Abstract
Purpose
The integrity of selective attention in people with aphasia (PWA) is currently unknown. Selective attention is essential for everyday communication, and inhibition is an important part of selective attention. This study explored components of inhibition—both intentional and reactive inhibition—during spoken-word production in PWA and in controls who were neurologically healthy (HC). Intentional inhibition is the ability to suppress a response to interference, and reactive inhibition is the delayed reactivation of a previously suppressed item.
Method
Nineteen PWA and 20 age- and education-matched HC participated in a Stroop spoken-word production task. This task allowed the examination of intentional and reactive inhibition by evoking and comparing interference, facilitation, and negative priming effects in different contexts.
Results
Although both groups demonstrated intentional inhibition, PWA demonstrated significantly more interference effects. PWA demonstrated no significant facilitation effects. HC demonstrated significant reverse facilitation effects. Neither group showed significant evidence of reactive inhibition, though both groups showed similar individual variability.
Conclusions
These results underscore the challenge interference presents for PWA during spoken-word production, indicating diminished intentional inhibition. Although reactive inhibition was not different between PWA and HC, PWA showed difficulty integrating and adapting to contextual information during language tasks.
Aphasia affects more than 1 million stroke and head injury survivors in the United States, and these survivors face daily life with debilitating impairments in communication. Aphasia has traditionally been defined as a loss of language representations (Jenkins, Jiménez-Pabón, Shaw, & Sefer, 1975). However, this view does not account for several language performance characteristics typically seen in individuals with aphasia (McNeil, 1982), including marked variability and susceptibility to manipulation (e.g., cueing and priming). For example, a person with aphasia (PWA) may not be able to name pencil one morning but does so easily a few hours later or once background noise has been eliminated. The semantic, lexical, or phonological representations for pencil are clearly not lost or deleted from memory, but the word-finding problem appears to relate to the processes used for constructing online representations needed to retrieve or produce the item. Therefore, what might account for the individual variability in language performance in PWA?
Attention, considered a variable and flexible resource for processing (Baddeley, 1993; Cowan, 1988; Kane & Engle, 2003), may be misallocated or diminished in aphasia and therefore impedes the process of building language representations (Hula & McNeil, 2008; McNeil, Odell, & Tseng, 1991; Murray, 2012; Tseng, McNeil, & Milenkovic, 1993). Attention and short-term memory are considered the two chief components of working memory (Engle, Tuholski, Laughlin, & Conway, 1999), or the ability to maintain and manipulate information for more complex cognitive processing. Within this framework, attention is theorized to increase the activation of relevant information and to actively inhibit irrelevant information and is allocated variably, depending on task context and the surrounding environment (Cohen, Dunbar, & McClelland, 1990; A. R. A. Conway & Engle, 1994; Cowan, 1988; Houghton & Tipper, 1994; Houghton, Tipper, Weaver, & Shore, 1996; Kane & Engle, 2003; Miyake et al., 2000). If attention is misallocated or otherwise limited, relevant inputs may not be activated and irrelevant inputs may not be suppressed, thus interfering with target language processes. Furthermore, items with strong activation pathways are believed to activate more automatically and require less attention, whereas weaker pathways may require more attention in order to reach activation threshold (Cohen et al., 1990).
Studies have shown that language performance when interference is present is significantly slowed in PWA relative to controls who are neurologically healthy (HC). For example, PWA perform significantly more slowly during picture naming while concurrently discriminating high and low tones compared with picture naming alone (e.g., Lim, McNeil, Doyle, Hula, & Dickey, 2012; Martin & Allen, 2008; Murray, Holland, & Beeson, 1997, 1998), and the difference between these experimental conditions is significantly exaggerated in PWA compared with HC. In other words, PWA appear to have exaggerated interference effects or slowing relative to HC when comparing a language task that includes interference with a stand-alone language task. These exaggerated interference effects for PWA may indicate a diminished capacity to inhibit irrelevant information compared with HC due to poorly controlled attention allocation (Hamilton & Martin, 2005; McNeil, Hula, & Sung, 2010; Wiener, Conner, & Obler, 2004; see Martin & Allen, 2008, for a review). Inhibition is generally understood to be the suppression of an action, behavior, or state of being (Dictionary.com, n.d.). According to Miyake et al. (2000), the construct of inhibition as it relates to cognition can be fragmented into several components, including intentional and reactive inhibition, both of which require varying degrees of attention.
Intentional Inhibition
Intentional inhibition is important when one is faced with simultaneous inputs. Intentional inhibition is evident when a more dominant, automatically activated stimulus (distractor) must be deliberately overcome to process a simultaneously presented weaker target. The resulting response is slowed and is called an interference effect, as previously described. Cohen et al. (1990; also see West & Alain, 1999) described separate activation pathways that respond to simultaneously presented stimuli as automatic (with assumed greater activation strength) and less automatic (less activation strength) pathways. By allocating more attention to the weaker, less automatic relevant pathway, activation of that pathway is boosted, allowing for greater focus on the input. At the same time, attention can be allocated to also actively inhibit the nontarget pathways (Cowan, 1988; Kane & Engle, 2003).
Intentional inhibition is frequently explored using the Stroop paradigm (Stroop, 1935), often presented as a color-word task.1 In this task, the participant is asked to name the ink or font color of an orthographically presented color word in three general conditions: congruent, incongruent, and neutral. In a congruent trial, the word and font color match (e.g., the word red in a red font) and the participant is expected to say “red.” In this condition, the weakly activated font color (red) receives a boost by the automatic activation of the word (red), as evidenced by faster response times compared with the neutral (control) condition. This boost in activation, called facilitation, is believed to require a relatively small degree of attention. In an incongruent trial, the word and font color are mismatched (e.g., the word red in a green font); the participant is expected to say “green.” In this condition, the automatically activated (prepotent) word (red) must be suppressed in favor of the more weakly activated font color (green), resulting in slower response times relative to the neutral (control) condition in which the participant names the color of symbols (e.g., %) or polygons (e.g.,  ; Dalrymple-Alford & Budayr, 1966). This is believed to be a more attention-demanding condition. In order to determine effects of intentional inhibition, conditions are compared; that is, the incongruent versus neutral condition assesses interference effects. The word and the color did not match; thus, the word interfered with the naming of the color. The congruent versus neutral condition assesses facilitation effects. The word and the color matched, thereby boosting the correct response (Verhaeghen & De Meersman, 1998). Once the more dominant color word has been suppressed in favor of the more weakly activated font color, the just-suppressed representation is not immediately available for reactivation; this phenomenon is known as reactive inhibition.
; Dalrymple-Alford & Budayr, 1966). This is believed to be a more attention-demanding condition. In order to determine effects of intentional inhibition, conditions are compared; that is, the incongruent versus neutral condition assesses interference effects. The word and the color did not match; thus, the word interfered with the naming of the color. The congruent versus neutral condition assesses facilitation effects. The word and the color matched, thereby boosting the correct response (Verhaeghen & De Meersman, 1998). Once the more dominant color word has been suppressed in favor of the more weakly activated font color, the just-suppressed representation is not immediately available for reactivation; this phenomenon is known as reactive inhibition.
Reactive Inhibition
Houghton et al. (1996) purported that reactive inhibition occurs after a distractor is actively suppressed. This suppression causes the representation to enter into a state below threshold, and the representation is not immediately available for reactivation and selection. If reactivation occurs, the resulting response is slowed. The reactive inhibition phenomenon is often tested using a negative priming experimental protocol, wherein the distractor on the first (prime) trial becomes the target on the second (probe) trial, also called repeated interference. One typically responds more slowly to a target stimulus that has just served as a distractor stimulus, creating a negative priming effect (Dalrymple-Alford & Budayr, 1966; Houghton & Tipper, 1994).
Working Memory and Impaired Inhibition
Studies of inhibitory function from within the theoretical framework of working memory have reported evidence of a relationship between diminished working memory capacity and diminished inhibitory function. That is, there are individual differences in inhibitory function related to the availability of cognitive resources (i.e., attention) for working memory. The greater the working memory capacity, the greater the inhibitory function (Grandjean & Collette, 2011; Kane & Engle, 2003). These findings augment the previous reports of Jonides and Nee (2006), who explored the neural mechanisms involved in intentional inhibition and its connection to working memory. The authors interpreted their research findings as evidence that people with limited working memory have greater difficulty in the presence of interference (A. R. A. Conway & Engle, 1994; Just & Carpenter, 1992). PWA are hypothesized to have deficits in working memory and difficulty resolving interference, as evidenced by studies of syntactic comprehension (e.g., Fassbinder et al., 2011), reading comprehension (e.g., McNeil et al., 2011), and listening comprehension (e.g., Wright, Downey, Gravier, Love, & Shapiro, 2007; see Wright & Fergadiotis, 2012, for a review).
Evidence of Impaired Attention in Aphasia
The markedly slower and less accurate responses for PWA in the presence of interference, compared with responses to stimuli presented without interference, have been interpreted by many as an indicator of limited or misallocated attention. Impairments in directing attention in PWA have been documented both behaviorally (e.g., Hunting Pompon, Kendall, & Moore, 2011) and electrophysiologically (e.g., Peach, Rubin, & Newhoff, 1994). Other studies have focused on interference effects in comprehension (e.g., Lim et al., 2012; Wiener et al., 2004) and word-retrieval tasks, often using a dual-task paradigm (e.g., Murray et al., 1997, 1998), and have concluded that, when attentional resources are divided for PWA, fewer attentional resources are available for managing interference and inhibiting distractions. In other words, if attention is limited in quantity or misallocated in its deployment in PWA, target language processes, such as retrieving the correct lexical item during conversation, may become challenging or impossible due to the presence of competing stimuli or increased task demands.
Some studies of PWA have attributed difficulty with language processes in the presence of distraction to diminished inhibition. For example, Wiener et al. (2004) examined interference and auditory comprehension in participants with Wernicke's aphasia using a numeric Stroop task with a manual response. Compared with HC, PWA demonstrated equivalent facilitation effects but significantly larger interference effects. The authors concluded that people with Wernicke's aphasia may not be able to adequately inhibit the automatically evoked items in favor of the less automatic items presented in the interference condition. These results are similar to those reported by Lim et al. (2012) in their reports of a picture-word interference task. However, the authors of these studies did not explicitly explore both intentional and reactive inhibition.
McNeil et al. (2010) studied interference and facilitation effects in PWA and HC using a Stroop task within the Computerized Revised Token Test–Reading (CRTT-R; McNeil et al., 2008). Overall, between-groups analyses showed that PWA were significantly slower and less accurate compared with HC and that they demonstrated greater interference effects. It is important to note that there was no interaction between the regular reading and neutral conditions when examining reading time, which was interpreted to indicate little to no evidence of slowed activation of the lexical representation for PWA. In addition, both groups showed significant facilitation effects when comparing the congruent and neutral conditions. Taken together, these results may indicate diminished inhibition in PWA, but components of inhibition were not addressed specifically.
Although interference is almost certainly a challenge for PWA, impairments in inhibition have been hypothesized but not studied systematically. To further understand the role of and potential limits in selective attention during spoken-word production, the present study sought to examine intentional inhibition as well as the presence and magnitude of reactive inhibition. Table 1 presents a summary of the conditions used to address the following research questions.
Table 1.
Each research question with condition comparisons and example stimuli.
| Condition | Con | Inc (prime) | Neu | Inc probe | Con probe |
|---|---|---|---|---|---|
| Question 1: Intentional inhibition | |||||
| Interference (Inc – Neu) and facilitation (Neu – Con) effects | Red in red | Red in green |  |
||
| Question 2: Reactive inhibition | |||||
| (a) Incongruent probe (repeated interference) versus incongruent prime | Red in green | Blue in red | |||
| (b) Incongruent probe versus congruent probe | Blue in red | Red in red | |||
| (c) Congruent probe versus novel congruent condition | Red in red | Red in red | |||
Note. Con = congruent; Neu = neutral; Inc = incongruent.
Is there a significant difference in intentional inhibition and facilitation in PWA compared with HC?
Is there a significant difference in the presence and magnitude of reactive inhibition in PWA compared with HC? This question was examined three ways: (a) Is reactive inhibition significantly different in PWA compared with HC when tested using an incongruent probe (repeated interference)? (b) Is reactive inhibition significantly different in PWA compared with HC when tested using a congruent probe? (c) Is reactive inhibition significantly different in PWA compared with HC when tested using a congruent probe compared with the novel congruent condition?
Method
Participants
Nineteen PWA (10 women, nine men) and 20 HC (13 women, seven men) met the study criteria described below.2 The average age of PWA was 56.3 years (SD = 13.1) and of HC was 57.5 years (SD = 9.0). The average years of education for PWA was 16.4 (SD = 3.4) and for HC was 16.0 (SD = 1.9). Table 2 summarizes participant demographic information.
Table 2.
Demographic and descriptive information (age in years, gender, and education in years) for people with aphasia (PWA) and controls who are neurologically healthy (HC), and, for PWA, months post onset (MPO) and presence of apraxia of speech (AOS) and dysarthria.
| PWA | Age | Gender | Education | MPO | AOS | Dysarthria | HC | Age | Gender | Education |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 67 | M | 15 | 23 | Mild | None | C1 | 41 | F | 16 |
| P2 | 50 | M | 20 | 8 | None | Moderate | C2 | 45 | F | 16 |
| P3 | 70 | M | 25 | 32 | Mild | None | C3 | 45 | F | 15 |
| P4 | 48 | F | 16 | 17 | None | None | C4 | 52 | F | 19 |
| P5 | 52 | M | 16 | 43 | Mild | None | C5 | 53 | F | 14 |
| P6 | 65 | M | 23 | 53 | None | None | C6 | 59 | F | 12 |
| P7 | 68 | F | 14 | 180 | None | None | C7 | 69 | M | 16 |
| P8 | 34 | M | 12 | 15 | Mild | None | C8 | 66 | M | 13 |
| P9 | 58 | M | 16 | 47 | Mild to moderate | None | C9 | 48 | M | 16 |
| P10 | 67 | F | 16 | 169 | None | None | C10 | 60 | F | 16 |
| P11 | 74 | M | 18 | 108 | None | None | C11 | 63 | M | 19 |
| P12 | 34 | F | 15 | 50 | None | None | C12 | 66 | F | 18 |
| P13 | 62 | F | 16 | 101 | None | None | C13 | 66 | F | 18 |
| P14 | 51 | F | 13 | 24 | Moderate | None | C14 | 61 | F | 14 |
| P15 | 69 | M | 17 | 126 | Moderate | Mild to moderate | C15 | 43 | F | 16 |
| P16 | 48 | F | 12 | 40 | None | None | C16 | 56 | M | 18 |
| P17 | 61 | F | 16 | 24 | Moderate | Mild | C17 | 60 | M | 16 |
| P18 | 62 | F | 18 | 159 | Mild to moderate | None | C18 | 67 | F | 16 |
| P19 | 30 | F | 14 | 19 | Moderate | None | C19 | 67 | F | 15 |
| C20 | 62 | M | 16 | |||||||
| M | 56.3 | 16.4 | 65.2 | M | 57.5 | 16.0 | ||||
| SD | 13.1 | 3.4 | 56.7 | SD | 9.02 | 1.85 |
Note. M = male; F = female.
PWA were recruited from the University of Washington (UW) Aphasia Registry, UW Speech and Hearing Clinic, area clinicians, and support groups. HC were recruited from the UW Communications Studies Participant Pool, the UW Speech and Hearing Clinic, and area support groups and via word of mouth. Participating PWA had a diagnosis of aphasia using the clinical criteria described by McNeil and Pratt (2001): a language-dominant hemisphere lesion resulting in acquired, multimodal language processing deficits. These impairments were objectified by the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983), Western Aphasia Battery (Kertesz, 1982), and the Standardized Assessment of Phonology in Aphasia (Kendall et al., 2010). The PWA were at least 6 months post onset left-hemisphere stroke with no prior neurologic event per participant or caregiver report and confirmed by clinical neurology and brain imaging reports. The presence and severity of dysarthria and apraxia of speech were determined by an experienced speech-language pathologist on the basis of samples of connected speech and repetition of single and multisyllabic real words (Duffy, 2005). Discriminatory diagnostic descriptors of apraxia of speech were based on Wambaugh, Duffy, McNeil, Robin, and Rogers (2006). No participant was identified to have severe apraxia of speech or dysarthria. The three participants who presented with speech profiles consistent with mild unilateral upper motor neuron dysarthria demonstrated speech characteristics such as mildly imprecise articulations and a unilaterally weak tongue. Participants in both groups were premorbidly right handed and native speakers of English per participant report, and their vision, screened as part of the study protocol, was better than 20/40 (corrected if necessary). Prospective participants were excluded for a self-reported history of psychiatric disturbance, schizophrenia or schizoaffective disorder, bipolar disorder, learning disability, developmental language delay, attention deficit disorder, a currently uncontrolled mood disorder or substance abuse, diffuse brain injury or disease, or hemianopia. In addition to vision screening, colorblindness screenings were completed for all participants during the screening portion of the study session (Snellen Vision Screen, Snellen, 1862; Ishihara Colorblindness Test, Ishihara, 1917). All participants also completed forward and backward digit spans with a verbal response (Wechsler Adult Intelligence Scale–Third Edition; Wechsler, 1997); Trail Making tests (Reitan, 1958); the Attention Questionnaire (from Attention Process Training-II; Sohlberg, Johnson, Paule, Raskin, & Mateer, 2001); and the regular reading, neutral, and incongruent subtests of the Computerized Revised Token Test–Reading (McNeil et al., 2008). Participants who performed at 80% accuracy or better on a Stroop color-word stimuli screen were permitted into the study. (The 80% accuracy cutoff was established to ensure that participants were able to perform the task at a better-than-chance level.) See Table 3 for results of descriptive measures.
Table 3.
Descriptive data (mean and standard deviation) for people with aphasia (PWA) and controls who are neurologically healthy (HC) on Trail Making Tests A and B (time in seconds; Reitan, 1958); forward and backward digit span scores on the Wechsler Adult Intelligence Scale–Third Edition (Wechsler, 1997); Attention Questionnaire scores (Sohlberg et al., 2001); and accuracy and efficiency scores for the regular reading, neutral, and incongruent subtests of the Computerized Revised Token Test–Reading (CRTT-R; McNeil et al., 2008).
| Group | Trail Making |
Digit span |
Attention Questionnaire | CRTT-R |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | Forward | Backward | Reading score | Reading efficiency | Neutral score | Neutral efficiency | Incongruent score | Incongruent efficiency | ||
| PWA | |||||||||||
| M | 47.4 | 159.1 | 5.68 | 3.11 | 22.42 | 13.76 | 4.63 | 13.82 | 5.14 | 13.52 | 3.65 |
| SD | 25.1 | 107.2 | 2.94 | 1.85 | 9.57 | 0.71 | 2.13 | 0.75 | 2.41 | 1.11 | 2.76 |
| HC | |||||||||||
| M | 23.8 | 49.9 | 11.20 | 7.60 | 11.55 | 14.49 | 8.81 | 14.52 | 9.09 | 14.41 | 8.48 |
| SD | 5.6 | 12.3 | 1.94 | 1.79 | 6.32 | 0.25 | 1.32 | 0.27 | 1.27 | 0.70 | 1.29 |
Equipment
The experiment was conducted using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA; Schneider, Eschman, & Zuccolotto, 2002) on a Dell (Round Rock, TX) Precision 870 computer and displayed using a Dell A5501 19-in. monitor with 20-ms response time and maximum resolution of 1280 × 1024 pixels. Participants wore an Audio-Technica (Tokyo, Japan) ATM 75 head microphone attached to a Psychology Software Tools serial response box (Model 200A). Participants also wore Audio-Technica QuietPoint ATH-ANC7 headphones (not plugged in) to dampen environmental sounds.
Stimuli
Five colors and color words (red, blue, green, pink, and white) were presented in 54-point Arial font centered on a gray background on a computer monitor. These five colors and color words were used in three basic stimuli types: congruent, neutral, and incongruent (refer to Table 1). As described previously, the congruent condition presented color words with matching font color (e.g., the word red in red font), the neutral condition presented a colored polygon ( ; approximately 2 in. in diameter), and the incongruent condition presented color words with a mismatched font color (e.g., the word red in green font). To test reactive inhibition, the incongruent trials served as primes for two probe stimuli types. Incongruent prime trials were followed by either (a) incongruent probe (repeated interference), where the prime trial distractor becomes the incongruent probe trial target (e.g., prime is the word red in green font; probe is the word blue in red font), or (b) congruent probe, where the prime trial distractor becomes a congruent probe (e.g., prime is the word red in green font; probe is the word red in red font).
; approximately 2 in. in diameter), and the incongruent condition presented color words with a mismatched font color (e.g., the word red in green font). To test reactive inhibition, the incongruent trials served as primes for two probe stimuli types. Incongruent prime trials were followed by either (a) incongruent probe (repeated interference), where the prime trial distractor becomes the incongruent probe trial target (e.g., prime is the word red in green font; probe is the word blue in red font), or (b) congruent probe, where the prime trial distractor becomes a congruent probe (e.g., prime is the word red in green font; probe is the word red in red font).
Procedures
The experiment comprised 340 trials total: 40 congruent trials, 40 neutral trials, 80 incongruent trials, 80 probe trials (40 incongruent probes, 40 congruent probes), and 100 neutral fillers. (The number of tokens was selected based on Hogge, Salmon, & Collette, 2008; Vitkovitch, Bishop, Dancey, & Richards, 2002; and Wiener et al., 2004.) Each incongruent trial served as a prime for one of the two types of probe trials. An unrelated neutral filler trial followed each of the 80 probe trials to control for episodic retrieval or lingering suppression effects. Twenty neutral filler trials followed half of the congruent trials to help conceal the prime–probe–neutral pattern. All trials were presented in 10 sets of 34 trials. Each of the 10 sets was presented in random order, and the 34 trials within each set were presented in pseudorandom order. Except for the prime–probe trial pairs, no stimulus was repeated in the immediate subsequent trial to allow dissipation of any priming effects (Hasher, Stoltzfus, Zacks, & Rypma, 1991; May, Kane, & Hasher, 1995). The color stimuli were also balanced; each color was represented nearly equally.
Participants sat about 20 in. from the monitor. Experimental instructions were presented on the computer screen as well as read aloud by the examiner: “Look at the fixation crosses. You will see words and symbols. Name the font color out loud, as quickly and accurately as you can.” The examiner also instructed participants to (a) look at the whole word or symbol, not just part of it, and (b) refrain from making any vocalization prior to response, making self-corrections, squinting or blurring vision, or using any other strategies that may prevent automatic word reading during the experiment. Prior to the testing session, participants completed one to three 34-item practice sets in order to become acquainted with the task and trial types, verify that they understood the experimental instructions, and bring performance to a personal maximum.
Within the experiment itself, the participants saw 250 ms of blank screen followed by 250 ms of three centered fixation crosses (appearing on the screen in the location and width of the color words) and then the trial stimulus. The stimulus remained on the screen until 1,000 ms after the onset of vocalization/response, as captured by the serial response box via head microphone. Stimuli presentation times and intertrial intervals were based on Hogge et al. (2008); May, Kane, and Hasher (1995); Titz, Behrendt, Menge, and Hasselhorn (2008); and others. This progression repeated for each of the 34 trials in each set. After each 34-trial set, the participant had a brief (<1 min) break before the start of the next set. The entire experiment was divided into two 5-set, 170-trial sessions, and all sessions were audio-recorded for intra- and interrater reliability purposes.
Following the experimental session, most participants were asked a general question about their experience of the experiment to capture whether they noticed a relationship between prime–probe trial sets or used any overt strategies during the experiment. One HC participant noted the prime–probe relationship, and one mentioned using a strategy in responding to the stimuli. Both participants were excluded from analyses.
Data Processing
During the experiment, the examiner used pen–paper score sheets to record accuracy data. Incorrect responses were defined as vocal hesitations or false starts, phonemic paraphasias, semantic substitutions, omissions, or responses shorter than 250 ms or outside 3 SD from each participant's mean response time for that condition (Wiener et al., 2004). Responses that needed to be repeated due to low microphone level or were incomplete due to high microphone level (trials advanced too quickly) were also omitted. Probe trials that followed an incorrect or omitted prime trial were not included in response latency analyses but were included in accuracy analyses. Using these accuracy criteria, 11.8% of trials were omitted for PWA and 2.6% were omitted for HC for the response latency analyses.
Data Analyses
For each research question, between-conditions differences in response latency and accuracy were determined for each participant. Between-conditions differences were important to calculate to explore how each participant performed relative to his or her own baseline in a word-retrieval task. The baseline condition, as in other Stroop studies, was the neutral condition. These individual participant differences between conditions were then compared between groups. Therefore, for Research Question 1, interference effects were determined for each participant by subtracting neutral from incongruent trials, and facilitation effects were determined by subtracting congruent from neutral trials. For Research Question 2, between-conditions differences were determined by subtracting (a) novel incongruent trials from incongruent probes, (b) congruent probes from incongruent probes, and (c) novel congruent trials from congruent probes.
After data were initially analyzed using an omnibus two-group by five-condition repeated measures analysis of variance, each research question was addressed separately using 10 planned comparisons (five response latency, five accuracy), with a corrected alpha level of .005. PWA showed more variability in response latencies compared with HC; therefore, raw response latencies were logarithmically transformed to meet the homogeneity of variance assumption. Response latency data for correct responses were analyzed using one-way, repeated measures analyses of variance. Accuracy data were analyzed using Mann–Whitney tests. Descriptive, within-group analyses involved paired-samples t tests for response latency and Wilcoxon tests for accuracy.
Results
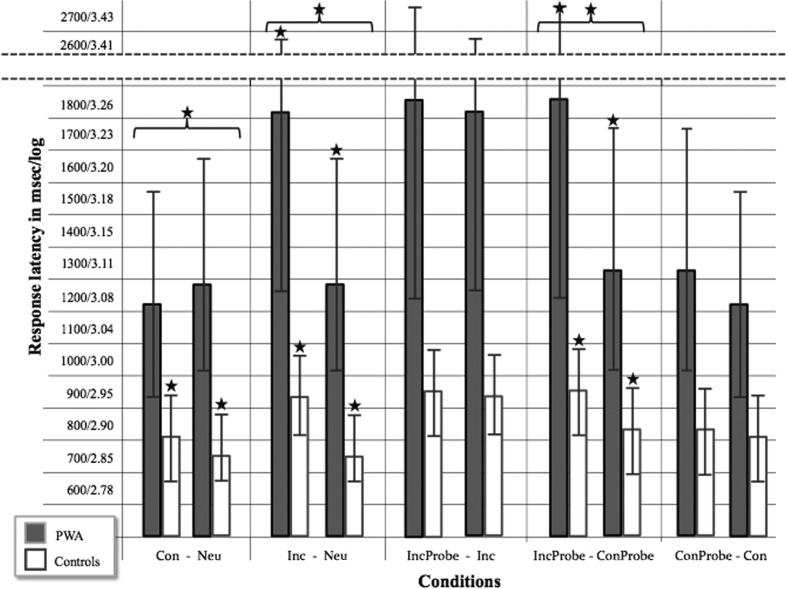
The omnibus analysis showed a statistically significant main effect of condition, F(1.752, 0.699) = 103.609, p < .05, and a statistically significant interaction of condition by group, F(1.752, 0.115) = 17.095, p < .05. Reliability analyses of 30% of each participant's accuracy scores resulted in 98.70% intrarater reliability and 98.92% interrater agreement. It is notable that there were no significant differences in any of the condition comparisons (all p > .05) when contrasting PWA with motor impairment and PWA with no motor impairment (see Table 5). Mean response latencies for each condition comparison are shown in Figure 1 and in Table 4. Significance levels for response latency and accuracy analyses are shown in Table 5.
Table 5.
Research questions with between-groups and within-group p values for all five research question condition comparisons.
| Variable | Question 1: Intentional inhibition |
Question 2: Reactive inhibition |
|||
|---|---|---|---|---|---|
| Facilitation (Neu – Con) | Interference (Inc – Neu) | (a) Repeated interference (Inc probe – Inc) | (b) Facilitation probe (Inc probe – Con probe) | (c) Comparing (Con probe – Con) | |
| Response latency between groups | <.005* | <.005* | .786 | <.005* | .034 |
| PWA | .017 | <.005* | .400 | <.005* | .006 |
| HC | <.005* | <.005* | .132 | <.005* | .038 |
| Accuracy between groups | .026 | .133 | .069 | .010 | .045 |
| PWA | .015 | .024 | .040 | <.005* | .047 |
| HC | .102 | .437 | .437 | .026 | 1.00 |
Note. Neu = neutral; Con = congruent; Inc = incongruent; PWA = people with aphasia; HC = controls who are neurologically healthy.
Significance level p < .005.
Figure 1.
Mean response latencies in milliseconds/log for both groups on all condition comparisons. Whiskers indicate 1 SD above and below the mean. Significance (p < .005) is indicated (a) between groups with bracket and star and (b) within groups with star over both conditions.
Table 4.
Mean (standard deviation) log-transformed response latencies and accuracy proportions for people with aphasia (PWA) and controls who are neurologically healthy (HC) for each condition.
| Group | Con | Neu | Inc | Inc probe | Con probe |
|---|---|---|---|---|---|
| PWA | |||||
| Response latency | 3.049 (0.121) | 3.076 (0.118) | 3.235 (0.174) | 3.243 (0.191) | 3.088 (0.134) |
| Accuracy | 0.971 (0.037) | 0.939 (0.073) | 0.894 (0.102) | 0.873 (0.094) | 0.958 (0.039) |
| HC | |||||
| Response latency | 2.853 (0.076) | 2.829 (0.061) | 2.919 (0.068) | 2.925 (0.071) | 2.862 (0.074) |
| Accuracy | 0.998 (0.008) | 0.993 (0.014) | 0.972 (0.039) | 0.980 (0.028) | 0.999 (0.006) |
Note. Con = congruent; Neu = neutral; Inc = incongruent.
Research Question 1: Intentional Inhibition and Facilitation
Statistically significant group-by-condition interactions were found for both planned comparisons that address this question. First, to address interference effects, statistically significant group-by-condition (response latency between the neutral and incongruent conditions) interactions were present, t(22.105) = 3.298, p < .005, ES(d) = 1.056. Both groups demonstrated interference effects; that is, incongruent condition response latency was significantly slower than neutral condition response latency. The within-group differences between these conditions were statistically significant for both groups. PWA performed significantly faster in the neutral condition than in the incongruent condition, t(18) = 8.066, p < .005. HC also performed significantly faster in the neutral condition than in the incongruent condition, t(19) = 13.610, p < .005. Although both groups demonstrated significant interference effects, these effects were more variable and proportionally significantly greater for PWA. After log transformation, PWA were 5.2% slower on the incongruent compared with the neutral condition, whereas HC were 3.2% slower on the incongruent compared with the neutral condition.
Addressing the facilitation effects, significant differences in response latency between neutral and congruent conditions were found between PWA and HC, t(29.833) = 4.303, p < .005. Within-group analyses showed that PWA were faster, but not significantly so, in the congruent condition compared with the neutral condition, t(18) = 2.605, p = .017. HC showed the opposite trend, performing significantly slower in the congruent condition compared with the neutral condition, t(19) = 3.928, p < .005.
No statistically significant group-by-condition interactions in accuracy were found when comparing the neutral and incongruent conditions, U(38) = 137, Z = 1.504, p = .133, ES(r) = .241, or the neutral and congruent conditions, U(37) = 119.500, Z = 2.230, p = .026, ES(r) = .357, in PWA compared with HC.
Research Question 2: Reactive Inhibition
Research Question 2a: Incongruent probe (repeated interference) versus incongruent prime. No statistically significant differences in response latency between the novel incongruent and incongruent probe (repeated interference) conditions were found when comparing PWA and HC, t(22.615) = 0.272, p = .786, ES(d) = 0.099. No significant within-group differences in this condition comparison were present. However, a post hoc analysis provided some additional information about these results (detailed in the Discussion). No significant group-by-condition interaction in accuracy measures was found when comparing the difference between the novel incongruent and incongruent probe conditions in PWA compared with HC, U(37) = 125.5, Z = 1.817, p = .069, ES(r) = .291.
Research Question 2b: Incongruent probe (repeated interference) versus congruent probe. Significant difference in response latency between the incongruent probe and congruent probe conditions were found when comparing PWA and HC, t(24.271) = 4.339, p < .005, ES(d) = 1.390. Within-group analyses showed that both groups performed significantly more slowly on the incongruent probe than on the congruent probe; PWA: t(18) = 7.890, p < .005; HC: t(19) = 7.538, p < .005. No significant group-by-condition interaction in accuracy was found when examining the difference between the incongruent probe and the congruent probe in PWA compared with HC, U(37) = 100.5, Z = 2.560, p = .010, ES(r) = .410.
Research Question 2c: Congruent probe versus novel congruent condition. No significant difference in response latency on the congruent probe and novel congruent conditions was found when comparing PWA and HC, t(21.639) = 2.268, p = .034, ES(d) = 0.745. Within-group analyses showed that PWA performed more slowly on the congruent probe compared with the novel congruent, t(18) = 3.067, p = .007. Likewise, HC performed more slowly on the congruent probe compared with the novel congruent, t(19) = 2.217, p = .038. Although both groups were slower on the congruent probe than the novel congruent, this difference was greater for PWA, though not statistically significant. No significant group-by-condition interaction in accuracy was present when examining the difference between the congruent probe and the novel congruent conditions in PWA compared with HC, U(37) = 125.5, Z = 2.004, p = .045, ES(r) = .321.
Discussion
The present study explored the presence of intentional and reactive inhibition in PWA compared with age- and education-matched HC. After finding the differences between baseline and experimental conditions for each participant, the results of this experiment suggest potentially impaired intentional inhibition during spoken-word production in PWA compared with HC, consistent with a diminished or misallocated attention explanation hypothesized to contribute to the word-retrieval impairments in aphasia. Furthermore, the interference and facilitation effects results may indicate impaired integration of information during language processes for PWA. Although reactive inhibition was not impaired for PWA in this experiment, some interesting trends were found for both groups in two of the reactive inhibition condition comparisons.
Research Question 1: Intentional Inhibition and Facilitation
Interference effects. PWA and HC performed similarly when interference was present: Both groups responded significantly more slowly to the incongruent condition compared with the neutral condition. In other words, when the color of the font and the word were mismatched, the participants' spoken-word response was slowed. However, PWA showed more variable and proportionately slower response latency during the incongruent condition relative to HC. Increased sensitivity to interference in PWA has also been reported by Hamilton and Martin (2005), Lim et al. (2012), McNeil, Kim, et al. (2010), Wiener et al. (2004), and others. The proportionally greater interference effects for PWA compared with HC could indicate limits in attention (and therefore limits in working memory capacity) allocated to managing interfering stimuli. In other words, if the overall level of activation resource is diminished, then one would expect greater impairment in responding to a stimulus when interference is present than in responding to a stimulus without interference.
Facilitation effects. In condition comparisons in which no interference was present (i.e., congruent versus neutral), PWA demonstrated no significant differences in response latency. It is surprising that HC responded significantly more quickly to the neutral condition compared with the congruent condition. Why did neither participant group take advantage of the match between word and font color, which should boost activation and speed response in the congruent condition? Congruent trials were the rarest of the basic trial types and therefore were least likely to be habituated by participants. This experiment comprised 23% congruent, 41% neutral, and 36% incongruent trials presented in pseudorandom order. HC may have habituated to the more frequent incongruent compared with congruent trials. For example, a number of HC reported things such as “When the color and the word matched, I double checked my response” or “When I saw the [neutral condition], it was a relief because I didn't have to worry about the word.” Therefore, although PWA as a group showed no significant difference between the neutral and congruent conditions, HC may have adapted their response strategy to accommodate the frequency of each condition type. This finding aligns with those of Tseng et al. (1993), who attributed the performance differences in PWA to an inability to appropriately allocate attention in evaluating the probability of conditions within the context of the experimental task. Studying HC and participants with Alzheimer's disease, Hogge et al. (2008) used a proportion of congruent, neutral, and incongruent trial types similar to that used in the present study and reported similar results (described as reverse facilitation effects).
According to Cohen et al. (1990), even processing considered more automatic, such as that required for the congruent condition, may become slower depending on the task context. Some have described these contextual factors as a “mental set,” or how the individual may differently apply suppression to the task given its context (Catena, Fuentes, & Tudela, 2002; Marí-Beffa, Fuentes, Catena, & Houghton, 2000). Torres-Quesada, Fúnes, and Lupiáñez (2013) called this phenomenon the proportion congruent effect and described how the proportion of congruent and incongruent trial types yields adaptations in cognitive control. Therefore, the relatively fewer congruent trials compared with neutral and incongruent trials may have changed the response strategy in HC but may not have had the same influence on PWA.
One might consider an alternative explanation to the present study's facilitation results. PWA were slow in retrieving the correct color word from perceptual input presented in this study in the neutral condition. When HC were faced with the neutral trial, they were significantly faster at retrieving the color word directly from the perceptual input. One might assume that the neutral condition would naturally be more difficult for PWA, regardless of contextual factors, and therefore expect the reported results for the neutral versus congruent condition. Because the present study did not include a regular reading condition, we cannot speak to the nature of the neutral condition responses relative to regular reading. However, a post hoc t test analysis of the participants' CRTT-R efficiency scores indicated that there were no significant between-groups differences (p = .37) when comparing the neutral and regular reading conditions. These scores indicate that the neutral condition may be a relatively good baseline measure for both participants.
In summary, given the proportionally greater interference effects and the insignificant group facilitation results shown by PWA, these participants may have impaired intentional inhibitory function, potentially due to (a) limited available or distributed attentional resources and/or (b) limited working memory capacity, as demonstrated by greater interference effects and an inability to integrate contextual information into their responses. This interpretation is supported by significant differences between PWA and HC scores on the working memory tasks (Trails B and backward digit span; both p < .0005).
Research Question 2: Reactive Inhibition
Research Question 2a: Testing reactive inhibition with an incongruent probe. In the present study, between-groups and within-group analyses revealed that neither HC nor PWA demonstrated significant reactive inhibition, or a slowing of response to a probe stimulus that has just served as a distractor (repeated interference). It is important to note that a statistical power analysis revealed that at least 333 participants would be required to have enough power at the .80 level to yield a significant difference in this group-by-condition contrast.
A post hoc analyses of within-group response latency data revealed that both groups were nearly evenly split between participants who demonstrated significant reactive inhibition via negative priming effects and participants who demonstrated significant positive priming effects on the incongruent probe (repeated interference) compared with the incongruent prime condition.3 Nine PWA demonstrated significant within-group negative priming effects (p = .001), and 11 HC demonstrated significant within-group negative priming effects (p = .002). Ten PWA demonstrated significant within-group positive priming effects (p = .013), and eight HC demonstrated significant within-group positive priming effects (p = .001). One HC demonstrated nearly equal response latencies in these conditions.
Many studies of reactive inhibition using negative priming have explored the presence, magnitude, or absence of the effect, but none have addressed positive priming (Andrés, Guerrini, Phillips, & Perfect, 2008; Filoteo, Rilling, & Strayer, 2002; Hogge et al., 2008; Houghton & Tipper, 1994; Houghton et al., 1996; Mayas, Fuentes, & Ballesteros, 2012; Titz et al., 2008; Vitkovitch et al., 2002). Other reports of facilitation during negative priming give accounts of stimuli that have been degraded (Kane, May, Hasher, Rahhal, & Stoltzfus, 1997), an absence of interference in the probe trial (Catena et al., 2002), or primes that consist of a “low level perceptual task” (e.g., letter search in target word; Marí-Beffa et al., 2000). None of these issues are consistent with the stimuli or methods involved in the present study and its findings on positive priming.
Puzzling and inconclusive findings related to reactive inhibition are not uncommon. Evidence of reactive inhibition may be influenced by instructions to participants about speed and accuracy of response, difficult prime trial selection, stimuli presentation duration, interstimulus interval duration, probe stimuli type, and advanced age of participants (Fox, 1995; Houghton & Tipper, 1994). All of these factors were well considered in this study. However, evidence of reactive inhibition may also differ from one individual to the next on the basis of hypothesized working memory capacity. A. R. A. Conway, Tuholski, Shisler, and Engle (1999) reported that study participants with larger working memory capacity (i.e., “high span”) demonstrated more consistent negative priming effects, whereas participants with more limited working memory capacity (i.e., “low span”) did not show consistent negative priming effects. In the present study, no significant differences were detected in working memory when comparing the negative and positive priming subgroups of the PWA and HC groups on Trails B and digit span backward working memory tasks. These measures, however, may not be sensitive enough to identify span differences between participant groups.
In summary, the results of Research Question 2a analyses reveal that PWA and HC performed similarly in reactive inhibition via repeated interference in this study, though some perplexing and highly variable within-group results are currently unexplained. Regardless of group, some participants demonstrated reactive inhibition (negative priming effects), whereas others demonstrated repeated interference facilitation (positive priming effects). These results are unique to the negative priming literature and may provide additional evidence of the wide individual variability reported therein. See the General Discussion for further interpretation of these results.
Research Question 2b: Testing reactive inhibition with a congruent probe. Results show that the two groups performed significantly differently in this test of reactive inhibition. Both PWA and HC showed a marked difference between the probe types: Responses to the congruent probe were significantly faster than responses to the incongruent probe (repeated interference). However, the difference between these conditions was much greater for PWA compared with HC, yielding a statistically significant between-groups difference. It is important to note that the individual variability within each group observed in the previous analyses was not observed in this comparison. In addition, PWA showed a significant within-group difference in accuracy scores when comparing these conditions: Incongruent probes were the least accurate of any condition and significantly less accurate than congruent probe responses. The diminished accuracy of PWA in the repeated interference condition highlights the challenge that interference presents during word finding relative to a congruent probe.
Although significant differences would be predicted between these condition comparisons, it is difficult to interpret the between-groups differences. If the previously discussed results are considered, wherein (a) PWA as a group showed no significant facilitation effects and HC showed significant reverse facilitation effects, (b) PWA showed significantly greater interference effects, and (c) both groups showed similar but widely variable responses related to reactive inhibition via negative priming, then the results that address this question are unclear at best. Because both interference effects and either facilitation or reverse facilitation effects are likely at play in the comparison addressing Research Question 2b, it is impossible to differentiate how much of each may be contributing to the overall result.
Research Question 2c: Comparing the congruent probe with the novel congruent trial. The results of this condition comparison were not statistically significant but are theoretically interesting for future exploration. Both PWA and HC performed more slowly on the congruent probes compared with the novel congruent condition, and PWA were relatively slower on the congruent probe compared with HC. Although these trends were not statistically significant and this group-by-condition comparison had enough statistical power at the .80 level, these results warrant discussion. Given previously discussed findings, reactive inhibition may not be at the root of this trend. However, it is possible that PWA had trouble switching from the interference context present in the prime trial to the facilitation present in the congruent probe trial. One way to consider this is through the lens of “conflict monitoring” (Botvinick, Braver, Barch, Carter, & Cohen, 2001) or “conflict adaptation” (Torres-Quesada et al., 2013), wherein the participant begins to monitor for conflict or interference once it has occurred. Therefore, if the prime trial is incongruent, the participant becomes unconsciously ready for another incongruent trial. In the present study, PWA's apparent greater difficulty adapting to a congruent probe when it is preceded by an incongruent prime may indicate a generally diminished ability to adapt to changes in task processing requirements.
It is difficult to say more about the results addressing Research Question 2c interpretations because the results are not statistically significant. However, the trend toward more substantial slowing on behalf of PWA inspires future research about the ability of PWA to resolve moment-to-moment linguistic conflicts.
General Discussion
Although the previous discussion offers some interpretations of the results, additional insight may be gained with additional integration. First, the response slowing observed during the novel incongruent condition is considered to be the result of a deliberate effort to suppress a distraction (or intentional inhibition), and the response delay related to the incongruent probe is considered to be the automatic, unintended suppression of a previously activated representation (or reactive inhibition; Andrés et al., 2008). Using Cohen et al.'s (1990) view of automatic and controlled processes, intentional interference requires greater attentional resources than the automatic inhibition seen in reactive inhibition (see also Engle, Conway, Tuholski, & Shisler, 1995). The results of the present study appear to be in line with the hypothesized variable attention demands of intentional versus reactive inhibition. Although PWA showed great difficulty with attention-demanding intentional inhibition, as a group they appear quite similar to HC in reactive inhibition, which is less attention demanding.
Some lesion data support the present findings, though in a general way. A number of studies (M. A. Conway & Fthenaki, 2003; Jonides & Nee, 2006; McDonald et al., 2005; Metzler & Parkin, 2000; Nelson, Reuter-Lorenz, Persson, Sylvester, & Jonides, 2009) report inhibition-related impairments in left-hemisphere regions that are highly connected and often implicated in aphasia. However, the striking similarity of the two participant groups in the present study when examining responses to the incongruent probe (repeated interference) condition does not motivate future study of reactive inhibition and lesion site. Instead, it underscores the influence of individual variability—variability that appears to be unaffected by the presence of aphasia—when attempting to capture the presence and magnitude of reactive inhibition.
The between-groups and within-group differences in interference and facilitation effects (or lack thereof) suggest diminished intentional inhibition and, therefore, attentional resources for PWA, as reported in previous studies (e.g., among others, Hula & McNeil, 2008; Murray, 2012). Diminished attention may also point to limited working memory capacity in aphasia (Wright & Fergadiotis, 2012). In the present study, diminished working memory capacity may be evidenced by PWA's apparent inability to integrate contextual information into their responses and potentially diminished moment-by-moment adaptability to the presence and absence of interference and may be underscored by performance on other working memory tasks (described earlier).
Study Limitations
A number of factors limit the interpretation of these results. First, the present study included a direct comparison of interference and facilitation probe trials (Research Question 2b). This type of comparison includes the potential for both interference and facilitation effects, making it impossible to understand the presence and magnitude of each. A neutral probe condition, in which a prime distractor becomes a color-polygon probe, would have been a useful addition to this study. The comparison of a neutral probe with an incongruent probe would have yielded better information about interference effects, and comparing a neutral probe with a congruent probe would have yielded better information about presence, absence, or reverse facilitation effects. Second, many but not all participants were asked about their experience of the experiment following its administration. This general and intentionally vague question helped identify two HC who used a strategy that is believed to have interfered with yielding interpretable results (e.g., one was able to predict probe stimuli), thus eliminating them from the analysis. There may have been other participants who used a strategy but did not report it. Asking participants in future studies about their approach to the experiment would help identify those participants who used a strategy and further explore how different strategies may affect participant performance.
Future Directions
In addition to addressing the limitations described previously, there are many potential directions for future study. For example, it would be valuable to include a priming condition using the same stimuli and timing parameters used in the present study. The resulting data could provide additional information about the nature of facilitation in aphasia, thus furthering understanding of its relationship to intentional and reactive inhibition, especially given the surprising repeated interference facilitation demonstrated by many participants. In addition, proportion effects of each condition may be addressed by providing two or more experimental sets, one that provides a high proportion of congruent stimuli and another that provides a high proportion of incongruent stimuli (see Lim et al., 2012; Tseng et al., 2008). A comparison of these proportion effects would provide additional information about how PWA change their performance within the context of these intentional and reactive inhibition tasks compared with HC.
Further, an exploration of “conflict adaptation” (Torres-Quesada et al., 2013) may result in further clarification of the influence of selective attention and its impairment in language processes in both PWA and HC. Last, because the present study tested participants using a closed semantic category, it would be interesting to explore interference and inhibition across semantic categories. A cross-category study of interference was undertaken by Lim et al. (2012). Building upon this study with conditions that address inhibition may yield useful information, especially if the study focuses on the semantic relation effects of a distractor.
Conclusions
PWA may have diminished or poorly controlled attention necessary for the working memory processes that manage distracting stimuli and contextual information related to language processes. The results of the present study could indicate that PWA may have diminished intentional inhibition and appear unable to inhibit distractions that accompany an intended language task. However, PWA and HC appear to have similarly variable demonstrations of reactive inhibition of previously activated distractions, which suggests that the underlying automatic mechanism of reactive inhibition may be shared by PWA and HC and cannot account for word-finding impairments in aphasia.
This study highlights the importance of understanding the impact of cognitive and linguistic competition and the mechanisms of selective attention in PWA in clinical environments and, more importantly, everyday life. How do we adequately measure an individual's ability in intentional inhibition and integrate contextual factors into language intervention? Although a few attention and working memory measures exist, they are not sufficiently sensitive to fully describe these impairments and the extent to which the impairments influence the presenting language impairments of PWA. The work ahead is essential: Build upon current understanding of the role that selective attention plays in language impairments, create more sensitive measures of attention for PWA, and establish more effective interventions for PWA—ones that reflect the naturally competitive processes of building language representations under finitely timed constraints of executive attention and working memory.
Acknowledgments
This research was supported by National Institute on Deafness and Other Communication Disorders Grant F31DC012457, awarded to Rebecca Hunting Pompon. The authors acknowledge Amanda Hendricks, Hallie Mass, Erin McDonald, Leslie Yoo, and all participants for their contributions to this study.
Funding Statement
This research was supported by National Institute on Deafness and Other Communication Disorders Grant F31DC012457, awarded to Rebecca Hunting Pompon. The authors acknowledge Amanda Hendricks, Hallie Mass, Erin McDonald, Leslie Yoo, and all participants for their contributions to this study.
Footnotes
There are alternate models of the Stroop phenomenon. For example, Roelofs and Hagoort (2002) described the GRAIN and WEAVER++ willed control/lexical activation models, both of which provide elegant explanations with varying degrees of evidence of the typical person's performance on a Stroop task. These models, however, do not delve into the processes that subserve the suppression of a distraction or its subsequent reactivation or adequately address the language performance variability seen in aphasia as well as models of working memory and selective attention—the focus of the present study.
Of the original 22 participants recruited for each group, two HC participants were excluded from final analysis because they reported using a strategy during the experiment. Two PWA did not complete the study protocol because they did not pass the Stroop color-word stimuli screening, and one PWA was eliminated because his Western Aphasia Battery, Boston Naming Test, and Standardized Assessment of Phonology in Aphasia scores were within normal limits.
For this post hoc analysis, participants within each group were separated into negative and positive priming subgroups. Response latency for each condition (incongruent probe and prime) was compared within each subgroup using pairwise t tests. These comparisons were statistically significant.
References
- Andrés P., Guerrini C., Phillips L. H., & Perfect T. J. (2008). Differential effects of aging on executive and automatic inhibition. Developmental Neuropsychology, 33, 101–123. [DOI] [PubMed] [Google Scholar]
- Baddeley A. D. (1993). Working memory or working attention. In Baddeley A. D., & Weiskrantz L. (Eds.), Attention: Selection, awareness and control; A tribute to Donald Broadbent (pp. 152–170). Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- Botvinick M. M., Braver T. S., Barch D. M., Carter C. S., & Cohen J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108, 624–652. [DOI] [PubMed] [Google Scholar]
- Catena A., Fuentes L. J., & Tudela P. (2002). Priming and interference effects can be disassociated in the Stroop task: New evidence in favor of automaticity of word recognition. Psychonomic Bulletin and Review, 9, 113–118. [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Dunbar K., & McClelland J. L. (1990). On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review, 97, 332–361. [DOI] [PubMed] [Google Scholar]
- Conway A. R. A., & Engle R. W. (1994). Working memory and retrieval: A resource-dependent inhibition model. Journal of Experimental Psychology: General, 123, 354–373. [DOI] [PubMed] [Google Scholar]
- Conway A. R. A., Tuholski S. W., Shisler R. J., & Engle R. W. (1999). The effect of memory load on negative priming: An individual differences investigation. Memory & Cognition, 27, 1042–1050. [DOI] [PubMed] [Google Scholar]
- Conway M. A., & Fthenaki A. (2003). Disruption of inhibitory control of memory following lesions to the frontal and temporal lobes. Cortex, 39, 667–686. [DOI] [PubMed] [Google Scholar]
- Cowan N. (1988). Evolving conceptions of memory storage, selective attention and their mutual constraints within the human information-processing system. Psychological Bulletin, 104, 163–191. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford E. C., & Budayr B. (1966). Examination of some aspects of the Stroop color-word test. Perceptual and Motor Skills, 23, 1211–1214. [DOI] [PubMed] [Google Scholar]
- Dictionary.com (n.d.). Inhibition. Retrieved from http://dictionary.reference.com/browse/inhibition
- Duffy J. R. (2005). Motor speech disorders: Substrates, differential diagnosis, and management (2nd ed.). St. Louis, MO: Mosby. [Google Scholar]
- Engle R. W., Conway A. R. A., Tuholski S. W., & Shisler R. J. (1995). A resource account of inhibition. Psychological Science, 6, 122–125. [Google Scholar]
- Engle R. W., Tuholski S. W., Laughlin J. E., & Conway A. R. A. (1999). Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General, 128, 309–331. [DOI] [PubMed] [Google Scholar]
- Fassbinder W., McNeil M. R., Dickey M. W., Lim K. Y., Pratt S., Kim A., … Szuminsky N. (2011). Developing a standardized measure of short-term memory and syntactic complexity: Results from subtests of the CRTT-R. Presented at the Clinical Aphasiology Conference, Fort Lauderdale, FL. [Google Scholar]
- Filoteo J. V., Rilling L. M., & Strayer R. L. (2002). Negative priming in patients with Parkinson's disease: Evidence for a role of the striatum in inhibitory attentional processes. Neuropsychology, 16, 230–241. [DOI] [PubMed] [Google Scholar]
- Fox E. (1995). Negative priming from ignored distractors in visual selection: A review. Psychonomic Bulletin and Review, 2, 145–173. [DOI] [PubMed] [Google Scholar]
- Grandjean J., & Collette F. (2011). Influence of response prepotency strength, general working memory resources, and specific working memory load on the ability to inhibit predominant responses: A comparison of young and elderly participants. Brain and Cognition, 77, 237–247. [DOI] [PubMed] [Google Scholar]
- Hamilton A. C., & Martin R. C. (2005). Dissociations among tasks involving inhibition: A single-case study. Cognitive, Affective and Behavioral Neuroscience, 5(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Hasher L., Stoltzfus E. R., Zacks R. T., & Rypma B. (1991). Age and inhibition. Journal of Experimental Psychology: Learning, Memory, and Cognition, 17, 163–169. [DOI] [PubMed] [Google Scholar]
- Hogge M., Salmon E., & Collette F. (2008). Interference and negative priming in normal aging and in mild Alzheimer's disease. Psychologica Belgica, 48(1), 1–23. [Google Scholar]
- Houghton G., & Tipper S. P. (1994). A model of inhibitory mechanisms in selective attention. In Dagenbach D., & Carr T. (Eds.), Inhibitory processes in attention, memory, and language (pp. 53–112). San Diego, CA: Academic Press. [Google Scholar]
- Houghton G., Tipper S. P., Weaver B., & Shore D. I. (1996). Inhibition and interference in selective attention: Some tests of a neural network model. Visual Cognition, 3, 119–164. [Google Scholar]
- Hula W. D., & McNeil M. R. (2008). Models of attention and dual-task performance as explanatory constructs in aphasia. Seminars in Speech and Language, 29, 169–187. [DOI] [PubMed] [Google Scholar]
- Hunting Pompon R., Kendall D. L., & Moore A. B. (2011). Examining attention and cognitive processing in participants with self-reported mild anomia. Aphasiology, 25, 800–812. [Google Scholar]
- Ishihara S. (1917). Tests for color-blindness. Handaya, Tokyo: Hongo Harukicho. [Google Scholar]
- Jenkins J. J., Jiménez-Pabón E., Shaw R. E., & Sefer J. W. (1975). Schuell's aphasia in adults: Diagnosis, prognosis and treatment (2nd ed.). Hagerstown, MD: Harper & Row. [Google Scholar]
- Jonides J., & Nee D. E. (2006). Brain mechanisms of proactive interference in working memory. Neuroscience, 139, 181–193. [DOI] [PubMed] [Google Scholar]
- Just M. A., & Carpenter P. A. (1992). A capacity theory of comprehension: Individual differences in working memory. Psychological Review, 99, 122–149. [DOI] [PubMed] [Google Scholar]
- Kane M. J., & Engle R. W. (2003). Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General, 132, 47–70. [DOI] [PubMed] [Google Scholar]
- Kane M. J., May C. P., Hasher L., Rahhal T., & Stoltzfus E. R. (1997). Dual mechanisms in negative priming. Journal of Experimental Psychology: Human Perception and Performance, 23, 632–650. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., & Weintraub S. (1983). The Boston Naming Test. Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Kendall D. K., del Toro C. M., Nadeau S., Johnson J., Rosenbek J., & Velozo C. (2010). The development of a standardized assessment of phonology in aphasia. Presented at the Clinical Aphasiology Conference, Isle of Palms, SC. [Google Scholar]
- Kertesz A. (1982). Western Aphasia Battery. New York, NY: Grune & Stratton. [Google Scholar]
- Lim K. Y., McNeil R. M., Doyle J. P., Hula D. W., & Dickey W. M. (2012). Conflict resolution and goal maintenance components of executive attention are impaired in persons with aphasia: Evidence from the picture-word interference task. Presented at the Clinical Aphasiology Conference, Lake Tahoe, CA. [Google Scholar]
- Marí-Beffa P., Fuentes L. J., Catena A., & Houghton G. (2000). Semantic priming in the prime task effect: Evidence of automatic semantic processing of distractors. Memory & Cognition, 28, 635–647. [DOI] [PubMed] [Google Scholar]
- Martin R. C., & Allen C. M. (2008). A disorder of executive function and its role in language processing. Seminars in Speech and Language, 29, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C., Kane M., & Hasher L. (1995). Determinants of negative priming. Psychological Bulletin, 118, 35–54. [DOI] [PubMed] [Google Scholar]
- Mayas J., Fuentes L. J., & Ballesteros S. (2012). Stroop interference and negative priming (NP) suppression in normal aging. Archives of Gerontology and Geriatrics, 54, 333–338. [DOI] [PubMed] [Google Scholar]
- McDonald C. R., Bauer R. M., Filoteo J. V., Grande L., Roper S. N., & Gilmore R. (2005). Attentional inhibition in patients with focal frontal lobe lesions. Journal of Clinical and Experimental Neuropsychology, 27, 485–503. [DOI] [PubMed] [Google Scholar]
- McNeil M. R. (1982). The nature of aphasia in adults. In Lass N. J., McReynolds L. V., Northern J., & Yoder D. E. (Eds.), Speech, language, and hearing (Vol. 2, pp. 692–740). Philadelphia, PA: Saunders. [Google Scholar]
- McNeil M. R., Hula W., & Sung J. E. (2010). Attention and working memory in aphasia. In Guendouzi J., Loncke F., & Williams M. J. (Eds.), The handbook of psycholinguistic and cognitive processes (pp. 549–575). New York, NY: Psychology Press. [Google Scholar]
- McNeil M. R., Kim A., Lim K. Y., Pratt S., Kendall D., Pompon R. H., … Dickey M. (2010). Automatic activation, interference and facilitation effects in persons with aphasia and normal adult controls on experimental CRTT-R-Stroop tasks. Presented at the Clinical Aphasiology Conference, Isle of Palms, SC. [Google Scholar]
- McNeil M. R., Odell K., & Tseng C. H. (1991). Toward the integration of resource allocation into a general theory of aphasia. Clinical Aphasiology, 20, 21–39. [Google Scholar]
- McNeil M. R., & Pratt S. R. (2001). Defining aphasia: Some theoretical and clinical implications of operating from a formal definition. Aphasiology, 15, 901–911. [Google Scholar]
- McNeil M. R., Pratt S. R., Fassbinder W., Dickey M. W., Kendall D., Lim K. Y., … Krieger D. (2011). Effects of linguistic complexity and executive attentional demands on sentence comprehension in persons with aphasia and normal controls: Exploring on-line and offline measures with two reading versions of the Computerized Revised Token Test. Presented at the Clinical Aphasiology Conference, Fort Lauderdale, FL. [Google Scholar]
- McNeil M. R., Pratt S. R., Szuminsky N., Sung J. E., Fossett T. R. D., Kim A. L., & Fassbinder W. (2008). Description and psychometric development of the Computerized Revised Token Test (CRTT): Test-retest reliability, concurrent and construct validity with comparison to three experimental reading versions (CRTT-R) in normal adults and persons with aphasia. Presented at the 2008 Clinical Aphasiology Conference, Jackson Hole, WY. [Google Scholar]
- McNeil M. R., Sung J. E., Pratt S., Szuminsky N., Kim A., Ventura M. B., … Musson N. (2008). Concurrent validation of the Computerized Revised Token Test (CRTT) and three experimental reading versions (CRTT-R) in normal elderly individuals and persons with aphasia. Presented at the Clinical Aphasiology Conference, Jackson Hole, WY. [Google Scholar]
- Metzler C., & Parkin A. J. (2000). Reversed negative priming following frontal lobe lesions. Neuropsychologia, 38, 363–379. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., & Wager T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Murray L. L. (2012). Direct and indirect treatment approaches for addressing short-term or working memory deficits in aphasia. Aphasiology, 26, 317–337. [Google Scholar]
- Murray L. L., Holland A. L., & Beeson P. M. (1997). Auditory processing in individuals with mild aphasia: A study of resource allocation. Journal of Speech, Language, and Hearing Research, 40, 792–808. [DOI] [PubMed] [Google Scholar]
- Murray L. L., Holland A. L., & Beeson P. M. (1998). Spoken language of individuals with mild fluent aphasia under focused and divided attention conditions. Journal of Speech, Language, and Hearing Research, 41, 213–227. [DOI] [PubMed] [Google Scholar]
- Nelson J. K., Reuter-Lorenz P. A., Persson J., Sylvester C.-Y. C., & Jonides J. (2009). Mapping interference resolution across task domains: A shared control process in left inferior frontal gyrus. Brain Research, 1256, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach R. K., Rubin S. S., & Newhoff M. (1994). A topographic event-related potential analysis of the attention deficit for auditory processing aphasia. Clinical Aphasiology, 22, 81–96. [Google Scholar]
- Reitan R. (1958). Validity of the train making test as an indicator of organic brain disease. Perceptual and Motor Skills, 8, 271–276. [Google Scholar]
- Roelofs A., & Hagoort P. (2002). Control of language use: Cognitive modeling of the hemodynamics of Stroop task performance. Cognitive Brain Research, 15, 85–97. [DOI] [PubMed] [Google Scholar]
- Schneider W., Eschman A., & Zuccolotto A. (2002). E-Prime reference guide. Pittsburgh, PA: Psychology Software Tools. [Google Scholar]
- Snellen H. (1862). Probebuchstaben zur Bestimmung der Sehschärfe. Utrecht, the Netherlands. [Google Scholar]
- Sohlberg M. M., Johnson L., Paule L., Raskin S. A., & Mateer C. A. (2001). Attention process training II: A program to address attentional deficits for persons with mild cognitive dysfunction (2nd ed.). Wake Forest, NC: Lash. [Google Scholar]
- Stroop J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662. [Google Scholar]
- Titz C., Behrendt J., Menge U., & Hasselhorn M. (2008). A reassessment of negative priming within the inhibition framework of cognitive aging: There is more in it than previously believed. Experimental Aging Research, 34, 340–366. [DOI] [PubMed] [Google Scholar]
- Torres-Quesada M., Fúnes M. J., & Lupiáñez J. (2013). Dissociating proportion congruent and conflict adaptation effects in a Simon-Stroop procedure. Acta Psychologica, 142, 203–210. [DOI] [PubMed] [Google Scholar]
- Tseng C. H., McNeil M. R., & Milenkovic P. (1993). An investigation of attention allocation deficits in aphasia. Brain and Language, 45, 276–296. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P., & De Meersman L. (1998). Aging and the Stroop effect: A meta-analysis. Psychology and Aging, 13, 120–126. [DOI] [PubMed] [Google Scholar]
- Vitkovitch M., Bishop S., Dancey C., & Richards A. (2002). Stroop interference and negative priming in patients with multiple sclerosis. Neuropsychologia, 40, 1570–1576. [DOI] [PubMed] [Google Scholar]
- Wambaugh J. L., Duffy J. R., McNeil M. R., Robin D. A., & Rogers M. A. (2006). ANCDS bulletin board: Treatment guidelines for acquired apraxia of speech; A synthesis and evaluation of the evidence. Journal of Medical Speech-Language Pathology, 14(2), xv–xxxiii. [Google Scholar]
- Wechsler D. (1997). Wechsler Adult Intelligence Scale–Third Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- West R., & Alain C. (1999). Event-related neural activity associated with the Stroop task. Cognitive Brain Research, 8, 157–164. [DOI] [PubMed] [Google Scholar]
- Wiener D. A., Conner L. T., & Obler L. K. (2004). Inhibition and auditory comprehension in Wernicke's aphasia. Aphasiology, 18, 599–609. [Google Scholar]
- Wright H. H., Downey R. A., Gravier M., Love T., & Shapiro L. P. (2007). Processing distinct linguistic information types in working memory in aphasia. Aphasiology, 21, 802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H. H., & Fergadiotis G. (2012). Conceptualising and measuring working memory and its relationship to aphasia. Aphasiology, 26, 258–278. [DOI] [PMC free article] [PubMed] [Google Scholar]