most common complications observed in the early period following LTx with incidence rates between 10% and 57%6,7. PGD is clinically and histologically analogous to the acute respiratory distress syndrome (ARDS)7,8, and results from a variety of often simultaneously contributing insults. It is characterized by diffuse pulmonary infiltrates with an abnormal oxygen requirement occurring within 72 hours of transplantation6,7. Histologic examination in PGD shows diffuse alveolar damage7. Severe PGD represents both the main risk factor for early mortality after LTx as well as a risk factor for the development of bronchiolitis obliterans syndrome, which is the primary late complication limiting long-term survival of LTx patients6,7,9. Therefore, interventions that reduce the rates of PGD could improve both short-term and long-term outcomes for LTx recipients. Management of MV may present an opportunity for such an intervention. Evolving approaches to MV for patients at risk for ARDS and patients with ARDS have resulted in tangible improvements in outcomes10–16. Lung-protective MV strategies incorporating low tidal volumes (VT) limit ventilator-induced lung injury (VILI), reduce morbidity in patients on MV and improve survival in patients with ARDS8,11,17–19. Guidelines embrace the use of lower VT in patients with ARDS17.
The benefits of a lung-protective MV strategy extend to patients at risk for ARDS13,20–23. Higher VT were associated with the development of ARDS in patients who came to the intensive care unit without ARDS but had risk factors for it22. Furthermore, in patients with no prior lung injury who received MV during cardiac surgery in the operating room, higher VT settings were associated with higher inflammatory mediator levels24. The IMPROVE study provided further evidence that even brief periods of intra-operative lung-protective ventilation result in lower rates of lung injury in surgical patients at intermediate to high risk of pulmonary complications25. While not specifically studied in the context of LTx, the tenets of lung-protective MV are likely generalizable to this conceptually similar setting, and in the absence of direct data, should inform MV strategies.
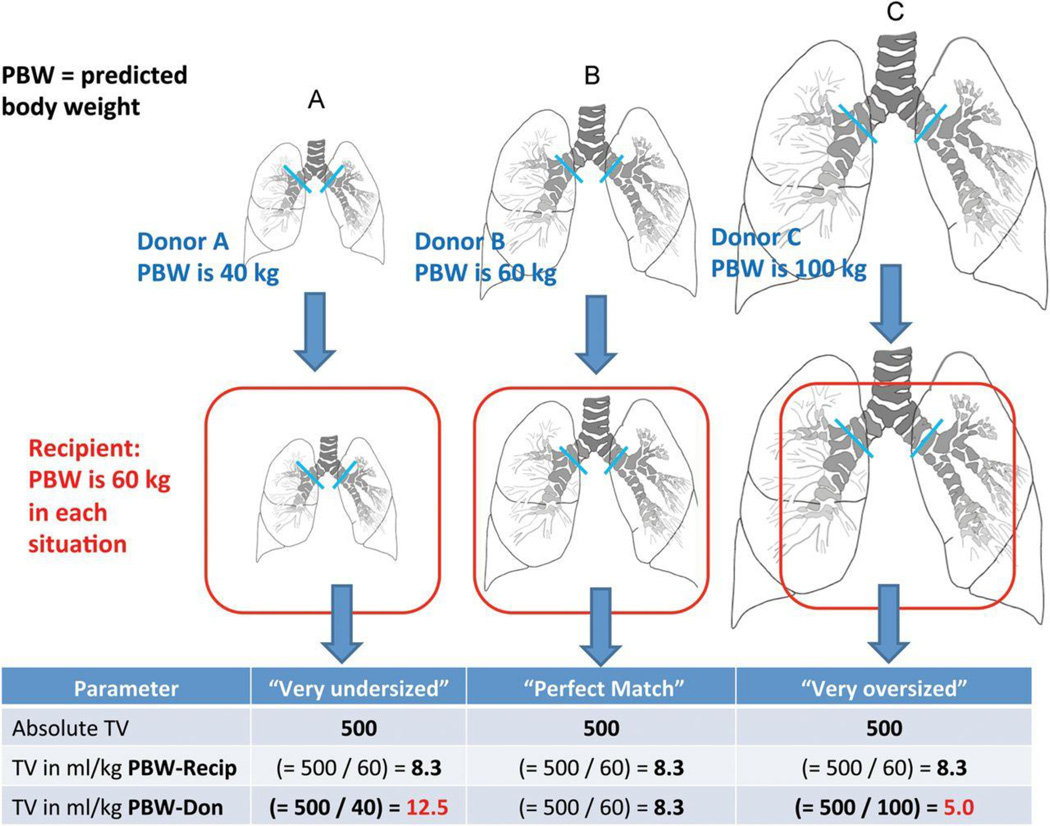
There are important differences between the LTx recipient and a general intra-operative or post-operative critically ill patient26,27. LTx recipients have mechanical impairments including: 1) a fresh thoracotomy wound that creates thoracic cage abnormalities, 2) frequent phrenic nerve dysfunction, and 3) pleural dysfunction28. The bronchial anastomoses sites and the allograft airway mucosa are prone to ischemia, poor healing, infection and subsequent anastomotic airway complications29. Another important aspect unique to LTx is that the size of the transplanted lungs can differ significantly from the size of the recipient’s thoracic cavity30–36, figure 1. In a study of bilateral LTx recipients, VT during MV were substantially higher if the allograft was undersized compared to oversized allografts, when VT were indexed to donor predicted body weight (as an estimate of the actual size of the allograft)37,38.
Figure 1.
Conceptual graphic on the possible effect of lung-size mismatch on mechanical ventilation tidal volumes expressed as mL/kg-predicted body weights of the donor. Reproduced with permission from Dezube et al37. Recip = Recipient; Don = Donor.
There are no randomized controlled trials (RCT) that address MV in the specific context of LTx. We will approach the review of MV of the LTx recipient by first providing a concise summary of potentially generalizable principles derived from key studies in critical care medicine and will then aim to synthesize these principles into strategies that incorporate the unique aspects of LTx12,39.
General principles
In the past MV strategies with VT of 10 to 15 mL/kg were commonly utilized both intraoperatively and in critically ill patients. VT of that size were believed to be necessary to prevent hypoxemia and atelectasis. However, mounting evidence from experimental and clinical studies consistently demonstrates that the application of high VT during MV may aggravate or cause lung injury40. MV using large VT can result in over-distention of alveoli and lead to ventilator-induced lung injury (VILI), which can amplify the risk for lung injury40,41. Lung-protective MV refers to the use of low VT and positive end-expiratory pressure (PEEP)11,18,19. The ARMA study (or tidal volume study), a RCT reported in 2000 by the NHLBI ARDS Network, provided landmark evidence to support a lung-protective MV strategy in the presence of ARDS11. Investigators in that trial examined an approach relating VT to estimated lung sizes expressed as milliliters (mL) per kilogram (kg) predicted body weight (PBW) and compared lung-protective low VT ventilation to conventional VT strategies11. VT targets of 6 mL/kg PBW and strategies limiting maximum allowable plateau pressure to 30 cm H2O were compared to VT targets of 12 mL/kg PBW with a maximum allowable plateau pressure of 50 cm H2O. The low VT strategy was associated with reduced 30-day mortality (31% versus 39.8%, p = 0.007)11. The timing of lung-protective ventilation is important for patients who already have ARDS10. ARDS patients who received lung-protective ventilation from the beginning of their lung injury had a lower mortality compared to patients who were initially given larger VT and then were changed to a protective strategy later in their ARDS course10. Each increase of 1 mL/kg PBW in initial VT was associated with a 23% increase in ICU mortality risk (adjusted hazard ratio 1.23, 95% confidence interval [CI] 1.06–1.44, p=0.008)10.
Open questions remain regarding the importance of limiting plateau pressure to < 30 cm H2O, limiting VT to 6 mLs/kg PBW, the optimal setting of PEEP and the role for recruitment maneuvers within the lung protective ventilation strategies for patients with ARDS18,19,42–47. However the benefits of a lung-protective MV strategy appear to extend even to patients without lung injury, but who are at risk for the development of ARDS13,20–23. Greater VT were associated with the development of ARDS in patients who came to the intensive care unit without ARDS but had risk factors for it20–22. In the context of donor management for transplant, a RCT compared low VT (6 mL/kg PBW) against a standard donor ventilation strategy (VT 10–12 mL/kg-PBW) and showed a significantly higher proportion of donor lungs from the low VT group could be utilized for LTx (54% versus 27%, P = 0.004)13. Based on the above evidence lung protective ventilation strategies should remain the preferred method of MV for most critically ill patients (with or without the presence of ARDS)17,22,23.
The principles of lung-protective low VT MV have recently been extended to even brief periods of MV, as required for general anesthesia during surgical procedures. Increasing evidence shows that in anesthetized patients without ARDS, lung-protective MV can lower the risk of pulmonary complications and ARDS24,25,48. The IMPROVE study, a RCT of lung-protective intra-operative MV, provided compelling evidence that lung-protective ventilation benefits surgical patients at intermediate to high risk of pulmonary complications25. The study demonstrated lower rates of pulmonary and extrapulmonary complications in the 7 days following surgery (27.5% versus 10.5%, p=0.001), when individuals received lung protective ventilation (VT = 6–8 mL/kg predicted body weight [PBW], PEEP = 6–8 cm H2O, and 30-second recruitment maneuvers of 30 cm H2O every 30 minutes) intraoperatively rather than conventional ventilation (VT = 10–12 mL/kg PBW, no PEEP, and no recruitment maneuvers)25. A recent meta-analysis of RCTs evaluated the effect of intraoperative lung-protective ventilation with lower VT on clinical outcomes in patients undergoing surgery48. This meta-analysis of 19 RCTs showed that anesthetized patients who received ventilation with lower VT during surgery had lower risks of lung injury and pulmonary infection than those who received conventional ventilation with higher VT48.
Lung transplant specific issues in mechanical ventilation of the recipient
Intraoperative considerations
There are several unique aspects regarding the intra-operative period during LTx49–53. Adult LTx can be performed with or without the use of cardiopulmonary bypass in the absence of severe pulmonary hypertension. An off bypass procedure is the preferred approach in many programs when feasible. Cardiopulmonary bypass is an independent predictor for the development of severe PGD in several studies6,38. To reduce the likelihood of requiring cardiopulmonary bypass, the least functional lung, as determined by preoperative quantitative ventilation and perfusion imaging, is usually resected and replaced first during a bilateral sequential LTx. Occasionally, patients with cystic fibrosis will have such voluminous purulent secretions that single lung ventilation, as required for an off-bypass LTx, can be difficult. Careful bronchoscopic airway clearance should be routinely done in the operating room before the start of the LTx in such patients with significant airway secretions. For a single LTx a lateral/anterior thoracotomy is performed. For a bilateral sequential LTx a clamshall incision or bilateral anterior thoracotomies are commonly used54. Alternatively, a median sternotomy can also be performed for bilateral lung transplantation on cardiopulmonary bypass. After implantation of the allograft it can be important to control the rate of reperfusion of the allograft by gradually releasing the clamp from the pulmonary artery to minimize reperfusion injury. During the period of single lung ventilation the entire cardiac output passes through the first implanted allograft, while the pulmonary artery on the contralateral side is clamped. Increased pulmonary blood flow results in greater sensitivity to develop VILI55. Consequently, careful attention to size of the VT can be especially important during this vulnerable period. We recommend VT of 6 mLs/kg-donor-PBW. The VT should be further adjusted for single lung ventilation by reducing VT approximately 50%. PEEP of +5 cm H2O should be used and in case of difficulties with oxygenation PEEP of up to +10 cm H2O can be considered. After rewarming of the allograft and following deflation episodes careful recruitment maneuvers to allow complete initial inflation are used by manual bag-inflation, while trying to avoid peak inspiratory pressure above 30 cm H2O. Since the lungs are visible in the operating field the anesthesiologist should be in close communication with the LTx-surgeon to assure that all atelectatic lungs areas are visibly seen as recruited. An association between increased FiO2 at reperfusion and a higher risk of severe PGD has been reported in several studies6,38. This suggests that using the lowest FiO2 to maintain appropriate partial pressure of oxygen in the arterial blood [(PaO2) > 70 mmHg] and hemoglobin oxygen saturations [(SpO2) > 92%] should be used. Many LTx recipients have significant pre-transplant chronic hypercarbia from their end-stage lung disease. Intraoperative permissive hypercapnia with pCO2 in pre-transplant range can be helpful to allow for optimal cerebral perfusion and to facilitate the use of low VT. However the allograft vasculature is often sensitive to elevated pCO2, which can cause vasoconstriction and elevated pulmonary arterial pressure and these factors need to be considered in the setting of permissive hypercapnia. Inhaled nitric oxide (iNO) or inhaled prostacyclin can be considered in case of pulmonary hypertension or to facilitate protective MV settings in case of significant PGD by improving oxygenation. However the routine use of iNO has no beneficial impact on outcomes56–58.
Several situations frequently necessitate the use of cardiopulmonary bypass during LTx. Patients with severe pulmonary hypertension, for example, are most safely transplanted on bypass. After allograft implantation while on bypass, protective resting ventilator settings should be used with VT 4–6 mLs/kg-donor-PBW (further reduced for single lung ventilation) and PEEP of +5 cm H2O. Before coming off cardiopulmonary bypass it can be helpful to bronchoscopically remove blood clots and secretions from the allograft airways to maximize allograft function and facilitate successful weaning from bypass59. More recently, veno-arterial extracorporeal membrane oxygenation (ECMO) has emerged as a valid alternative method of support and was associated with decreased rates of pulmonary and renal complications, as compared with cardiopulmonary bypass60. Occasionally the chest remains open following the LTx61. If pressure-assist-control MV modes are used in that setting, the pressure control should be carefully adjusted to assure lung protective low VT, as increased respiratory system compliance with an open chest is possible. Table 1 summarizes recommendations for the intraoperative MV of the LTX recipient.
Table 1.
Recommendations for intraoperative mechanical ventilation
| Off CPB transplant | On CPB |
|---|---|
|
|
CPB: Cardiopulmonary bypass; PEEP: Positive end expiratory pressure; FiO2: Fraction of inspired oxygen; PaO2: partial pressure of oxygen in the blood; PaCO2: Partial pressure of carbon dioxide in the blood
Postoperative considerations
The goals of controlled MV immediately following LTx are to protect the allografts from injury while improving function and facilitating early weaning and extubation.
Bilateral Lung Transplant
A bilateral LTx is the most common LTx in the modern era3. There are limited data on MV after a LTx, however, a murine model of LTx demonstrated that the mode of mechanical ventilation applied during the early phase of reperfusion influenced the severity of PGD62. A protective ventilatory strategy that minimized pulmonary mechanical stress by low VT was associated with less PGD and improved lung function after LTx. The study concluded that VILI might be an under-recognized phenomenon that contributes significantly to PGD after LTx and that protective ventilatory strategies with low VT could potentially lead to improved outcomes after LTx62. In a single-center observational cohort study, the implementation of a management guideline for respiratory and hemodynamic status within the first 72 hours after LTx resulted in less severe PGD63. The respiratory portion of the protocol was based on a lung-protective low VT ventilation strategy63. The study also gave parameters for hemodynamic support that emphasized the use of vasoactive drugs over fluid administration to maintain a lower central venous pressure63,64.
In an international survey of the LTx community, the majority of respondents indicated a preference for using lung-protective approaches to mechanical ventilation after LTx4. Low VT based on recipient characteristics were frequently chosen4. Donor characteristics often were not considered and frequently were not known by the team managing mechanical ventilation after LTx4. In a single-center study, the relationship between donor-recipient lung size mismatch and postoperative MV VT in a cohort of bilateral LTx patients was evaluated, figure 1. VT settings were expressed as absolute values (in mL) and also as fractions of recipient and donor PBW37. Postoperative absolute VT settings were comparable between subsets of patients with undersized, matched, and oversized allografts, and VT settings according to recipient PBW was also similar. VT settings according to donor PBW, however, revealed significant differences between undersized, matched, and oversized subsets (11.4 ± 3.1 versus 9.4 ± 1.2 versus 8.1 ± 2.1, respectively; P < 0.05)37. Thus, during mechanical ventilation after bilateral LTx, patients with undersized allografts received relatively greater VT compared to those with oversized allografts when VT was related to donor PBW (as an estimate of the actual allograft size). Postoperatively, a single-center report linked hyperinflation of undersized allografts (i.e., donor lungs smaller than recipient thorax) to an increased risk of early allograft failure65. The results of other studies have demonstrated that patients with undersized allografts had worse outcomes, specifically increased rates of PGD, tracheostomy and resource utilization30,38. In an ancillary study to the LTx outcomes group, an undersized allograft was associated with a significantly increased risk of ISHLT grade 3 PGD after bilateral LTx38. Furthermore, a series of studies revealed an association between undersized allografts and risk of first-year mortality30–36,38,66–69. The mechanisms associating an undersized allograft with a higher risk of PGD and a higher risk of first-year mortality are unclear. Hyperinflation of significantly undersized allografts by VT set according to recipient characteristics could increase the risk of VILI. A hypothesis generated from these investigations of lung size mismatch and clinical outcomes after LTx is that a lung-protective mechanical ventilation strategy based on estimates of the allograft size (i.e., donor PBW) could be protective for patients with undersized allografts. A clinical trial of allograft protective mechanical ventilation with VT settings of 6 mL/kg donor PBW compared with routine mechanical ventilation after LTx could test this hypothesis70. Although a majority of respondents to a survey did not consider donor characteristics they indicated that they might modify MV settings if they knew the donor characteristics4; thus we recommend that donor characteristics should be communicated to and known by the team managing the MV4,30,38,66. This could be especially important in case of size reduced and lobar transplants71,72.
When there is severe PGD, mechanical ventilation may not be able to safely meet the LTx recipients’ needs in terms of oxygenation and minute ventilation, and the ventilator settings needed may be harmful to the allograft. Many LTx centers use veno-venous ECMO as rescue strategy for severe PGD73–75. The advantages of using VV-ECMO are that it allows using protective ventilator settings and minimizing sedation73–75. Ventilator rest settings on VV-ECMO commonly use very low VT of approximately 4 mL/kg (donor PBW) with PEEP 5–8 cm H2O76,77. There is a prospective trial in progress testing whether ultra-protective ventilation using a tidal volume of 3 mL/kg combined with extracorporeal carbon dioxide removal will improve outcomes in severe ARDS compared with conventional low-VT ventilation78. Furthermore, if a single dual-lumen bicaval cannula can be utilized for VV-ECMO, physical therapy and mobilization can occasionally be resumed.
Some patients fail extubation or have complications that require longer duration of mechanical ventilation or VV-ECMO. In these cases early tracheostomy is often performed79–81. This allows for safe weaning trials that lessen the risk of airway complications from repeated intubations and constant high pressure on the bronchial anastomoses79–81. Patients also have better comfort, oral hygiene, clearance of pulmonary secretions and a lower risk of vocal cord injury.
Single Lung Transplants
Single LTx represent a minority of procedures done in the modern era3. When managing these patients, it is important to consider that the native lung has end-stage disease from different etiologies and should not be relied upon to share the volumes and pressures during mechanical ventilation equally with the allograft. In idiopathic pulmonary fibrosis (IPF) the native lung is less compliant than the allograft, and most of the VT will likely go to the more compliant allograft. Lung-protective ventilator VT should be reduced, and we prefer an initial VT of 4–6 mL/kg of the donor’s PBW. Liberalization of VT may be necessary to minimize patient sedation and to allow for early extubation. Recipients of a single LTx for IPF can also have an IPF flare in the native lung triggered by the LTx surgery. This can lead to more severe hypoxemia from shunt physiology through a very non-compliant IPF lung. Recipients of a single LTx for COPD on the other hand have a very compliant native lung, which has severe expiratory airflow obstruction. This can lead to over-distention of the recipient’s native lung from dynamic hyperinflation and auto-PEEP. Here an approach to mechanical ventilation that maximizes expiratory time, by using a short inspiratory time, a low respiratory rate and a VT that allows for full expiration are important. If these difficulties cannot be managed with conventional mechanical ventilation, patients may require independent lung ventilation with a double-lumen endotracheal tube and different ventilator settings for each lung5. However independent lung ventilation generally requires heavy sedation and a preferable approach can be to utilize VV-ECMO, or extracorporeal CO2 elimination as a rescue strategy, as discussed above.
Bronchial Anastomoses
A key aspect unique to LTx is the presence of the bronchial anastomoses. Anastomotic airway complications occur in approximately 10–20% of LTx recipients and often present both acute and long-term problems29,82–87. Anastomotic airway complications include infection, stenosis and dehiscence29,82–87. In general, the bronchial circulation is not restored during transplant, and ischemia of the transplanted airway and airway mucosa frequently occur after LTx29,88. Thus the bronchial anastomoses sites are prone to poor healing, infection and anastomotic airway complications. There may be collateral flow from the pulmonary circulation, but the pulmonary circulation has relatively low vascular pressure and thus the magnitude of collateral flow is probably small. Therefore, positive pressure mechanical ventilation could potentially impair perfusion to transplanted airways, especially when high inflation pressures are required. In addition any allograft parenchymal pathology such as PGD, infection or rejection will reduce the pulmonary flow to the major bronchi and thereby impair anastomotic healing. Alternatively, it is possible that PEEP may increase perfusion through microscopic collateral vessels by redistributing blood flow from the pulmonary vessels which in this setting could be acting as a vascular capacitance bed. This theory is supported by a dog model of LTx without restoration of the bronchial arterial circulation, where increasing the PEEP from 5 to 10 cm H2O was associated with increased retrograde bronchial mucosal blood flow to the bronchial anastomoses89. However positive pressure ventilation can also contribute to bronchial wall and anastomotic stress. High airway pressures and prolonged ventilation times have been linked to the risk for anastomotic airway complications in some studies, however not in others82,85,90. The concern regarding high airway pressures and anastomotic airway complications are likely reflected in the responses on approaches to peak inspiratory pressure (PIP) and PEEP during MV after LTx in an international survey4. Almost all respondents (91%) reported routinely assessing airway pressures and most had a peak inspiratory pressure (PIP) limit4. The median limit was 30 cm H2O (IQR 30–35 cm H2O). The PIP limit differed significantly between volume assist/control (VAC) users and pressure assist/control (PAC) users (median 35 [IQR 35–40] versus median 30 [IQR 20–35], p = 0.002). In that survey the maximum acceptable PEEP level after LTx averaged 11 cm H2O (IQR 10–12.5 cmH2O)4. However, there is little evidence guiding optimal setting of PEEP and PIP for the LTx recipient and regarding how much pressure is too much for the anastomoses.
Modes of ventilation
Immediately after surgery there are many different providers and support staff involved in the management of the MV of the LTx recipient. An international survey indicated that the ventilator settings were determined by intensivists in 50% of centers, pulmonologists in 42%, surgeons in 28%, anesthesiologists in 26%, and respiratory therapists in several instances (multiple answers were allowed)4. Approximately equal percentages of respondents reported using pressure assist/control (PAC) ventilation (37%) and volume assist/control (VAC) ventilation (35%)4. This requires careful attention to the ventilator inputs and outputs as different providers have different preferences and levels of experience with specific ventilator modes. VAC modes are most likely to have consistent tidal volumes but require attention to peak and plateau airway pressures. PAC modes can avoid high peak but not transpulmonary pressures, sometimes providing larger VT than intended. We emphasize that limiting peak inspiratory pressures does not assure that transpulmonary pressure remains in a lung protective range, except during general anesthesia or deep sedation. Therefore, we prefer the VAC or pressure regulated volume control (PRVC) modes, rather than PAC, during the period of controlled mechanical ventilation in the ICU. Management guidelines have been successfully implemented at individual LTx centers and can help to facilitate a consistent approach to mechanical ventilation of the LTx recipient4,63. Table 2 summarizes recommendations for the postoperative MV of the LTX-recipient.
Table 2.
Recommendations for post-operative mechanical ventilation
| No PGD | PGD |
|---|---|
|
In addition to “no PGD” recommendations:
|
PGD: Primary graft dysfunction; PEEP: Positive end expiratory Pressure; FiO2: Fraction of inspired oxygen; PaO2: partial pressure of oxygen in the blood; PaCO2: Partial pressure of carbon dioxide in the blood; VV ECMO: venovenous extracorporeal membrane oxygenation; PBW: Predicted body weight. PRVC: pressure regulated volume controlled
Summary
Lung transplantation is a very specialized field with unique surgical and medical aspects. The principles of lung protective ventilation have a strong evidence base in patients at risk for or with ARDS. Much of the recommendations presented in this review of lung transplant recipient mechanical ventilation are extrapolated from data in the general patient populations, because of the close relationship between PGD and ARDS, as well as the general influence of anesthesia on the respiratory system. All LTx recipients are at risk for PGD, which is similar to ARDS, and should receive mechanical ventilation according to the principles of lung-protective ventilation with low tidal volumes. In our opinion the low tidal volume strategy should be based on donor characteristics (i.e. donor predicted body weight as a parameter reflecting the actual allograft size), rather than based on LTx recipient characteristics.
References
- 1.Grossman RF, Frost A, Zamel N, et al. Results of single-lung transplantation for bilateral pulmonary fibrosis. The Toronto Lung Transplant Group. N Engl J Med. 1990;322:727–733. doi: 10.1056/NEJM199003153221104. [DOI] [PubMed] [Google Scholar]
- 2.Eberlein M, Garrity ER, Orens JB. Lung allocation in the United States. Clin Chest Med. 2011;32:213–222. doi: 10.1016/j.ccm.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 4. Beer A, Reed RM, Bolukbas S, et al. Mechanical ventilation after lung transplantation. An international survey of practices and preferences. Ann Am Thorac Soc. 2014;11:546–553. doi: 10.1513/AnnalsATS.201312-419OC. •International survey of the lung transplant community to determine ventilator management strategies in lung transplant recipients.
- 5.Lucangelo U, Del Sorbo L, Boffini M, et al. Protective ventilation for lung transplantation. Curr Opin Anaesthesiol. 2012;25:170–174. doi: 10.1097/ACO.0b013e32834fdb54. [DOI] [PubMed] [Google Scholar]
- 6. Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. ••This 10-center prospective cohort study identified risk factors for grade 3 PGD. History of donor smoking, higher FiO2 during allograft reperfusion, use of cardiopulmonary bypass and pulmonary arterial hypertension were independent predictors of severe PGD.
- 7.Lee JC, Christie JD. Primary graft dysfunction. Clin Chest Med. 2011;32:279–293. doi: 10.1016/j.ccm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 10. Needham DM, Yang T, Dinglas VD, et al. Timing of Low Tidal Volume Ventilation and ICU Mortality in ARDS: A Prospective Cohort Study. Am J Respir Crit Care Med. 2014 doi: 10.1164/rccm.201409-1598OC. ••This is a prospective study of 482 patients with ARDS and 11,558 twice-daily tidal volume assessments. Higher tidal volumes shortly after ARDS onset were associated with a greater risk of ICU mortality. Per each 1 ml/kg PBW increase in initial tidal volume there was a 23% increase in ICU mortality risk (adjusted hazard ratio, 1.23; 95% confidence interval [CI], 1.06–1.44; P = 0.008).
- 11.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 12.Klesney-Tait JA, Eberlein M. Lung protective ventilation in donors: an ounce of prevention. Chest. 2014;146:4–6. doi: 10.1378/chest.14-0163. [DOI] [PubMed] [Google Scholar]
- 13.Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. Jama. 2010;304:2620–2627. doi: 10.1001/jama.2010.1796. [DOI] [PubMed] [Google Scholar]
- 14.Brower RG, Rubenfeld GD. Lung-protective ventilation strategies in acute lung injury. Crit Care Med. 2003;31:S312–S316. doi: 10.1097/01.CCM.0000057909.18362.F6. [DOI] [PubMed] [Google Scholar]
- 15.Diaz JV, Brower R, Calfee CS, et al. Therapeutic strategies for severe acute lung injury. Crit Care Med. 2010;38:1644–1650. doi: 10.1097/CCM.0b013e3181e795ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fessler HE, Brower RG. Protocols for lung protective ventilation. Crit Care Med. 2005;33:S223–S227. doi: 10.1097/01.ccm.0000155919.53727.d5. [DOI] [PubMed] [Google Scholar]
- 17.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 18.Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janz DR, Ware LB. Approach to the Patient with the Acute Respiratory Distress Syndrome. Clin Chest Med. 2014;35:685–696. doi: 10.1016/j.ccm.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajic O, Frutos-Vivar F, Esteban A, et al. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31:922–926. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 22. Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. Jama. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. •• A meta-analysis of 20 studies that evaluated lower vs higher tidal volumes in surgical and ICU patients without ARDS concluded that lower volumes were associated with reduced rates of lung injury, mortality, pneumonia, and atelectasis.
- 23.Serpa Neto A, Simonis FD, Barbas CS, et al. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive Care Med. 2014;40:950–957. doi: 10.1007/s00134-014-3318-4. [DOI] [PubMed] [Google Scholar]
- 24.Zupancich E, Paparella D, Turani F, et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. J Thorac Cardiovasc Surg. 2005;130:378–383. doi: 10.1016/j.jtcvs.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 25. Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. •• This was a multicenter, randomized, parallel group trial of intraoperative ventilation strategies in patients undergoing abdominal surgery with elevated risk of pulmonary complications. It showed lower rates of major complications, pneumonia, and length of stay in the lung protective ventilation group as compared to non-protective ventilation.
- 26.Lau CL, Patterson GA, Palmer SM. Critical care aspects of lung transplantation. J Intensive Care Med. 2004;19:83–104. doi: 10.1177/0885066603261509. [DOI] [PubMed] [Google Scholar]
- 27.Schuurmans MM, Benden C, Inci I. Practical approach to early postoperative management of lung transplant recipients. Swiss Med Wkly. 2013;143:w13773. doi: 10.4414/smw.2013.13773. [DOI] [PubMed] [Google Scholar]
- 28.Arndt A, Boffa DJ. Pleural space complications associated with lung transplantation. Thorac Surg Clin. 2015;25:87–95. doi: 10.1016/j.thorsurg.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Machuzak M, Santacruz JF, Gildea T, et al. Airway Complications After Lung Transplantation. Thorac Surg Clin. 2015;25:55–75. doi: 10.1016/j.thorsurg.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Eberlein M, Arnaoutakis GJ, Yarmus L, et al. The effect of lung size mismatch on complications and resource utilization after bilateral lung transplantation. J Heart Lung Transplant. 2012;31:492–500. doi: 10.1016/j.healun.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Eberlein M, Bolukbas S, Reed RM. eComment. Gender mismatching in lung transplantation: lung size mismatch is the issue! Interact Cardiovasc Thorac Surg. 2013;16:435–436. doi: 10.1093/icvts/ivt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eberlein M, Bolukbas S, Reed RM. Bilateral lobar lung transplantation and size mismatch by pTLC-ratio. Eur J Cardiothorac Surg. 2013;44:394–395. doi: 10.1093/ejcts/ezt004. [DOI] [PubMed] [Google Scholar]
- 33.Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and survival after single and bilateral lung transplantation. Ann Thorac Surg. 2013;96:457–463. doi: 10.1016/j.athoracsur.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 34. Eberlein M, Reed RM, Maidaa M, et al. Donor-recipient size matching and survival after lung transplantation. A cohort study. Ann Am Thorac Soc. 2013;10:418–425. doi: 10.1513/AnnalsATS.201301-008OC. •This analysis of the SRTR lung transplant database in the post lung allocation score era showed that donor to recipient lung size matching (pTLC ratio) is an independent predictor of death in the first year after LTx. Specifically the more undersized the allograft was the higher the risk of one year mortality.
- 35.Eberlein M, Reed RM, Permutt S, et al. Parameters of donor-recipient size mismatch and survival after bilateral lung transplantation. J Heart Lung Transplant. 2012;31:1207.e1207–1213.e1207. doi: 10.1016/j.healun.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Reed RM, Eberlein M. Sizing strategies in heart and lung transplantation: you cannot manage what you do not measure. Future Cardiol. 2014;10:303–306. doi: 10.2217/fca.14.17. [DOI] [PubMed] [Google Scholar]
- 37.Dezube R, Arnaoutakis GJ, Reed RM, et al. The effect of lung-size mismatch on mechanical ventilation tidal volumes after bilateral lung transplantation. Interact Cardiovasc Thorac Surg. 2013;16:275–281. doi: 10.1093/icvts/ivs493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant. 2014 doi: 10.1016/j.healun.2014.09.030. •Ancillary study to the Lung Transplant Outcomes Group Study analyzing 812 patients bilateral lung transplant recipients. Oversized donor lungs were independently associated with decreased odds of grade 3 primary graft dysfunction.
- 39.Klesney-Tait JA, Parekh K. Diamonds in the rough: identification of usable donor lungs. Am J Respir Crit Care Med. 2013;188:410–412. doi: 10.1164/rccm.201306-1185ED. [DOI] [PubMed] [Google Scholar]
- 40.Dreyfuss D, Soler P, Basset G, et al. High Inflation Pressure Pulmonary Edema: Respective Effects of High Airway Pressure, High Tidal Volume, and Positive End-expiratory Pressure. American Review of Respiratory Disease. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 41.Slutsky AS, Ranieri VM. Ventilator-Induced Lung Injury. New England Journal of Medicine. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 42.Jaswal DS, Leung JM, Sun J, et al. Tidal volume and plateau pressure use for acute lung injury from 2000 to present: a systematic literature review. Crit Care Med. 2014;42:2278–2289. doi: 10.1097/CCM.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan E, Wilcox ME, Brower RG, et al. Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med. 2008;178:1156–1163. doi: 10.1164/rccm.200802-335OC. [DOI] [PubMed] [Google Scholar]
- 44.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. Jama. 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 45.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 46.Miller RR, 3rd, Macintyre NR, Hite RD, et al. Point: should positive end-expiratory pressure in patients with ARDS be set on oxygenation? Yes. Chest. 2012;141:1379–1382. doi: 10.1378/chest.12-0155. discussion 1386-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt GA. Counterpoint: should positive end-expiratory pressure in patients with ARDS be set based on oxygenation? No. Chest. 2012;141:1382–1384. doi: 10.1378/chest.12-0157. discussion 1384–1386. [DOI] [PubMed] [Google Scholar]
- 48.Gu WJ, Wang F, Liu JC. Effect of lung-protective ventilation with lower tidal volumes on clinical outcomes among patients undergoing surgery: a meta-analysis of randomized controlled trials. Cmaj. 2014 doi: 10.1503/cmaj.141005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baez B, Castillo M. Anesthetic considerations for lung transplantation. Semin Cardiothorac Vasc Anesth. 2008;12:122–127. doi: 10.1177/1089253208319871. [DOI] [PubMed] [Google Scholar]
- 50.Castillo M. Anesthetic management for lung transplantation. Curr Opin Anaesthesiol. 2011;24:32–36. doi: 10.1097/ACO.0b013e328341881b. [DOI] [PubMed] [Google Scholar]
- 51.Miranda A, Zink R, McSweeney M. Anesthesia for lung transplantation. Semin Cardiothorac Vasc Anesth. 2005;9:205–212. doi: 10.1177/108925320500900303. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg AL, Rao M, Benedict PE. Anesthetic implications for lung transplantation. Anesthesiol Clin North America. 2004;22:767–788. doi: 10.1016/j.atc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Singh H, Bossard RF. Perioperative anaesthetic considerations for patients undergoing lung transplantation. Can J Anaesth. 1997;44:284–299. doi: 10.1007/BF03015367. [DOI] [PubMed] [Google Scholar]
- 54.Parekh K, Patterson GA. Technical considerations in adult lung transplantation. Semin Thorac Cardiovasc Surg. 2004;16:322–332. doi: 10.1053/j.semtcvs.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 55.Broccard AF, Hotchkiss JR, Kuwayama N, et al. Consequences of vascular flow on lung injury induced by mechanical ventilation. Am J Respir Crit Care Med. 1998;157:1935–1942. doi: 10.1164/ajrccm.157.6.9612006. [DOI] [PubMed] [Google Scholar]
- 56.Meade MO, Granton JT, Matte-Martyn A, et al. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am J Respir Crit Care Med. 2003;167:1483–1489. doi: 10.1164/rccm.2203034. [DOI] [PubMed] [Google Scholar]
- 57.Khan TA, Schnickel G, Ross D, et al. A prospective, randomized, crossover pilot study of inhaled nitric oxide versus inhaled prostacyclin in heart transplant and lung transplant recipients. J Thorac Cardiovasc Surg. 2009;138:1417–1424. doi: 10.1016/j.jtcvs.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 58.Yerebakan C, Ugurlucan M, Bayraktar S, et al. Effects of inhaled nitric oxide following lung transplantation. J Card Surg. 2009;24:269–274. doi: 10.1111/j.1540-8191.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 59.Eberlein M, Parekh K, Hansdottir S, et al. Plastic bronchitis complicating primary graft dysfunction after lung transplantation. Ann Thorac Surg. 2014;98:1849. doi: 10.1016/j.athoracsur.2014.06.079. [DOI] [PubMed] [Google Scholar]
- 60.Bermudez CA, Shiose A, Esper SA, et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann Thorac Surg. 2014;98:1936–1943. doi: 10.1016/j.athoracsur.2014.06.072. [DOI] [PubMed] [Google Scholar]
- 61.Shigemura N, Orhan Y, Bhama JK, et al. Delayed chest closure after lung transplantation: techniques, outcomes, and strategies. J Heart Lung Transplant. 2014;33:741–748. doi: 10.1016/j.healun.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 62.de Perrot M, Imai Y, Volgyesi GA, et al. Effect of ventilator-induced lung injury on the development of reperfusion injury in a rat lung transplant model. J Thorac Cardiovasc Surg. 2002;124:1137–1144. doi: 10.1067/mtc.2002.125056. [DOI] [PubMed] [Google Scholar]
- 63. Currey J, Pilcher DV, Davies A, et al. Implementation of a management guideline aimed at minimizing the severity of primary graft dysfunction after lung transplant. J Thorac Cardiovasc Surg. 2010;139:154–161. doi: 10.1016/j.jtcvs.2009.08.031. •• This study showed that the implementation of an evidence-based guideline for managing respiratory and hemodynamic status of lung transplant recipients led to a reduction in severity of primary graft dysfunction.
- 64.McIlroy DR, Pilcher DV, Snell GI. Does anaesthetic management affect early outcomes after lung transplant? An exploratory analysis. Br J Anaesth. 2009;102:506–514. doi: 10.1093/bja/aep008. [DOI] [PubMed] [Google Scholar]
- 65.Kozower BD, Meyers BF, Ciccone AM, et al. Potential for detrimental hyperinflation after lung transplantation with application of negative pleural pressure to undersized lung grafts. J Thorac Cardiovasc Surg. 2003;125:430–432. doi: 10.1067/mtc.2003.139. [DOI] [PubMed] [Google Scholar]
- 66.Eberlein M, Diehl E, Bolukbas S, et al. An oversized allograft is associated with improved survival after lung transplantation for idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2013;32:1172–1178. doi: 10.1016/j.healun.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Eberlein M, Permutt S, Brown RH, et al. Supranormal expiratory airflow after bilateral lung transplantation is associated with improved survival. Am J Respir Crit Care Med. 2011;183:79–87. doi: 10.1164/rccm.201004-0593OC. [DOI] [PubMed] [Google Scholar]
- 68.Eberlein M, Permutt S, Chahla MF, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012;141:451–460. doi: 10.1378/chest.11-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eberlein M, Reed RM. Letter by Eberlein and Reed regarding article, "transplantation for idiopathic pulmonary arterial hypertension: improvement in the lung allocation score era". Circulation. 2014;129:e457. doi: 10.1161/CIRCULATIONAHA.113.004888. [DOI] [PubMed] [Google Scholar]
- 70.Diamond JM, Ahya VN. Mechanical ventilation after lung transplantation. It's time for a trial. Ann Am Thorac Soc. 2014;11:598–599. doi: 10.1513/AnnalsATS.201403-104ED. [DOI] [PubMed] [Google Scholar]
- 71.Shigemura N, Bhama J, Bermudez C, et al. Lobar lung transplantation: emerging evidence for a viable option. Semin Thorac Cardiovasc Surg. 2013;25:95–96. doi: 10.1053/j.semtcvs.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 72.Shigemura N, D'Cunha J, Bhama JK, et al. Lobar lung transplantation: a relevant surgical option in the current era of lung allocation score. Ann Thorac Surg. 2013;96:451–456. doi: 10.1016/j.athoracsur.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 73.Castleberry AW, Hartwig MG, Whitson BA. Extracorporeal membrane oxygenation post lung transplantation. Curr Opin Organ Transplant. 2013 doi: 10.1097/MOT.0b013e328365197e. [DOI] [PubMed] [Google Scholar]
- 74. Hartwig MG, Walczak R, Lin SS, et al. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg. 2012;93:366–371. doi: 10.1016/j.athoracsur.2011.05.017. • This was a retrospective, single-center study of patients requiring VV ECMO after lung transplantation. ECMO provided a reasonable support option with improved outcomes compared to conventional managment.
- 75.Rehder KJ, Turner DA, Hartwig MG, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care. 2013;58:1291–1298. doi: 10.4187/respcare.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Del Sorbo L, Goffi A, Goligher E, et al. Setting Mechanical ventilation in ARDS patients during VV-ECMO: where are we? Minerva Anestesiol. 2014 [PubMed] [Google Scholar]
- 77.Marhong JD, Telesnicki T, Munshi L, et al. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc. 2014;11:956–961. doi: 10.1513/AnnalsATS.201403-100BC. [DOI] [PubMed] [Google Scholar]
- 78.Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (approximately 3 mL/kg) combined with extracorporeal CO2 removal versus 'conventional' protective ventilation (6 mL/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–856. doi: 10.1007/s00134-012-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feltracco P, Milevoj M, Alberti V, et al. Early tracheostomy following lung transplantation. Transplant Proc. 2011;43:1151–1155. doi: 10.1016/j.transproceed.2011.01.154. [DOI] [PubMed] [Google Scholar]
- 80.Padia SA, Borja MC, Orens JB, et al. Tracheostomy following lung transplantation predictors and outcomes. Am J Transplant. 2003;3:891–895. doi: 10.1034/j.1600-6143.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 81.Waller EA, Aduen JF, Kramer DJ, et al. Safety of percutaneous dilatational tracheostomy with direct bronchoscopic guidance for solid organ allograft recipients. Mayo Clin Proc. 2007;82:1502–1508. doi: 10.1016/S0025-6196(11)61094-X. [DOI] [PubMed] [Google Scholar]
- 82.Murthy SC, Blackstone EH, Gildea TR, et al. Impact of anastomotic airway complications after lung transplantation. Ann Thorac Surg. 2007;84:401–409. 409.e401–404.e401. doi: 10.1016/j.athoracsur.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 83.Porhownik NR. Airway complications post lung transplantation. Curr Opin Pulm Med. 2013;19:174–180. doi: 10.1097/MCP.0b013e32835d2ef9. [DOI] [PubMed] [Google Scholar]
- 84.Puchalski J, Lee HJ, Sterman DH. Airway complications following lung transplantation. Clin Chest Med. 2011;32:357–366. doi: 10.1016/j.ccm.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009;6:79–93. doi: 10.1513/pats.200808-094GO. [DOI] [PubMed] [Google Scholar]
- 86.Thistlethwaite PA, Yung G, Kemp A, et al. Airway stenoses after lung transplantation: incidence, management, and outcome. J Thorac Cardiovasc Surg. 2008;136:1569–1575. doi: 10.1016/j.jtcvs.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 87.Weder W, Inci I, Korom S, et al. Airway complications after lung transplantation: risk factors, prevention and outcome. Eur J Cardiothorac Surg. 2009;35:293–298. doi: 10.1016/j.ejcts.2008.09.035. discussion 298. [DOI] [PubMed] [Google Scholar]
- 88.Widdicombe J. New perspectives on basic mechanisms in lung disease. 4. Why are the airways so vascular? Thorax. 1993;48:290–295. doi: 10.1136/thx.48.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yokomise H, Cardoso PF, Kato H, et al. The effect of pulmonary arterial flow and positive end-expiratory pressure on retrograde bronchial mucosal blood flow. J Thorac Cardiovasc Surg. 1991;101:201–208. [PubMed] [Google Scholar]
- 90.Date H, Trulock EP, Arcidi JM, et al. Improved airway healing after lung transplantation. An analysis of 348 bronchial anastomoses. J Thorac Cardiovasc Surg. 1995;110:1424–1432. doi: 10.1016/S0022-5223(95)70065-X. discussion 1432–1423. [DOI] [PubMed] [Google Scholar]