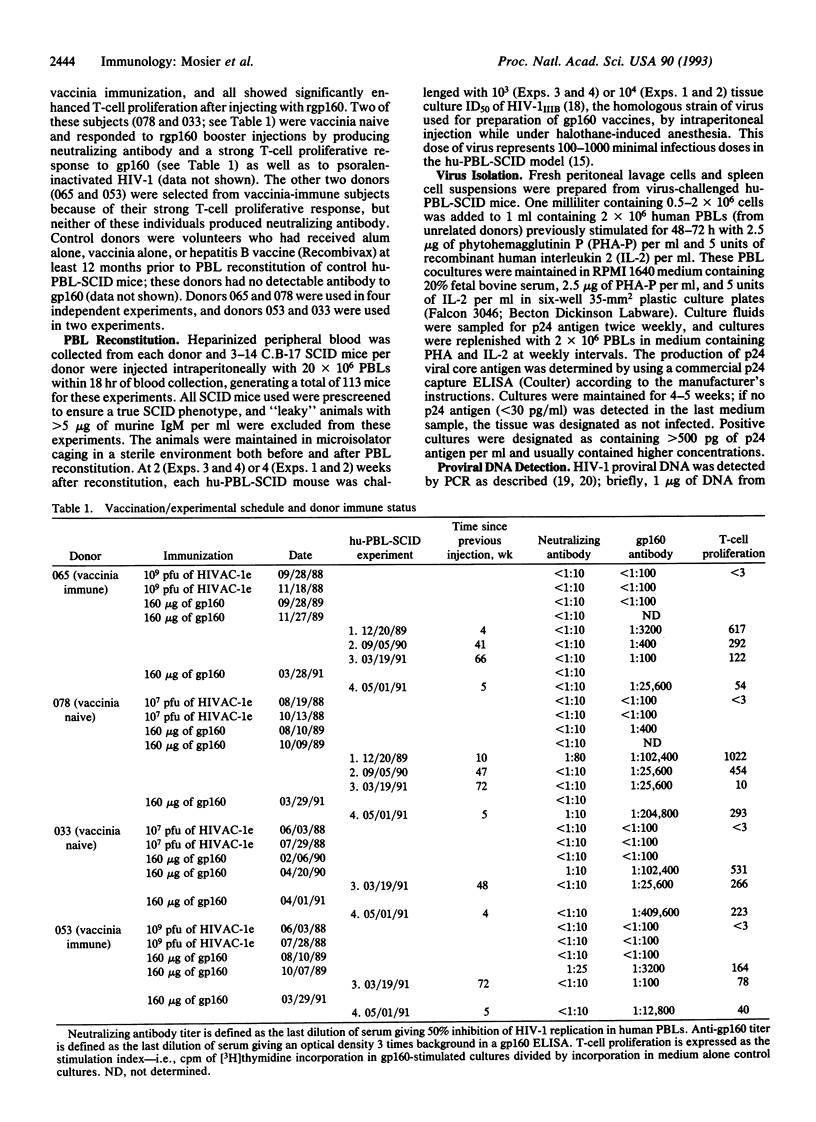
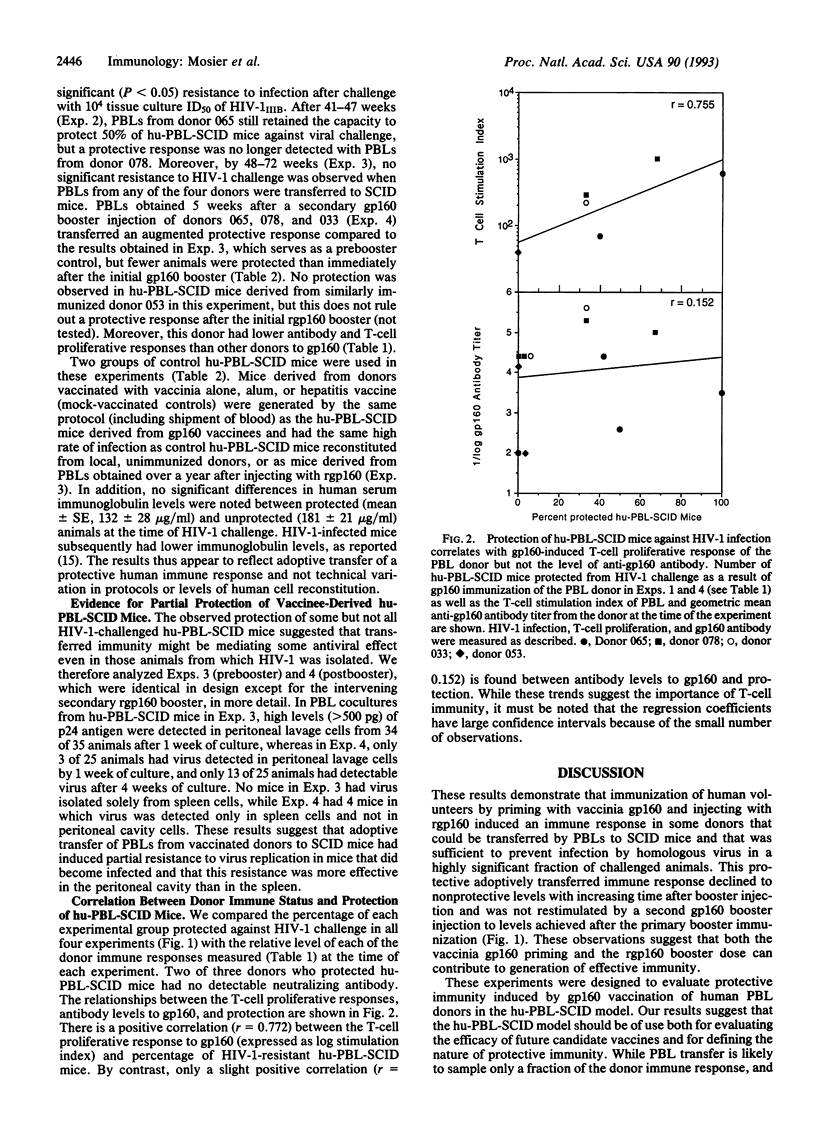
Abstract
SCID mice reconstituted with adult human peripheral blood leukocytes (hu-PBL-SCID mice) make antigen-specific human antibody responses following secondary immunization and can be infected with human immunodeficiency virus 1 (HIV-1), suggesting that they might prove useful for evaluating protective immunity to HIV-1 following vaccination of PBL donors. HIV-seronegative volunteers were immunized with vaccinia expressing HIV-1LAV-1/Bru 160-kDa envelope glycoprotein (vaccinia gp160) and subsequently given booster injections of recombinant gp160 protein (rgp160). Their PBLs were used at intervals of 4-72 weeks after booster injections to construct hu-PBL-SCID mice, which were then challenged with 10(2)-10(3) minimal animal infectious doses of highly homologous HIV-1IIIB. Control hu-PBL-SCID mice were constructed from donors receiving vaccinia, alum, or hepatitis B vaccine. Protection against virus infection was defined as the absence of HIV-1 by culture and no detection of proviral genomes following PCR amplification. Control animals were highly susceptible to HIV infection. By contrast, hu-PBL-SCID mice reconstituted with cells from three of four donors immunized with vaccinia gp160 and recently injected with rgp160 showed no evidence of HIV-1 infection by culture or PCR assays. With increasing time after rgp160 injection, the ability of vaccine-derived hu-PBL-SCID mice to resist HIV-1 infection diminished. These results demonstrate that a potentially protective human immune response was stimulated by this HIV gp160 immunization protocol and show the utility of the hu-PBL-SCID model in the rapid evaluation of candidate vaccines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur L. O., Bess J. W., Jr, Waters D. J., Pyle S. W., Kelliher J. C., Nara P. L., Krohn K., Robey W. G., Langlois A. J., Gallo R. C. Challenge of chimpanzees (Pan troglodytes) immunized with human immunodeficiency virus envelope glycoprotein gp120. J Virol. 1989 Dec;63(12):5046–5053. doi: 10.1128/jvi.63.12.5046-5053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P. W., Gregory T. J., Riddle L., Nakamura G. R., Champe M. A., Porter J. P., Wurm F. M., Hershberg R. D., Cobb E. K., Eichberg J. W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990 Jun 14;345(6276):622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- Clerici M., Via C. S., Lucey D. R., Roilides E., Pizzo P. A., Shearer G. M. Functional dichotomy of CD4+ T helper lymphocytes in asymptomatic human immunodeficiency virus infection. Eur J Immunol. 1991 Mar;21(3):665–670. doi: 10.1002/eji.1830210319. [DOI] [PubMed] [Google Scholar]
- Cooney E. L., Collier A. C., Greenberg P. D., Coombs R. W., Zarling J., Arditti D. E., Hoffman M. C., Hu S. L., Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991 Mar 9;337(8741):567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Wyand M. S., Kodama T., Ringler D. J., Arthur L. O., Sehgal P. K., Letvin N. L., King N. W., Daniel M. D. Vaccine protection against simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6353–6357. doi: 10.1073/pnas.86.16.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin R., Graham B. S., Greenberg S. B., Tacket C. O., Belshe R. B., Midthun K., Clements M. L., Gorse G. J., Horgan B. W., Atmar R. L. The safety and immunogenicity of a human immunodeficiency virus type 1 (HIV-1) recombinant gp160 candidate vaccine in humans. NIAID AIDS Vaccine Clinical Trials Network. Ann Intern Med. 1991 Jan 15;114(2):119–127. doi: 10.7326/0003-4819-114-2-119. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Powell D., Lightfoote M., Koenig S., Fauci A. S., Benn S., Rabson A., Daugherty D., Gendelman H. E., Hoggan M. D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986 Jul 1;164(1):280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. J., Jr, Peters R., Gravell M., Johnson B. K., Jensen F. C., Carlo D. J., Salk J. Observations after human immunodeficiency virus immunization and challenge of human immunodeficiency virus seropositive and seronegative chimpanzees. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3348–3352. doi: 10.1073/pnas.88.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Kieny M. P., Pinter A., Barre-Sinoussi F., Nara P., Kolbe H., Kusumi K., Chaput A., Reinhart T., Muchmore E. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. L., Fultz P. N., McClure H. M., Eichberg J. W., Thomas E. K., Zarling J., Singhal M. C., Kosowski S. G., Swenson R. B., Anderson D. C. Effect of immunization with a vaccinia-HIV env recombinant on HIV infection of chimpanzees. Nature. 1987 Aug 20;328(6132):721–723. doi: 10.1038/328721a0. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Kosowski S. G., Dalrymple J. M. Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature. 1986 Apr 10;320(6062):537–540. doi: 10.1038/320537a0. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Hoth D. F. Development and testing of AIDS vaccines. Science. 1988 Jul 22;241(4864):426–432. doi: 10.1126/science.3293212. [DOI] [PubMed] [Google Scholar]
- Kwok S., Mack D. H., Mullis K. B., Poiesz B., Ehrlich G., Blair D., Friedman-Kien A., Sninsky J. J. Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol. 1987 May;61(5):1690–1694. doi: 10.1128/jvi.61.5.1690-1694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Spector S., Spector D., Kipps T. J., Fox R. I., Carson D. A., Cooper N., Richman D. D. Studies of HIV infection and the development of Epstein-Barr virus-related B cell lymphomas following transfer of human lymphocytes to mice with severe combined immunodeficiency. Curr Top Microbiol Immunol. 1989;152:195–199. doi: 10.1007/978-3-642-74974-2_23. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. On the SCIDs? Nature. 1989 Mar 16;338(6212):211–211. doi: 10.1038/338211b0. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B., Spector D. H., Spector S. A. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991 Feb 15;251(4995):791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Namikawa R., Kaneshima H., Lieberman M., Weissman I. L., McCune J. M. Infection of the SCID-hu mouse by HIV-1. Science. 1988 Dec 23;242(4886):1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Orentas R. J., Hildreth J. E., Obah E., Polydefkis M., Smith G. E., Clements M. L., Siliciano R. F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990 Jun 8;248(4960):1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Redfield R. R., Birx D. L., Ketter N., Tramont E., Polonis V., Davis C., Brundage J. F., Smith G., Johnson S., Fowler A. A phase I evaluation of the safety and immunogenicity of vaccination with recombinant gp160 in patients with early human immunodeficiency virus infection. Military Medical Consortium for Applied Retroviral Research. N Engl J Med. 1991 Jun 13;324(24):1677–1684. doi: 10.1056/NEJM199106133242401. [DOI] [PubMed] [Google Scholar]
- Spector S. A., Hsia K., Denaro F., Spector D. H. Use of molecular probes to detect human cytomegalovirus and human immunodeficiency virus. Clin Chem. 1989 Aug;35(8):1581–1587. [PubMed] [Google Scholar]
- Sutjipto S., Pedersen N. C., Miller C. J., Gardner M. B., Hanson C. V., Gettie A., Jennings M., Higgins J., Marx P. A. Inactivated simian immunodeficiency virus vaccine failed to protect rhesus macaques from intravenous or genital mucosal infection but delayed disease in intravenously exposed animals. J Virol. 1990 May;64(5):2290–2297. doi: 10.1128/jvi.64.5.2290-2297.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Cheynier R., Desportes I., Leonard R., Fouchard M., Reveil B., Ittele D., Lurhuma Z., Mbayo K. A group specific anamnestic immune reaction against HIV-1 induced by a candidate vaccine against AIDS. Nature. 1988 Apr 21;332(6166):728–731. doi: 10.1038/332728a0. [DOI] [PubMed] [Google Scholar]




