Abstract
Progressive multifocal leukoencephalopathy (PML) is an often-fatal demyelinating disease of the CNS that usually develops in immunocompromised individuals due to reactivation of quiescent JC virus (JCV). There are only a few reports of JCV infection in the human spinal cord. PML-like demyelinating lesions have been documented in the brains of simian immunodeficiency virus (SIV)-infected macaques. To determine whether Simian virus 40 (SV40) can infect and cause PML lesions in spinal cords of immunosuppressed macaques, we examined archival spinal cord samples from 15 SIV-infected rhesus monkeys with AIDS and SV40 infection of the brain. Among those, 6 (40%) had SV40-infected cells in spinal cord, including 1 with PML-like lesions, 1 with PML-like lesions and meningoencephalitis, 2 with meningoencephalitis, 1 with gray matter gliosis and 1 with no lesions. One animal with a large PML-like lesion had extensive demyelination and SV40 infection of astrocytes, oligodendrocytes and meningeal cells. None of the 6 animals had SV40-infected spinal cord neurons. These observations indicate that like JCV in immunosuppressed humans, SV40 can infect glial cells and cause PML-like lesions in the spinal cord of immunosuppressed rhesus macaques. Rhesus macaques could serve as an animal model to study polyomavirus infection and pathogenesis in the spinal cord.
Keywords: Gray matter, Progressive multifocal leukoencephalopathy (PML), Rhesus macaques, Simian immunodeficiency virus (SIV), Simian virus 40 (SV40), Spinal cord, meningoencephalitis
INTRODUCTION
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the CNS that usually develops in immunosuppressed individuals due to reactivation of the quiescent human polyomavirus JC (JCV) (1, 2). Simian virus 40 (SV40) has 69% sequence homology with JCV. As with JCV in human populations, asymptomatic lifelong SV40 infection is common in rhesus monkeys (3–5). Moreover, reactivation of SV40 has been documented in 2% to 3% of simian immunodeficiency virus (SIV)-infected rhesus macaques with demyelinating brain lesions; this is analogous to JCV-induced PML (4, 6). PML lesions have been well characterized in JCV-infected individuals and SV40-infected rhesus macaques (3, 6–8). Both JCV and SV40 infect CNS glial cells (oligodendrocytes and astrocytes) and, in some instances, neurons (5, 9–11). PML lesions are often located in cerebral subcortical white matter but they can also occur in the white matter of cerebellum and brain stem. Recent human and rhesus PML studies have shown that lesions can extend from white matter into the adjacent gray matter (5, 11). However, there are only a few reports of JCV infection and PML lesions in the spinal cord in humans (12–16).
Here, we report for the first time, SV40 infection in the spinal cord of 6 SIV-infected rhesus macaques from the New England Primate Research Center (NEPRC), including 2 with PML-like lesions.
MATERIALS AND METHODS
Animals
A total of 1324 records of SIV-infected macaques were available from a database of pathology records at the NEPRC from 1996–2012. Cases were selected for further analysis based on prior identification of SV40-associated disease, including PML-like CNS disease and SV40-associated nephritis. SV40 cases in rhesus macaque (Macaca mulatta) were SIV-infected with end-stage simian AIDS, defined by the presence of opportunistic infections or other AIDS-defining lesions. Selected cynomolgus macaques (Macaca fascicularis) included those with SV40 CNS and/or renal infection associated with immunosuppressive drug regimens after kidney-transplantation. In addition, 3 rhesus macaques previously studied from 1990–91 were also included in this cohort for further analysis (17). One or, when available, 2 cervical spinal cord sections were used for SV40 analyses for all of the animals.
The animals had been maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Harvard Medical School, and were housed at the NEPRC, Harvard Medical School, Southborough, Massachusetts, and cared for by its veterinary staff.
Immunohistochemistry
Immunoperoxidase staining for SV40 alone was performed on 5-µm-thick formalin-fixed paraffin-embedded sections using the standard avidin-biotin peroxidase complex technique (Dako, Carpinteria, CA). In brief, the sections were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 5 minutes followed by antigen retrieval achieved by microwaving for 20 minutes in citrate buffer. The sections were treated with Dako Protein Block for 10 minutes and incubated overnight at 4°C with a specific anti-SV40 large T antigen (T Ag) antibody (IgG1, Oncogene, La Jolla, CA), which does not cross react with small T Ag. The antibody was used at a dilution of 1:25,600 or for 1 hour at room temperature; a mouse monoclonal anti-SV40 VP1 (PAB597, generous gift from Dr. Walter Atwood) (18) was used at a dilution of 1:1000 (Table 1). Slides were then incubated with the secondary antibody (biotinylated horse anti-mouse, Vector Laboratories, Burlingame, CA) diluted 1:200 for 30 minutes, followed by a 30-minute incubation with Vectastain ABC Elite (Vector). Tissue sections were washed and developed with DAB Chromogen (Dako) and counterstained with Mayer’s hematoxylin. Isotype matched irrelevant controls were tested on all tissues.
Table 1.
Antibodies Used for Immunochemistry and Immunofluorescence Experiments
| Antigen; product name |
Host | Isotype | Target | Source |
|---|---|---|---|---|
| PAB597 | Mouse | NA | SV40 VP1 | Walter Atwood, Brown University, Providence, RI |
| SV40 T Ag (Pab 101); sc-147 | Mouse | IgG | SV40 T Ag | Santa Cruz Biotechnology, Santa Cruz, CA |
| SV40 T Ag; sc-20800 (v-300) | Rabbit | IgG | SV40T Ag | Santa Cruz Biotechnology, |
| GFAP; Z0334 | Rabbit | IgG | Astrocytes | Dako, Carpinteria, CA |
| SV40 T Ag | Mouse | IgG1 | SV40T Ag | Oncogene, La Jolla, CA |
| CNPase; ab50739 | Chicken | IgY | Myelin/Oligo-dendrocytes | Abcam, Cambridge, MA |
Ag, antigen; CNPase, 2',3'-cyclic-nucleotide 3'-phosphodiesterase; GFAP, glial fibrillary acidic protein; Pab, polyclonal antibody; SV40 T Ag, Simian virus 40 T antigen.
Immunofluorescence
Immunofluorescence staining assays were performed as previously described (19). In multiple staining experiments, primary antibodies from different species (mouse monoclonal, rabbit and chicken polyclonal) were used with appropriately matched (species and isotype) secondary antibodies (Table 1). These antibodies were conjugated to Alexa Fluor 350, 488 & 568 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Negative controls included omission of the primary antibodies and the use of sections from individuals known to be free of SV40-infected cells.
RESULTS
Macaque SV40-spinal Cord Study Cohort
As recently reported by our group, 21 immunosuppressed macaques were identified from NEPRC records and archives with SV40 infection in the brain (21/1324, 1.6%). Fourteen of the 21 animals with SV40 CNS disease had SV40-positive neurons (14/21, 67%) (5). Of 21 animals, 15 had spinal cord sections archived that were available for analysis. Six of the 15 animals had SV40-positive cells in the spinal cord (6/15, 40%). Four of the 6 animals with spinal cord infection had cerebral PML (4/6, 66.7%) (5), including 2 with meningoencephalitis; 2 of the 6 animals had meningoencephalitis without PML (2/6, 33.3%); and 4 of the 6 animals had SV40-positive neurons in the brain (4/6, 66.7%) (Table 2). All but 1 animal were killed because of neurologic signs.
Table 2.
Patterns of Brain and Spinal Cord SV40 Infection of 6 SIV-infected Rhesus Macaques
| Brain | Spinal cord | ||||||
|---|---|---|---|---|---|---|---|
| Animal # | Lesion pattern |
Demyeli- nation |
Neuronal SV40 infection |
Euthanasia due to neurological signs |
SV40 infected cell type |
Demyeli- nation |
Lesion |
| 1 | PML | Yes | Yes | Yes | Glial cells in GM | No | None |
| 2 | PML | Yes | Yes | Yes | Glial cells in WM and GM; Meningeal cells | Yes (large) | PML and ME |
| 3 | PML, ME | Yes | Yes | Yes | Glial cells in GM | No | Gliosis |
| 4 | ME | No | Yes | Yes | Glial cells in WM and GM; Meningeal cells | No | ME; Perivascular lymphocytic cuff in meninges |
| 5 | PML,ME | Yes | No | No | Glial cells in WM and GM; Meningeal cells | Yes (small) | PML |
| 6 | ME | No | No | Yes | Glial cells in GM | Negative | ME; Perivascular lymphocytic cuff in GM and meninges |
PML, Progressive multifocal leukoencephalopathy; ME, Meningoencephalitis or meningitis; GM, Gray matter; WM, White matter; SV40, Simian virus 40.
Distribution of SV40 in the Spinal Cord
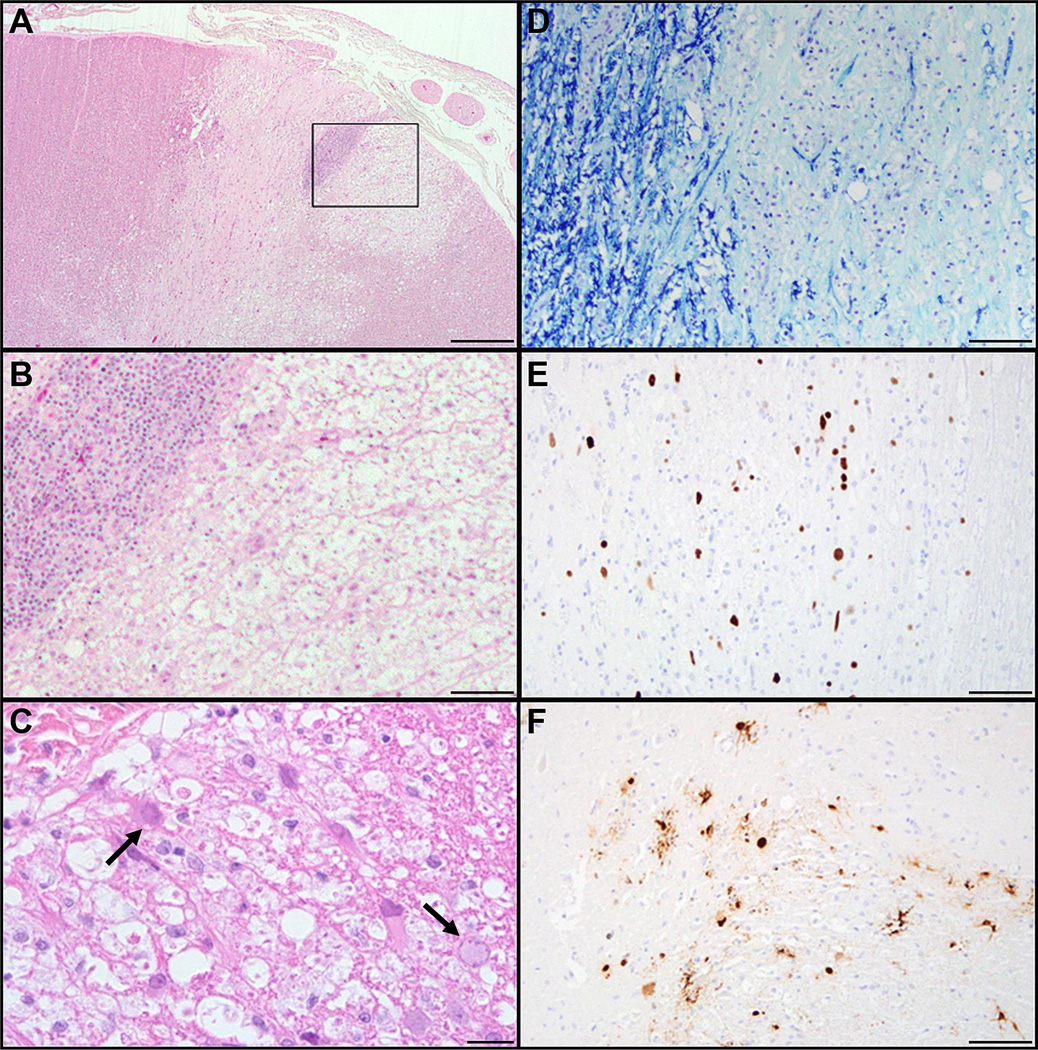
To analyze the tissue distribution of viral infection, spinal cord sections from 15 CNS SV40-positive animals were subjected to SV40 large T Ag immunohistochemistry and Luxol fast blue staining for myelin. Six of the 15 animals had SV40-positive cells within the spinal cord; 2 (animals #2 and #5) had demyelination with classic PML-like lesions in the cord Histopathological and immunohistochemical examination of cases with spinal cord involvement revealed mild to moderate numbers of SV40-infected glial cells in gray and white matter and in meningeal cells despite the absence of demyelinating lesions in 4 of the 6 animals. Of these 4 animals, 2 had perivascular lymphocytic infiltrates in leptomeninges (animal # 4; Table 2) and also in gray matter in close proximity to SV40-positive cells (animal # 6; Table 2); 1 had gliosis in close proximity to SV40-infected cells in gray matter (animal # 3; Table 2); and 1 had isolated SV40-infected glial cells in gray matter but no specific lesion (animal #1; Table 2). The remaining 2 animals had PML-like lesions, including 1 (animal #2; Table 2) with an extensive lesion expanding from the central canal area into adjacent gray matter dorsal horn and white matter (Fig. 1A, B). These areas were characterized by marked loss of myelin, multiple swollen axons (axonal spheroids), necrotic glial cells, foamy macrophages and cellular debris (Fig. 1B, C). The extensive nature of demyelination was indicated by Luxol fast blue staining that showed marked pallor due to myelin loss (Fig. 1D). Multiple blood vessels in the affected area lined by reactive endothelial cells were surrounded by large numbers of lymphocytes and macrophages (Supplementary Fig. 1).
Figure 1.
Histopathology of SV40 infection of spinal cord. (A-C) Hematoxylin and eosin-stained sections show extensive demyelination, necrosis and perivascular lymphoplasmacytic cuffing. (C) There are 5- to 7-µm amphophilic intranuclear viral inclusions within glial cells (black arrows). (D) Luxol fast blue stain for myelin demonstrates extensive myelin loss. (E, F) Immunohistochemical stains for SV40 large T Ag- (E) and SV40 VP1 (F) -positive cells reveal comparable expression of early and late SV40 viral proteins. Scale bars: A, 500 µm; B, 100 µm; C; 20 µm, D-F, 100 µm.
Frequently, glial cells (oligodendrocytes and astrocytes) within and at the periphery of the lesion had enlarged nuclei with marginated chromatin and glassy eosinophilic to amphophilic intranuclear viral inclusions (Fig. 1C, black arrows); these stained positively by immunohistochemistry for both SV40 large T Ag (Fig. 1E) and VP1 protein (Fig. 1F).
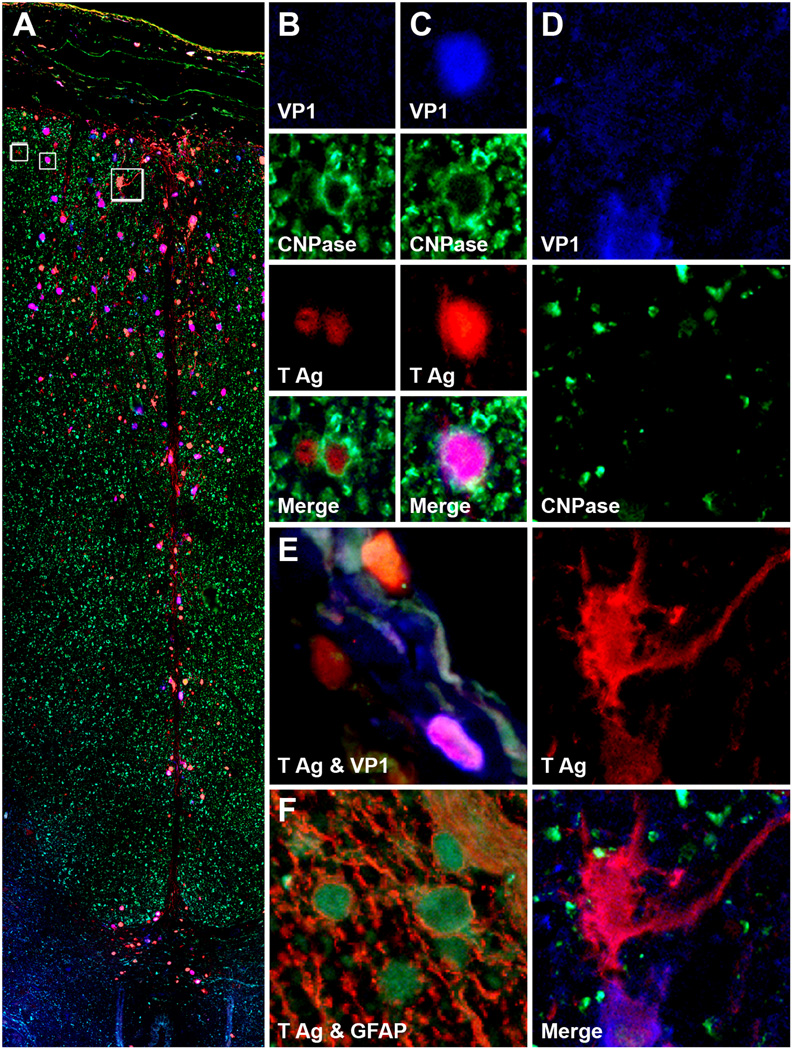
Triple immunofluorescence assay demonstrated that both oligodendrocytes and astrocytes were targeted by SV40 in spinal cord. The majority of the infected cells were oligodendrocytes (as demonstrated by 2',3'-cyclic-nucleotide 3'-phosphodiesterase (CNPase) staining (Fig. 2A-D), but there were also a few CNPase-negative SV40-infected cells with astrocytic phenotype (Fig. 2A, D), as demonstrated by glial fibrillary acidic protein immunohistochemistry (Fig. 2F). Some astrocytes expressed both T Ag and VP1 (Fig. 2E), while some expressed T Ag only (Fig. 2D). In 3 cases, some meningeal cells were also infected, expressing either T Ag alone or both T Ag and VP1. In animal #2, SV40 infection seemed to follow a track from the outside (where infected cells were more numerous) to the inside of the cord, consisting mostly of astrocytes processes in contact with each other. The 2 animals with PML lesions in the spinal cord also had PML lesions in the brain (Table 2) None of the 6 animals had SV40-positive neurons in the spinal cord, as determined by double immunohistochemistry with antibodies against SV40 T Ag and microtubule associated protein-2, a neuronal marker (data not shown).
Figure 2.
Large lesion of progressive multifocal leukoencephalopathy in spinal cord. (A-D) Triple immunofluorescence assay for 2',3'-cyclic-nucleotide 3'-phosphodiesterase (CNPase, Alexa Fluor® 488), SV40 T Ag (Alexa Fluor® 568) and VP1 (Alexa Fluor® 350) in monkey #2 shows SV40-infected cells expressing T antigen (T Ag) alone (red), VP1 alone (blue) or both T Ag and VP1 (purple) in the leptomeninges and along the posterior fissure of the spinal cord (A). Magnification, separate channels and merge for the 2 boxes on the left in (A) are shown in (B) and (C). The boxes contain SV40-infected oligodendrocytes (CNP-ase-positive) expressing T Ag alone (B) or T Ag and VP1 (C). (D) Magnification, separate channels and merge for the box on the right in (A) are shown in 4 panels on the right of the montage. This box shows a T Ag-positive SV40-infected cell not stained for CNPase that has an astrocyte phenotype. (E, F) Similarly to other SV40-infected cells, meningeal cells (E) express both T Ag and VP1 separately or simultaneously. A double immunofluorescence assay for T Ag (green) and glial fibrillary acidic protein (GFAP, red) confirms that the majority of SV40-infected cells are astrocytes (F).
DISCUSSION
To our knowledge, there are no published reports of PML-like lesions or SV40 infection in spinal cords of non-human primates, even though there are a few accounts of its occurrence in humans (12–16). Only 4 animals from 6 SV40-positive spinal cord cases had demyelinating lesions of PML in the brain (5), and among those, 1 had extensive classic PML lesions in the spinal cord. Also, 5 of the 6 cases with SV40 infection of spinal cord were reported with neurological signs, including partial hemiparesis, consistent with spinal cord involvement. The animal with extensive demyelination and PML-like lesion in spinal cord had SV40 infection of astrocytes, oligodendrocytes and meningeal cells. It has been shown that replication-incompetent recombinant SV40 vectors can transduce microglial cells in vitro (20, 21). In JCV-induced PML, phagocytic macrophages may contain myelin debris containing SV40 virions; therefore, it is possible that this may also occur in SV40-associated demyelinating lesions. None of the examined animals had SV40 infection in spinal cord neurons although 4 of the animals had neuron infection in their brain, suggesting a different viral pathogenesis in spinal cord (Table 2). The very low frequency at which SV40 infection causes PML-like lesions in spinal cord (2/1327, 0.15%) in our study cohort could be due the fact that only 15/21 CNS SV40-positive animals had available archival tissues and the samples were restricted to cervical spinal cord areas. Moreover, in all cases only limited cervical cord sections were available.
There have been only 5 published cases of human PML affecting both brain and spinal cord of immunosuppressed individuals (12–16). A recent study reported an isolated JCV myelitis without any clinical nor radiological signs of involvement of other CNS areas in a patient with acute myeloid leukemia receiving rituximab for the treatment for autoimmune hemolytic anemia (22). To date, there have been 548 natalizumab- and 1 dimethylfumarate-treated patients with multiple sclerosis (MS) who developed PML (23). This underscores the importance of investigating and characterizing JCV infection in the entire CNS (24–30). Because MS frequently affects the spinal cord, PML should be considered in the differential diagnosis of all MS patients treated with immunomodulatory medications associated with an increased risk of PML.
Why PML is less prevalent in spinal cord compared to brain both in humans and non-human primates remains unclear. One may speculate that in experimental studies involving non-human primates, animals reach study endpoints for death before the infection extends to the spinal cord. Also, as for JCV in humans, SV40 may favor cerebral infection, or lesions may escape detection on postmortem examination if only few randomly selected spinal cord levels are examined. Other possibilities are that spinal cord PML lesions are overlooked clinically in the setting of concomitant cerebral involvement, or that immune clearance of virus may be more efficient in spinal cord (13).
SV40 involvement of the spinal cord should be considered in immunosuppressed macaques exhibiting paresis. As is the case for the study of PML in the brain, rhesus macaques could serve as an animal model to study polyomavirus infection and pathogenesis in the spinal cord.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Ron Desrosiers for availability to archived tissue. We thank Kristen Toohey for graphic assistance.
This work was supported by NIH grants T32 OD011064 (SW) and New England Primate Research Center Base grant P51OD011103-51. IJK was supported in part by NIH grants R01 NS 047029 and NS 074995. The funding agencies had no input in the investigations or analysis of the results.
Footnotes
Conflict of Interest: Dr. Koralnik receives royalties for chapters in the online textbook UpToDate. Drs. Kaliyaperumal, Wüthrich and Westmoreland declare that they have no competing interests.
REFERENCES
- 1.Gheuens S, Wuthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Ann Rev Pathol. 2013;8:189–215. doi: 10.1146/annurev-pathol-020712-164018. [DOI] [PubMed] [Google Scholar]
- 2.Wollebo HS, White MK, Gordon J, et al. Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol. 2015;77:560–570. doi: 10.1002/ana.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dang X, Wüthrich C, Axthelm MK, et al. Productive simian virus 40 infection of neurons in immunosuppressed Rhesus monkeys. J Neuropathol Exp Neurol. 2008;67:784–792. doi: 10.1097/NEN.0b013e318180f0d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horvath CJ, Simon MA, Bergsagel DJ, et al. Simian virus 40-induced disease in rhesus monkeys with simian acquired immunodeficiency syndrome. Am J Pathol. 1992;140:1431–1440. [PMC free article] [PubMed] [Google Scholar]
- 5.Kaliyaperumal S, Dang X, Wüthrich C, et al. Frequent infection of neurons by SV40 virus in SIV-infected macaque monkeys with progressive multifocal leukoencephalopathy and meningoencephalitis. Am J Pathol. 2013;183:1910–1917. doi: 10.1016/j.ajpath.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon MA, Ilyinskii PO, Baskin GB, et al. Association of simian virus 40 with a central nervous system lesion distinct from progressive multifocal leukoencephalopathy in macaques with AIDS. Am J Pathol. 1999;154:437–446. doi: 10.1016/S0002-9440(10)65290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Major EO, Amemiya K, Tornatore CS, et al. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wüthrich C, Cheng YM, Joseph JT, et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2009;68:15–25. doi: 10.1097/NEN.0b013e3181912570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wüthrich C, Dang X, Westmoreland S, et al. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol. 2009;65:742–748. doi: 10.1002/ana.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wüthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2012;71:54–65. doi: 10.1097/NEN.0b013e31823ede59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer W, Chamberlain W, Horenstein S. Spinal demyelination in progressive multifocal leukoencephalopathy (Abstract) Neurology. 1969;19:287–288. [Google Scholar]
- 13.Bernal-Cano F, Joseph JT, Koralnik IJ. Spinal cord lesions of progressive multifocal leukoencephalopathy in an acquired immunodeficiency syndrome patient. J Neurovirol. 2007;13:474–476. doi: 10.1080/13550280701469178. [DOI] [PubMed] [Google Scholar]
- 14.Shintaku M, Matsumoto R, Sawa H, et al. Infection with JC virus and possible dysplastic ganglion-like transformation of the cerebral cortical neurons in a case of progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2000;59:921–929. doi: 10.1093/jnen/59.10.921. [DOI] [PubMed] [Google Scholar]
- 15.Takeda S, Yamazaki K, Miyakawa T, et al. Progressive multifocal leukoencephalopathy showing extensive spinal cord involvement in a patient with lymphocytopenia. Neuropathology. 2009;29:485–493. doi: 10.1111/j.1440-1789.2008.00981.x. [DOI] [PubMed] [Google Scholar]
- 16.von Einsiedel RW, Fife TD, Aksamit AJ, et al. Progressive multifocal leukoencephalopathy in AIDS: a clinicopathologic study and review of the literature. J Neurol. 1993;240:391–406. doi: 10.1007/BF00867351. [DOI] [PubMed] [Google Scholar]
- 17.Ilyinskii PO, Daniel MD, Horvath CJ, et al. Genetic analysis of simian virus 40 from brains and kidneys of macaque monkeys. J Virol. 1992;66:6353–6360. doi: 10.1128/jvi.66.11.6353-6360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashok A, Atwood WJ. Contrasting roles of endosomal pH and the cytoskeleton in infection of human glial cells by JC virus and simian virus 40. J Virol. 2003;77:1347–1356. doi: 10.1128/JVI.77.2.1347-1356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang X, Koralnik IJ. A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene. J Gen Virol. 2006;87:2533–2537. doi: 10.1099/vir.0.81945-0. [DOI] [PubMed] [Google Scholar]
- 20.Cordelier P, Strayer DS. Using gene delivery to protect HIV-susceptible CNS cells: inhibiting HIV replication in microglia. Virus Res. 2006;118:87–97. doi: 10.1016/j.virusres.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Cordelier P, Morse B, Strayer DS. Targeting CCR5 with siRNAs: using recombinant SV40-derived vectors to protect macrophages and microglia from R5-tropic HIV. Oligonucleotides. 2003;13:281–294. doi: 10.1089/154545703322616961. [DOI] [PubMed] [Google Scholar]
- 22.Stich O, Herpers M, Keil A, et al. JC virus myelitis without cerebral involvement in acute myeloid leukemia. Eur J Neurol. 2011;18:e143–e144. doi: 10.1111/j.1468-1331.2011.03480.x. [DOI] [PubMed] [Google Scholar]
- 23.Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372:1476–1478. doi: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- 24.Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab--unforeseen consequences. N Engl J Med. 2005;353:414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 25.Boster AL, Nicholas JA, Topalli I, et al. Lessons learned from fatal progressive multifocal leukoencephalopathy in a patient with multiple sclerosis treated with natalizumab. JAMA Neurol. 2013;70:398–402. doi: 10.1001/jamaneurol.2013.1960. [DOI] [PubMed] [Google Scholar]
- 26.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 27.Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 28.Métivier D, Arnaud FX, Dutasta F, et al. Immune reconstitution inflammatory syndrome in a patient treated with natalizumab presenting progressive multifocal leukoencephalopathy. Diagn Interv Imaging. 2013;94:101–103. doi: 10.1016/j.diii.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Schippling S, Kempf C, Büchele F, et al. JC virus granule cell neuronopathy and GCN-IRIS under natalizumab treatment. Ann Neurol. 2013;74:622–626. doi: 10.1002/ana.23973. [DOI] [PubMed] [Google Scholar]
- 30.Wüthrich C, Popescu BF, Gheuens S, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in a patient with multiple sclerosis: a postmortem study. J Neuropathol Exp Neurol. 2013;72:1043–1051. doi: 10.1097/NEN.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.