Abstract
Purpose
The two-step floating catchment area (2SFCA) method of measuring access to care has never been used to study cancer disparities in Appalachia. First, we evaluated the 2SFCA method in relation to traditional methods. We then examined the impact of access to mammography centers and primary care on late stage breast cancer diagnosis and receipt of adjuvant hormonal therapy.
Methods
Cancer registries from Pennsylvania, Ohio, Kentucky, and North Carolina were linked with Medicare data to identify the stage of breast cancer diagnosis for Appalachia women diagnosed between 2006–2008. Women eligible for adjuvant therapy had stage I, II, or III diagnosis; mastectomy or breast conserving surgery; and hormone-receptor positive breast cancers. Geographically weighted regression (GWR) was used to explore non-stationarity in the demographic and spatial access predictor variables.
Results
Over 21% of 15,299 women diagnosed with breast cancer had late-stage (stages III–IV) diagnosis. Predictors included age at diagnosis (odds ratio [OR], 0.86; P < 0.001), insurance status (OR, 1.32; P < 0.001), county primary care to population ratio (OR, 0.95; P < 0.001), and primary care 2SFCA score (OR, 0.96; P = 0.006). Only 46.9% of eligible women received adjuvant hormonal therapy, and predictors included comorbidity status (OR, 1.18; P = 0.047), county economic status (OR, 1.32; P = 0.006), and mammography center 2SFCA scores (OR, 1.12; P = 0.021).
Conclusion
Methodologically, the 2SFCA method offered the greatest predictive validity of the access measures examined. Substantively, rates of late stage breast cancer diagnosis and adjuvant hormonal therapy are substandard in Appalachia.
INTRODUCTION
The Appalachia region of the United States has reduced health outcomes and treatment patterns across a number of diseases, including breast cancer.1–3 Because many areas of Appalachia have lower socioeconomic status.4 and occupy rural, mountainous terrain, reduced access to care is often implicated in the region’s cancer disparities.5
Spatial access to care is traditionally measured using either provider to population ratios or by computing the travel time between patient and provider.6 Both methods have limitations, however. Provider to population ratios use fixed geographic boundaries (e.g., counties) that do not reflect actual patient behaviors, while travel time fails to account for supply and demand factors.7 More recently, the two-step floating catchment area (2SFCA) method was developed to overcome these limitations.8 Despite its improvement over traditional measures of spatial access to care, the 2SFCA method has never been used to study cancer outcomes or treatment patterns in Appalachia.
We recently evaluated the impact of different 2SFCA parameter options when measuring access to mammography centers and primary care physicians in Appalachia. Here, we used a linked central cancer registry and Medicare dataset across four Appalachian states to evaluate the relationship between spatial access to care and two important clinical indicators for breast cancer—late stage diagnosis and receipt of adjuvant hormonal therapy. Late stage breast cancer diagnosis leads to fewer treatment options and increased mortality9 and is more prevalent in lower socioeconomic, rural, and black populations.10–12 Adjuvant hormonal therapy is recommended for hormone receptor positive patients after either breast conserving surgery or mastectomy.13,14 Lower socioeconomic status is also associated with reduced rates of adjuvant hormonal therapy.15
First, we focused on the methodological aspects of spatial access to care by evaluating the predictive ability of the 2SFCA method compared to traditional spatial access approaches. We then focused on the substantive clinical outcomes of interest in the Appalachia region. We used geographically weighted regression (GWR) to examine whether the influence of demographic or spatial access predictor variables on stage of breast cancer diagnosis or receipt of adjuvant hormonal therapy differed throughout the study region.
METHODS
This research was approved by the institutional review board at the University of Michigan.
STUDY POPULATION
We used PA, OH, KY, and NC Central Cancer Registry (CCR) datasets to identify 15,299 women diagnosed with breast cancer between 2006 and 2008 who lived in Appalachia counties, defined by the Appalachia Regional Commission (ARC). To examine receipt of hormonal therapy, CCR datasets were linked with Medicare claims to further limit the sample to patients with Medicare Part D enrollment; diagnosis during year 2007; stage I, II, or III diagnosis;16 confirmed mastectomy or breast conserving surgery; and hormone-receptor positive breast cancers; resulting in 834 women eligible for adjuvant hormonal therapy.17 The methods used to link CCR and Medicare datasets have been previously described.18 Briefly, Medicare claims and cancer registry data were linked by Center for Medicare and Medicaid Services contractors with a probabilistic match algorithm based on a finders file supplied from the cancer registry data. The standard matching string consisted of Social Security number, last name and the first 3 characters of the first name.
DEFINITION OF VARIABLES
Dependent Variables
Breast cancer stage at diagnosis and receipt of adjuvant hormonal therapy were the two dependent variables. Similar to previous research,19 early stage diagnosis was defined as stages 0, I, or II, while late stage was defined as stages III and IV. Patients with prescription codes for tamoxifen, anastrozole, letrozole, or exemestane were defined as having received adjuvant hormonal therapy.13
Spatial Access Measures
Three spatial access methods were evaluated: 1) county provider to population ratios, 2) travel time to closest provider, and 3) the 2SFCA method.
Provider to population ratios were calculated for primary care physicians and mammography centers. The American Medical Association (AMA) Physician Masterfile from 2008 was used to identify primary care physicians, which we defined as having specialties of Family Practice, General Practice, Internal Medicine, and General Pediatrics.20 The number of primary care physicians in each county was divided by that county’s population, using counties and populations from the 2010 Census, to yield a ratio for each county. All 2008 U.S. Food and Drug Administration (FDA) accredited mammography centers in Appalachia counties of the study region were identified. The number of mammography centers in each county was divided by that counties population of women age 45 and older. Although 2008 guidelines called for mammography screening beginning at age 40, the 2010 Census used age groups 25–34 and 35–44. We chose to use women age 45 and older. Each patient was assigned a primary care ratio and a mammography center ratio based on their county of residence.
For travel time calculations, the physician and mammography centers were geocoded using ArcGIS 10.1. Exact office addresses were available for 8,039 of the 9,483 physicians. For the remaining 1,444 physicians, the population weighted centroid of the census tract associated with the physician’s office was geocoded. All 1,181 mammography centers had geocodable addresses. We used ArcGIS Network Analyst extension to calculate the driving time between the closest physician and mammography center and the population weighted centroid of every census block group in the study region. For reference, census block groups are generally composed of between 600 and 3,000 people. Each patient was assigned a travel time to their closest primary care physician and mammography center based on the census block group of their residence.
More detailed methodological explanations of the 2SFCA method are described elsewhere.21 To complete the first step of the 2SFCA, we created service provider to population ratios using each provider and the sum of all the census block group populations (or populations of females 45 and older for mammography centers) within that provider’s catchment area. Catchment areas were set to 60 minutes to reflect the rural nature of Appalachia.22 Step two then moved to each block group population center and identified all service providers within the designated 60 minute catchment around that block group. The step one ratios within each population’s catchment were summed, resulting in each block group having a primary care access score and a mammography center access score.
Two critiques of the original 2SFCA method are its failure to vary catchment sizes based on population needs and its failure to account for distance decay within catchments.23 We added a distance decay function by breaking catchments into four time zones: 0–10 minutes, 11–20 minutes, 21–30 minutes, and 31–60 minutes.23 Two sets of weights were chosen for the distance decay function, corresponding to fast decay (weights 1, 0.60, 0.25, 0.05) and slow decay (weights 1, 0.80, 0.55, 0.15).21 Distance decay was applied at both steps.
We also varied the catchment size at each step of the 2SFCA, using McGrail’s (2012) approach. At step one, the technique uses a set of rules to determine whether the distance decay weights are applied (thereby expanding or reducing a service provider’s coverage area). No step one decay weight was applied if the travel time between a provider and population was less than 10 minutes, if the provider was one of the 25 closest (5 closest for mammography centers) to that population center, or if the population center had a population less than 5,000 and less than half the population of the service provider’s town.21 For step two, catchments were varied by only including the closest 100 primary care physicians and only the closest 20 mammography centers.21 Thus, each census block group in the study region was given an original 2SFCA score without any distance decay or varied catchments, a slow decay 2SFCA score with the variable catchment rules, and a fast decay 2SFCA score with the variable catchment rules. Patients were matched to 2SFCA scores by the census block group of their address.
Demographic and Additional Independent Variables
We included age, insurance status, race/ethnicity, and county economic status along with the spatial access measures for the late stage breast cancer diagnosis model. Age was categorized as younger than 50, 50–64, or 65 or older. Insurance status was split into five categories: private, Medicare, Medicaid, uninsured, and unknown. Race/ethnicity was defined as white, black, or Hispanic/other. We used the Appalachia Regional Commission’s (ARC) designation for labeling counties as either attainment, competitive, transitional, at-risk, or distressed.24 The ARC uses the three-year average unemployment rate, the per capita market income, and the poverty rate to calculate an average for every county in the nation, and then ranks each county into quintiles to yield the final economic status descriptor.
When creating the model for receipt of adjuvant hormonal therapy, we included age, race/ethnicity, county economic status, cancer stage, and comorbidity index along with the spatial access measures. Age was defined as 65–69, 70–74, 75–79, and older than 80. Race/ethnicity and county economic status were defined as described in the above model. Cancer stage was categorized as stage I, II, or III, reflecting those patients eligible for adjuvant hormonal therapy. The Charlson Comorbidity Index, which creates a weighed score of comorbidity, was constructed from Medicare claims data.25
DATA ANALYSIS
Initial exploratory analysis and goodness-of-fit statistics were used to determine which spatial access and demographic variables to include in the logistic regression models predicting late stage breast cancer diagnosis and receipt of adjuvant hormonal therapy.26,27 Goodness-of-fit statistics included the corrected Akaike’s Information Criterion (AICc) and the area under the receiver operating characteristic curve (AUC). AICc values lower than 3 indicate better model fit.27 An AUC value of 1 indicates that a model perfectly predicts the dependent variable, while an AUC value of 0.5 indicates random chance.28 Adjusted R squared, a measure of variation explained by the chosen predictor variables, was also used to compare models.26
Once an appropriate model was specified for the traditional logistic regression and the logistic GWR, we looked for evidence of spatial non-stationarity of the predictor variables. Briefly, GWR includes geographic coordinates at each study observation to construct a series of local regressions, resulting in unique predictor coefficients at each study observation. Traditional global logistic regression creates one coefficient for each predictor variable that is assumed to be stationary across the entire study region. We used a fixed Gaussian kernel type and the golden selection search to minimize the AICc when selecting bandwidth size for the logistic GWR. GWR 4.0 software was used for the logistic GWR (available at https://geodacenter.asu.edu/gwr_software). Fotheringham et al. (2002) provide a more detailed methodological overview of the GWR approach.
We also examined descriptive statistics of the local coefficients in the logistic GWR to identify large minimum to maximum ranges, existence of both positive and negative coefficient values, skewness, or any other indicators of spatial non-stationarity. When spatial non-stationarity was found, we mapped the coefficients onto the geographic study area to examine varying geographic effects of each predictor variable.
RESULTS
There were 15,299 women living in Appalachia counties of PA, OH, KY, and NC diagnosed with breast cancer between years 2006 and 2008. Table 1 provides descriptive and chi-square statistics of the study variables characterized by early and late stage breast cancer diagnosis. The majority of women had early stage diagnosis (78.8%), were older than 65 (mean age = 71.1, SD = 10.4), and were insured by Medicare (65.5%). Although race/ ethnicity was not included in Table 1, most women were white (95.3%). Race/ethnicity was not associated with stage at diagnosis (X2 = 4.26, p = 0.119). The fast decay 2SFCA scores for primary care and mammography centers showed a stronger relationship to stage of diagnosis than slow decay and original 2SFCA scores in both chi-square analysis and subsequent regression modeling, and for brevity are the only 2SFCA scores shown in Table 1. 2SFCA scores are broken into quintiles for ease of interpretation, with larger scores (and larger quintiles) indicating greater spatial access to care.
Table 1.
Characteristics of study patients by breast cancer stage at diagnosis.
| Factors | Total cases | % | % Early stage | % Late Stage | p-value |
|---|---|---|---|---|---|
| 15,299 | 78.8 | 21.2 | |||
| Age | |||||
| <50 | 501 | 3.3 | 64.5 | 35.5 | <0.001 |
| 50 – 64 | 3,242 | 21.2 | 80.9 | 19.1 | |
| 65+ | 11,556 | 75.5 | 78.8 | 21.2 | |
| Insurance Status | |||||
| Private | 4,309 | 28.2 | 81.8 | 18.2 | <0.001 |
| Medicare | 10,027 | 65.5 | 78.6 | 21.4 | |
| Medicaid | 544 | 3.6 | 71.1 | 28.9 | |
| Uninsured | 112 | 0.7 | 68.8 | 31.3 | |
| Unknown | 307 | 2 | 59.3 | 40.7 | |
| County Economic Status | |||||
| Attainment | 0 | 0 | 0 | 0 | <0.001 |
| Competitive | 3,351 | 21.9 | 80.6 | 19.4 | |
| Transitional | 9,519 | 62.2 | 78.7 | 21.3 | |
| At-risk | 1,482 | 9.7 | 78.3 | 21.7 | |
| Distressed | 947 | 6.2 | 73.5 | 26.5 | |
| Driving Time to Closest Primary Care (minutes) | |||||
| <10 | 13,010 | 85.0 | 79.2 | 20.8 | 0.031 |
| 10 – <20 | 1,863 | 12.2 | 76.8 | 23.2 | |
| 20 – <30 | 383 | 2.5 | 74.2 | 25.8 | |
| 30 – <40 | 41 | 0.3 | 85.4 | 14.6 | |
| 40 – <50 | 1 | <0.001 | 100 | 0 | |
| 50+ | 1 | <0.001 | 100 | 0 | |
| Driving Time to Closest Mammography Center (minutes) | |||||
| <10 | 9,332 | 61.0 | 79.3 | 20.7 | 0.337 |
| 10 – <20 | 4,008 | 26.2 | 78.3 | 21.7 | |
| 20 – <30 | 1,417 | 9.3 | 76.7 | 23.2 | |
| 30 – <40 | 422 | 2.8 | 79.4 | 20.6 | |
| 40 – <50 | 97 | 0.6 | 78.4 | 21.6 | |
| 50+ | 23 | 0.2 | 82.6 | 17.4 | |
| Ratio of Primary Care Physicians / County Population | |||||
| 1st Quintile | 3,104 | 20.3 | 75.9 | 24.1 | <0.001 |
| 2nd Quintile | 3,055 | 20.0 | 77.5 | 22.5 | |
| 3rd Quintile | 3,065 | 20.0 | 80.0 | 20.0 | |
| 4th Quintile | 3,364 | 22.0 | 79.3 | 20.7 | |
| 5th Quintile | 2,711 | 17.7 | 81.4 | 18.6 | |
| Ratio of Mammography Centers / County Population of Females Age 45 and older | |||||
| 1st Quintile | 3,185 | 20.8 | 79.3 | 20.7 | <0.001 |
| 2nd Quintile | 3,050 | 19.9 | 81.9 | 18.1 | |
| 3rd Quintile | 3,352 | 21.9 | 78.6 | 21.4 | |
| 4th Quintile | 2,735 | 17.9 | 77.4 | 22.6 | |
| 5th Quintile | 2,977 | 19.5 | 76.4 | 23.6 | |
| 2SFCA Fast Decay Scores to Primary Care | |||||
| 1st Quintile | 3,059 | 20.0 | 76.4 | 23.6 | <0.001 |
| 2nd Quintile | 3,060 | 20.0 | 79.0 | 21.0 | |
| 3rd Quintile | 3,063 | 20.0 | 80.5 | 19.5 | |
| 4th Quintile | 3,057 | 20.0 | 78.1 | 21.9 | |
| 5th Quintile | 3,060 | 20.0 | 79.9 | 20.1 | |
| 2SFCA Fast Decay Scores to Mammography Centers | |||||
| 1st Quintile | 3,060 | 20.0 | 79.0 | 21.0 | 0.130 |
| 2nd Quintile | 3,061 | 20.0 | 79.6 | 20.4 | |
| 3rd Quintile | 3,060 | 20.0 | 79.7 | 20.3 | |
| 4th Quintile | 3,061 | 20.0 | 77.4 | 22.6 | |
| 5th Quintile | 3,057 | 20.0 | 78.2 | 21.8 |
2SFCA, Two-Step Floating Catchment Area
Table 2 provides descriptive and chi-square statistics characterized by receipt of adjuvant hormonal therapy for the 834 eligible women living in Appalachia during 2007. The majority of women (53.1%) did not receive adjuvant hormonal therapy. Most women were white (97.1%), and race/ethnicity was again omitted from the table for brevity. Unlike the stage at diagnosis analysis, the slow decay 2SFCA scores showed a stronger relationship to receipt of adjuvant hormonal therapy compared to fast decay and original 2SFCA scores, and are the only 2SFCA scores shown in Table 2.
Table 2.
Characteristics of study patients by receipt of adjuvant hormonal therapy (AT).
| Factors | Total cases | % | % Had AT | % No AT | p-value |
|---|---|---|---|---|---|
| 834 | 46.9 | 53.1 | |||
| Age | |||||
| 65 – 69 | 172 | 20.6 | 48.8 | 51.2 | 0.159 |
| 70 – 74 | 195 | 23.4 | 40.5 | 59.5 | |
| 75 – 79 | 208 | 24.9 | 51.4 | 48.6 | |
| 80+ | 259 | 31.1 | 46.7 | 53.3 | |
| Charlson Comorbidity Index | |||||
| 0 | 535 | 64.1 | 43.9 | 56.1 | 0.110 |
| 1 | 199 | 23.9 | 52.8 | 47.2 | |
| 2 | 57 | 6.8 | 47.4 | 52.6 | |
| 3+ | 43 | 5.2 | 55.8 | 44.2 | |
| County Economic Status | |||||
| Attainment | 0.0 | 0.0 | 0.0 | 0.0 | 0.025 |
| Competitive | 110 | 13.2 | 40.0 | 60.0 | |
| Transitional | 571 | 68.5 | 45.9 | 54.1 | |
| At-risk | 100 | 12.0 | 51.0 | 49.0 | |
| Distressed | 53 | 6.4 | 64.2 | 35.8 | |
| Driving Time to Closest Primary Care (minutes) | |||||
| <10 | 706 | 84.7 | 47.3 | 52.7 | 0.386 |
| 10 – <20 | 99 | 11.9 | 44.4 | 55.6 | |
| 20 – <30 | 26 | 3.1 | 50.0 | 50.0 | |
| 30 – <40 | 3 | 0.4 | 0.0 | 100.0 | |
| 40 – <50 | 0 | 0.0 | 0.0 | 0.0 | |
| 50+ | 0 | 0.0 | 0.0 | 0.0 | |
| Driving Time to Closest Mammography Center (minutes) | |||||
| <10 | 522 | 62.6 | 46.9 | 53.1 | 0.576 |
| 10 – <20 | 201 | 24.1 | 48.8 | 51.2 | |
| 20 – <30 | 81 | 9.7 | 40.7 | 59.3 | |
| 30 – <40 | 22 | 2.6 | 54.5 | 45.5 | |
| 40 – <50 | 6 | 0.7 | 50.0 | 50.0 | |
| 50+ | 2 | 0.2 | 0.0 | 100.0 | |
| Ratio of Primary Care Physicians / County Population | |||||
| 1st Quintile | 174 | 20.9 | 49.4 | 50.6 | 0.609 |
| 2nd Quintile | 157 | 18.8 | 48.4 | 51.6 | |
| 3rd Quintile | 174 | 20.9 | 46.0 | 54.0 | |
| 4th Quintile | 168 | 20.1 | 48.8 | 51.2 | |
| 5th Quintile | 161 | 19.3 | 41.6 | 58.4 | |
| Ratio of Mammography Centers / County Population of Females Age 45+ | |||||
| 1st Quintile | 170 | 20.4 | 40.0 | 60.0 | 0.339 |
| 2nd Quintile | 176 | 21.1 | 50.6 | 49.4 | |
| 3rd Quintile | 183 | 21.9 | 47.0 | 53.0 | |
| 4th Quintile | 141 | 16.9 | 48.2 | 51.8 | |
| 5th Quintile | 164 | 19.7 | 48.8 | 51.2 | |
| 2SFCA Slow Decay Scores to Primary Care | |||||
| 1st Quintile | 165 | 19.8 | 40.6 | 59.4 | 0.259 |
| 2nd Quintile | 172 | 20.6 | 45.9 | 54.1 | |
| 3rd Quintile | 162 | 19.4 | 53.1 | 46.9 | |
| 4th Quintile | 166 | 19.9 | 48.2 | 51.8 | |
| 5th Quintile | 169 | 20.3 | 46.7 | 53.3 | |
| 2SFCA Slow Decay Scores to Mammography | |||||
| 1st Quintile | 168 | 20.1 | 41.1 | 58.9 | 0.037 |
| 2nd Quintile | 172 | 20.6 | 42.4 | 57.6 | |
| 3rd Quintile | 162 | 19.4 | 51.9 | 48.1 | |
| 4th Quintile | 167 | 20.0 | 44.3 | 55.7 | |
| 5th Quintile | 165 | 19.8 | 55.2 | 44.8 |
2SFCA, Two-Step Floating Catchment Area
Goodness-of-fit statistics revealed that the model with variables age, insurance status, fast decay 2SFCA primary care score, and county primary care to population ratio was the best predictor of late stage breast cancer diagnosis. Table 3 shows the resulting parameter estimates for the global logistic regression using these variables to predict stage at diagnosis. Travel time to the closest primary care provider was not a significant predictor in the global logistic regression (OR, 1.051; p = 0.241) and did not improve overall model fit. The mammography center spatial access scores did not fit the model as well as the primary care spatial access scores, as measured by AICc, AUC, and adjusted r2.
Table 3.
Parameter estimates for the global logistic regression model predicting late stage breast cancer diagnosis* and receipt of adjuvant hormonal therapy+.
| Variable* | β | s.e. | z-statistic | Sig. | Exp(β) | 95% C.I. for Exp(β) |
|---|---|---|---|---|---|---|
| Intercept | −1.151 | 0.123 | 88.267 | <0.001 | 0.316 | |
| Age | −0.146 | 0.038 | 15.103 | <0.001 | 0.864 | 0.803 – 0.930 |
| Insurance Status | 0.276 | 0.026 | 110.749 | <0.001 | 1.318 | 1.252 – 1.388 |
| 2SFCA score- Primary Care, Fast Decay | −0.036 | 0.014 | 7.509 | 0.006 | 0.962 | 0.935 – 0.989 |
| County Primary Care / Population Ratio | −0.056 | 0.014 | 14.837 | <0.001 | 0.946 | 0.919 – 0.973 |
| Variable+ | β | s.e. | z-statistic | Sig. | Exp(β) | 95% C.I. for Exp(β) |
| Intercept | −1.431 | 0.353 | 16.464 | <0.001 | 0.239 | |
| Charlson Comorbidity Index | 0.168 | 0.084 | 3.948 | 0.047 | 1.183 | 1.002 – 1.395 |
| County Economic Status | 0.280 | 0.101 | 7.620 | 0.006 | 1.323 | 1.085 – 1.615 |
| 2SFCA score - Mammography Center, Slow Decay | 0.115 | 0.050 | 5.340 | 0.021 | 1.122 | 1.018 – 1.236 |
2SFCA, Two-Step Floating Catchment Area
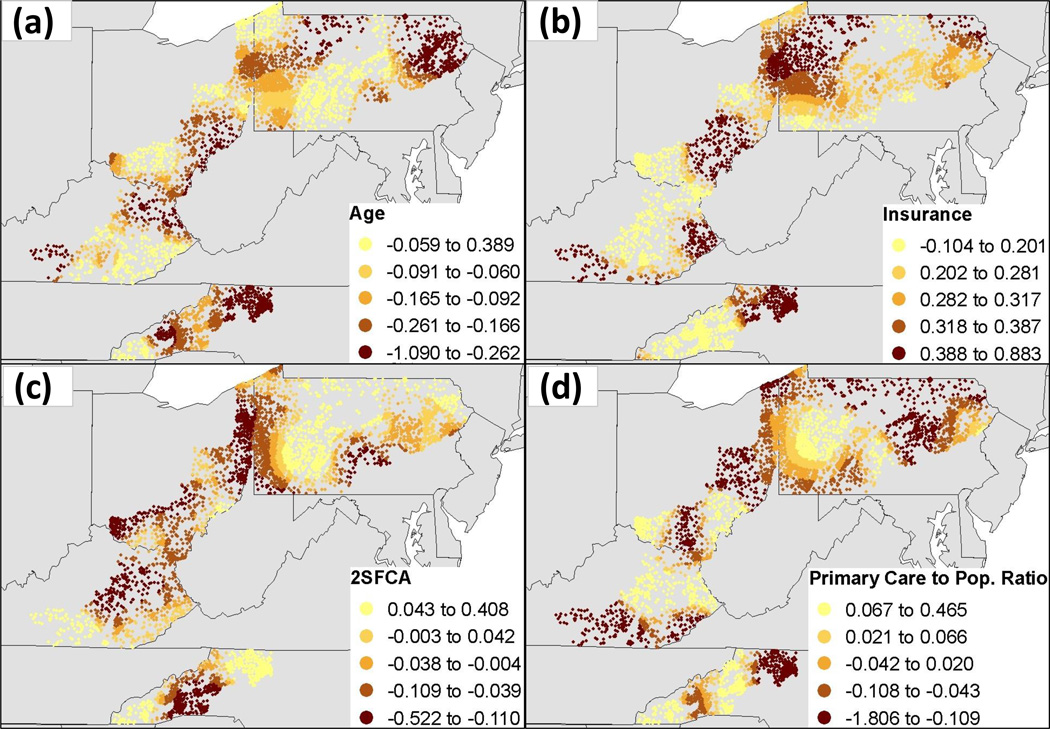
Descriptive statistics for the identical logistic GWR model predicting stage at diagnosis are presented in Table 4. The range of coefficient estimates, including both positive and negative values for each predictor variable, suggests spatial non-stationarity across the study region. Goodness-of-fit statistics showed that the logistic GWR model performed better than the global logistic regression model when predicting late stage breast cancer diagnosis (Table 5). The logistic GWR model had a lower AICc (15,590.5 vs. 15,680.6), higher AUC (0.623 vs. 0.562), and higher adjusted r2 (0.033 vs 0.009), demonstrating better model performance because of spatial non-stationarity in the final predictor variables (Table 5). This non-stationarity is displayed in Figure 1, where the effects of the final predictor variables clearly vary throughout the four state study region.
Table 4.
Summary statistics of the parameter estimates for the logistic GWR models for late stage breast cancer diagnosis*a and receipt of adjuvant hormonal therapy+b.
| Variable* | Minimum | 1st Quartile | Median | 3rd Quartile | Maximum |
|---|---|---|---|---|---|
| Intercept | −4.532 | −1.860 | −1.276 | −0.709 | 1.725 |
| Age | −1.090 | −0.229 | −0.126 | −0.078 | 0.389 |
| Insurance Status | −0.104 | 0.231 | 0.299 | 0.370 | 0.883 |
| 2SFCA score - Primary Care, Fast Decay | −0.522 | −0.083 | −0.020 | 0.023 | 0.408 |
| County Primary Care / Population Ratio | −1.806 | −0.078 | −0.022 | 0.057 | 0.465 |
| Variable+ | Minimum | 1st Quartile | Median | 3rd Quartile | Maximum |
| Intercept | −1.555 | −1.437 | −1.318 | −1.287 | −1.261 |
| Charlson Comorbidity Index | 0.131 | 0.155 | 0.181 | 0.186 | 0.193 |
| County Economic Status | 0.217 | 0.237 | 0.252 | 0.291 | 0.324 |
| 2SFCA score - Mammography Center, Slow Decay | 0.100 | 0.102 | 0.105 | 0.108 | 0.121 |
2SFCA, Two-Step Floating Catchment Area
36.9 km bandwidth
541.5 km bandwidth
Table 5.
Comparison of fit between global logistic regression and GWR models for late stage breast cancer diagnosis*a and receipt of adjuvant hormonal therapy+b.
| Model* | n | ke | −2 log likelihood | AICc | AUC ± s.e. | r2 (adj.) |
|---|---|---|---|---|---|---|
| Global Logistic | 15,299 | 5.0 | 15,670.6 | 15,680.6 | 0.562 ± 0.006 | 0.009 |
| GWR | 15,299 | 144.1 | 15,299.6 | 15,590.5 | 0.623 ± 0.006 | 0.033 |
| Model+ | n | ke | −2 log likelihood | AICc | AUC ± s.e. | r2 (adj.) |
| Global Logistic | 834 | 4.0 | 1,135.0 | 1,143.0 | 0.583 ± 0.20 | 0.016 |
| GWR | 834 | 5.2 | 1,133.4 | 1,143.9 | 0.587 ± 0.20 | 0.017 |
n, number of patients; ke, effective number of parameters; AICc, corrected Akaike's Information Criterion; AUC, area under the receiver operating characteristic curve.
36.9 km bandwidth
541.5 km bandwidth
Figure 1.
Local coefficient estimates for the logistic GWR model predicting late stage breast cancer diagnosis across variables (a) age, (b) insurance status, (c) two-step floating catchment area (2SFCA) score, and (d) county primary care to population ratio.
The model with variables Charlson comorbidity index, county economic status, and slow decay 2SFCA mammography score was the best predictor of adjuvant hormonal therapy receipt. The parameter estimates for the resulting global logistic regression model are shown in Table 3. Travel time to the nearest mammography center (OR, 0.954; p = 0.621) and the county mammography center to women age 45 and older ratio (OR, 0.985; p = 0.792) did not improve overall model fit. Unlike the stage at diagnosis model, the mammography access scores resulted in a better fitting model than using primary care access scores. The receipt of adjuvant therapy model also used the slow decay 2SFCA score rather than the fast decay 2SFCA score, based on improved AICc, AUC, and adjusted r2 values.
Descriptive statistics for the identical logistic GWR model predicting receipt of adjuvant hormonal therapy are presented in Table 4. The smaller coefficient ranges and lack of positive and negative values indicate that the effect of the predictor variables were more stationary across the study region. Goodness-of-fit statistics demonstrated that the logistic GWR model was no better than the global logistic regression model in predicting receipt of adjuvant hormonal therapy. There were no meaningful differences between the AICc (1,143.9 vs 1,143.0), AUC (0.587 vs 0.583), and adjusted r2 values (0.017 vs 0.016) of the GWR model compared to the global logistic regression approach (Table 5). As a result, we did not map the geographic variability of the logistic GWR parameter coefficients because we found no evidence of spatial non-stationarity in the predictor variables
DISCUSSION
Our analysis of breast cancer patients in Appalachia PA, OH, KY, and NC made several substantive clinical findings. Over 20% of women were diagnosed with late stage breast cancer. Age and insurance status predicted late stage diagnosis, as well as the spatial access to care measures of fast-decay 2SFCA primary care score and primary care to county population ratio. In our sample, only 46.9% of eligible women received adjuvant hormonal therapy following breast cancer surgery. Patients’ comorbidity and county economic status predicted receipt of adjuvant therapy. The slow-decay 2SFCA mammography center score also predicted receipt of adjuvant therapy.
Our analysis also made several important methodological findings. The analysis showed the superior predictive validity of the 2SFCA method compared with the traditional spatial access measure of driving time from patient to provider. We also demonstrated the necessity of considering spatial non-stationarity across study areas. A GWR approach was more appropriate for our late stage diagnosis model than a traditional global regression.
Previous research using similar early (0–II) and late (III–IV) stage classification found late stage breast cancer diagnosis in anywhere from 13 percent of cases in Kentucky29 16.7 percent of cases in Georgia,19 and 17.3 percent of cases in Appalachia counties of Pennsylvania, Ohio, and Kentucky.30 The national average from Surveillance, Epidemiology, and End Result data is 16 percent. Our 21.2 percent finding continues the trend of increased late stage diagnosis in Appalachia.
Prior research was inconclusive as to whether spatial access to care increased risk of late stage diagnosis, with some research29 finding that greater travel time to mammography centers did increase risk, while other research9,31 found no increase due to longer travel times. Our work adds clarity by using a more advanced spatial access method than travel time, the 2SFCA method, and by including access to primary care instead of only mammography centers. For our study population, a fast-decay 2SFCA primary care score was a better predictor of late stage diagnosis than 2SFCA mammography scores, travel time to mammography centers, or travel time to primary care providers. This result supports the finding that primary care providers are often a crucial gateway to breast cancer care.32 Stage at diagnosis is a relatively early event in the course of breast cancer identification and treatment, and we hypothesize that access to primary care services is therefore particularly important when considering stage at diagnosis as an outcome measure. Similarly, we hypothesize that fast-decay primary care scores were a better predictor than slow-decay scores because primary care services are more regular and thus require closer geographic proximity than more specialized services. We also found that lower primary care to county population ratios predicted late stage diagnosis. This ratio may reflect the increased risk of late stage diagnosis among lower socioeconomic groups,33 as it was strongly correlated with county economic status (p < 0.001).
Only 46.9 percent of eligible women in our sample received adjuvant hormonal therapy of tamoxifen, anastrozole, letrozole, or exemestane. This is considerably lower than the 67 percent reported for an analysis of patients in California, Florida, Illinois and New York,34 and the 64 percent reported in an analysis of low-income patients throughout North Carolina.35 Again, the 2SFCA method was a better predictor of adjuvant hormonal therapy than travel time. In this model, slow decay 2SFCA mammography scores were a better predictor of treatment than primary care 2SFCA scores. We hypothesize that it is access to these more specialized cancer care services, rather than primary care providers, that impact later stages of care, such as our measure of adjuvant hormonal therapy. Previous research has found that access to specialized cancer care services improves cancer outcomes and reduces treatment complications.36 We also hypothesize that slow-decay scores were better predictors than fast-decay scores because closer geographic proximity (as was the case for fast-decay primary care scores) is not as important for less regular, specialized cancer care services.
There are both methodological and clinical strengths to this study. To the best of our knowledge, this is the first study that uses the more advanced 2SFCA method to measure spatial access to care in Appalachia. Because Appalachia is largely rural and mountainous, and many areas experience physician shortages,37 accurately measuring spatial access to care is essential. Another strength was our GWR approach. Logistic GWR allowed us to visualize the varying effects of our included predictor variables, such as the finding that having Medicaid, being uninsured, or having an unknown insurance status are more important predictors of late stage diagnosis in northeastern PA than 2SFCA scores. Clinically, our study benefits from the inclusion of both access to primary care services and mammography centers. Access to care is multifaceted, with variables contributing differentially depending on the outcome or treatment pattern studied.8
There are also important limitations to the study. We were not able to obtain provider data for states bordering our study area. We did include bordering populations when calculating the 2SFCA scores, but additional provider data would have further reduced any edge effects in our spatial access calculations. Additionally, we used the closest provider and mammography centers to patients, not the actual service providers. For patients with more restrictive or no insurance, this could represent a significant discrepancy. We also did not included non-automobile transportation, which is possible to account for in the 2SFCA method.38
Overall, we found disparities wihtin our sample of breast cancer patients in Appalachia. The 2SFCA method offered the best predictive ability of all access measures. GWR was useful in identifying spatial non-stationarity in our model predicting late stage breast cancer diagnosis. More research is needed to understand patient behaviors in relation to various spatial access measures, which would help elucidate the assumptions inherent in each approach. Future research is also needed to examine our unexpected finding that higher comorbidity scores had a positive effect on receipt of adjuvant hormonal therapy, which was beyond the scope of this study. Additional research is also needed to identify the clinical decisions that lead to reduced rates of adjuvant hormonal therapy among women in Appalachia.
Acknowledgments
Acknowledgement of Support:
This research was funded by the National Cancer Institute and the NIH Office on Women's Health through grant 1 R21 CA168479 (Balkrishnan, PI)
Footnotes
Disclaimers:
None of the authors have any conflicts of interests to disclose.
References
- 1.Blackley D, Behringer B, Zheng S. Cancer mortality rates in Appalachia: descriptive epidemiology and an approach to explaining differences in outcomes. J Community Health. 2012;37(4):804–813. doi: 10.1007/s10900-011-9514-z. [DOI] [PubMed] [Google Scholar]
- 2.Reiter PL, Katz ML, Paskett ED. Correlates of HPV vaccination among adolescent females from Appalachia and reasons why their parents do not intend to vaccinate. Vaccine. 2013;31:3121–3125. doi: 10.1016/j.vaccine.2013.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sergeev AV. Stroke mortality disparities in the population of the Appalachian Mountain region. Ethn Dis. 2013;23(3):286–291. [PubMed] [Google Scholar]
- 4.Pollard K, Jacobson LA. The Appalachian Region: A Data Overview from the 2006–2010 American Community Survey. Appalachia Regional Commission. 2012 Retrieved from http://www.arc.gov/research/researchreportdetails.asp?REPORT_ID=96.
- 5.Paskett ED, Fisher JL, Lengerich EJ, et al. Disparities in underserved white populations: the case of cancer-related disparities in Appalachia. Oncologist. 2011;16(8):1072–1081. doi: 10.1634/theoncologist.2011-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F. Measurement, Optimization, and Impact of Health Care Accessibility: A Methodological Review. Ann Assoc Am Geogr. 2012;102(5):1104–1112. doi: 10.1080/00045608.2012.657146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo W, Wang F. Measures of spatial accessibility to health care in a GIS environment: synthesis and a case study in the Chicago region. Environment and Planning B. 2003;30(6):865–884. doi: 10.1068/b29120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Luo W. Assessing spatial and nonspatial factors for healthcare access: towards an integrated approach to defining health professional shortage areas. Health Place. 2005;11:131–146. doi: 10.1016/j.healthplace.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Henry KA, Boscoe FP, Johnson CJ, et al. Breast cancer stage at diagnosis: Is travel time important? J. Community Health. 2011;1(36):933–942. doi: 10.1007/s10900-011-9392-4. [DOI] [PubMed] [Google Scholar]
- 10.Amey CH, Miller MK, Albrecht SL. The role of race and residence in determining stage at diagnosis of breast cancer. Journal of Rural Health. 1997;13(2):99–108. doi: 10.1111/j.1748-0361.1997.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 11.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantz PM, Mujahid M, Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. American Journal of Public Health. 2006;96(12):2173–2178. doi: 10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 14.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: Status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 15.Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 16.Johnson CH, Adamo M. SEER Program Coding and Staging Manual 2007. Bethesda, MD: National Cancer Institute, NIH Publication 07-5581; 2007. [Google Scholar]
- 17.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RT, Camacho FT, Balkrishnan R, et al. Use of cancer registry data for research on patterns of breast cancer care of individuals with Medicaid insurance. J Clin Oncol. 2005;24:533s. (suppl; abstr 6021) [Google Scholar]
- 19.Markossian TW, Hines RB. Disparities in Late Stage Diagnosis, Treatment, and Breast Cancer-Related Death by Race, Age, Rural Residence Among Women in Georgia. Women & Health. 2012;52(4):317–333. doi: 10.1080/03630242.2012.674091. [DOI] [PubMed] [Google Scholar]
- 20.Camacho F, Hwang W, Kern T, et al. Receipt of Regular Primary Care and Early Cancer Detection in Appalachia. The Journal of Rural Health. 2014 doi: 10.1111/jrh.12097. [DOI] [PubMed] [Google Scholar]
- 21.McGrail MR. Spatial accessibility of primary health care utilising the two step floating catchment area method: an assessment of recent improvements. Int J Health Geogr. 2012;16:11–50. doi: 10.1186/1476-072X-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrail MR, Humphreys JS. A new index of access to primary care services in rural areas. Aust NZ J Pub Health. 2009;33:418–423. doi: 10.1111/j.1753-6405.2009.00422.x. [DOI] [PubMed] [Google Scholar]
- 23.Luo W, Whippo TL. Variable catchment sizes for the two-step floating catchment area (2SFCA) method. Health Place. 2012;18:789–795. doi: 10.1016/j.healthplace.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Appalachian Regional Commission (ARC) Economic Assessment of Appalachia. County Economic Status in Appalachia, FY 2012. 2012 Retrieved from http://www.arc.gov/research/MapsofAppalachia.asp?MAP_ID=55.
- 25.D’Hoore W, Bouckaert A, Telquin C. Practical considerations on the use of the Charlson Comorbidity Index with administrative data bases. J Clin Epidemiol. 1996;49:1429–1433. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 26.Fotheringham AS, Brunsdon C, Charlton ME. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships. Chichester, UK: Wiley; 2002. p. 269. [Google Scholar]
- 27.Windle MJS, Rose GA, Devillers R, et al. Exploring spatial non-stationarity of fisheries survey data using geographically weighted regression (GWR): an example from the Northwest Atlantic. ICES Journal of Marine Science. 2010;67:145–154. [Google Scholar]
- 28.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 29.Huang B, Dignan M, Han D, et al. Does Distance Matter? Distance to Mammography Facilities and Stage at Diagnosis of Breast Cancer in Kentucky. The Journal of Rural Health. 2009;25:366–371. doi: 10.1111/j.1748-0361.2009.00245.x. [DOI] [PubMed] [Google Scholar]
- 30.Anderson RT, Yang TC, Matthews SA, et al. Breast Cancer Screening, Area Deprivation, and Later-Stage Breast Cancer in Appalachia: Does Geography Matter? Health Services Research. 2014;49:546–567. doi: 10.1111/1475-6773.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lian M, Struthers J, Schootman M. Comparing GIS-Based Measures in Access to Mammography and Their Validity in Predicting Neighborhood Risk of Late-Stage Breast Cancer. PLoS ONE. 2012;7(8):e43000. doi: 10.1371/journal.pone.0043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roetzheim RG, Ferrante JM, Lee JH, et al. Influence of primary care on breast cancer outcomes among Medicare beneficiaries. Ann Fam Med. 2012;10(5):401–411. doi: 10.1370/afm.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruffin M, Gorenflo F, Woodman B. Predictors of screening for breast, cervical, colorectal, and prostatic cancer among community-based primary care practices. J Am Board FamMed. 2000;13(1):1–10. doi: 10.3122/jabfm.13.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Yen TWF, Czypinski LK, Sparapani R, et al. Socioeconomic Factors Associated with Adjuvant Hormonal Therapy Use in Older Breast Cancer Survivors. Cancer. 2007;117(2):398–405. doi: 10.1002/cncr.25412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimmick G, Anderson R, Camacho F, et al. Adjuvant Hormonal Therapy Use Among Insured, Low-Income Women With Breast Cancer. Journal of Clinical Oncology. 2009;27(21):3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: Importance in quality of cancer care. Journal of Clinical Oncology. 2000;18:2327–2340. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 37.Halverson J, Lin M, Harner E. An analysis of disparities in health status and access to health care in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2004. [Google Scholar]
- 38.Mao L, Nekorchuk D. Measuring spatial accessibility to healthcare for populations with multiple transportation modes. Health Place. 2013;24:115–122. doi: 10.1016/j.healthplace.2013.08.008. [DOI] [PubMed] [Google Scholar]