Abstract
Background
Widespread HIV screening and access to highly-active antiretroviral treatment (HAART) has been deemed cost-effective using mathematical models, though its population-level implementation has raised questions regarding cost, value and feasibility. We aimed to determine the cost-effectiveness of HAART scale-up in British Columbia (BC), Canada compared to scenarios of constrained treatment access.
Methods
Using comprehensive linked population-level data, we populated a dynamic, compartmental transmission model to simulate the HIV/AIDS epidemic in BC from 1997–2010. HIV incidence, prevalence, mortality, costs (in 2010$CDN) and quality-adjusted life years (QALYs) were estimated. Incremental cost-effectiveness ratios (ICERs) were calculated from societal and third party payer (TPP) perspectives to compare actual practice (true numbers of individuals accessing HAART) to scenarios of constrained expansion (75% and 50% probability of accessing HAART). Structural and parameter uncertainty was investigated.
Findings
Actual practice resulted in 263 and 676 averted incident cases compared to the 75% and 50% HAART access scenarios, respectively. From a TPP perspective, actual practice resulted in ICERs of $23,679/QALY versus 75% access, and $24,250/QALY versus 50% access. From a societal perspective, actual practice was cost-saving within the study period, and, resulted in savings of $25.1M compared to the 75% access scenario, and $65.5M compared to the 50% access scenario with an extended time horizon (2035).
Interpretation
HAART expansion in BC has decreased HIV-related morbidity, mortality and transmission. Resulting ICERs for actual practice, derived within a limited timeframe, were within common cost-effectiveness thresholds, and were cost-saving from a societal perspective.
1.0 Introduction
People living with HIV/AIDS (PLHIV) initiating modern highly active antiretroviral therapy (HAART) regimens typically achieve viral suppression within six months [1], triggering CD4 count recovery, thus preventing the development of AIDS and related premature mortality, to a point where PLHIV are now approaching near-normal life spans [2]. Furthermore, there is now wide consensus that plasma HIV-1 RNA level is the strongest predictor of the risk of HIV transmission [3] and that HAART induced suppression of viral replication is highly effective in reducing HIV transmission [4]. HAART was initially demonstrated to be cost-effective on the basis of individual benefit alone [5]. Earlier treatment initiation to simultaneously prevent new infections and benefit the patient, referred to as “Treatment as Prevention (TasP)”, has since been deemed cost-effective to cost-saving in mathematical models, and alongside a randomized controlled trial [6,7].
These developments have fueled the enthusiasm for the deployment of TasP as a means to curb the morbidity, mortality and transmission of HIV globally. Indeed, this has been a driving force for the recently proposed UN 90-90-90 target [8], calling for 90% of HIV-infected individuals to be diagnosed worldwide, 90% of them to receive HAART, and 90% of them to achieve sustained viral suppression. Meeting the 90-90-90 target by 2020 would be expected to dramatically alter the course of the HIV/AIDS pandemic, transforming it into a sporadic endemic condition by 2030 [8]. Nonetheless, ongoing investment is required to sustain efforts to combat the epidemic. A shift, or decrease in focus could lead to increased incidence, which perpetuates the epidemic and compounds its public health and economic burden.
Critically, the cost-effectiveness of a TasP-oriented public health response to HIV/AIDS has yet to be assessed at a population-level in a real-world setting. While prior ecological studies have demonstrated the population-level epidemiological effects of expanded HAART access to treatment [9,10], the vast majority of economic evaluations of HIV screening and treatment are projections based on collections of parameters from multiple sources, and often optimistic, hypothetical scenarios of public health response and health system performance. Here, we have taken advantage of an extensive population-level linked data and surveillance systems to determine the cost-effectiveness of HAART scale-up as observed in the province of British Columbia (BC), Canada from 1997–2010. BC was the epicenter of the HIV/AIDS epidemic in Canada at the outset; however the rate of new case reports per 100,000 population has since fallen below most Canadian provinces [11], a result at least in part attributed to universal coverage of drug and other healthcare costs among PLHIV, and aggressive efforts to scale-up access to HAART [10].
We adapted a dynamic compartmental mathematical model to reproduce the dynamics of the HIV epidemic during the HAART era, and compared observed outcomes to hypothetical scenarios in which access to HAART was constrained to 75% and 50% of actual HAART uptake. Additionally, we considered alternative scenarios altering non-HAART medical costs for those out of treatment, HIV screening rates and risk behaviours, and an extended time horizon to determine the long-term effects of the expansion of HAART access in BC. We hypothesized that actual practice of HAART scale-up was cost-effective compared to the hypothetical scenarios of 50% and 75% actual HAART access, and that both behavioural factors as well as aspects of health service delivery had an important impact on the course of the HIV/AIDS epidemic in BC.
2.0 Methods
2.1 Study Design
We adapted and extended an existing deterministic transmission model previously used to estimate the health benefits and costs of expanded HIV screening and HAART in the United States [12]. We integrated linked, population-level epidemiologic, clinical and economic data to estimate HIV prevalence, incidence, quality-adjusted life-years (QALYs), health care costs and incremental cost-effectiveness ratios (ICERs) associated with actual practice (or observed HAART scale-up) in BC from 1997–2010, compared to outcomes that would have been observed under hypothetical scenarios of constrained access to HAART. The study was approved by the University of British Columbia/Providence Health Care’s research ethics board.
Hypothetical scenarios were constructed such that the probability of HAART entry for treatment-eligible individuals was decreased to 75% and 50% of observed access, respectively. We note that treatment access constraints are not intended to result in the number reaching treatment equaling 75% and 50% of the observed access scenario; by restricting the probability of accessing treatment, the pool of diagnosed and treatment-eligible individuals increased in the hypothetical scenarios, thus increasing the actual numbers of person- years on HAART. Treatment access constraints are intended to represent reductions in the instantaneous probability of an individual accessing HAART in contacts with physicians. The resulting hypothetical scenarios are intended to approximate expected levels of treatment uptake in the absence of a range of province-wide initiatives to inform and engage physicians in HIV treatment, including the biannual HIV/Antiretroviral Update [13].
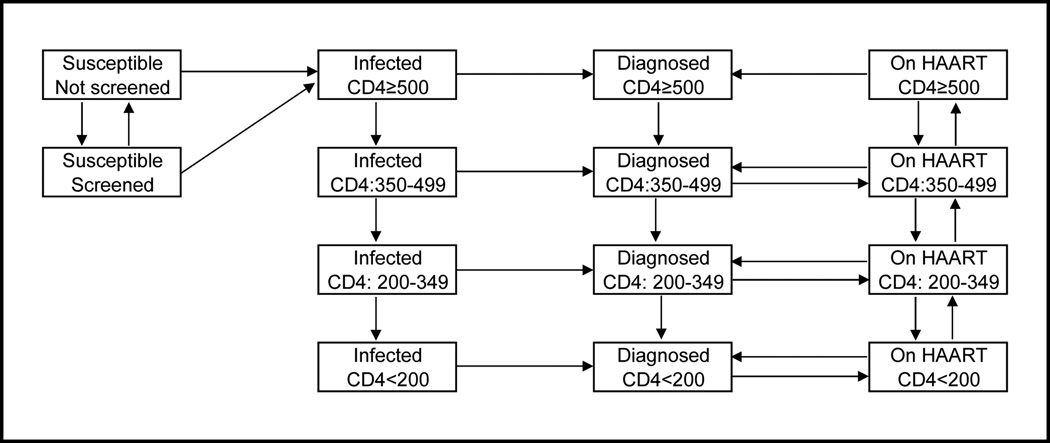
A schematic of the model and dynamics is presented in Figure 1. The adult population of BC aged 15–64 were partitioned into compartments on the basis of HIV risk behavior (men who have sex with men (MSM), injection drug users (IDU), MSM-IDU, and heterosexual) screening status (screened in past 12 months or not) and HIV infection status. Among those HIV-infected, individuals were further classified as infected, diagnosed, and on HAART, and partitioned according to the CD4 cell count (CD4≥500 ppm3, 350–499, 200–349, <200). Health state transitions occurred at monthly intervals. It’s important to note that our model captures individuals that are both infected and never diagnosed (the second ‘column’ of compartments in Figure 1), and diagnosed but do not enter treatment (third column of compartments). Both sets of states feature non-zero rates of transition to mortality, leaving open the possibility of death prior to HAART access – as observed in real-world settings. We further note that the population of diagnosed patients is dynamic, and composed, at any given time, of HAART-experienced and HAART-naïve PLHIV.
Figure 1. Model Diagram.
The model schematic represents movement of the population of BC aged 15–64, both susceptible and HIV-positive. The population was stratified into four complete and mutually-exclusive groups: [MSM, IDU, MSM/IDU and Heterosexual]; the model diagram demonstrates movement for individuals in each of these strata. Further, individuals can transition to mortality from any of the model states (transitions not shown). A more comprehensive description of the model, with specific references to parameters used for each transition, is provided in the supplementary appendix.
We simulated the HIV epidemic by first instantiating the risk group size and HIV prevalence levels based on Q4 1995 BC data and population figures from the public health agency of Canada and BC stats [10, 14,15], thus using 1996 as an instantiation period for the model. We calibrated our model to replicate the true number of PLHIV on HAART in BC at the annual midpoint (actual number of individuals receiving a HAART in June of each calendar year) by adjusting the probability that a diagnosed individual in diagnosed compartment i entered treatment at a given CD4-based state of disease progression at time t (αit). Alternative scenarios of constrained access were created by multiplying αit by constants (0.75, 0.50 respectively) to restrict access to treatment.
Following calibration to match the known annual number of PLHIV on HAART, the model was validated to ensure key epidemiological parameters approximated known or externally-estimated figures. We focused on the size of the HIV-negative population (aged 15–65); overall prevalence; overall prevalence of diagnosed cases; overall incidence; incidence by risk group (MSM, IDU, Heterosexual); and annual mortality among PLHIV.
Cost-effectiveness analysis was conducted according to well-established methods and conformed to guidelines on cost-effectiveness analyses and dynamic transmission modeling [16]. We considered a third-party, or government-payer perspective, as well as a societal perspective, also taking into account costs borne by the individual. All costs were presented in 2010$CDN, and discounted costs and QALYs at an annual rate of 3% in scenarios which projected beyond the timeframe of the primary analysis. Key parameters are presented in Table 1, with further detail regarding model construction, parameterization, calibration and validation provided in the supplementary appendix.
Table 1.
Epidemiological results of dynamic transmission model: British Columbia, 1997–2010
| Observed HAART Access | 75% of Observed Access | 50% of Observed Access | ||||
|---|---|---|---|---|---|---|
| No. Patient-Years on HAART | 46,350 | 42,765 | 36,986 | |||
| Incident Cases of HIV | ||||||
| Overall | 6,230 | 6,493 | 6,906 | |||
| MSM | 2,509 | 2,599 | 2,745 | |||
| IDU* | 1,629 | 1,719 | 1,855 | |||
| HETERO | 2,091 | 2,176 | 2,307 | |||
| Deaths among PLHIV | ||||||
| Overall | 3,193 | 3,272 | 3,394 | |||
| MSM | 994 | 1,009 | 1,034 | |||
| IDU* | 1,645 | 1,701 | 1,786 | |||
| HETERO | 554 | 562 | 574 | |||
| HIV Prevalence (2010) | ||||||
| Overall | 11,326 | 11,523 | 11,825 | |||
| MSM | 4,662 | 4,738 | 4,859 | |||
| IDU* | 3,429 | 3,473 | 3,537 | |||
| HETERO | 3,236 | 3,312 | 3,429 | |||
PLHIV: People living with HIV; MSM: Men who have sex with men; IDU: Injection drug users; HETERO: Heterosexuals.
Includes both MSM/IDU and IDU.
2.2 The force of HIV infection
We incorporated HIV transmission through heterosexual contact, homosexual contact and needle sharing associated with injection drug use. Heterosexual contact occurs within risk groups (for example, both partners are heterosexual, or both are injection drug users) and across groups (for example, a (female) injection drug user with a (male) heterosexual, or a (female) heterosexual with a MSM partner). We assumed proportional mixing, in which individuals could be infected by those of other risk groups, but the probability of infection was proportional to the level of contact (sexual or injection) between groups. The probability of HIV transmission between two persons depended on the infected person’s HIV risk behavior classification, disease status and treatment status and the uninfected person’s HIV risk behavior classification. Specifically, the rate of heterosexual and homosexual transmission was a function of the number of sexual partnerships, condom use and transmission probability per partnership. The model captures HIV transmission through needle sharing in a similar manner, as a function of the annual number of injections, average needle-sharing rates and probability of transmission per shared needle. Further, these probabilities were allowed to change over time according to proxies of injection and sexual risk behavior – specifically, rates of methadone maintenance treatment (MMT) uptake [17] and non-HIV sexually transmitted disease [18] during the study period. The model also accounts for changes in risky behavior due to effective HIV screening and counseling.
2.3 Disease progression
Disease progression was differentiated among those on HAART and not on HAART, and estimated as a function of CD4 count, stratified into the four categories noted above. We derived monthly transition rates by adapting a previously-published BC population-level analysis of individuals on HAART, estimated using a multivariate multi-state Markov model [19]. We adapted the model to account for changes in transition rates by calendar year, and allowed for transitions out of treatment, in addition to CD4 improvement and deterioration in treatment (as indicated by arrows in Figure 1). Otherwise, individuals progressed according to the natural history of HIV, for which transition rates were drawn from the published literature [20].
2.4 Costs and quality adjusted life years
The costs of HAART and non-HAART medical care among PLHIV were estimated using linked individual-level health administrative data for all known PLHIV in BC [15]. Time-dependent estimates of these costs, stratified by CD4 category and HIV risk group (IDU and non-IDU) were adapted from prior studies on the study population, and included HAART and non-HAART medication and pharmacy dispensation costs, the costs of physician billing for outpatient care, and the costs of hospitalization [21,22]. We also considered cost savings due to HAART-attributable productivity gains among PLHIV within a societal perspective, adapting employment data from a population-level study in Denmark [23]. We applied QALY weights derived from the peer-reviewed literature for HIV-negative individuals and PLHIV in and out of treatment, adjusting for injection drug use (further details in the supplementary appendix).
2.5 Sensitivity analysis
We considered alternative scenarios to test structural and parameter uncertainty (pertaining to movement in the model, and the point estimates of parameters dictating these movements, respectively), and otherwise contextualize the impact of observed HAART uptake in BC during the study period. First, in order to determine the incremental impact of HIV screening, we reduced screening rates by 25% and 50% while maintaining the observed level of access to HAART. Second, we held injection and sexual risk behaviors constant at 1996 levels, with observed levels of HAART access, and also considered a scenario whereby injection risk behavior decreased at levels implied by MMT initiation rates, and sexual risk behaviors were held constant at 1996 levels – as opposed to the increase implied by provincial non-HIV STD rates; a relative ‘best case scenario’ in terms of changes in risk behaviours over time. Third, we considered alternate assumptions regarding the accumulation of non-HAART medical costs while out of treatment. Our initial assumption of equal costs for those in- and out- of HAART was necessitated by the fact that the costs of medical care for the undiagnosed are necessarily unobserved. This was a conservative assumption, as prior studies implemented higher out-of-treatment non-HAART costs for individuals with CD4<350. We implemented alternate scenarios using proportionally higher out-of-treatment non-HAART costs according to Long et al. [12], to determine the impact of this assumption. Finally, we extended the model’s time horizon an additional 25 years, holding all key model parameters constant at 2010 levels, accounting for projected population growth estimates [14], to capture the potential second and third order transmission benefits of HAART. We note that, following the IAS-USA guidelines, British Columbia has encouraged access to HAART at diagnosis since 2010, a fact which was captured in our analyses with extended time horizons. Otherwise, we tested the impact of adjustments to parameters for the efficacy of HAART on HIV transmission through sexual contact, and the percentage reduction in the number of sexual partners after HIV diagnosis (results presented in the supplementary appendix).
2.6. Role of the Funding Source
This study was funded by the BC Ministry of Health-funded ‘Seek and treat for optimal prevention of HIV & AIDS’ pilot project. The funder had no direct role in the conduct of the analysis or the decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3.0 Results
The model-estimated annual number of PLHIV on HAART was calibrated to within a mean of 0.7 people compared to the observed number of PLHIV on HAART at the midpoint of each calendar year, and validated to reproduce observed or externally-estimated outcomes with a high level of precision. Further details on the results of the calibration and validation of the model are presented in the supplemental appendix.
Epidemiological estimates from the competing scenarios are presented in Table 2. Constraining the probability of accessing treatment to 75% of observed access resulted in 3,585 fewer patient years in treatment (92.3% of observed access, or a 7.7% reduction), and 9,364 fewer patient years on HAART (79.8% of observed access, or a 20.2% reduction) in the 50% constrained access scenario. The model predicted 6,230 incident cases during the study period, 3,193 deaths and 11,326 prevalent cases at the end of 2010 in the ‘observed HAART scale-up’ scenario. With treatment constrained to 75% of observed access, there were an additional 263 incident cases, 79 deaths and 197 prevalent cases at the end of 2010. Constraining treatment to 50% of observed access led to an additional 676 incident cases, 201 deaths and 499 prevalent cases at the end of 2010.
Table 2.
Results of incremental cost-effectiveness analysis
| PLHIV | PLHIV | Total Population | Total Population | ||
|---|---|---|---|---|---|
| HAART costs $CDN (Millions) |
Non-HAART costs $CDN (Millions) |
Costs $CDN (Billions) |
QALYs (Millions) |
ICER1 | |
| Societal Perspective2 | |||||
| Observed HAART access | 646.10 | 873.32 | 104.606 | 40.578 | |
| 75% of Observed access | 598.00 | 934.36 | 104.613 | 40.578 | D |
| 50% of Observed access | 519.72 | 1032.44 | 104.623 | 40.576 | D |
| Third Party Payer Perspective3 | |||||
| Observed HAART access | 646.10 | 1,205.83 | 104.939 | 40.578 | |
| 75% of Observed access | 598.00 | 1,241.38 | 104.920 | 40.578 | 23,679 |
| 50% of Observed access | 519.72 | 1,298.32 | 104.889 | 40.576 | 24,250 |
| Alternative non-HAART medical costs4 | |||||
| Observed HAART access | 646.10 | 1,436.25 | 105.169 | 40.578 | |
| 75% of Observed access | 598.00 | 1,504.88 | 105.183 | 40.578 | D |
| 50% of Observed access | 519.72 | 1,619.12 | 105.209 | 40.576 | D |
| Proportional decrease in HIV screening rates in hypothetical scenarios5 | |||||
| Observed HAART access | 646.10 | 1,205.83 | 104.939 | 40.578 | |
| Observed access, 75% observed screening | 632.51 | 1,221.15 | 104.933 | 40.577 | 5,920 |
| Observed access, 50% observed screening | 616.11 | 1,238.78 | 104.927 | 40.576 | 6,380 |
| No change in HIV risk behaviours over time | |||||
| Observed HAART access | 646.10 | 1,205.83 | 104.939 | 40.578 | |
| Observed access, constant injection risks6 | 687.74 | 1,449.96 | 105.096 | 40.576 | D |
| Observed access, constant sexual risks7 | 639.73 | 1,186.78 | 104.923 | 40.579 | DT |
| Observed access, constant inj., sex.risks6,7 | 680.72 | 1,427.83 | 105.078 | 40.576 | D |
D: Observed scale-up is a dominant strategy: lower costs, higher QALYs; DT: Observed scale-up is a dominated strategy: higher costs, lower QALYs.
. Incremental Cost-Effectiveness Ratio of Observed HAART scale-up versus 75%, 50% of Observed Scale-up’ scenario: ICER = (Costobserved − Cost75%/50% observed)/(QALYobserved − QALY75%/50% observed).
. Accounting for productivity gains among PLHIV, attributable to HAART engagement.
. Only direct medical costs are included.
. Incremental non-HAART medical costs for the untreated.
. Assumes HIV screening rate in hypothetical treatment scale-up scenarios are also 75%, 50% of observed rates.
. Scenario maintains HAART accessibility, but assumes no decrease in the number of shared injections.
. Scenario maintains HAART accessibility, but assumes no increase in the rate of unprotected heterosexual and homosexual sex.
For the period 1997–2010, from a third party payer perspective (TPP), we estimated that a total of $CDN1.852 billion was spent on healthcare for PLHIV, 34.9% of which was attributable to HAART (Table 3). In comparison, hypothetical scenarios of 75% and 50% of observed HAART scale-up resulted in $CDN1.839 and $CDN1.818 billion, respectively, with 32.5% and 28.6% attributable to HAART. Notably, non-HAART costs increased from $CDN1.206 to $CDN1.241 and $CDN1.298 billion from observed scale-up to hypothetical 75% and 50% HAART scale-up scenarios, respectively. Observed HAART access resulted in approximately 1,000 additional QALYs compared to the ‘75% observed access’ scenario and 2,000 QALYs greater than the ‘50% observed access’ scenario. As a result, from a TPP perspective, ‘observed HAART access’ cost $23,679 per QALY gained, compared to the ‘75% observed access’ scenario, and $24,250 per QALY gained compared to the ‘50% observed access’ scenario. These figures are 44.5% of current Canadian GDP per capita (2013: $US51,911; $53,214 in 2010$CDN), making observed HAART scale-up highly cost-effective according to stated WHO thresholds for cost-effectiveness [24]. Considering a societal perspective, productivity gains due to HAART access more than offset the additional costs of treatment, resulting in ‘Observed HAART access’ being a dominant strategy (lower total costs, higher QALY gains).
Sensitivity analysis on the rate of HIV screening suggested that reduced rates of HIV screening may have had at least as large an effect on public health and economic outcomes as constraints on treatment. In particular, holding HAART access constant, 75% of observed screening resulted in a slightly lower QALY loss (40.578M − 40.577M=1,000 QALYs, noting that QALY gain figures represent the total population of BC aged 15–64), resulting in an ICER of $5,920 per QALY gained for observed versus 75% of observed HIV screening (Table 3). A greater constraint on screening (50% of observed) resulted in a greater QALY loss (40.578M−40.576M=2,000 QALYs). Constrained screening resulted in lower total costs as a result of a lower number of diagnosed cases accessing HAART.
Holding HIV risk behaviours constant had a profound impact on public health and economic outcomes. Without the observed decreases in injection risk behaviours, the model estimated an increment of C$42M in HAART costs and a detriment of approximately 2,000 QALYs (Table 3). In contrast, had high risk sexual behaviours remained constant (as opposed to their observed increase), the model estimated lower healthcare costs resulting from decreased HIV incidence, and an additional 1,000 QALYs gained within the study population.
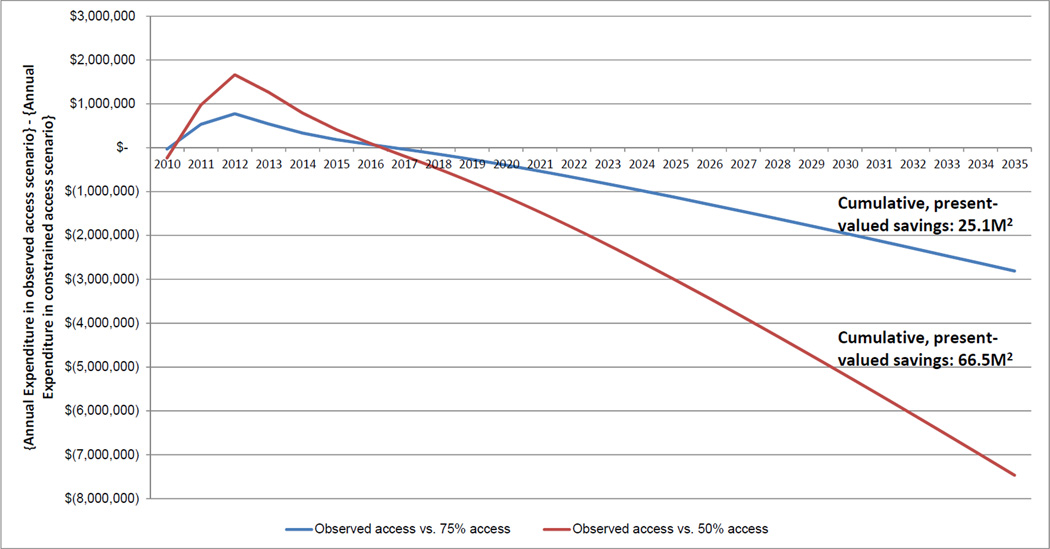
Finally we extended the time horizon an additional 25 years to determine the long-term implications of observed versus constrained access. Considering a societal perspective, and holding all model parameters constant at 2010 levels, we estimated that annual expenditure on PLHIV in the actual practice scenario fell below the constrained access scenarios from 2017, with annual savings increasing to $2.8M (vs 75% access) and $7.5M (vs. 50% access) by 2035 (Figure 2). Observed HAART access resulted in savings of $25.1M and $66.5M in present value compared to 75% and 50% HAART access scenarios, respectively.
Figure 2. Projected difference in annual, undiscounted expenditures for PLHIV1: HAART scale-up observed in 1997–2010 vs. hypothetical constrained access scenarios.
1. Using a societal perspective, accounting for workforce productivity gains among PLHIV. Plotted differences in annual expenditures are not discounted. An amount in parentheses in x-axis indicates negative numbers. 2. Presented in 2010$CDN, discounted at an annual rate of 3%. This represented the difference in the sum of total expenditure, 1997–2035, for the ‘Observed Access’ scenario, compared to ‘75% Access’ and ‘50% Access’, respectively.
4.0 Discussion
Using perhaps the most comprehensive linkage of population-level surveillance, disease registry and health administrative data available, our results suggest that actual practice of HIV testing and treatment in BC resulted in 263 averted incident cases compared to the 75% HAART access scenario, and 676 averted cases compared to the 50% access scenario between 1997 and 2010. Within the study timeframe and using a TPP perspective, actual practice led to substantially greater QALY gains, resulting in ICERs of $23,679/QALY compared to 75% expansion, and $24,250/QALY compared to 50% expansion.
Furthermore, from a societal perspective, actual practice was cost-saving over the study horizon. We note that, aside from the retrospective nature of the study, which captured periods of clinical-guideline-constrained access and earlier, poorer-quality HAART regimens, the limited time horizon makes these results highly conservative, as the public health benefits of HAART – and particularly averted incident cases and mortality – are realized over the long term. Extending the time horizon to 2035 indicated that actual practice – as delivered in 2010 - should lead to a present-valued savings of $25.1M in total cumulative costs compared to the 75% access scenario, and a savings of $66.5M compared to the 50% access scenario. The higher initial costs of expanded treatment observed in Figure 2 were rapidly diminished due to decreases in inpatient care costs, productivity gains and infections averted, resulting in lower annual costs for the ovserved access scenario by 2017. Recent results from the Strategic Timing of Antiretroviral Treatment (START) study, demonstrating individual-level health benefits from starting antiretroviral treatment at diagnosis, will be influential in further expanding access to HAART internationally.
We note that projections with an extended time horizon are based on 2010-level HAART and non-HAART medical costs, and do not account for the potential decreases in HAART costs brought about by generic formulations. Such a shift could decrease absolute HIV-related expenditures, but perhaps also diminish incremental savings in analyses like ours if the savings outweigh additional costs brought on by the more expensive 2nd- and 3rd-line regimens that long-term HAART users may require to sustain viral suppression for a near-normal lifespan. This is one of a number of questions – many of which centered around choosing interventions to optimize the HIV care cascade - that will need to be addressed to inform resource allocation decisions to achieve the 90-90-90 treatment target established by UNAIDS [8]. Locally, these decisions will continue to be reliant upon evidence derived from the extensive, and exemplary, data systems established in BC.
Our sensitivity analysis constraining screening rates assumed less HIV testing than observed (keeping people 'undiagnosed' for longer periods) with HAART access set as in the baseline 'observed HAART access' scenario. We found that maintaining - and increasing - the level of HIV testing was of similar importance to increasing access to HAART.
Despite the overwhelmingly positive impacts of increases in HIV screening and treatment engagement, it is clear that changes in underlying risk behaviours had substantial impacts on the epidemic in BC. First, it appears that large increases in access to MMT, which coincided with the discovery and proliferation of HAART use [17], had an exceptionally strong impact on the HIV/AIDS epidemic, and perhaps greater than previously thought. This impact of MMT on the epidemic further demonstrates the urgent need to prioritize delivery of maintenance-oriented treatment, which remains constrained internationally [25]. The decline in HIV incidence among IDUs was also likely influenced by the introduction of needle exchange and supervised consumption rooms, and may have further benefited from substitution to non-injection drugs use [26]. To be clear, our model has no means of distinguishing the respective magnitude of impact of these effects on injection risk behavior and subsequent HIV incidence among IDU in the province, a topic requiring further study.
Otherwise, our model suggests increasing levels of unprotected sex, deduced from increasing non-HIV infectious disease rates [18], have clearly had a substantially negative effect on the HIV epidemic, and compromised the potential gains of increased HAART access, particularly among MSM. We note that we are not able to confirm population-level sexual disinhibition as a direct driver of new infections, but rather use the most relevant ecological proxy available to inform behavioral parameters in our model; an aspect which was necessary to achieve the level of accuracy in validation that we’d attained. Otherwise, it is worth noting that the PARTNERS randomized controlled trial has indicated HAART is equivalently efficacious in preventing HIV transmission among sero-discordant MSM couples and heterosexual couples [27]. In concentrated epidemics situated in high- and middle-income settings, far more is needed to identify effective test-and-treat campaigns. Alternative strategies will be required to reach this otherwise healthy population of young MSM, within which the incidence of HIV/AIDS has emerged as the highest in North America Pre-exposure prophylaxis (PreP) has been touted as a viable prevention strategy among MSM and other high-risk populations [28], however its value - relative to interventions for testing and HAART access for PLHIV - in settings with relatively low levels of undiagnosed HIV and unsuppressed viremia should be established with real-world data to be considered for larger-scale implementation. Unbiased determinations of relative value would necessarily need to be made with a QALY-based analysis –taking into account not only infections averted, but also the reductions in HIV-related morbidity and mortality associated with HAART in PLHIV.
Deterministic compartmental models are useful for modeling average behavior of disease epidemics in large populations. While agent-based approaches may be preferred when stochastic effects (eg. extinction of disease in small populations), complex interactions between behaviour and disease, or distinctly non-random mixing patterns are present, previously-published comparisons have revealed a high level of concordance between model types [29]. Our model was validated on ten key aspects of the HIV epidemic in BC, and we are thus confident in the validity of our results. While our model is parameterized with largely local source data to simulate the BC HIV/AIDS epidemic, the structure of the model is generalizable, with prior applications to the US, China and South Africa [12,29]. Results are nonetheless specific to the BC setting, and will vary according to the epidemiological and systemic factors shaping localized HIV epidemics.
This analysis was not without limitations. First, similar to other models used in cost-effectiveness analyses of HIV screening and treatment [12], infectivity was modeled indirectly through CD4-based disease progression stages. Second, while the probability of transmission in early HIV infection has been debated and deemed not as high as initially thought [30], its long-term impact on reduction of incidence was determined to be minimal.61 While our model did not account for these temporal transmission dynamics, it was able to produce risk-group specific incidence estimates and reproduce key aspects of the HIV epidemic at the population-level in BC with a high degree of precision. Third, while the total susceptible population in BC was reproduced accurately, in- and out-migration of PLHIV is not currently observed directly within our linked population-level database, and was assumed to be equal to zero. Otherwise, in the absence of representative local sources, data on productivity loss were adapted from another jurisdiction, and thus must be regarded as substantially lower-quality than that used to calculate TPP costs. While we believe our linked database to be among the highest quality and most comprehensive available worldwide, these remain areas for further inquiry. Fourth, while drug resistance was not explicitly modeled in our study, It was accounted for in that the cost and disease progression estimates were derived from statistical analyses that captured the full population of BC PLHIV - including those with multi-drug resistance. The input values used in the CEA were fitted values from regression models which included MDRT as a covariate in both progression and cost models, and the annual population of drug resistant individuals was used in generating the model inputs - in other words, these inputs were weighted averages of CD4 progression and cost for non-resistant and resistant clients - balancing also other relevant covariates [19,21,22]. Further, rates of drug resistance are low in BC, and concentrated amongst pre-HAART-era treatment initiators [21]. Fifth, in the extended time horizon, it is likely that cost estimates were conservative given stabilization of HAART costs since 2007, the high relative costs of individuals initiating treatment in the pre-HAART era, and the decreasing proportion of these individuals within the broader HIV-positive population [21]. Finally, a full probabilistic sensitivity analysis was not executed, and indeed is not recommended in dynamic transmission modeling due to the implicit objective nature of model calibration to actual practice [16]
The expansion of HAART in BC has resulted in substantial decreases in morbidity and mortality as well as a reduction in new HIV diagnoses. Resulting ICERs, derived within a limited timeframe, were well within the range of societal willingness to pay for an incremental QALY gain, and were cost-saving from a societal perspective. Further efforts should be directed to evaluate the long term impact of this strategy, as well as to validate these results in other settings, including low resource settings and generalized epidemics.
Supplementary Material
Research in context.
Evidence before this study
We executed a search of British Columbia provincial data sources and reports to populate and calibrate our model to best reflect the dynamics of the HIV epidemic in BC during the study period. This included an extensive search of the peer-reviewed literature (British Columbia; Vancouver; HIV; AIDS; antiretroviral therapy; highly active antiretroviral therapy; injection drug use; men who have sex with men). Furthermore, we executed a literature search for cost-effectiveness analyses and mathematical modeling analyses to inform model selection and development (pubmed search keyword: HIV; AIDS; antiretroviral therapy; highly active antiretroviral therapy; cost-effectiveness; economic evaluation; mathematical modelling).
Added value of this study
The cost-effectiveness of a Treatment as Prevention (TasP)-oriented public health response to HIV/AIDS has yet to be assessed at a population-level in a real world setting. The vast majority of studies demonstrating the value of HIV screening and treatment are based on often optimistic projections of public health response and health system performance. We have taken advantage of an extensive population-level linked data and surveillance systems to calibrate a mathematical model to represent the provincial HIV epidemic during the study period, and consider the cost-effectiveness of actual practice versus hypothetical scenarios of less aggressive efforts to scale-up access to highly active antiretroviral therapy (HAART).
Implications of all the available evidence
We found that the aggressive scale-up of HAART observed in British Columbia averted incident HIV cases, prevented HIV-related mortality, was cost-effective within the study period, and cost-saving when accounting for costs of foregone productivity. These results confirm the value of aggressive scale-up of HAART access as a public health strategy to reduce morbidity, mortality and transmission of HIV/AIDS, and should serve to focus efforts on the implementation of HIV testing and treatment initiatives to maximize the benefits of HAART.
Acknowledgements
This study was funded by the BC Ministry of Health-funded ‘Seek and treat for optimal prevention of HIV & AIDS’ pilot project. The funder had no direct role in the conduct of the analysis or the decision to submit the manuscript for publication. Bohdan Nosyk is a Michael Smith Foundation for Health Research Scholar. Dr. Viviane Lima is a Michael Smith Foundation for Health Research Scholar and CIHR New Investigator.
We acknowledge Michelle Olding for assistance with manuscript preparation, as well as all BCMoH and Vancouver Coastal Health Decision Support Staff involved in data access and procurement, including Monika Lindegger, Clinical Prevention Services, BC Centre for Disease Control; Al Cassidy, BC Ministry of Health Registries and Joleen Wright and Karen Luers, Vancouver Coastal Health decision support. We would also like to acknowledge Ciro Panessa, Nancy South and Mark Gilbert for their contributions to the STOP/HIV AIDS study group.
Julio Montaner is supported with grants paid to his institution by the British Columbia Ministry of Health and by the US National Institutes of Health (R01DA036307). He has also received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. Viviane D Lima has received limited unrestricted funding, paid to her institution, from GlaxoSmithKline.
The STOP HIV/AIDS Study Group is comprised of the following
Rolando Barrios, Vancouver Coastal Health Authority
Patty Daly, Vancouver Coastal Health Authority
Reka Gustafson, Vancouver Coastal Health Authority
Perry RW Kendall, British Columbia Ministry of Health
Gina McGowan, British Columbia Ministry of Health
Irene Day, BC Centre for Excellence in HIV/AIDS
Kate Heath, BC Centre for Excellence in HIV/AIDS
Robert S Hogg, BC Centre for Excellence in HIV/AIDS
Julio SG Montaner, BC Centre for Excellence in HIV/AIDS
Bohdan Nosyk, BC Centre for Excellence in HIV/AIDS
Footnotes
Author Contributions: BN designed the study, assisted with analysis and wrote the first draft of the article. JM led the analysis and assisted with study design and interpretation of results. RSH, VDL, and JSGM aided in study design, interpretation of results, and provided critical revisions to the article. JSGM led the procurement of the database. All authors approved the final draft.
Declaration of Interests: All other authors have no conflicts of interest to declare.
References
- 1.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 2.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS one. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedberg KA, Scharfstein JA, Seage GR, III, Losina E, Weinstein MC, Craven DE, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. Journal of the American Medical Association. 1998;279(2):130–136. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 6.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):1077–1078. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 7.Walensky RP, Cohen MS, Freedberg KA. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2014;370(6):581–582. doi: 10.1056/NEJMc1314998. [DOI] [PubMed] [Google Scholar]
- 8.UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. [June 1st, 2015];2014 Accessed at: www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. [Google Scholar]
- 9.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montaner JS, Lima VD, Harrigan PR, Lourenço L, Yip B, Nosyk B, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV Treatment as Prevention” experience in a Canadian setting. PloS one. 2014;9(2):e87872. doi: 10.1371/journal.pone.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogg GS, Nosyk B, Harrigan PR, Lima VD, Chan K, Heath K, et al. Rates of new infections in British Columbia continue to decline at a faster rate than in other Canadian regions HIV Medicine. 2013;14:581–582. doi: 10.1111/hiv.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med. 2010;153(12):778–789. doi: 10.1059/0003-4819-153-12-201012210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HIV/AIDS BCfE. Fall HIV/Antiretroviral Update. Vancouver, BC: 2014. 2014 November 24th, 2014. [Google Scholar]
- 14.BC Statistics. Population Estimates. [cited January 2015];2014 Available from: http://www.bcstats.gov.bc.ca/StatisticsBySubject/Demography/PopulationEstimates.aspx.
- 15.Nosyk B, Montaner JSG, Colley G, Lima VD, Chan K, Yip B, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: A population-based retrospective cohort study. Lancet Infectious Diseases. 2014;S1473-3099(13):70254–70258. doi: 10.1016/S1473-3099(13)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, et al. Dynamic transmission modeling: A report of the ISPOR-SMDM modeling good research practices task force-5. Value Health. 2012;15:828–834. doi: 10.1016/j.jval.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nosyk B, Marsh DC, Sun H, Schechter MT, Anis AH. Trends in methadone maintenance treatment participation, retention and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. Journal of substance abuse treatment. 2010;39(1):22–31. doi: 10.1016/j.jsat.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 18.BC Centre for Disease Control. HIV and Sexually Transmitted Infections 2010. Vancouver: BC Centre for Disease Control; 2010. [Google Scholar]
- 19.Nosyk B, Min J, Lima VD, Yip B, Hogg RS, Montaner JS, et al. HIV-1 disease progression during highly active antiretroviral therapy: an application using population-level data in British Columbia: 1996–2011. JAIDS. 2013;63(5):653–659. doi: 10.1097/QAI.0b013e3182976891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellors JW, Muñoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Nosyk B, Yip B, Lima VD, Hogg RS, Montaner JSG STOP HIV/AIDS Study Group. Antiretroviral drug costs and prescription patterns in British Columbia, Canada: 1996–2011. Med Care. 2014;52(4):362–369. doi: 10.1097/MLR.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nosyk B, Lima V, Colley G, Yip B, Hogg RS, Montaner JS. Costs of health resource utilization among HIV-positive individuals in British Columbia, Canada: Results from a population-level study. Pharmacoeconomics. 2015 Mar;33(3):243–253. doi: 10.1007/s40273-014-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legarth R, Omland LH, Kronborg G, Larsen CS, Pedersen C, Pedersen G, et al. Employment status in persons with and without HIV infection in Denmark: 1996–2011. AIDS. 2014;28(10):1489–1498. doi: 10.1097/QAD.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 2010 ART Guidelines for adults and adolescents – evidence map. [cited; Available from: http://www.who.int/hiv/topics/treatment/evidence/en/index.html.
- 25.Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2010;382(9904):1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 26.Marshall BD, Milloy MJ, Wood E, Montaner JS, Kerr T. Reduction in overdose mortality after the opening of North America's first medically supervised safer injecting facility: a retrospective population-based study. Lancet. 2011;377(9775):1429–1437. doi: 10.1016/S0140-6736(10)62353-7. [DOI] [PubMed] [Google Scholar]
- 27.Rodger A, Bruun T, V C, J L, Vernazza P, Collins S, et al. HIV transmission risk through condomless sex if HIV positive partner is on supressive ART: PARTNER study. Boston: CROI; 2014. [Google Scholar]
- 28.Beyrer C, Bekker LG, Pozniak A, Barré-Sinoussi F. Pre-exposure prophylaxis works--it's time to deliver. Lancet. 2015;385(9977):1482–1484. doi: 10.1016/S0140-6736(15)60724-3. [DOI] [PubMed] [Google Scholar]
- 29.The HIV Modelling Consortium Treatment as Prevention Editorial Writing Group. HIV Treatment as Prevention: models, data, and questions – towards evidence-based decision-making. PLoS Medicine. 2012;9(7):e1001259. doi: 10.1371/journal.pmed.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eaton JW, Hallett TB. Why the proportion of transmission during early-stage HIV infection does not predict the long-term impact of treatment on HIV incidence. PNAS. 2014;111(45):16202–16207. doi: 10.1073/pnas.1323007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.