What are the neural signatures of consciousness? Sitt et al. assess the suitability of putative electrophysiological markers of consciousness for distinguishing patients in a vegetative state from those in a minimally conscious or conscious state. Low-frequency power, EEG complexity, and information exchange constitute reliable, partially independent, markers of consciousness.
Keywords: consciousness, vegetative state, unresponsive wakefulness syndrome, minimally conscious state, EEG
Abstract
In recent years, numerous electrophysiological signatures of consciousness have been proposed. Here, we perform a systematic analysis of these electroencephalography markers by quantifying their efficiency in differentiating patients in a vegetative state from those in a minimally conscious or conscious state. Capitalizing on a review of previous experiments and current theories, we identify a series of measures that can be organized into four dimensions: (i) event-related potentials versus ongoing electroencephalography activity; (ii) local dynamics versus inter-electrode information exchange; (iii) spectral patterns versus information complexity; and (iv) average versus fluctuations over the recording session. We analysed a large set of 181 high-density electroencephalography recordings acquired in a 30 minutes protocol. We show that low-frequency power, electroencephalography complexity, and information exchange constitute the most reliable signatures of the conscious state. When combined, these measures synergize to allow an automatic classification of patients’ state of consciousness.
Introduction
Despite intense experimental and theoretical efforts, the neural signatures of conscious processing remain a highly debated issue: are they to be found in early (Supèr et al., 2001; Pins, 2003; Koivisto et al., 2006; Melloni et al., 2007) or late (Sergent et al., 2005; Gaillard et al., 2009) responses to sensory stimulations? Is conscious processing systematically associated with re-entrant top–down processing (Lamme et al., 2000; Pascual-Leone et al., 2001)? Does consciousness depend on a massive exchange of information across distant cortical sites (Lumer et al., 1999; Vuilleumier et al., 2001; Rees et al., 2002; Marois et al., 2004; Gaillard et al., 2009) with prefrontal cortex and cingulate cortices acting as critical communication hubs (Sahraie et al., 1997; Dehaene et al., 2001; Lau et al., 2006)? Or, instead, can it emerge solely from localized reverberating activity (Lamme et al., 2000; Pins, 2003)? Even more, can it be directly associated to the global integration of information in the brain (Tononi, 2008)? Several models share the view that global information integration and coordination of distant areas may be two specific properties of conscious processes (Lamme et al., 2000; Tononi, 2008; Dehaene et al., 2011).
The identification of reliable signatures of conscious processing goes beyond theoretical research, and has critical practical implications in the clinic. In particular, detecting these signatures is essential for patients with disorders of consciousness who, even during wakefulness, are seemingly unable to communicate. To better describe these disorders, a distinction has been introduced between the vegetative state (VS) and the minimally conscious state (MCS). Both exhibit similarly preserved arousal, but MCS patients show signs of intentional behaviour whereas VS patients remain largely unresponsive. Yet, even in the latter case, science and modern medicine remain hard-pressed to decide whether a patient diagnosed as VS may still be conscious but unable to communicate (Laureys, 2005; Schnakers et al., 2009b; Laureys et al., 2012). For instance, Owen et al. (2006) have shown that functional MRI could detect consciousness in some VS patients and, in a few cases, even restore communication (Monti et al., 2010). Similar results may be obtained using scalp EEG, a more economical and practical technique that can be easily applied at bedside (Cruse et al., 2011; Faugeras et al., 2011; Goldfine et al., 2011). We recently introduced a measure (weighted symbolic mutual information, wSMI) that evaluates long-distance cortical information sharing from scalp EEG and discriminates VS and MCS patients (King et al., 2013b). Another recent method based on the quantification of the complexity in the EEG evoked responses to transcranial magnetic stimulation pulses has also been shown to efficiently detect consciousness in patients with disorders of consciousness (Casali et al., 2013). Nevertheless, there is still no consensus amongst clinicians as to which measure may be best suited to detect residual consciousness. Part of this results from the fact that the putative signatures of consciousness have been proposed and tested in independent studies, in distinct populations and with different criteria and methodologies.
The multiplicity of theoretical proposals and the practical need to improve the diagnosis of patients with disorders of consciousness call for a systematic assessment of the ability of each of these measures to index states of consciousness. To address this issue, we provide an extensive analysis of empirically and theoretically derived measures of conscious activity that can be extracted from a large database of EEG recordings in patients with disorders of consciousness. We show that local low-frequency power and complexity measures, together with information exchange, are markers of conscious states. These measures and their respective fluctuations can be used in synergy in an automatic classification system to improve the diagnosis of VS patients.
Materials and methods
Patients
Patients underwent EEG recording to make a clinical electrophysiological evaluation of their conscious state. During this evaluation patients were presented with the ‘Local-Global’ auditory protocol (Bekinschtein et al., 2009), which is designed to elicit event-related potentials that help assess patients’ present and future state of consciousness (Fischer et al., 2004; Naccache et al., 2005; Wijnen et al., 2007; Bekinschtein et al., 2009; Schnakers et al., 2009a; Faugeras et al., 2011, 2012). The Ethical Committee of the Pitié-Salpêtrière approved this research under the French label of ‘Recherche en soins courants’ (routine care research). Patients were recorded at least 24 h after sedation to maximize their arousal and their cognitive abilities during the auditory stimulation. Other drugs that could potentially influence EEG such as myorelaxants or anti-epileptic drugs were not controlled, but note that these drugs are not specifically related to one of the three patient groups. Recordings were performed by trained electrophysiologists (F.F, B.R and L.N). EEG quality and ongoing activity were verified before starting the recording. Patients that showed seizures or any clearly identifiable abnormal activity were not recorded. We performed a total of 173 patient recordings. Six recordings were discarded because they presented <200 non-artefacted trials (see below). The remaining 167 valid recordings were acquired from 113 distinct patients (79 males and 34 females, sex ratio = 2.32), aged from 16 to 83 years (mean = 48 ± 17 years). Patients were recorded one to six times. Their aetiologies matched those typically observed in disorders of consciousness: anoxia (24%), intracranial haemorrhage (35%), traumatic brain injury (24%); and other aetiologies (17%) (see Supplementary Table 4 for details). Our cohort had variable delays separating the incident and the acquisition of the EEG [mean = 178 days since onset of disorders of consciousness; median = 35 days; standard deviation (SD) = 532 days; earliest = 6 days; latest = 4383 days].
Healthy subjects
Experiments were approved by the Ethical Committee of the Pitié-Salpêtrière Hospital. All 14 healthy subjects [mean age = 21.3 ± 2.9; sex-ratio (male:female) = 2.5] gave written informed consent.
Behavioural assessment of consciousness
Clinical evaluation of consciousness was based on the French version of the Coma Recovery Scale Revised (CRS-R) scale (Schnakers et al., 2009b), and careful neurological examination by trained neurologists (F.F., B.R., L.N.). This scale consists of 23 items forming six subscales addressing auditory, visual, motor, oromotor, communication and arousal functions. CRS-R subscales are comprised of hierarchically arranged items. The scale enables a distinction between conscious states (CS), MCS and VS (Schnakers et al., 2009b). Clinical examination and behavioural assessment were systematically performed immediately before EEG recording.
Auditory stimulation
Subjects were stimulated auditorily using the ‘Local-Global’ protocol (Bekinschtein et al., 2009). In this protocol, patients were presented with a series of sounds that contain regularities at two different hierarchical levels. The deviations from these regularities evoke event-related potentials that are useful to evaluate the cognitive state of the patients. See Supplementary material for a complete description of the protocol.
High-density scalp electroencephalography recordings
EEG recordings were sampled at 250 Hz with a 256-electrode geodesic sensor net (EGI) referenced to the vertex. Recordings were band-pass filtered (from 0.2 to 45 Hz). Trials were then segmented from −200 ms to +1336 ms relative to the onset of the first sound. Trials with voltages exceeding ±150 µV, eye-movements activity exceeding ±80 µV and eye-blinks exceeding ±150 µV were rejected. Trials were baseline corrected over the first 200 ms window preceding the onset of the first sound. Electrodes with a rejection rate superior to 20% across trials were rejected and were interpolated. Trials with >20 corrected electrodes were rejected. The remaining trials were digitally transformed to an average reference. All these processing stages were performed using the EGI Waveform Tools Package.
Connectivity measures were based on a spatial Laplacian transformation of the EEG—a computation also known as the Current Source Density estimate (Kayser et al., 2006). This transformation roughly consists, in subtracting from each electrode, the activity of its neighbouring electrodes. This has the main advantage of increasing spatial resolution and minimizing the influence of common sources on multiple distant electrodes.
Calculation of putative electroencephalography measures of consciousness
We calculated the entire set of EEG measures independently for each individual subject, trial and for every electrode (n = 256) or pair of electrodes (n = 32 640). Connectivity measures were summarized by calculating the median value from each electrode to all of the other scalp (non-facial) electrodes. Note that, given the spatial distribution of the electrodes, this procedure enhances the weight of long-distance connections on the computed measure. As a final result, all measure distributions ended up with the same number of values per subject and trial. Finally, for each subject, these values were again collapsed to two scalars by considering the mean and the standard deviation across trials.
Except for event-related potential measures, analyses were performed on EEG data recorded during the time period when subjects were presented with undifferentiated series of tones (from 200 ms before the onset of the first tone to the onset of the fifth tone). Thus, all trials were pooled independently of the condition of auditory stimulation.
See Supplementary material for a detailed description of each measure and its computation.
Statistics
Topographical analysis
Topographical analyses were performed using a similar approach to the method above. Pair-wise comparisons across clinical states were performed with Mann-Whitney U-tests on each electrode separately. Robust regression analyses were performed across the four groups (VS, MCS, CS, and healthy controls) to test for the monotony of the changes observed across states of consciousness. Statistical significance is reported at two levels of significance (P < 0.01 and P < 0.05, uncorrected for the number of electrodes tested).
Univariate receiver operating characteristic
Pair-wise comparisons were performed across the three clinical groups (VS, MCS and CS), leading, for each measure, to three statistical comparisons (VS/MCS, MCS/CS, and VS/CS). We implemented a non-parametric statistical method (Mann-Whitney U-test) and report the effect size as the empirical area under the curve (AUC) from an empirical receiver operating curve analysis. See Supplementary methods for the computation of the AUC.
Multivariate pattern analyses
In the present case, each analysis aimed at predicting the clinically-defined state of consciousness (VS, MCS or CS) of each subject from the EEG-based measures of conscious processing. For this purpose, we used a linear Support Vector Classifier (Pedregosa et al., 2011) with a probabilistic output calibration. See Supplementary material for a detailed description of the computational steps.
Results
We analysed a large set of 181 high-density 256-channel EEG recordings, corresponding to all 167 patients admitted at the Pitié-Salpêtrière Hospital and referred to us in the 2008–10 period for a clinical evaluation of their state of consciousness. In total, 75 VS recordings, 68 MCS recordings, and 24 brain-injured but conscious patients (CS) were recordings were acquired. Fourteen additional recordings were obtained from healthy control subjects. Recordings were acquired in the ‘Local Global’ protocol, a 30-min experimental protocol designed to probe the depth of processing of auditory regularities (Bekinschtein et al., 2009) (Fig. 1). This procedure allowed us to jointly quantify the event-related potentials evoked by exogenous stimuli and the presence of endogenous fluctuations in the EEG, while maximizing and homogenizing the patients’ attention and vigilance. For each recording, we systematically extracted a set of measures organized according to a theory-driven taxonomy (Table 1). Full details and motivations for each measure can be found in the Supplementary material. Here we briefly describe the most relevant aspects of each measure.
Figure 1.
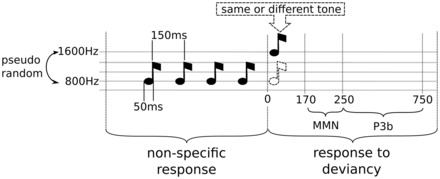
A multi-dimensional approach to categorize states of consciousness. Description of the experimental protocol used for patient auditory stimulation. Spectral and information-theory measures were computed in the early time window (which is identical for all trials), whereas event-related potentials were computed on the late window (response specific to the trial condition).
Table 1.
Taxonomy of EEG-derived measures used to categorize states of consciousness
| Event-related potentials |
‘Ongoing’ activity |
||||
|---|---|---|---|---|---|
| Early components | Late components | Single electrode |
Across electrodes |
||
| Spectrum | Information theory | Spectrum | Information theory | ||
| P1 | P3a | Power in frequency bands | Permutation entropy | Phase lag index | Weighted symbolic mutual information |
| MMN | P3b | Spectral summaries | K complexity | Amplitude envelope correlation | |
| CNV | Spectral entropy | Imaginary coherence | |||
Measures can be conceptually organized along several dimensions: first, we distinguish measures of stimulus processing from measures computed from ongoing EEG activity. The former (event-related potentials) are further subdivided into early versus late components. The latter are classified according to the theoretical background used to derive the measure: (i) local dynamics versus connectivity: some measures are computed within each electrode, whereas others index the interactions between electrodes; and (ii) spectral (Fourier frequency analysis) versus Information Theory. Finally, for each measure, intertrial average (computed as the mean across trials) or intertrial fluctuations (computed as the standard deviation) were studied.
We propose to organize EEG measures according to several major dimensions (Table 1). A first distinction separates measures of the processing of external stimuli (auditory event-related potentials) from measures that are not locked to the stimuli and rather reflect ongoing brain activity. Current theories of consciousness differ in their attribution of conscious awareness of an external stimulus to early or late brain responses (Dehaene et al., 2011). Therefore, we classified event-related potentials according to their latency (early, <250 ms versus late, >300 ms). Measures of ongoing EEG activity were further classified based on: (i) dynamics of brain signals at a single electrode site versus assessment of functional connectivity between two brain sites; and (ii) whether the measure is based on spectral frequency content or on information-theoretic estimates of signal complexity, which do not presume strong specific hypotheses about the frequency content of the signal. For each measure we computed, the intertrial average, which reflects the overall mean value during the half-hour recording session, and the fluctuation of this measure (SD) during the recording period, which tests the hypothesis that stability may be a hallmark of consciousness (Schurger et al., 2010).
Topographical differences across measures and groups
First we inspected whether differences in amplitude and spatial distribution of the computed measures could discriminate patients’ state of consciousness. This analysis also aims at evaluating whether different measures present specific scalp topographies, and thus (i) highlight optimal recording regions for clinical practice; and (ii) suggest different underlying neural systems. Figure 2 depicts a representative set of measures; the full set of topographies is presented in Supplementary Figs 1–4. The specific results obtained for each marker is discussed below.
Figure 2.
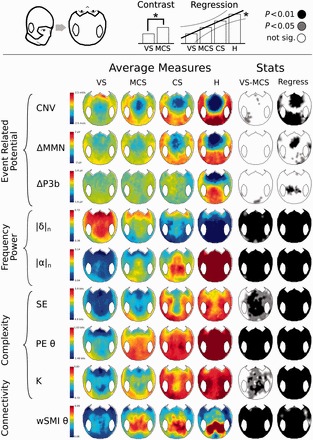
Scalp topography of the most discriminatory measures. The topographical 2D projection (top = front) of each measure [contingent negative variation (CNV), mismatch negativity (MMN) and P300b (ΔP3b), normalized power in delta (|δ|n) and alpha (|α|n) bands, spectral entropy (SE), permutation entropy in theta band (PEθ), Komolgorov-Chaitin Complexity (K) and weighted symbolic mutual information (wSMIθ)] is plotted for each state of consciousness (columns). The fifth column indicates whether the VS and MCS patients were significantly different from one another (black = P < 0.01, light grey = P < 0.05, white = not significant, uncorrected for the number of electrodes tested). The sixth column shows the statistics of a regression analysis of the measure across the four states of consciousness (VS < MCS < CS < healthy controls (H). Black: P < 0.01, light grey: P < 0.05, white: not significant, uncorrected for the number of electrodes tested).
Event-related topographies show low sensitivity to discriminate patient groups
Only two of seven sound-related potentials (Bekinschtein et al., 2009) differed significantly between VS and CS patients (Supplementary Fig. 1). None of them could significantly discriminate VS and MCS patients. The MMN and the P300, two event-related potentials, which both signal the detection of violations of auditory regularities, only showed modest differences between the groups of patients. Although the MMN discriminated VS from CS and MCS from CS, confirming that it increases when consciousness recovers (Wijnen et al., 2007), it did not discriminate VS from MCS. More surprisingly, univariate, electrode-by-electrode statistics of the P300 topography also failed to reveal any discrimination of VS from MCS. This finding, which partially contradicts earlier results with smaller patient samples (Bekinschtein et al., 2009), may be ascribed to large interindividual variability in EEG topography (King et al., 2013a), as discrimination improves when using multivariate decoding (see below).
Theta and alpha band power efficiently index the state of consciousness
Contrary to event-related potentials, most of the power spectrum measures seemed to be efficient indices of consciousness states. These measures appeared maximally informative for electrodes over the parietal region (Supplementary Fig. 2). All spectral measures showed monotonic effects from VS to CS patients groups, and 6 of 10 succeeded in discriminating VS from MCS. Within the lower frequencies, normalized delta (1–4 Hz) showed decreasing power from VS to CS, significantly separating VS from non-VS patients. On the contrary, normalized theta (4–8 Hz) and normalized alpha power (8–13 Hz) increased significantly from VS to CS. Both frequency bands showed increasing power in parietal regions and significantly discriminated VS from the other groups of patients. Normalized beta power failed to discriminate between VS and MCS patients, but significantly discriminated CS from the other groups of patients.
Because of these opposing variations of low (delta) and higher (alpha and above) frequencies, spectral summaries such as the median spectral frequency, which summarize the relative distribution of power in the frequency spectrum, were particularly efficient. The spectral entropy analysis showed that CS and MCS patients presented a less predictable spectral structure (higher entropy) than VS patients.
Electroencephalography complexity increases with conscious state
Algorithmic (or Kolmogorov-Chaitin) complexity (K) estimates the complexity of a sequence based on its compressibility. Several theories predict that the complexity of information integration (Tononi, 2008) or distributed processing (Dehaene et al., 2011) is elevated during conscious states. In agreement with this prediction, we found that the computed measure of complexity, based on EEG compression, increased in patients with a higher clinical state of consciousness. This measure significantly discriminated VS from MCS patients, particularly for a set of electrodes over the parietal region (Supplementary Fig. 3). A complementary mathematical approach is permutation entropy, which evaluates the regularity of the probabilistic distributions of temporal patterns in the signal (Bandt and Pompe, 2002). Permutation entropy can be computed in distinct frequency bands; we found that permutation entropy-based measures were particularly efficient in the theta frequency range, discriminating VS from the other groups. Again, a greater value of permutation entropy, especially over centro-posterior regions (Fig. 2), indicating a more complex and unpredictable distribution, indexed a higher state of consciousness.
Information sharing across brain regions indexes conscious state
Similar to the spectral measures at a single recording site, measures of functional connectivity between two recording sites proved particularly efficient at lower frequencies (Supplementary Fig. 4). Amongst the spectral connectivity measures, only the phase-locking index in the delta band was significant, progressive deficits in consciousness being accompanied by greater delta synchrony. Connectivity measures based on information theory, such as weighted symbolic mutual information, demonstrated a higher sensitivity, as previously reported (King et al., 2013b), inter-electrode information exchanges increased from VS to CS. VS patients presented significant lower weighted symbolic mutual information than both MCS and CS patients in the theta and alpha bands, consistent with the theoretical notion that loss of consciousness in VS reflects an impaired exchange of information across brain areas, and particularly over medium-to-long cortico-cortical distances (King et al., 2013b).
Quantifying the electroencephalography differences between groups of patients with disorders of consciousness
Although the above analysis evaluated the topographical differences across groups, judging discrimination capacity in sensor space while correcting for multiple comparisons over the large number of available electrodes poses a difficult statistical problem. To reduce dimensionality and quantify the discriminative power of each measure, we summarized spatial information by considering the average over all scalp electrodes or, in the case of event-related potentials, over predefined electrodes forming a priori regions of interest (see Supplementary material for details of region of interest selection). All individual trial measures were summarized in their intertrial average and in their fluctuation (SD) during the recording period. In the case of event-related measures, we also applied a systematic within-subject decoding approach for local and global responses to auditory novelty, following the procedure described in King et al. (2013a), which enhanced the discrimination of these conditions. This approach ultimately yielded a total of 92 measures per subject (see Supplementary Tables 1 and 2 for numerical summaries of each measure in each group).
To investigate which measures showed significant differences across groups of patients, we implemented receiver operator curves (Supplementary Fig. 5) and quantified classification performance with the AUC. A strict criterion based on false discovery rate (FDR) was applied to correct for multiple comparisons (92 values × three tests: VS–MCS, MCS–CS and VS-CS). An AUC of 50% corresponds to chance classification. An AUC >50% implies that the measure increases with conscious state (i.e. MCS > VS), whereas AUC <50% implies a decrease with conscious state (i.e. MCS < VS).
Average spectrum, complexity, connectivity, and global responses to novelty provide efficient signatures of consciousness
Analysis of the discrimination performance of the EEG measures revealed that the most discriminative measure was weighted symbolic mutual information, which separated VS from MCS (wSMIθ: AUC = 74 ± 4%, PFDR < 0.0001) and CS patients (wSMIθ: AUC = 78 ± 6%, PFDR < 0.001). Power spectrum measures also performed well: increased normalized delta power separated VS from MCS (AUC = 31 ± 4%, PFDR < 0.001) and from CS patients (AUC = 19 ± 4%, PFDR < 0.0002), whereas the converse occurred in higher frequency bands, with decreased normalized alpha power segregating VS from MCS (AUC = 72 ± 4%, PFDR < 0.0002) and from CS patients (AUC = 85 ± 5%, PFDR < 0.0001). A third variable affording accurate classification was permutation entropy in theta frequency range, discriminating VS from the other groups (permutation entropyθ: MCS > VS, AUC = 72 ± 4%, PFDR < 0.0002, CS > VS, AUC = 83 ± 5%, PFDR < 0.0001). Finally, the decoding of the global effect (the event-related potential response to deviant auditory sequences; Bekinschtein et al., 2009; King et al., 2013a) also separated VS from MCS (AUC = 62 ± 5%, PFDR < 0.05) and CS patients (AUC = 81 ± 6%, PFDR < 0.0002).
We tested whether the discrimination performance of the EEG measures was consistent across the different aetiologies of patients. To this end, for each EEG measure, we performed an ANOVA with factors of consciousness state (three levels: VS, MCS, CS) and aetiology (four levels: traumatic brain injury, anoxia, stroke, and other). We observed main effects of state of consciousness for the same measures that showed an efficient state discrimination (Supplementary Table 5). However, no marker showed a main effect of aetiology or an interaction between state and aetiology after FDR correction for multiple comparisons (Supplementary Table 5). Similarly, we tested the effect of delay from the date of the accident. We categorized the patients according to the delay as acute (<21 days) or chronic (>21 days). Again, a main effect of state of consciousness was found for most of the successful measures but no measure showed a main effect of delay or an interaction after FDR correction for multiple comparisons (Supplementary Table 6).
One issue is that the vigilance level can be confounded with VS/MCS differences. Indeed, the clinical assessment of the CRS-R subscore corresponding to the arousal level showed a small increase in the MCS group as compared to the VS group (mean ± SEM, MCS: 1.53 ± 0.07, VS: 1.30 ± 0.05, P = 0.013). To rule out this potential confound and isolate the variations specifically due to consciousness, we replicated the previous analysis in the subset of VS and MCS patients with CRS-R arousal subscore of 1, corresponding to patients that presented eye opening only to stimulation (VS: 52 recordings, MCS: 32 recordings). Performance of the EEG measures was essentially unchanged in this vigilance-controlled subset of patients as compared to the overall comparison (Supplementary Fig. 6 and Supplementary Table 3).
Fluctuation of measures across trials convey independent information
The stability of evoked activity has been proposed as a marker of consciousness (Schurger et al., 2010). We then hypothesized that EEG variability over time, quantified as the fluctuations of a given measure across trials, might add independent information about consciousness, relative to its mere average value. The results confirmed that EEG fluctuations had important discriminative information (Fig. 3). Interestingly, we did not always observe a positive correlation between the classifying power of a given measure based on its average and on its fluctuations. Figure 4 provides a graphical comparison of discrimination power based on the mean or the fluctuation of each measure for the MCS/VS contrast. Different types of measures can be distinguished. Some simply fail to separate these two groups (e.g. low gamma power). Others show a significant increase in both the average and the fluctuation over time for MCS compared to VS (this is the case for power in theta, alpha and beta bands, median spectral frequency and wSMIθ). Yet other measures exhibit dissociation between average and fluctuation. In particular, the state of consciousness is associated with a high average but a low fluctuation of algorithmic (or Kolmogorov-Chaitin) complexity (K), permutation entropy (PE)θ and PEα, indicating that a stable and lasting increase in complexity and entropy reflects a conscious patient. Conversely, the state of consciousness is associated with a low average and high fluctuation of power and phase-locking index in the delta band: while fluctuating slow cortical potentials, covering the delta range, are typical in the normal conscious brain (He et al., 2009), stable and intense delta waves are a sign of unconsciousness in anaesthesia and deep sleep (Franks, 2008). Finally, the remaining measures were discriminative only for averages (i.e. spectral entropy) or for fluctuations across trials (i.e. phase-locking index in beta and alpha bands). The latter finding may suggest that consciousness implies a constantly fluctuating stream of transiently phase-locked brain states.
Figure 3.
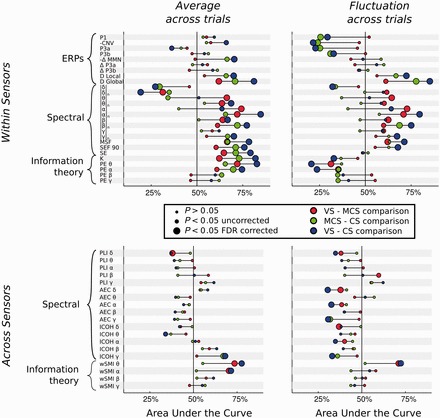
Discrimination power for all measures. Each line provides a summary report of its respective measure. The meaning of each acronym can be found in the Supplementary material. The measures are ordered according to the taxonomy presented in Table 1. The location of each dot corresponds to the AUC for a pair-wise comparison between two states of consciousness (see ‘Materials and methods’ section). Chance level corresponds to AUC = 50% (central vertical line). An AUC>50% suggests that the corresponding measure is correlated with the state of consciousness (from VS to MCS and CS). An AUC <50% suggests that the corresponding measure is anti-correlated with the state of consciousness. Dot colour and size indicate the type and significance of the comparison (see legend). The red colour highlights the minimal contrast between the MCS and VS states of consciousness. As the contingent negative variation and MMN are negative EEG components, their respective AUC was computed after changing the sign of their amplitudes.
Figure 4.
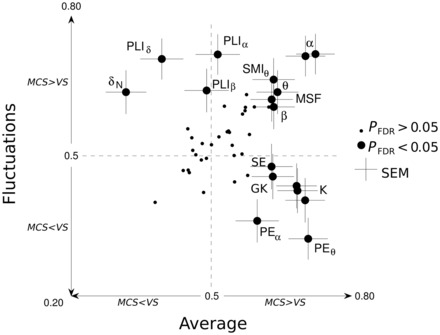
Summary of the measures discriminating VS and MCS patients. Each measure is plotted in a 2D graph. Acronyms meanings can be found in the Supplementary material. The x-axis indicates discriminatory power for each measure’s average across trials, whereas the y-axis indicates discriminatory power for their respective fluctuations across trials. For instance, the Kolmogorov-Chaitin complexity (K) measure appears in the bottom right quadrant, suggesting that its average value is significantly higher in MCS than in VS, whereas its standard deviation, conversely, is higher in VS than in MCS. Large circles indicate significant measures (PFDR < 0.05). Non-significant measures are indicated with small dots.
Combining measures improves discrimination
We next examined whether these EEG measures could be combined to improve discrimination of the different states of consciousness, particularly in the crucial VS/MCS comparison. One goal was to determine whether these markers act in concert, and be thus combined to improve discrimination between groups or whether these measures are redundant. In this latter case, the best measure would provide information comparable to the entire set of measures. To this aim we used a multivariate classification method based on a support vector machine (Pedregosa et al., 2011). The support vector machine was applied to all VS and MCS recordings. To avoid over-fitting, the support vector machine was repeatedly fitted and evaluated on independent data sets using stratified nested cross-validation. It took as input a measure or set of measures, and output the estimated probability for a given recording to belong to the VS group. Results showed that when the classifier was set to use the best cross-validated single measure the performance reached an AUC = 71 ± 4%, for the VS-MCS comparison. By contrast, when using the whole set of measures, AUC was significantly higher than when using the best single measure: VS-MCS: AUC = 78 ± 4% (P < 0.001). In other words, measures were not entirely redundant, and the support vector machine provided an efficient way to identify the combination of markers that leads to a better discrimination of the patients states of consciousness.
Automatic classification of patients’ state of consciousness
A main clinical objective of this work was to develop an automatic system that potentially helps physicians to detect consciousness in non-communicating patients. For this, we evaluated the performance of the support vector machine classifier to differentiate VS patients from MCS patients, i.e. to reliably identify the presence of a clinically detectable state of consciousness. The classifier achieved above-chance levels of accuracy [χ2 (2, n = 143) = 26.7, P = 10−7]: 67% (50 of 75) of VS-diagnosed patients and 76% (52 of 68) of MCS-diagnosed patients were classified in their respective clinical categories solely from their brain activity (Fig. 5). To test for robustness, we also evaluated whether the same classifier, trained to distinguish VS from MCS patients, generalized to CS patients and healthy subjects. The great majority of these recordings (89%, 34 of 38) were classified as conscious (MCS rather than VS).
Figure 5.
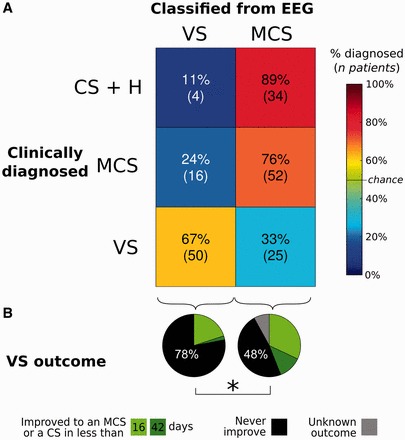
Comparison of EEG-based classification with clinical diagnosis and patients’ outcome. (A) Confusion matrix showing, on the y-axis, the clinical diagnosis (VS, MCS or CS/Healthy), and on the x-axis, the prediction using the automatic classifier based on EEG measures (VS or MCS). The number of recordings and their respective percentages within each clinical state category are reported in each cell. For VS and MCS patients EEG-based classification matches the clinical diagnosis in a majority of cases. Using the same classifier (trained to predict VS or MCS state) the top cells show the predicted condition for CS and healthy subjects. The majority of these recordings were classified as MCS. Non-matching cells can suggest inappropriate classifications, but may also indicate that EEG measures are detecting information unseen by clinicians. (B) The pie charts show the clinical outcome of the VS patients, as a function of whether EEG measures classified them as VS or in a higher state of consciousness (MCS or CS). The probability of recovery was significantly higher (P = 0.02) for patients classified into a higher state of consciousness than for patients predicted to be truly VS.
To train our classifier, we relied on clinical labels (VS and MCS) that derive solely from behavioural observations and need not provide a perfect ‘gold standard’ (Owen et al., 2006). A disagreement between the automatic classification and the clinical label may represent an error of the classifier, but it may also indicate the presence of EEG-based information not accessible to the clinician. To investigate whether the information derived from neurophysiological activity may improve diagnosis, we tested whether VS patients classified as MCS from their EEG activity would later show signs of intentional behaviour that may have been missed at the time of the recording. Most patients classified as VS on both clinical and EEG-based criteria showed no signs of regaining consciousness in the six weeks following EEG recording [recovery <21 days: 10 (20%); recovery within 21–42 days: 1 (2%); no recovery: 39 (78%)]. By contrast, among the clinically VS patients classified as MCS based on their EEG activity, the proportion of those who later showed signs of consciousness significantly increased [recovery <21 days: 8 (32%); recovery within 21–42 days: 3 (12%); no recovery: 12 (48%); unknown: 2 (8%)].The number of subjects who recovered consciousness was significantly higher for VS patients classified as MCS than for those classified as VS [χ2(2, n = 73) = 4.99, P = 0.025]. It should be emphasized that clinical assessment at the time of the recording, based on the full CRS-R or its subscores, neither predicted the VS patients’ recovery nor the automatic classification category (Supplementary material). We then investigated which of the EEG measures that successfully identified the state of consciousness were also efficient in predicting the recovery of VS patients. Results show that the patients recovering from VS (recovery <42 days), relative to non-recovering ones, tended to exhibit a higher contingent negative variation, higher normalized power in the theta frequency band, and smaller phase-locking index in the delta frequency band. These results should be considered exploratory, however, given that these results achieved significance only at an uncorrected P < 0.05 level (Supplementary Table 7).
In summary, within a behaviourally indistinguishable group of clinical VS patients, neurophysiological measures provided information about the future improvement of consciousness, suggesting a better functional status at the time of recording than the one indicated by the clinical diagnosis.
Discussion
We systematically evaluated putative signatures of consciousness in a large data set of high-density bedside EEG recordings of patients suffering or recovering from disorders of consciousness. An important feature of our study is that we included all patients with disorders of consciousness within a ∼2-year period, thus ensuring an unbiased sampling and maximizing clinical relevance. By testing 92 candidate measures arising from previous empirical and theoretical research, we showed that patients’ EEG contains many useful features that discriminate between VS and CS. Each of these measures can thus index consciousness, either directly, or indirectly through its consequences on arousal, instruction understanding, active maintenance of stimuli and instructions in working memory, and task monitoring, etc. Crucially, only a few of these measures were effective in discriminating the minimal contrast between VS and MCS patients. We focus the discussion on this subset of measures, which seem most relevant to the objective identification of conscious processing from the patterns of EEG activity.
Spectral power analysis revealed that alpha and theta power was significantly lower in VS than in MCS patients, whereas delta power showed the opposite pattern. Similar increases in low-frequency oscillations are a classical observation in coma and deep sleep (Posner et al., 2007). Here we demonstrate their relevance to distinguish VS from MCS patients, as recently reported in smaller groups of patients (Fellinger et al., 2011; Lehembre et al., 2012; Lechinger et al., 2013). The fronto-parietal topography of these spectral effects is consistent with a crucial role of fronto-parietal networks in a ‘global workspace’ mediating a serial stream of conscious states at theta-like frequencies (100–300 ms per state) (Alkire et al., 2008; Vanhaudenhuyse et al., 2010; Dehaene et al., 2011; Laureys et al., 2012). This regional hypothesis should be confirmed with cortical source analysis, which was not attempted here given the difficulty of obtaining an accurate source model in patients with massive brain and skull damage (King et al., 2011).
Spectral measures also exhibited greater fluctuations in MCS than in VS patients. This finding agrees with the definition of MCS as a fluctuating state (Giacino et al., 2005) and shows that, contrariwise, a stable state of increased delta and reduced alpha-theta power is a solid sign of unconsciousness. Finally, the structure of the EEG spectrum, as measured by spectral entropy, was increased in MCS and CS as opposed to VS patients. This finding was previously reported to be relevant only in the acute stage and in a much smaller subset of patients (14 VS versus 9 MCS) (Gosseries et al., 2011).
Multiple EEG measures of signal complexity (spectral entropy, permutation entropy, algorithmic complexity) discriminated VS from MCS patients. Indeed, both the average and the intertrial stability of EEG complexity increased monotonically with the patients’ state of consciousness. This result confirms that the complexity of cortical activity indexes consciousness, as explicitly formulated for instance by the dynamic core model of Tononi (2008). According to this model and related ones (Seth et al., 2011), a minimal level of complexity in neuronal signals is required to encode a rich and differentiated representation, thus singling it out from the vast repertoire of potential contents of consciousness. Regions specifically implicated in the coding of conscious representations would thus show higher complexity during conscious than during unconscious states, as observed here.
We found that consciousness was indexed not only by a high information complexity, but also by a stability of this complexity, with reduced fluctuations during the 30-min recording. This result extends to spontaneous EEG the findings of a recent functional MRI study showing that neural activation patterns are more reproducible when evoked by a visible than by an invisible stimulus (Schurger et al., 2010). We hypothesize that this phenomenon reflects one property of the conscious brain, which is to achieve both a reproducible perception of identical sensory stimuli while keeping an internal stream of computations of roughly constant complexity even in the absence of sensory stimulation.
One of the most striking differences between patients was an increase in functional connectivity measures around the theta (4–8 Hz) frequency band (wSMIθ) in MCS as compared to VS patients (also see King et al., 2013b). This result strengthens previous findings relating long-distance synchronization with conscious states in patients with disorders of consciousness in similar frequency bands (Schiff et al., 2005; Lehembre et al., 2012; Leon-Carrion et al., 2012). In addition to generalizing this finding to a large data set, our results clarify the topography of this large-scale increase in communication. We observed a maximal effect over mesio-parietal areas, in close agreement with recent work showing the crucial role of precuneus and posterior cingulate as ‘hubs’ in a long-distance cortical network that may underlie conscious integration (Schiff et al., 2005; Vogt and Laureys, 2005). Future research should investigate whether a more detailed description of this functional connectivity network, possibly aided by directional connectivity analyses such as Granger causality (Gaillard et al., 2009; Barrett et al., 2012; Lee et al., 2013), may also help to distinguish these clinical groups.
The importance of long-distance cortical communication and signal complexity in consciousness fits with a set of perturbational transcranial magnetic stimulation-EEG studies conducted in patients with disorders of consciousness (Rosanova et al., 2012), sleep (Massimini et al., 2005) and anaesthesia (Ferrarelli et al., 2010), which revealed that the prolonged propagation of transcranial magnetic stimulation-induced activation to distant sites systematically correlates with consciousness. More recently a quantification of the structure of EEG-evoked responses to the transcranial magnetic stimulation pulse has been presented (Casali et al., 2013). In a small cohort of normal subjects and patients, this measure, coined pertubational complexity index, was found to discriminate conscious state across a broad range of physiological, pharmacological and pathological conditions. Further research should test whether a similar measure can be obtained without the transcranial magnetic stimulation excitation (Sitt et al., 2013). It may not be irrelevant that, in the present study, long-distance communication and complexity were measured while patients were stimulated with a series of auditory tones. Our results may partially reflect the brain state evoked by these stimuli, thus paralleling those triggered by a transcranial magnetic stimulation pulse. Recording EEG while patients are stimulated with a challenging set of auditory stimuli, as we did here, presents several advantages, including increased vigilance and the capacity to simultaneously evaluate evoked and spontaneous brain activity patterns. Nevertheless, future research will be needed to disentangle which of our measures would continue to be discriminative when applied to resting-state EEG.
We also found larger fluctuations of functional connectivity in MCS than in VS patients, in particular in phase-locking index (from delta to beta) and in weighted symbolic mutual information (wSMIθ). Recent studies conducted with epileptic patients with implanted electrodes (SEEG) for presurgical mapping reported that loss of consciousness during the transition from partial simple to partial complex seizures was marked by a sudden excessive increase in cortico-cortical and thalamo-cortical synchrony (Lambert et al., 2012; Arthuis et al., 2009). Our results lead to the testable prediction that this excessive synchrony, also observed in propofol anaesthesia (Supp et al., 2011), would cause a marked decrease of information complexity, which would in turn mark the loss of consciousness.
EEG studies using active cognitive tasks to probe conscious states often use event-related potentials as their main outcome measure (Bekinschtein et al., 2009; Schnakers et al., 2009a). Indeed, the auditory regularities violation task used here was designed to dissociate the automatic and supposedly unconscious MMN from the P3b complex associated with conscious access. We previously demonstrated that this P3b component, which indexes the detection of the violation of a global regularity, can be useful as a specific marker of consciousness in individual patients, sometimes in advance of a clinical diagnosis (Faugeras et al., 2011, 2012). The present results, however, indicate that this traditional event-related potential averaging approach lacks sensitivity in comparison with other EEG-based measures. Event-related potential components may still contribute importantly to diagnosis, but mostly when analysed with a multivariate pattern analysis procedure that deals with interindividual differences (King et al., 2013a), particularly in patients whose event-related potential topography may be significantly distorted relative to the normal population.
The present results also show that event-related potential fluctuations across trials provide a sensitive measure of conscious state. Consistent with a previous functional MRI study (Schurger et al., 2010), consciousness was characterized by more stereotyped responses to external stimuli. This result could also reflect the fact that VS patients presented a greater power of spontaneous low-frequency EEG signals, non-phase-locked with incoming stimuli and thus interfering with the reproducibility of evoked signals.
Our conclusions are supported by a large sample of patients with various aetiologies, robust methods controlling for multiple comparisons, and a focus on the minimal contrast between VS and MCS patients. It could be argued that differences in vigilance, rather than consciousness, also distinguish MCS and VS patients. Indeed, the CRS-R subscore of vigilance, measured just before the recording session, was slightly larger in MCS than in VS patients. However, most of the identified measures that distinguished VS from MCS patients remained significant when contrasting individuals with matched levels of vigilance. Thus, vigilance per se is unlikely to contribute to the identification of the relevant measures.
A significant gain in discrimination was obtained by combining several EEG measures. This is an important result for both theoretical and clinical reasons. Theoretically, this gain of information suggests that our markers tap onto distinct and dissociable features of conscious states. From the medical perspective, our results stress the usefulness of combining a subset of EEG measures (spectral, informational and connectivity-based).
Although most patients were correctly decoded on the basis of their EEG measures, the number of disagreements between the automated and clinical diagnoses remained too high for individual diagnosis. In particular, 24% of MCS patients, who manifested clear-cut behavioural signs of consciousness on some occasions, were classified as VS. We note, however, that the present study was based on a single half-hour EEG recording. As the minimal conscious state is defined by the presence of fluctuating signs, assessed by repeated clinical evaluation, it seems entirely possible that our examination was performed at a moment when these patients’ consciousness lapsed. In the future, multiple sessions or even 24-h EEG recordings might yield an improved clinical discrimination. Although our accuracy in discriminating patients is roughly comparable to that reported by other groups (Schnakers et al., 2008; Fellinger et al., 2011; Gosseries et al., 2011; Fingelkurts et al., 2012; Lehembre et al., 2012), these previous studies used smaller sample sets and were focused on quantifying the power of a single EEG measure in discriminating pre-selected groups of well-differentiated patients (e.g. non-overlapping CRS-R scores for MCS and VS groups). The present study, by contrast, tackles the more difficult problem of identifying residual consciousness in a serial cohort of all patients admitted to our clinic. Structural MRI was also reported to be efficient in discriminating patients (Fernández-Espejo et al., 2011). Further work will explore if the combination of multimodal (MRI + EEG) information can achieve a better performance.
Conversely, 33% of clinically VS patients were classified as MCS by their EEG patterns. Not all of these misclassifications may be errors, however. Rather, in a subset of clinically defined VS patients, our decoder demonstrably discovered information indicating a better functional status, as indicated by a higher rate of clinically detectable recovery in the months after EEG recording. This result fits with previous functional MRI findings indicating that the vegetative state is not a homogeneous category, and that some VS patients may actually be minimally or even fully conscious (Owen et al., 2006).
In conclusion, because EEG is a time-resolved, low-cost, widespread, and easily repeatable method, it may supplement MRI or functional MRI to identify VS patients with residual consciousness or with a potential for future recovery. Our results point to the possibility that a reduced set of EEG measures, selected for example on the basis of their individual discrimination power, and potentially computed from a few scalp electrodes, could ultimately serve as a reliable bedside tool to probe consciousness in patients with disorders of consciousness.
Supplementary Material
Acknowledgements
The authors would like to thank to Gael Varoquaux, Marcello Massimini and Aaron Schurger for useful discussions. This study is dedicated to the patients and to their close relatives.
Glossary
Abbreviations
- AUC
area under the curve
- CRS-R
Coma Recovery Scale Revised
- CS
conscious state
- MCS
minimally conscious state
- VS
vegetative state
Funding
This work was supported by INSERM, CEA, by a senior ERC grant ‘NeuroConsc’ and the Spoelbech foundation (S.D.), by the James S. McDonnell Foundation (collaborative activity award), by the Fondation pour la Recherche Médicale (FRM) (‘Equipe FRM 2010’ grant to L.N.), Institut pour le Cerveau et la Moëlle épinière (ICM Institute, Paris, France), by the program ‘‘Investissements d'avenir’’ ANR-10-IAIHU-06 and by A-HP, by the Direction Generale de l’Armement (DGA) (J.-R.K.), by Stic-Amsud grant ‘RTBRAIN’ (J.D.S.), by the James McDonell Foundation 21st Century Science Initiative in Understanding Human Cognition- Scholar Award (M.S.) and AXA Research Fund to I.E.K.
Supplementary material
Supplementary material is available at Brain online.
References
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthuis M, Valton L, Régis J, Chauvel P, Wendling F, Naccache L, et al. Impaired consciousness during temporal lobe seizures is related to increased long-distance corticalsubcortical synchronization. Brain. 2009;132:2091–101. doi: 10.1093/brain/awp086. [DOI] [PubMed] [Google Scholar]
- Bandt C, Pompe B. Permutation entropy: a natural complexity measure for time series. Phys Rev Lett. 2002;88:1–4. doi: 10.1103/PhysRevLett.88.174102. [DOI] [PubMed] [Google Scholar]
- Barrett AB, Murphy M, Bruno MA, Noirhomme Q, Boly M, Laureys S, et al. Granger causality analysis of steady-state electroencephalographic signals during propofol-induced anaesthesia. PLoS One. 2012;7:e29072. doi: 10.1371/journal.pone.0029072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L, Naccache L. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci USA. 2009;106:1672–7. doi: 10.1073/pnas.0809667106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med. 2013;5:198ra105. doi: 10.1126/scitranslmed.3006294. [DOI] [PubMed] [Google Scholar]
- Cruse D, Chennu S, Chatelle C, Bekinschtein TA, Fernández-espejo D, Pickard JD, et al. Bedside detection of awareness in the vegetative state: a cohort study. Lancet. 2011;378:2088–94. doi: 10.1016/S0140-6736(11)61224-5. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–27. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein TA, Galanaud D, Puybasset L, et al. Probing consciousness with event-related potentials in the vegetative state. Neurology. 2011;77:264–8. doi: 10.1212/WNL.0b013e3182217ee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein T, Galanaud D, Puybasset L, et al. Event related potentials elicited by violations of auditory regularities in patients with impaired consciousness. Neuropsychologia. 2012;50:403–18. doi: 10.1016/j.neuropsychologia.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Fellinger R, Klimesch W, Schnakers C, Perrin F, Freunberger R, Gruber W, et al. Cognitive processes in disorders of consciousness as revealed by EEG time-frequency analyses. Clin Neurophysiol. 2011;122:2177–84. doi: 10.1016/j.clinph.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Fernández-Espejo D, Bekinschtein T, Monti MM, Pickard JD, Junque C, Coleman MR, et al. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage. 2011;54:103–12. doi: 10.1016/j.neuroimage.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci USA. 2010;107:2681–6. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Bagnato S, Boccagni C, Galardi G. EEG oscillatory states as neuro-phenomenology of consciousness as revealed from patients in vegetative and minimally conscious states. Conscious Cogn. 2012;21:149–69. doi: 10.1016/j.concog.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Fischer C, Luaute J, Adeleine P, Morlet D. Predictive value of sensory and cognitive evoked potentials for awakening from coma. Neurology. 2004;63:669–73. doi: 10.1212/01.wnl.0000134670.10384.e2. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Gaillard R, Dehaene S, Adam C, Clémenceau S, Hasboun D, Baulac M, et al. Converging intracranial markers of conscious access. PLoS Biol. 2009;7:e1000061. doi: 10.1371/journal.pbio.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K. Diagnostic and prognostic guidelines for the vegetative and minimally conscious states. Neuropsychol Rehabil. 2005;15:166–74. doi: 10.1080/09602010443000498. [DOI] [PubMed] [Google Scholar]
- Goldfine AM, Victor JD, Conte MM, Bardin JC, Schiff ND. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin Neurophysiol. 2011;122:2157–68. doi: 10.1016/j.clinph.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosseries O, Schnakers C, Ledoux D, Vanhaudenhuyse A, Bruno MA, Demertzi A, et al. Automated EEG entropy measurements in coma, vegetative state/unresponsive wakefulness syndrome and minimally conscious state. Funct Neurol. 2011;26:25–30. [PMC free article] [PubMed] [Google Scholar]
- He BJ, Raichle ME. The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci. 2009;13:302–9. doi: 10.1016/j.tics.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006;117:348–68. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- King JR, Bekinschtein T, Dehaene S. Comment on “Preserved feedforward but impaired top-down processes in the vegetative state”. Science. 2011;334:1203. doi: 10.1126/science.1210012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Faugeras F, Gramfort A, Schurger A, El Karoui I, Sitt JD, et al. Single-trial decoding of auditory novelty responses facilitates the detection of residual consciousness. Neuroimage. 2013a;83:726–38. doi: 10.1016/j.neuroimage.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Sitt JDD, Faugeras F, Rohaut B, El Karoui I, Cohen L, et al. Information sharing in the brain indexes consciousness in noncommunicative patients. Curr Biol. 2013b;23:1914–9. doi: 10.1016/j.cub.2013.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto M, Revonsuo A, Lehtonen M. Independence of visual awareness from the scope of attention: an electrophysiological study. Cereb Cortex. 2006;16:415–24. doi: 10.1093/cercor/bhi121. [DOI] [PubMed] [Google Scholar]
- Lambert I, Arthuis M, McGonigal A, Wendling F, Bartolomei F. Alteration of global workspace during loss of consciousness: a study of parietal seizures. Epilepsia. 2012;53:2104–10. doi: 10.1111/j.1528-1167.2012.03690.x. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Lau HC, Passingham RE. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc Natl Acad Sci USA. 2006;103:18763–8. doi: 10.1073/pnas.0607716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci. 2005;9:556–9. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Laureys S, Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage. 2012;61:478–91. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Lechinger J, Bothe K, Pichler G, Michitsch G, Donis J, Klimesch W, et al. CRS-R score in disorders of consciousness is strongly related to spectral EEG at rest. J Neurol. 2013;260:2348–56. doi: 10.1007/s00415-013-6982-3. [DOI] [PubMed] [Google Scholar]
- Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–75. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehembre R, Marie-Aurélie B, Vanhaudenhuyse A, Chatelle C, Cologan V, Leclercq Y, et al. Resting-state EEG study of comatose patients: a connectivity and frequency analysis to find differences between vegetative and minimally conscious states. Funct Neurol. 2012;27:41–7. [PMC free article] [PubMed] [Google Scholar]
- Leon-Carrion J, Leon-Dominguez U, Pollonini L, Wu M-H, Frye RE, Dominguez-Morales MR, et al. Synchronization between the anterior and posterior cortex determines consciousness level in patients with traumatic brain injury (TBI) Brain Res. 2012;1476:22–30. doi: 10.1016/j.brainres.2012.03.055. [DOI] [PubMed] [Google Scholar]
- Lumer ED, Rees G. Covariation of activity in visual and prefrontal cortex associated with subjective visual perception. Proc Natl Acad Sci USA. 1999;96:1669–73. doi: 10.1073/pnas.96.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R, Yi DJ, Chun MM. The neural fate of consciously perceived and missed events in the attentional blink. Neuron. 2004;41:465–72. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27:2858–65. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med. 2010;362:579–89. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Naccache L, Puybasset L, Gaillard R, Serve E, Willer JC. Auditory mismatch negativity is a good predictor of awakening in comatose patients: a fast and reliable procedure. Clin Neurophysiol. 2005;116:988–89. doi: 10.1016/j.clinph.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–2. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in python. J Mach Learn Res. 2011;12:2825–30. [Google Scholar]
- Pins D. The neural correlates of conscious vision. Cereb Cortex. 2003;13:461–74. doi: 10.1093/cercor/13.5.461. [DOI] [PubMed] [Google Scholar]
- Posner JB, Saper CB, Schiff N, Plum F. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–70. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Gosseries O, Casarotto S, Boly M, Casali AG, Bruno MA, et al. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain. 2012;135(Pt 4):1308–20. doi: 10.1093/brain/awr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraie A, Weiskrantz L, Barbur JL, Simmons A, Williams SCR, Brammer MJ. Pattern of neuronal activity associated with conscious and unconscious processing of visual signals. Proc Natl Acad Sci USA. 1997;94:9406–11. doi: 10.1073/pnas.94.17.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, Rodriguez-Moreno D, Kamal A, Kim KHS, Giacino JT, Plum F, et al. fMRI reveals large-scale network activation in minimally conscious patients. Neurology. 2005;64:514–23. doi: 10.1212/01.WNL.0000150883.10285.44. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Ledoux D, Majerus S, Damas P, Damas F, Lambermont B, et al. Diagnostic and prognostic use of bispectral index in coma, vegetative state and related disorders. Brain Inj. 2008;22:926–31. doi: 10.1080/02699050802530565. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Perrin F, Schabus M, Hustinx R, Majerus S, Moonen G, et al. Detecting consciousness in a total locked-in syndrome: an active event-related paradigm. Neurocase. 2009a;15:271–7. doi: 10.1080/13554790902724904. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009b;9:35. doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurger A, Pereira F, Treisman A, Cohen JD. Reproducibility distinguishes conscious from non-conscious neural representations. Science. 2010;327:97–9. doi: 10.1126/science.1180029. [DOI] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci. 2005;8:1391–400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- Seth AK, Barrett AB, Barnett L. Causal density and integrated information as measures of conscious level. Philos Trans A Math Phys Eng Sci. 2011;369:3748–67. doi: 10.1098/rsta.2011.0079. [DOI] [PubMed] [Google Scholar]
- Sitt JD, King JR, Naccache L, Dehaene S. Ripples of consciousness. Trends Cogn Sci. 2013;17:552–4. doi: 10.1016/j.tics.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Supèr H, Spekreijse H, Lamme VA. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) Nat Neurosci. 2001;4:304–10. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- Supp GG, Siegel M, Hipp JF, Engel AK. Cortical hypersynchrony predicts breakdown of sensory processing during loss of consciousness. Curr Biol. 2011;21:1988–93. doi: 10.1016/j.cub.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull. 2008;215:216–42. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133(Pt 1):161–71. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–17. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Sagiv N, Hazeltine E, Poldrack RA, Swick D, Rafal RD, et al. Neural fate of seen and unseen faces in visuospatial neglect: a combined event-related functional MRI and event-related potential study. Proc Natl Acad Sci USA. 2001;98:3495–500. doi: 10.1073/pnas.051436898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen VJM, van Boxtel GJM, Eilander HJ, de Gelder B. Mismatch negativity predicts recovery from the vegetative state. Clin Neurophysiol. 2007;118:597–605. doi: 10.1016/j.clinph.2006.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


