Abstract
A 57-year-old man with a 21 year history of Parkinson's disease underwent bilateral subthalamic nucleus deep brain stimulator (DBS) placement. One week postoperatively he developed an acute left subdural hematoma from a fall with significant displacement of the DBS leads. It was promptly evacuated, the patient slowly recovered neurologically, and the leads moved near the original position. Six months of stimulation therapy attained 50% reduction in symptoms. This case report demonstrates the movement of DBS leads due to brain shift and ability to come back to previous location once the brain shift is corrected.
Keywords: Deep brain stimulation, Electrode displacement, Lead displacement, Lead shift, Subdural hematoma
1. Introduction
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is proven to be beneficial in alleviating motor symptoms in well-selected patients with advanced Parkinson's disease (PD)[1]. As with any other surgical procedure, certain risks exist, such as intracranial hematoma, infection, and hardware malfunction [1, 2]. This case report describes development of an acute subdural hematoma (SDH) necessitating evacuation after placement of bilateral STN DBS. It was possible to preserve the electrodes in this patient during the hematoma evacuation, and despite significant initial shift of the electrodes, the patient ultimately received excellent benefits from the stimulation therapy.
2. Case history
This patient was a 57-year-old man with a 21 year history of PD. His symptoms were progressive bradykinesia, rigidity, shuffling gait and bilateral hand resting tremor, low speech volume and bothersome motor fluctuations, despite maximal tolerated medication therapy. His regimen included pramipexole 1.5 mg in the morning and 1 mg three times a day, and carbidopalevodopa 50-200 mg five times daily plus carbidopa-levodopa extended-release 50-200 mg bedtime, amantadine 100 mg three times daily. He had intolerance to bromocriptine, trihexyphenidyl and entacapone. His co-morbidities included hypertension, and he was not taking any antiplatelet or anticoagulation agents.
Preoperative assessment for DBS candidacy included Unified Parkinson's Disease Rating Scale (UPDRS) Part III (motor part) assessment with anti-parkinsonian medication off for 12 hours prior the exam and at 30–60 minutes after taking the medications, neuropsychiatric testing to rule out major cognitive deficits, and MRI of the brain to rule out structural lesions.
The patient's preoperative UPDRS Part III score was 30 out of 104 off medications and 19 out of 104 after taking medications. Symptoms on the left side were worse by 3 points. Preoperative neuropsychiatric evaluation demonstrated only mild cortical slowing. MRI of the brain did not demonstrate any significant structural lesions. Preoperative planning was performed using Framelink software (Medtronic Navigation, Louisville, CO, USA). Trajectories were planned out on gadolinium enhanced volumetric brain MRI and effort was made to avoid sulci and blood vessels. Patient underwent bilateral STN DBS electrode (Model 3389, Medtronic, MN, USA) placement using stereotactic framed technique with intraoperative microelectrode recording and macrostimulation, in a previously described fashion[3]. Postoperative head CT scan demonstrated minimal pneumocephalus and satisfactory position of the electrodes (Fig. 1). The patient was discharged on the second postoperative day.
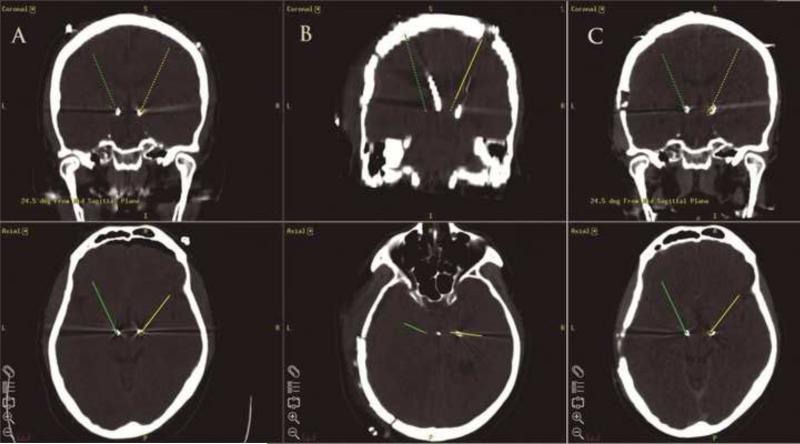
Fig. 1.
Volumetric coronal (top row) and axial (bottom row) CT head images merged with the intraoperative coordinate plan in Framelink environment (Medtronic Navigation, Louisville, CO, USA). (A) Immediately post-deep brain stimulator (DBS) placement images demonstrating satisfactory placement of bilateral subthalamic nucleus DBS. (B) Immediately after the evacuation of both hematomas. (C) Immediately before the implanted pulse generator placements. (Note: right and left side designation is reversed from the conventional).
One week later, he sustained a fall with development of severe headache and confusion. Head CT scan demonstrated an acute left hemispheric SDH with 1 cm midline shift and significant displacement of the DBS leads (Fig. 2). He underwent an emergent craniotomy with evacuation of the SDH. Skin incision was made and the skin flap was elevated so as to not damage the existing stimulator leads, which were secured in a subgaleal pocket medial to the craniotomy incision. It was possible to turn the bone flap and perform decompression without involving the DBS burr hole and the locking mechanism. Two days later patient became more lethargic and head CT scan revealed SDH re-accumulation causing mass effect (Fig. 2). The SDH was promptly evacuated via initial craniotomy with same technique for preservation of the leads. The patient was followed by serial CT scans and underwent a long rehabilitation course. The DBS leads shifted away from the original target with the shift of the brain. They then returned close to the initial position after resolution of brain shift (Fig. 3). After the craniotomies and a course of rehabilitation the patient had some worsening of his PD symptoms and had UPDRS motor scores of 36 off medication. Three months after the craniotomies bilateral internal pulse generators (Activa SC, Medtronic) were placed and the patient was programmed shortly after. His UPDRS Part III scores returned to the baseline of 30 points. Six months after the continuous bilateral STN DBS therapy the UPDRS Part III score was 15 off medication with bilateral improvement of motor symptoms (Table 1).
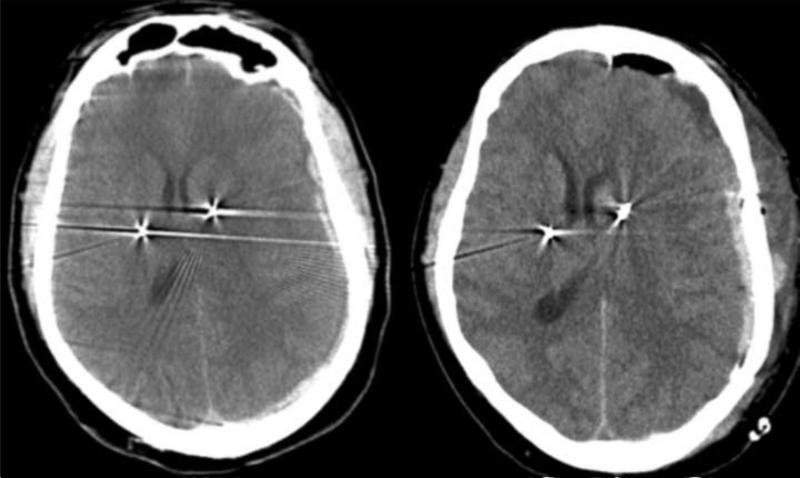
Fig. 2.
Axial CT scan of the head demonstrating left acute subdural hematoma with a midline and electrode shift (left). Subdural hematoma re-accumulated 2 days later, resulting in further shift (right).
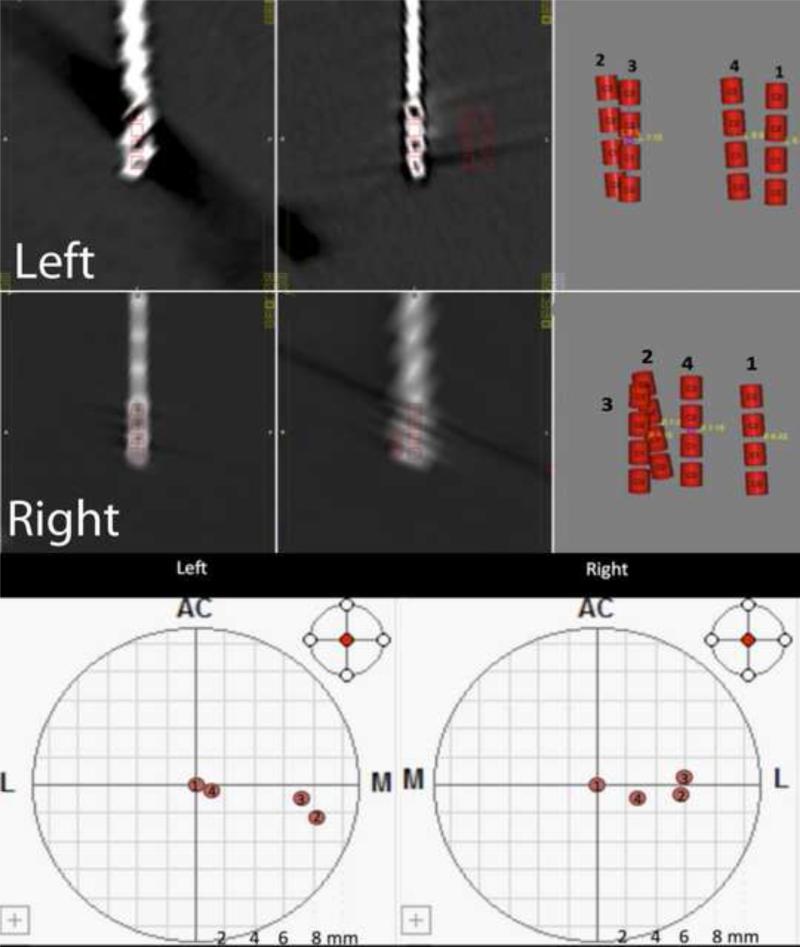
Fig. 3.
Using CRAnialVault Explorer (CRAVE) software (Vanderbilt University, Nashville, TN, USA), the precise positions of the lead contacts were located in Cartesian stereotactic space in relation to the midcommisural line. Lead positions post deep brain stimulator placement (1), after the first (2) and second (3) hematomas and after the resolution of the shift (4) are demonstrated bilaterally.
Table 1.
Unified Parkinson's Disease Rating Scale Part III scores
| Timeline | 11/12/2010 | 10/2011 | 01/2012 | 7/2012 |
|---|---|---|---|---|
| DBS status | Pre-DBS assessment | Post-DBS leads placement, craniotomies and rehabilitation | Prior to first programming | DBS therapy on for 6 months |
| UPDRS Part III scores: off medications | 30 | 36 | 30 | 15 |
| UPDRS Part III scores: on medications | 19 | Not performed | Not performed | Not performed |
DBS = deep brain stimulator, UPDRS = Unified Parkinson's Disease Rating Scale.
Postoperative CT scan merged with the intraoperative plan on the planning software and Table 2 show the final intraoperative coordinates with respect to the midcommisural line. Deviation of the leads towards the right is seen in the middle. Final lead position at 6 months postoperatively is close to the initial positioning. Using CRAnialVault Explorer (CRAVE) (Vanderbilt University, Nashville, TN, USA)[4], we obtained precise evaluation of the initial, post-hematoma and final lead positions on each side. Figure 3 and Table 2 show initial versus final coordinates and total change in two (2D) and three (3D) dimensions (XY and XYZ) using CRAVE software. The total change in 2D (XY) space was 1.56 mm on the left and 3.82 mm on the right. The total change in 3D space was 2.42 mm on the left and 3.82 mm on the left. Clearly, a larger net change occurred on the right side. Nevertheless, we must also account for the fact that the brain itself is not in its precise pre-hematoma position. This could explain that despite persistence difference between initial and final electrode positions the patient greatly benefited from the stimulation therapy.
Table 2.
Coordinates of leads after stimulator placement, after the shift from the subdural hematoma mass effect and the final position
| Date | Left | Net change | Right | Net change | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X (mm) | Y (mm) | Z (mm) | 2D (XY) | 3D (XYZ) | X (mm) | Y (mm) | Z (mm) | 2D (XY) | 3D (XYZ) | |
| 22 June | –10.34 | –0.83 | –4.84 | 0 | 0 | 11.5 | –1.36 | –4.1 | 0 | 0 |
| 5 July | –1.37 | –3.69 | –0.78 | 9.83 | 10.05 | 17.21 | –0.65 | –4.01 | 5.74 | 5.74 |
| 16 July | –2.7 | –1.8 | –1.3 | 7.7 | 8.48 | 17.35 | –1.95 | –4.155 | 5.88 | 5.88 |
| 8 August | –8.35 | –1.037 | –3.47 | 1.56 | 2.42 | 15.13 | –0.15 | –4.11 | 3.82 | 3.82 |
2D = two dimensions, 3D = three dimensions.
3. Discussion
Intracranial bleeding post-electrode insertion falls into the category of operation-related complication of DBS[2]. It is also one of the most dreaded of complications and occurs at a rate of about 2–3%[2, 5, 6]. Significant neurological impairment, however, occurs in less than 1% of patients overall[2]. Specifically, SDH have been reported in this setting. Series of SDH requiring no surgery or burr hole drainage with hardware preservation and successful DBS therapy have also been reported [7]. With the leads in place a full craniotomy poses a more significant surgical challenge than burr hole drainage. The incision must be planned in a fashion that will allow future healing and prevent dehiscence and hardware exposure through the scalp. In our patient the location of the left sided DBS system incision was in line with the craniotomy incision and after careful opening we were able to medialize the electrode loops away from the operative site without displacing the leads. Craniotomy with navigational pre-planning prior to DBS insertion has been described in the past, but we have not seen published reports describing performing a large craniotomy with DBS already in place[8]. With SDH in direct proximity to the burr hole and the electrode, evacuation and decompression must avoid the burr hole containing the hardware if possible, but usually needs to be tailored partially around it.
However, with such significant lead displacement the question arises if an effort to preserve the leads in an emergent situation should be undertaken. Our patient returned to his baseline preoperative UPDRS off medications score of 30 by the time of the first programming session. Six months after the stimulation therapy patient's off medications score was 15, better than preoperative on medications score and his overall improvement in PD symptoms was 50%. His left side remained slightly more symptomatic than the right (Table 1). His final settings are summarized in Table 3. In a previously reported series of four patients post-DBS SDH created a significant lead displacement as well. After burr hole drainage in three and conservative treatment in one patient the leads eventually returned to their optimal position and the patients had a good clinical response to stimulation, however in a delayed fashion (4–18 months)[7]. Our patient has also demonstrated that with the significant displacement and subsequent return of the leads to near their initial position, stimulation success may still be achieved.
Table 3.
Final deep brain stimulator settings
| Laterality | Contacts | Amplitude | Frequency (Hz) | Pulse width (μs) |
|---|---|---|---|---|
| Left | 2+ 0– | 2.1 | 185 | 120 |
| Right | 1+ 2– 3– | 3.5 | 185 | 120 |
Here, we also demonstrated the ability to track lead positioning over multiple CT scan series and accurately measure lead displacement and subsequent return to placement using the CRAVE software program. The technology allowed us a unique opportunity to see precise lead locations over time and observe relationships in Cartesian space (Fig. 3). Final displacement was 1.56 mm on the left in the XY plane posterior-medially. On the right most of shift was lateral in the X plane (3.6 mm). It proved that bipolar stimulation was more beneficial on the right to avoid the lateral side effects from the stimulation of the internal capsule fibers, whereas on the left we were able to achieve optimal settings with monopolar stimulation at lower amplitudes (Table 3).
4. Conclusion
Both STN DBS electrodes demonstrated significant shift from the intended positions. Potential implications of lead displacement are loss of efficacy and increase in adverse side effects. Our patient demonstrates that with allowed time, after the space occupying lesion and associated shift have resolved, radiographic position of the leads returned to near their initial position. Moreover, the patient experienced significant benefit from the bilateral DBS therapy on follow-up.
Highlights.
We were able to perform full craniotomy for hematoma evacuation with preservation of with pre-existing deep brain stimulators in place.
Significant displacement of the electrodes secondary to brain shift did not preclude successful deep brain stimulation therapy.
Radiographic and 3D coordinate analysis confirmed eventual return of the electrode tips near their target positions.
Acknowledgements
The CRAVE software used in this study has been developed at Vanderbilt University with support of the NIH (NIH-R01-EB006136,“Computer-assisted functional neurosurgery”, PI B.M Dawant)”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- 1.Deuschl G, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 2.Chan DT, et al. Complications of deep brain stimulation: a collective review. Asian J Surg. 2009;32(4):258–63. doi: 10.1016/S1015-9584(09)60404-8. [DOI] [PubMed] [Google Scholar]
- 3.Rezai AR, et al. Surgery for movement disorders. Neurosurgery. 2008;62(Suppl 2):809–38. doi: 10.1227/01.neu.0000316285.52865.53. discussion 838-9. [DOI] [PubMed] [Google Scholar]
- 4.D'Haese PF, et al. CranialVault and its CRAVE tools: a clinical computer assistance system for deep brain stimulation (DBS) therapy. Med Image Anal. 2012;16(3):744–53. doi: 10.1016/j.media.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boviatsis EJ, et al. Surgical and hardware complications of deep brain stimulation. A seven-year experience and review of the literature. Acta Neurochir (Wien) 2010;152(12):2053–62. doi: 10.1007/s00701-010-0749-8. [DOI] [PubMed] [Google Scholar]
- 6.Beric A, et al. Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg. 2001;77(1-4):73–8. doi: 10.1159/000064600. [DOI] [PubMed] [Google Scholar]
- 7.Oyama G, et al. Delayed clinical improvement after deep brain stimulation-related subdural hematoma. Report of 4 cases. J Neurosurg. 2011;115(2):289–94. doi: 10.3171/2011.3.JNS101424. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi K, et al. Simulation to Locate Burr Hole Sites in a Patient for Deep Brain Stimulation Surgery and Clipping of Intracranial Aneurysm. Neuromodulation. 2012 doi: 10.1111/j.1525-1403.2012.00466.x. [DOI] [PubMed] [Google Scholar]