Abstract
Objective
Cognitive measures that are sensitive to biological markers of Alzheimer disease (AD) pathology are needed in order to (a) facilitate preclinical staging, (b) identify individuals who are at the highest risk for developing clinical symptoms and (c) serve as endpoints for evaluating the efficacy of interventions. The present study assesses the utility of two cognitive composite scores of attentional control and episodic memory as markers for preclinical AD pathology in a group of cognitively normal older adults (N = 238), as part of the Adult Children Study.
Method
All participants were given a baseline cognitive assessment and follow-up assessments every 3 years over an 8-year period, as well as a lumbar puncture within two years of the initial assessment to collect cerebrospinal fluid (CSF) and a PET-PIB scan for amyloid imaging.
Results
Results indicated that attentional control was correlated with levels of Aβ42 at the initial assessment whereas episodic memory was not. Longitudinally, individuals with high CSF tau exhibited a decline in both attention and episodic memory over the course of the study.
Conclusion
These results indicate that measures of attentional control and episodic memory can be utilized to evaluate cognitive decline in preclinical AD and provide support that CSF tau may be a key mechanism driving longitudinal cognitive change.
Keywords: Attention, Episodic Memory, Longitudinal Study, Alzheimer Disease, Biological Markers, Amyloid
Introduction
There is substantial evidence indicating that Alzheimer disease (AD) pathology (amyloid plaques and neurofibrillary tangles) can accumulate for decades before the onset of clinically detectable symptoms (Bateman et al., 2012; Price et al., 2009). This pattern suggests the presence of a lengthy preclinical stage of the disease during which pathology accrues without detectable influence on standard clinical measures. Thus, considerable research effort has been devoted to identifying cognitive and biological markers that are indicative of preclinical AD in order to best predict who will eventually develop clinical symptoms. Many biomarkers of the preclinical disease process have been identified. For example, a decrease in levels of amyloid-beta 42 (Aβ42) in the cerebrospinal fluid (CSF), which serves as a marker of amyloid deposition in the brain, and an increase in CSF levels of tau and p-tau181, which serve as a marker of neurodegeneration, have been observed in the earliest stages of AD (Fagan et al., 2007). In addition to CSF markers, it is also possible to image amyloid plaques directly using positron emission tomography (PET) and the amyloid tracer, Pittsburgh compound-B (PIB).
Despite the predictive power of these biomarkers, there remain a substantial number of individuals who do not develop clinical symptoms even though they exhibit substantial AD pathology at autopsy (Price et al., 2009). This heterogeneity in clinical progression could be due to variations in where a specific individual falls on the continuous spectrum of preclinical AD. Thus, there has been considerable emphasis on detecting subtle cognitive changes that are related to preclinical AD pathology in order to provide a more specific characterization of AD risk. Specifically, recent recommendations from the NIA suggest that slight cognitive deficits, in conjunction with evidence of amyloidosis and neurodegeneration define stage 3 (highest risk) of preclinical AD (Sperling et al., 2011). To this end, numerous recent studies have been reported that aim to elucidate the relationships between AD biomarkers and outcomes on concurrent cognitive tests. Indeed, a meta-analysis by Hedden, Oh, Younger, and Patel (2013) indicated that subtle effects of amyloid burden can be detected on measures of episodic memory and executive functioning. These results suggest that such constructs can serve as sensitive cognitive markers of preclinical AD.
It is important to note that although recent reports have found evidence for a relationship between levels of AD biomarkers and concurrent cognitive performance (Aschenbrenner et al., 2015; Duchek et al., 2013; Hedden et al., 2013; Rodrigue et al., 2012; Sperling et al., 2013), a substantial number of other studies have reported null effects (Aizenstein et al., 2008; Fagan et al., 2009; Nebes et al., 2013; Storandt, Head, Fagan, Holtzman, & Morris, 2012; Storandt, Mintun, Head, & Morris, 2009; Vemuri et al., 2009). At present, it is unclear whether the differing patterns of results are due to the relative sensitivity of the various cognitive tasks that are employed or due to subject specific variations across samples in protective factors such as cognitive reserve (Stern, 2009). Regardless, it is clear that developing cognitive measures that are consistently related to early AD biomarkers is critical.
In addition to episodic memory, accumulating evidence suggests that measures of attentional control are sensitive cognitive markers of very mild AD (Balota & Faust, 2001; Perry & Hodges, 1999; Twamley, Ropacki, & Bondi, 2006). Attentional control refers to the ability to direct attention toward relevant information and control irrelevant information in the environment and is particularly stressed when there are multiple competing dimensions that need to be controlled. For example, the Stroop color naming task (Stroop, 1935) is a classic paradigm used to measure attentional control. In this task, individuals are presented with a series of color words (e.g., “red”) written in colored font and are asked to name the color of the word while ignoring the word itself. Differences in performance between congruent trials (where the color and the word overlap) and incongruent trials (where the color and the word are different) can serve as a measure of attentional control.
Recently, attentional control tasks have also been shown to be sensitive to preclinical AD processes in asymptomatic individuals (Aschenbrenner et al., 2015; Balota et al. 2010; Duchek et al., 2009, 2013; Gordon et al., 2015). Although attention seems to be less consistently measured in studies on AD, there is evidence to suggest it is more consistently related to preclinical AD processes, compared to other cognitive processes. Specifically, in a review by Twamley et al. (2006), the authors note that 71% of studies that measured attention found relationships with preclinical AD compared to 57% for verbal learning and 50% for memory. Similarly, in the meta-analysis conducted by Hedden et al. (2013) only 60% (47 datasets) included a general measure of executive function, only some of which measured attentional control directly. Furthermore, only 15 datasets were reported that included longitudinal changes in cognition, many of which analyzed PIB and episodic memory. Taken together, it is clear that both episodic memory and attentional control merit further evaluation as sensitive cognitive markers of preclinical AD.
Although most studies of cognitive-biomarker relationships have been cross-sectional in nature, a few studies have examined longitudinal cognitive changes as a function of amyloid burden primarily measured with imaging techniques (e.g., Doraiswamy et al., 2012; Ellis et al., 2013; Ewers et al., 2012; Landau et al., 2012; Lim et al., 2013; Resnick et al., 2010; Storandt et al., 2009; Villemagne et al., 2011). In contrast, while several studies have been reported regarding CSF markers and cognition, most have examined prediction of later progression to dementia (Craig-Schapiro et al., 2010; Fagan et al., 2007; Li et al., 2007). Only a few studies have examined the relationship between CSF biomarkers and longitudinal cognitive change, and these studies have yielded variable findings including no relationship (Stomrud et al., 2010; Rolstad et al., 2013), correlations only with Aβ42 (Li et al., 2014; Lo et al., 2011) or ptau (Glodzik et al., 2011) to correlations with multiple biomarkers (Roe et al., 2013).
We had two primary goals in the present study. First, we assessed the sensitivity of attentional control and episodic memory composite scores to baseline levels of markers for AD pathology, particularly CSF measures (tau and Aβ42) and PET-PIB binding. We focus specifically on tau and Aβ42, given that these measures often account for independent variance and may reflect different underlying pathological processes (Aschenbrenner et al. 2015; Storandt et al. 2012). Importantly, we extend the results of the aforementioned meta-analysis by also evaluating the sensitivity of attention to levels of tau-related biomarkers, which was not examined by Hedden et al. (2013). We expected that both composite scores would be sensitive to the biomarkers at baseline based on the available literature. Second, and more importantly, we evaluated via longitudinal analyses the extent to which the two cognitive composite scores exhibited decline over time as a function of the same baseline biomarkers.
Method
Participants
All participants were recruited through the Charles F. and Joanne Knight Alzheimer Disease Research Center at Washington University in St. Louis as part of the Adult Children Study. This study was designed to longitudinally follow a group of cognitively normal middle-aged adults (age 45-74 years of age at study entry) on antecedent markers and risk factors of AD, including the presence or absence of a family history of AD. The Washington University Human Research Protection office approved this study.
All participants were assessed for symptoms of dementia by trained physicians using the Clinical Dementia Rating scale (Morris, 1993). To be included in the present analyses, participants were required to be CDR 0 at the time of the first cognitive assessment (hereafter referred to as “baseline assessment”) and also have received a lumbar puncture to collect CSF within 2 years of the initial cognitive assessment. Any individual who did not receive a lumbar puncture within 2 years of the baseline cognitive assessment was excluded (N = 30), leaving 238 individuals for further analysis. Similarly, any individual who did not receive a PET scan within two years of the first assessment was excluded from the PIB analysis leaving 228 individuals.
Cognitive Battery
Participants were given a series of computerized and paper and pencil tasks to measure different facets of cognition. Performance on selected tests from the battery was combined to form two cognitive composite scores. The first composite measured episodic memory and was created from the total number of items correctly recalled from the 3 free recall trials of the Selective Reminding Test (Grober, Buschke, Crystal, Bang, & Dresner, 1988), a weighted sum of the easy and hard trials from the associate learning subtest of the Wechsler Memory Scale (Wechsler & Stone, 1973), and the number of correctly recalled units from the Logical Memory Delayed Recall task (Wechsler, 1997). These standard neuropsychological tests of episodic memory have shown strong sensitivity to AD dementia (see Morris et al., 1991; Storandt & Hill, 1989)
The second composite measured attentional control and was formed from computerized versions of the Stroop color naming task (Spieler, Balota, & Faust, 1996; Stroop, 1935), the Simon task (Castel, Balota, Hutchison, Logan, & Yap, 2007; Simon, 1969), and consonant-vowel/odd-even (CVOE) task-switching paradigm (Tse, Balota, Yap, Duchek, & McCabe, 2010). Each of these attentional control tasks is discussed briefly below and more detailed information is provided elsewhere (Duchek et al., 2009).
Stroop Color Naming
The Stroop task consisted of 4 color words (yellow, red, blue and green) and 4 neutral items (bad, legal, poor and deep). The words and colors were crossed to form 36 congruent trials (each color word printed in its corresponding color nine times), 36 incongruent trials (each color word printed in each of the other colors three times), and 32 neutral trials (each neutral word printed twice in each color). Participants made a vocal response into a microphone and an experimenter coded each response as correct, incorrect or microphone error (false starts, stutters or soft responses).
Simon Task
Participants were presented with a left or right pointing arrow and were asked to indicate which direction the arrow was pointing by pressing a key on the left or right side of the keyboard. On each trial, the arrow could appear in the middle, left or right side of the computer screen and participants were instructed to ignore the spatial location of the stimulus when making their response. Forty trials were congruent (the arrow direction was the same as the location on the screen), 40 incongruent (the arrow direction was opposite that of the screen) and 40 were neutral trials (the arrow was displayed in the center).
CVOE Task-Switching
Participants were presented with a letter-number stimulus (e.g., “A14) accompanied by one of two response cues. “CV” indicated they should classify the letter as either a consonant or a vowel and “OE” indicated they should classify the number as odd or even. During the switch block1, the cues alternated such that the response cue switched every two trials. Participants completed 60 trials consisting of 30 switch trials (where the response cue was the opposite from the previous trial) and 30 non-switch trials (where the response cue was the same as the previous trial). The stimulus and the cue appeared simultaneously and remained until a response was made. The next trial began immediately after each response.
Formation of the Composite Scores
The episodic memory composite was created by standardizing each participant's raw score on each task to the mean and standard deviation of the groups’ first time completing that particular test. The z-scores were then averaged together and a higher score indicated better performance. The attentional control composite was formed in the same way using accuracy from the incongruent trials only from each task2. These are trials that place the strongest demands on attentional control.
Cerebrospinal Fluid Assessment
CSF was collected using methods described previously (Fagan et al. 2007). After an overnight fasting period, 20-30 mL were collected and then alliquoted (500 μL) and stored at −84° C in polypropylene tubes. Analyses were conducted after a single thaw using ELISA (INNOTEST, Fujirebio [formerly Innogenetics], Ghent, Belgium). Scores were examined for outliers and one was identified and excluded from further analysis (tau level greater than 6 SDs from the group mean).
PET-PIB Imaging
Imaging methods have been fully described elsewhere (Su et al., 2013). A tissue mask for each region of interest was generated using Freesurfer segmentation (Fischl et al., 2004) and binding potentials were calculated using Logan graphical analysis with cerebellar gray matter as a reference region. A mean cortical binding potential (MCBP) was created by averaging across the following regions: left and right lateral orbitofrontal, inferior parietal, precuneus, rostral middle frontal, superior frontal, superior temporal, and middle temporal. After examining the distribution of MCBP, one individual was excluded as an outlier (score greater than 5 SDs from the group mean). It should be noted that this was the same individual who was excluded from the CSF analyses due to high tau.
Statistical Analysis
Each composite score was analyzed separately using the lme4 package in R (Bates, Machler, Bolker & Walker, 2014). Our model selection strategy proceeded in two steps. We first tested a model that included main effects of baseline age, family history (coded 0 for negative and 1 for positive), APOE genotype (coded 0 for absence of an ε4 allele and 1 for the presence of at least one allele), education, years in study (hereafter referred to as “time”) baseline CSF tau and Aβ42, the interaction between the CSF markers and time as well as the interaction between tau and Aβ42. These model terms were always retained because they serve as tests of our theoretically motivated hypotheses. In the second step, we added all additional two-way interactions between the covariates (e.g., age, APOE) with time in order to examine which other variables may moderate the longitudinal change. Any interaction terms from this step that were not significant were removed from the final model. We feel this procedure strikes the best balance between testing theoretical terms of interest (specifically, the interaction of CSF biomarkers with time) and forming overly complex statistical models that may capitalize on chance fluctuations in the data. A separate model was specified in the same manner that substituted the CSF markers with PET-PIB.
Degrees of freedom for the approximate t tests were calculated using the Satterthwaite method implemented in the “lmerTest package” (Kuznetsova, Brockhoff, & Christensen, 2014). All models included random intercepts and slopes for time across subjects unless otherwise noted. Finally, because the residuals were non-normally distributed, due to the skewness of the cognitive composites, for our inferential tests of the fixed effects, we calculated the standard errors from a non-parametric (case-resampling) bootstrap procedure. This approach is most robust to model misspecification compared with other bootstrapping techniques (Davison & Hinkley, 1997)3.
Results
Sample Characteristics
Demographic information on our sample is provided in Table 1. For descriptive purposes, we have indicated the proportion of individuals with “abnormal” Aβ42 and tau based on recently published cut offs (Vos et al. 2013) although we use the continuous measures for the current analyses.
Table 1.
Demographic characteristics, mean (SD) at initial assessment
| Variable | Mean (SD) |
|---|---|
| Age | 61.2 years (7.9) |
| Education | 16.1 years (2.5) |
| % FH positive | 57% |
| % APOE ε4 positive | 36% |
| CSF Tau | 242 pg / ml (105) |
| CSF Aβ42 | 668 pg / ml (242) |
| MCBP | .22 (.29) |
| Time from LP | .49 years (.55) |
| Time from PET scan | .53 years (.50) |
| Number of Assessments | 2.2 (1.6) |
| Time between Attention Assessments | 2.91 years (1.51) |
| Time between Memory Assessments | 2.97 years (1.63) |
| % Abnormal Aβ42 | 19% |
| % Abnormal Tau | 18% |
| Time in Study | 3.7 years (2.8) |
| MMSE | 29.2 (1.1) |
Note: FH = Family History, CSF = cerebrospinal fluid, MMSE = Mini Mental State Exam, LP = Lumbar Puncture, MCBP = mean cortical binding potential. Abnormal Aβ42 was defined as less than 459 pg / ml and abnormal tau was defined as greater than 339 pg / ml.
Attention Composite Analysis
In order to avoid the undue influence of extreme outliers, we first calculated the estimated slope of the attention score over time for each participant. We then eliminated any individual who exhibited a slope (i.e., decline over time) that was greater than 3 standard deviations from the average slope of the sample, which removed 4 participants4. These analyses included 233 participants and of those 170 completed at least one follow up cognitive assessment. Given our interest in both baseline and longitudinal differences, individuals with only one assessment (the baseline assessment) were included in order to provide a better estimate of initial differences. It is not problematic for individuals with only one observation to be included when data are analyzed within the multi-level modeling framework (Snijders & Bosker, 1999).
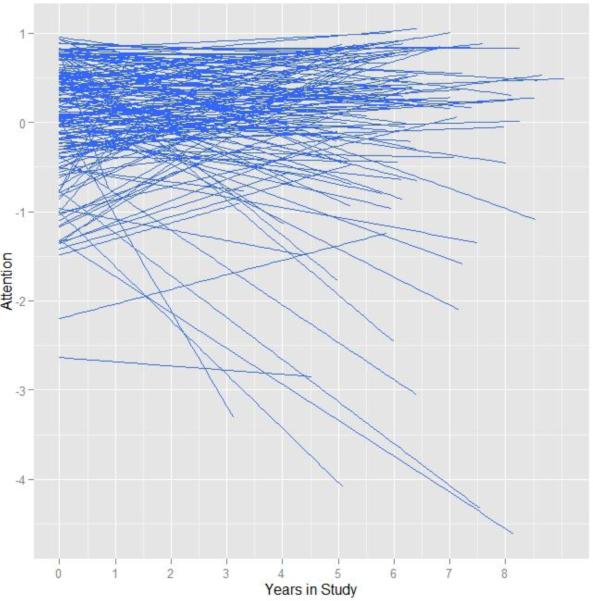
For descriptive purposes, we first calculated the intra-class correlation (ICC) from a random intercept only model to provide an estimate of between and within person variability. The ICC was .33 indicating that 33% of the variation in the attention composite was due to cross sectional differences, leaving 67% of the variability due to within person change. We next added fixed and random effects of time to assess the magnitude of individual differences in rate of change. The inclusion of random slopes for time provided a significant increment in model fit over the random intercepts only model (χ2(3) = 47.43, p = < .001), indicating substantial individual differences in rate of change. Figure 1 displays a spaghetti plot of the entire sample in order to visually convey these individual differences. The random effects confidence interval for time was −.192 to .164, which indicates that 95% of our sample was predicted to have a slope between those values.
Figure 1.
Spaghetti plots of individual predicted trajectories in the attention composite over time.
We next entered our predictor variables and their interactions in a single model as described above. The parameter estimates from this model are provided in Table 2.
Table 2.
Parameter estimates from the analysis of CSF and attentional control
| Variable | Estimate (SE)a | Degrees of Freedomb | t-valuec | p-value |
|---|---|---|---|---|
| Intercept | .0435 (.066) | 298.2 | .662 | 0.508 |
| Age | −.0578 (.040) | 231.6 | −1.449 | 0.149 |
| Education | .0332 (.018) | 339.4 | 1.823 | 0.069 |
| Time | −.0040 (.012) | 196.8 | −.328 | 0.743 |
| APOE genotype | −.0461 (.098) | 237.6 | −.470 | 0.639 |
| FH | .0446 (.081) | 245.6 | .549 | 0.583 |
| Tau | −.0943 (.072) | 335.4 | −1.304 | 0.193 |
| Aβ42 | .1383 (.046) | 331.5 | 2.975 | 0.003 |
| Education * Time | .0173 (.007) | 205.6 | 2.550 | 0.012 |
| Tau * Time | −.0308 (.015) | 186.4 | −2.068 | 0.040 |
| Aβ42 * Time | .0115 (.058) | 197.3 | .198 | 0.843 |
| Tau * Aβ42 | −.0106 (.014) | 233.4 | −.777 | 0.438 |
Note: Age and the CSF markers were standardized within the sample. Education was centered at 16 years (the average of the sample). Time reflects years since the initial assessment. APOE genotype was coded 0 for absence of and 1 for the presence of the ε4 allele. Family history was coded 0 for no history and 1 for positive history.
Standard error was calculated from the standard deviation of the bootstrapped distribution based on 5000 replications.
Degrees of Freedom estimated using the Satterthwaite method
Estimate divided by the bootstrapped standard error
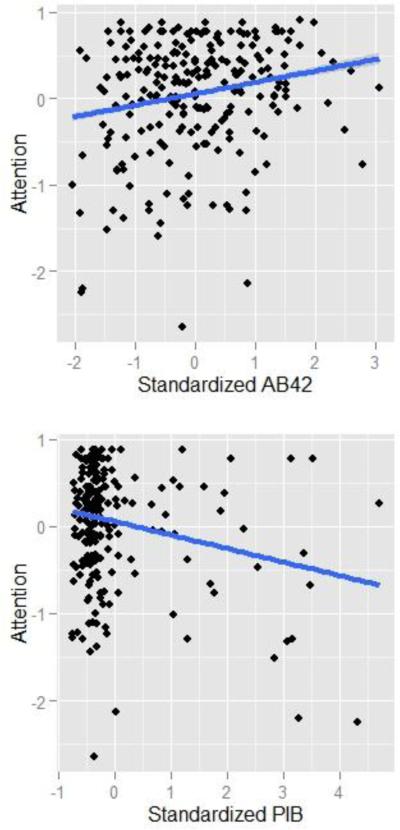
Importantly for the present work, baseline levels of CSF were significantly related to attention performance at the initial assessment such that individuals with lower levels of Aβ42 exhibited lower baseline performance (Figure 2, β = .14, p = .003). In contrast, tau levels at baseline did not correlate with concurrent performance although the effect was in the expected direction (β = −.09, SE = .072, p = .19). In addition, APOE genotype also did not predict attention performance, however our recent results have shown that the effect of APOE on cognitive performance is mediated by levels of Aβ42 (Aschenbrenner et al. 2015), thus APOE did not exert an influence above and beyond Aβ42 in these data.
Figure 2.
Relationship between cognition and Aβ42 (Top Panel) and PET-PIB (Bottom Panel) at baseline assessment.
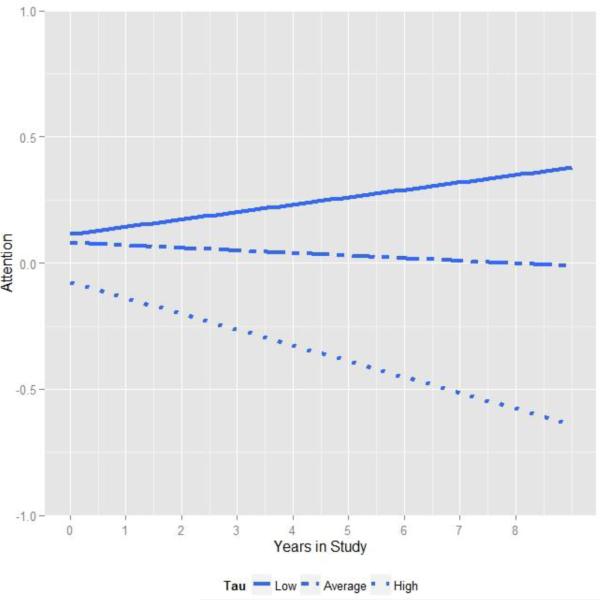
Importantly, turning to longitudinal change, our analysis revealed a significant interaction between tau and time, indicating that individuals with the highest levels of tau at baseline declined significantly on the attention composite over the course of the study. This relationship is depicted in Figure 3, which plots change in attention at three tertiles of tau. As shown, when tau is relatively high, the decline in the attention score was particularly dramatic. None of the other factors, in particular neither Aβ42 nor the tau by Aβ42 interaction, interacted with time indicating that longitudinal changes in the cognitive composites in the present sample were driven primarily by tau5.
Figure 3.
Relationship between attention and time in study varies as a function of baseline CSF tau. For visualization, regression lines are plotted at the lowest tertile of tau (solid line, less than 190 pg/ ml), middle tertile (dashed line) and highest tertile (dotted line, greater than 276 pg / ml).
The results of the second model, using PIB as a predictor rather than the CSF biomarkers, are presented in Table 3. The effect of PIB on baseline attention performance was statistically significant using a one-tailed test which is justified by the a priori predicted directionality of the effect (i.e. higher PIB values should impair cognition). Despite this, there was no detectable influence of PIB on longitudinal change.
Table 3.
Parameter estimates from the analysis of PIB and attentional control
| Variable | Estimate (SE)a | Degrees of Freedomb | t-valuec | p-value |
|---|---|---|---|---|
| Intercept | .107 (.071) | 255.12 | 1.51 | 0.132 |
| Age | −.0774 (.043) | 217.18 | −1.82 | 0.07 |
| Education | .0368 (.019) | 281.54 | 1.948 | 0.052 |
| Time | −.0126 (.014) | 156.11 | −0.884 | 0.378 |
| APOE genotype | −.09 (.093) | 228.18 | −.97 | 0.333 |
| FH | −.042 (.09) | 228.54 | −.468 | 0.64 |
| PIB | −.1161 (.066) | 276.02 | −1.763 | 0.079d |
| Education * Time | .0159 (.008) | 167.36 | 2.011 | 0.046 |
| PIB * Time | −.0111 (.019) | 152.34 | −.57 | 0.57 |
Note: Age and the CSF markers were standardized within the sample. Education was centered at 16 years (the average of the sample). Time reflects years since the initial assessment. APOE genotype was coded 0 for absence of and 1 for the presence of the ε4 allele. Family history was coded 0 for no history and 1 for positive history. PIB = Pittsburgh compound B
Standard error was calculated as the standard deviation of the bootstrapped distribution based on 5000 replications.
Degrees of Freedom estimated using the Satterthwaite method
Estimate divided by the bootstrapped standard error
This comparison is significant using a one-tailed test.
Episodic Memory Composite
The memory assessment was given separately from the attention assessment and the assessments were on average 53 days apart. Since we were interested in how episodic memory changes within the same time frame as the attention measures, we excluded 17 participants who received the episodic memory tests more than 1 year before or after the attention assessment. It should be noted that inclusion or exclusion of these participants had minimal effects on the outcomes. As before, we also screened for individuals with extreme slopes, which removed 5 individuals.
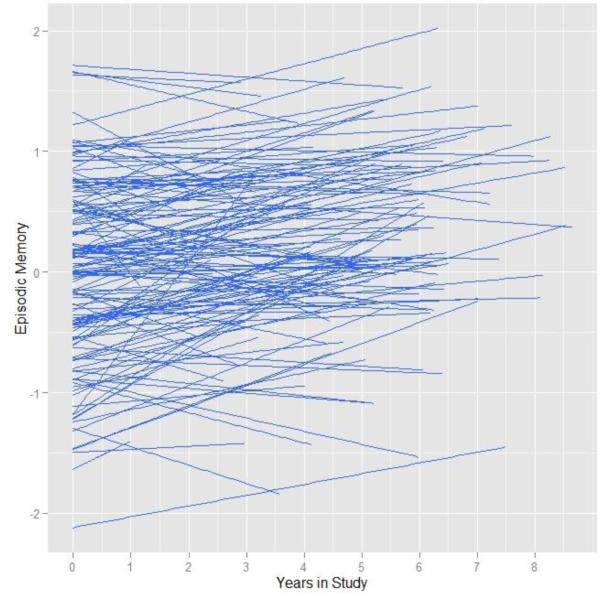
The intra-class correlation was .74 indicating the bulk of the variation was carried by cross-sectional differences. As shown in Figure 4, the overall trend was for scores on episodic memory to increase over time, which is consistent with a practice effect on certain episodic memory tasks (Galvin et al., 2005). This observation was confirmed by a simple model including fixed and random effects for time, which revealed a positive and significant slope over time, β = .051, SE = .007, p < .001, with a random effects confidence interval of 0 to .103.
Figure 4.
Spaghetti plots of individual predicted trajectories in the episodic memory composite over time.
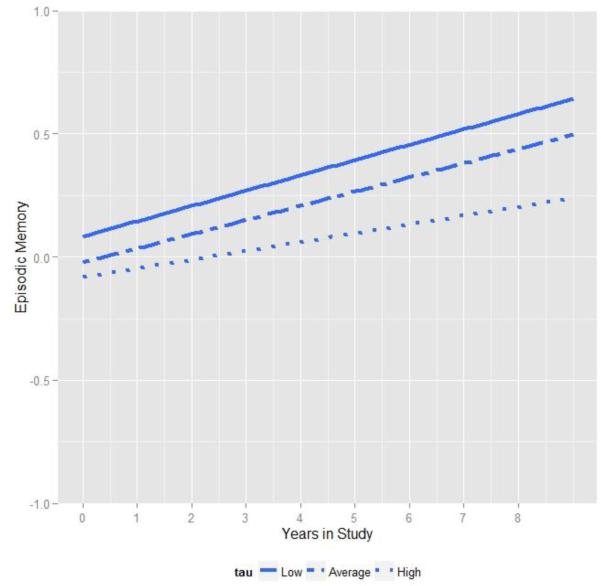
Parameters from a model that included all baseline risk factors and CSF are presented in Table 4. As shown, none of the risk factors including APOE, family history, tau or Aβ42 significantly correlated with baseline performance in episodic memory. However, in terms of the longitudinal changes, we again observed that levels of CSF tau were significantly related to the magnitude of change in memory performance over time depicted in Figure 5. As shown, although everyone increased in performance over the course of the study (likely due to practice effects), individuals with relatively high levels of baseline tau exhibited a slightly attenuated increase relative to the rest of the sample. As with the attention composite, the 3-way interaction among tau, Aβ42 and time was not significant.
Table 4.
Parameter estimates from the analysis of CSF and episodic memory
| Variable | Estimate (SE)a | Degrees of Freedomb | t-valuec | p-value |
|---|---|---|---|---|
| Intercept | −.0159 (.079) | 223.21 | −.202 | 0.840 |
| Age | −.0888 (.050) | 205.16 | −1.785 | .076 |
| Education | .0385 (.019) | 209.23 | 2.014 | .045 |
| Time | .053 (.007) | 97.73 | 7.104 | < .001 |
| APOE genotype | −.0151 (.116) | 203.58 | −0.13 | .896 |
| FH | .0526 (.100) | 209.45 | .524 | .601 |
| Tau | −.0243 (.064) | 217.16 | −.381 | .703 |
| Aβ42 | −.0489 (.058) | 220.19 | −.838 | .403 |
| Education * Time | .0006 (.003) | 104.92 | 1.95 | .054 |
| Tau * Time | −.0151 (.007) | 87.02 | −2.15 | .034 |
| Aβ42 * Time | .0063 (.008) | 106.14 | .756 | .451 |
| Tau * Aβ42 | .0886 (.052) | 203.74 | 1.708 | .089 |
Note: Age and the CSF markers were standardized within the sample. Education was centered at 16 years (the average of the sample). Time reflects years since the initial assessment. APOE genotype was coded 0 for absence of and 1 for the presence of the ε4 allele. Family history was coded 0 for no history and 1 for positive history.
Standard error was calculated from the standard deviation of the bootstrapped distribution based on 5000 replications.
Degrees of Freedom estimated using the Satterthwaite method
Estimate divided by the bootstrapped standard error
Figure 5.
Relationship between episodic memory and time in study varies as a function of baseline CSF tau. For visualization, regression lines are plotted at the lowest tertile of tau (solid line, less than 190 pg/ ml), middle tertile (dashed line) and highest tertile (dotted line, greater than 276 pg / ml).
Table 5 lists the parameters from the model that included PIB. Similar to the attention analysis, PIB did not significantly predict baseline performance nor longitudinal change.
Table 5.
Parameter estimates from the analysis of PIB and episodic memory
| Variable | Estimate (SE)a | Degrees of Freedomb | t-valuec | p-value |
|---|---|---|---|---|
| Intercept | .004 (.079) | 205.48 | .050 | 0.480 |
| Age | −.092 (.051) | 192.88 | −1.79 | .038 |
| Education | .049 (.019) | 191.56 | 2.53 | .0006 |
| Time | .048 (.007) | 285.74 | 6.601 | < .001 |
| APOE genotype | .023 (.116) | 195.46 | .198 | .422 |
| FH | .036 (.103) | 196.2 | .349 | .364 |
| PIB | 0.066 (.059) | 202.25 | 1.12 | .132 |
| PIB * Time | −.011 (.009) | 280.26 | −1.25 | .106 |
Note: Age and the CSF markers were standardized within the sample. Education was centered at 16 years (the average of the sample). Time reflects years since the initial assessment. APOE genotype was coded 0 for absence of and 1 for the presence of the ε4 allele. Family history was coded 0 for no history and 1 for positive history. PIB = Pittsburg compound B
Standard error was calculated as the standard deviation of the bootstrapped distribution based on 5000 replications.
Degrees of Freedom estimated using the Satterthwaite method
Estimate divided by the bootstrapped standard error
Discussion
The primary goals of the present report were to provide a systematic evaluation of cross-sectional differences in two cognitive constructs as a function of AD pathology measured with well-established biomarkers, and more importantly, to analyze whether baseline biomarkers would predict the magnitude of cognitive change over time. Our results returned two primary findings. First, baseline Aβ42 was correlated with concurrent attention performance consistent with our prior research. Second, levels of tau in the CSF significantly predicted longitudinal change in both the attention and episodic memory composites. We discuss each of these issues in turn.
Individuals who exhibit slight deficits on sensitive cognitive measures, in addition to possessing indicators of AD pathology are possibly at the greatest risk of developing symptomatic AD. This is supported by Vos et al. (2013) who used a composite score of 3 episodic memory tests to define stage 3 of preclinical AD and showed the rate of progression to dementia was greatest for individuals in stage 3 over a 5-year period. Past literature has focused to a large extent on episodic memory performance given that brain regions that underlie episodic memory retrieval appear particularly vulnerable to the accumulation of AD pathology (e.g., Buckner et al., 2005). However, a growing body of evidence suggests that measures of attentional control are also sensitive cognitive markers and indeed the relationship between memory and attention processes has already been well-established (Balota & Duchek, 2015; Craik & Lockhart, 1972).
Our attention composite was highly related to levels of Aβ42 at baseline assessment. Although past results using attentional control measures (Aschenbrenner et al., 2015) showed sensitivity to both CSF markers separately, we did not replicate the relationship with tau in the present sample. This is possibly because our sample consists of individuals who are younger (mean age = 61 years) than our past report (mean age = 68 years). Concurrent tau-cognition relationships are possibly more likely to be detected in older individuals, who are further along in the disease process.
The attention composite was also related to levels of PIB at baseline when using a one-tailed test. In contrast, the episodic memory composite score was not reliably related to either the CSF markers or the PIB measure at the initial assessment in this sample. This is concordant with the literature in showing that relationships between episodic memory and concurrent AD biomarkers are subtle and difficult to detect (Hedden et al., 2013). Whether our inability to detect a concurrent relationship between amyloid and memory is due to differences in power or the relatively younger age range of our sample remains to be determined. However, it is interesting to note that the relationship with Aβ42 was significant for attentional control and larger than the same relationship with episodic memory (z = 2.53, p < .05) in the present sample. Thus, in order to stage individuals within the preclinical AD continuum according to recent recommendations (Sperling et al., 2011), attentional control measures may serve as a more promising target for defining stage 3 “subtle cognitive impairment”, and should be considered in cognitive assessment batteries. Clearly, larger samples are important to better establish this possibility.
Most importantly, both the attention and episodic memory composite exhibited changes in longitudinal trajectories that were related to baseline tau. Specifically, individuals with high levels of tau exhibited robust declines in attention relative to the rest of the cohort. Similarly, although the net result was for episodic memory to increase over time, individuals with high tau levels exhibited an attenuated increase in performance over the course of the study. As previously mentioned, the majority of studies to date have examined the relationship of PET-PIB to longitudinal changes in cognition and this study is important in extending those findings to CSF markers.
In contrast to tau, neither Aβ42 nor PET-PIB predicted longitudinal change in either cognitive composite. It is particularly noteworthy that we did not detect a relationship between PIB and cognitive decline because these two measures have been shown to be correlated in past reports even in cognitively healthy controls (Doraiswamy et al., 2012; Ellis et al., 2013; Storandt et al., 2009). One possible reason for this discrepancy is, as noted, the present sample is relatively young compared to previous studies. The three studies cited above, for example, examined individuals who were in their early to mid seventies.
Prior research has also provided evidence for a relationship between CSF markers and cognitive decline (e.g., Fagan et al. 2007; Roe et al. 2013). It is interesting to note that the best predictor is often the tau / Aβ42 ratio which conflates the two main effects as well as the interaction between these two biomarkers. Indeed, when we examined the ratio in the present data, the results showed a reliable effect on baseline performance as well as on longitudinal change in attentional control (β = −.11, p = .009). However, when we decompose the ratio into the constituent parts (as we did in the reported analyses) the baseline differences were driven by Aβ42 and the longitudinal effect by tau. This is consistent with recent evidence modeling the temporal changes in CSF biomarkers which suggests that Aβ42 plateaus while individuals are still in the asymptomatic stage of AD but tau continues to increase into mild cognitive impairment (Bertens, Knol, Scheltens, & Visser, 2014). Thus, it may be the continued increase of tau throughout the early stages of the disease that ultimately drives cognitive decline. Our results are also consistent with a temporal ordering of AD biomarkers, in which Aβ42 becomes abnormal first (and drives the baseline correlation with attentional control), followed by amyloid deposition measured with PET-PIB followed by changes in tau (which drives longitudinal change across multiple cognitive domains). Clearly, this interpretation is post-hoc and further modeling efforts should strive to test these hypotheses more directly.
It is important to consider these findings within the framework of neurobiological changes that occur in early and pre-clinical AD. As mentioned, focus is often devoted to medial temporal regions, but substantial PIB deposition appears in prefrontal and parietal regions (Klunk et al., 2004; Mintun et al., 2006), regions that are often implicated in attention based tasks (e.g., Banich et al., 2000; Kane & Engle, 2002; Vanderhasselt, De Raedt, & Baeken, 2009). Indeed, accumulation of biomarkers have functional and behavioral consequences for these regions even in otherwise cognitively healthy adults (Duchek et al., 2013; Gordon et al. 2015). Given that attention is heavily linked to episodic memory processes (McCabe, Roediger, McDaniel, Balota, & Hambrick, 2010), it is possible that early disruption of attention systems can be detected at the same time or potentially before substantial accumulation of pathology leads to clinically detectable effects on memory.
Despite its strengths, there are a few limitations to this study that should be noted. We performed a large number of statistical comparisons without including any correction to p-values. We addressed this issue by testing only theoretically justified higher-order interactions involving time in study and utilizing composite scores of cognition rather than individual test scores. In addition, many of our participants had only two assessment points which may have limited our power. As more data and assessments become available, future work should carefully address the shape of trajectories (e.g., linear vs. quadratic) as well as testing for the presence of higher order interactions among the targeted variables. Furthermore, future studies should also consider correlated age-related changes such as lewy body accumulation6. Finally, CSF biomarkers and PET PIB measures have differential reliability (Mattsson et al. 2011; Su et al. 2013), and because of this, one should be cautious in arguing for a null effect of Aβ42 and PIB on cognitive change.
Conclusion
AD biomarkers have the potential to provide critical insight into the preclinical AD process and to help stage individuals based on relative risk of developing dementia. CSF markers predict concurrent deficits in attentional control as well as longitudinal declines in both attention and memory. Both attentional control measures and episodic memory provide insight into the cognitive consequences of preclinical AD and should continue to be evaluated as sensitive screening instruments for preclinical staging as well as cognitive endpoints for treatment outcomes. Future work should be conducted with increased emphasis on longitudinal change in these cognitive constructs to best characterize the preclinical AD profile.
Acknowledgements
This research was supported by the National Institute on Aging grants T32 AG000030 and P01-AG26276, National Institute of Neurological Disorders and Stroke grant 5P30NS048056, and Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS). We thank our participants and the Clinical Core of the Knight Alzheimer Disease Research Center at Washington University for providing clinical evaluations.
Footnotes
This task also included “pure” blocks in which participants only made consonant-vowel decision or odd-even decisions. We do not consider these trials in the present composite because it is unlikely to rely on attentional control mechanisms to the same extent as the switch block.
For the attention composite, we also evaluated the efficacy of a response latency measure. We formed a similar composite as the accuracy measure but utilizing latencies rather than accuracy scores. This measure did not exhibit any sensitivity to AD biomarkers which is consistent with Balota et al. (2010) in demonstrating that accuracy measures tend to be more sensitive indicators of preclinical AD pathology.
Another common technique for dealing with non-normal residuals is to apply a nonlinear transformation of the dependent variable. We did so, using a natural log transform, which substantially improved the normality of the residuals. However, because this technique yielded the same inferences as the bootstrap method, we choose only to present the bootstrap results in order to maintain the interpretability of the original scale (as opposed to making inferences on the transformed scale).
We also examined other outlier screening strategies including removing participants who exhibited an attention score above 5 SDs from the group mean at any test point, removing only the particular observations that were greater than 5 SDs from the mean, as well as no screening at all. Importantly, results from all techniques were qualitatively identical.
Although we did not detect the 3-way interaction among tau, Aβ42 and time, we conducted an exploratory analysis of the influence of tau at high and low levels of Aβ42 defined using a mean split. Although the tau by time interaction was not significant at either level of Aβ42, the beta weight was numerically larger at low levels of Aβ42 (β = −.033, p = .11) compared to high Aβ42 (β = −.023, p = .22). Given the reduction in sample size due to the mean split, these analyses should be considered descriptive only.
One such age-related disease is cerebrovascular disease. We had several measures of cerebrovascular health available on these participants. First, as part of the clinical assessment, participants report occurrence of stroke or transient ischemic attack. In our sample, 3 reported TIA and 5 reported stroke. We eliminated these participants and re-ran our analyses and the same pattern of significant effects was found. Second, for 143 participants we had information of white matter hyperintensities (WMHs). We examined the correlation between WMH and CSF tau and found no relationship after controlling for age. Furthermore, there was no evidence of a time × WMH × tau interaction, although with a limited sample we may be underpowered to detect that effect.
The authors have no conflicts of interest to declare.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of Neurology. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. doi:10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner AJ, Balota DA, Tse C-S, Fagan AM, Holtzman DM, Benzinger TLS, Morris JC. Alzheimer disease biomarkers, attentional control, and semantic memory retrieval: Synergistic and mediational effects of biomarkers on a sensitive cognitive measure in non-demented older adults. Neuropsychology. 2015 doi: 10.1037/neu0000133. http://doi.org/10.1037/neu0000133. [DOI] [PMC free article] [PubMed]
- Balota DA, Duchek JM. Remembering: Attributions, Processes, and Control in Human Memory. Psychology Press; New York: 2015. Attention, Variability, and Biomarkers in Alzheimer's Disease. pp. 285–303. [Google Scholar]
- Balota DA, Faust M. Attention in dementia of the Alzheimer's type. Handbook of Neuropsychology. 2001;6:51–80. [Google Scholar]
- Balota DA, Tse C-S, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer's type in a healthy control sample: The power of errors in stroop color naming. Psychology and Aging. 2010;25:208–218. doi: 10.1037/a0017474. doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Magin R. fMri studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of cognitive neuroscience. 2000;12 doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. New England Journal of Medicine. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–7. 2014 http://CRAN.R-project.org/packagelme4.
- Bertens D, Knol DL, Scheltens P, Visser PJ. Temporal evolution of biomarkers and cognitive markers in the asymptomatic, MCI, and dementia stage of Alzheimer's disease. Alzheimer's & Dementia. 2014 doi: 10.1016/j.jalz.2014.05.1754. doi: 10.1016/j.jalz.2014.05.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. doi:10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ. Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer's disease: Evidence for disproportionate selection impairments in the Simon task. Neuropsychology. 2007;21:170–182. doi: 10.1037/0894-4105.21.2.170. doi: 10.1037/0894-4105.21.2.170. [DOI] [PubMed] [Google Scholar]
- Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Holtzman DM. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biological Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Davison AC, Hinkley DV. Bootstrap Methods and Their Application. Cambridge University Press; 1997. [Google Scholar]
- Delacourte A, Sergeant N, Champain D, Wattez A, Maurage C-A, Lebert F, David J-P. Nonoverlapping but synergetic tau and APP pathologies in sporadic Alzheimer's disease. Neurology. 2002;59(3):398–407. doi: 10.1212/wnl.59.3.398. [DOI] [PubMed] [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, Dale AM. Amyloid-β–associated clinical decline occurs only in the presence of elevated p-tau. Archives of Neurology. 2012;69(6):709–713. doi: 10.1001/archneurol.2011.3354. http://doi.org/10.1001/archneurol.2011.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD, Pontecorvo MJ. Amyloid- assessed by florbetapir F 18 PET and 18-month cognitive decline: A multicenter study. Neurology. 2012;79:1636–1644. doi: 10.1212/WNL.0b013e3182661f74. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Thomas JB, Snyder AZ, Rich P, Benzinger TLS, Ances BM. Relationship between Stroop performance and resting state functional connectivity in cognitively normal older adults. Neuropsychology. 2013;27(5):516–528. doi: 10.1037/a0033402. doi: 10.1037/a0033402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Tse C-S, Holtzman DM, Fagan AM, Goate AM. The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer's disease. Neuropsychology. 2009;23(6):746–758. doi: 10.1037/a0016583. doi: 10.1037/a0016583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KA, Lim YY, Harrington K, Ames D, Bush AI, Darby D, Maruff P. Decline in cognitive function over 18 months in healthy older adults with high amyloid-β. Journal of Alzheimer's Disease. 2013;34(4):861–871. doi: 10.3233/JAD-122170. [DOI] [PubMed] [Google Scholar]
- Ewers M, Insel P, Jagust WJ, Shaw L, Trojanowski J JQ, Aisen P, Weiner MW. CSF biomarker and PIB-PET-derived beta-amyloid signature predicts metabolic, gray matter, and cognitive changes in nondemented subjects. Cerebral Cortex. 2012;22(9):1993–2004. doi: 10.1093/cercor/bhr271. http://doi.org/10.1093/cercor/bhr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, Holtzman DM. Cerebrospinal fluid tau and ptau 181 increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer's disease. EMBO Molecular Medicine. 2009;1:371–380. doi: 10.1002/emmm.200900048. http://doi.org/10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Archives of Neurology. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. http://doi.org/10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Fischl B. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Powlishta KK, Wilkins K, McKeel DW, Xiong C, Grant E, Morris JC. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Archives of Neurology. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- Glodzik L, de Santi S, Tsui WH, Mosconi L, Zinkowski R, Pirraglia E, de Leon MJ. Phosphorylated tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiology of Aging. 2011;32(12):2131–2141. doi: 10.1016/j.neurobiolaging.2009.12.026. http://doi.org/10.1016/j.neurobiolaging.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Zacks JM, Blazey T, Benzinger TLS, Morris JC, Fagan AM, Balota DA. Task-evoked fMRI changes in attention networks are associated with preclinical Alzheimer's disease biomarkers. Neurobiology of Aging. 2015 doi: 10.1016/j.neurobiolaging.2015.01.019. http://doi.org/10.1016/j.neurobiolaging.2015.01.019. [DOI] [PMC free article] [PubMed]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for demenita by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychonomic Bulletin & Review. 2002;9(4):637–671. doi: 10.3758/bf03196323. http://doi.org/10.3758/BF03196323. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Långström B. Imaging Brain Amyloid in Alzheimer's Disease with Pittsburgh Compound-B. Annals of Neurology. 2004;55(3):306–319. doi: 10.1002/ana.20009. http://doi.org/10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerT-est: Tests for random and fixed effects for linear mixed effects models (lmer objects of lme4 package). R Package Version 2.0 –11. 2014 http://CRAN.R-project.org/packagelmerTest.
- Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, Jagust WJ. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Annals of Neurology. 2012;72(4):578–586. doi: 10.1002/ana.23650. http://doi.org/10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Millard SP, Peskind ER, Zhang J, Yu C-E, Leverenz JB, Montine TJ. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. JAMA Neurology. 2014;71(6):742–751. doi: 10.1001/jamaneurol.2014.445. http://doi.org/10.1001/jamaneurol.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Montine TJ. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment A follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- Lim YY, Pietrzak RH, Ellis KA, Jaeger J, Harrington K, Ashwood T, Maruff P. Rapid decline in episodic memory in healthy older adults with high amyloid-β. Journal of Alzheimer's Disease. 2013;33:675–679. doi: 10.3233/JAD-2012-121516. doi: 10.3233/JAD-2012-121516. [DOI] [PubMed] [Google Scholar]
- Lo RY, Hubbard AE, Shaw LM, Trojanowski JQ, Peterson RC, Aisen PS, Jagust WJ. Longitudinal change of biomarkers in cognitive decline. Archives of Neurology. 2011;68(10):1257–1266. doi: 10.1001/archneurol.2011.123. http://doi.org/10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Persson S, Arai H, Batish SD, Bernardini S, Blennow K. The Alzheimer's Association external quality control program for cerebrospinal fluid biomarkers. Alzheimer's & Dementia. 2011;7:386–395e6. doi: 10.1016/j.jalz.2011.05.2243. doi:10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology. 2010;24(2):222–243. doi: 10.1037/a0017619. http://doi.org/10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, LaRossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Morris JC. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. doi:10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Storandt M, Rubin EH, Price JL, Grant EA, Berg L. Very mild Alzheimer's disease: Informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991;41:469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- Mungas D, Tractenberg R, Schneider JA, Crane PK, Bennett DA. A 2-process model for neuropathology of Alzheimer's disease. Neurobiology of Aging. 2014;35:301–308. doi: 10.1016/j.neurobiolaging.2013.08.007. http://doi.org/10.1016/j.neurobiolaging.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebes RD, Snitz BE, Cohen AD, Aizenstein HJ, Saxton JA, Halligan EM, Klunk WE. Cognitive aging in persons with minimal amyloid-β and white matter hyperintensities. Neuropsychologia. 2013;51:2202–2209. doi: 10.1016/j.neuropsychologia.2013.07.017. http://doi.org/10.1016/j.neuropsychologia.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease: A critical review. Brain: A Journal of Neurology. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Buckles VD, Roe CM, Xiong C, Grundman M, Morris JC. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiology of Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. http://doi.org/10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, Wong DF. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Rieck JR, Hebrank AC, Diaz-Arrasita R, Park DC. B-Amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Dreyfus DM, Morris JC. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Rolstad S, Berg AI, Bjerke M, Johansson B, Zetterberg H, Wallin A. Cerebrospinal fluid biomarkers mirror rate of cognitive decline. Journal of Alzheimer's Disease. 2013;34:949–956. doi: 10.3233/JAD-121960. doi: 10.3233/JAD-121960. [DOI] [PubMed] [Google Scholar]
- Simon JR. Reactions toward the source of stimulation. Journal of Experimental Psychology. 1969;81:174–176. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel Analysis: A introduction to basic and advanced multilevel modeling. SAGE Publications; 1999. [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. http://doi.org/10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, Pontecorvo MJ. Amyloid deposition detected with florbetapir F 18 (18FAV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiology of Aging. 2013;34:822–831. doi: 10.1016/j.neurobiolaging.2012.06.014. http://doi.org/10.1016/j.neurobiolaging.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer's type. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. http://doi.org/10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Archives of Neurology. 2010;67(2):217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- Storandt M, Head D, Fagan AM, Holtzman DM, Morris JC. Toward a multifactorial model of Alzheimer disease. Neurobiology of Aging. 2012;33:2262–2271. doi: 10.1016/j.neurobiolaging.2011.11.029. http://doi.org/10.1016/j.neurobiolaging.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Hill RD. Very mild senile dementia of the Alzheimer type II: Psychometric test performance. Archives of Neurology. 1989;46:383–386. doi: 10.1001/archneur.1989.00520400037017. [DOI] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-β peptide deposition identified with Pittsburgh compound B. Archives of Neurology. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. doi:10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. http://doi.org/10.1037/h0054651. [Google Scholar]
- Su Y, D'Angelo GM, Vlassenko AG, Zhou G, Snyder AZ, Marcus DS, Benzinger TLS. Quantitative analysis of PiB-PET with Freesurfer ROIs. PLoS One. 2013;8:e73377. doi: 10.1371/journal.pone.0073377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C-S, Balota DA, Yap MJ, Duchek JM, McCabe DP. Effects of healthy aging and early stage dementia of the Alzheimer's type on components of response time distributions in three attention tasks. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. doi:10.1037/a0018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SAL, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. Journal of the International Neuropsychological Society. 2006;12:707–735. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhasselt M-A, De Raedt R, Baeken C. Dorsolateral prefrontal cortex and Stroop performance: tackling the lateralization. Psychonomic Bulletin & Review. 2009;16(3):609–612. doi: 10.3758/PBR.16.3.609. http://doi.org/10.3758/PBR.16.3.609. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Jack CR. MRI and CSF biomarkers in normal, MCI, and AD subjects Diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. doi:10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chételat G, Ellis KA, Mulligan RS, Bourgeat P, Rowe CC. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Annals of Neurology. 2011;69(1):181–192. doi: 10.1002/ana.22248. http://doi.org/10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Fagan AM. Preclinical Alzheimer's disease and its outcome: A longitudinal cohort study. The Lancet Neurology. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual: Wechsler Memory Scale-Revised. Psychological Corporation; San Antonio, Texas: 1987. [Google Scholar]
- Wechsler D, Stone CP. Manual: Wechsler Memory Scale. Psychological Corporation; New York: 1973. [Google Scholar]
- Xiong C, Roe CM, Buckles V, Fagan A, Holtzman D, Balota D, Morris JC. Role of family history for Alzheimer biomarker abnormalities in the Adult Children Study. Archives of Neurology. 2011;68(10):1313–1319. doi: 10.1001/archneurol.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]