Abstract
Background
Left ventricular (LV) trabeculation is highly variable between individuals, is increased in some diseases (e.g. congenital heart disease or cardiomyopathies), but its significance in population representative individuals is unknown.
Objectives
To determine if excessive LV trabeculation in population representative subjects is associated with preceding changes in cardiac volumes and function.
Methods
The extent of trabeculation, expressed as the ratio of non-compacted to compacted (NC/C) myocardium was measured for technical reasons on cardiac magnetic resonance (CMR) long-axis cine images in 2742 subjects in the Multi-Ethnic Study of Atherosclerosis (mean age 68.7 years, 52.3% women, 56.4% with hypertension, 16.8% with diabetes) at the exam 5. These were considered in quintiles of trabeculation extent, with quintile 5’s NC/C 2.46 – 5.41. We determined the relationship between maximal NC/C ratio and preceding change (9.5 year between exam 1 and 5) in end-systolic volume indexed to the body surface area (ESVi). Secondary analysis assessed associations between maximal NC/C and preceding changes in end-diastolic volume indexed to the body surface area (EDVi) and ejection fraction (EF).
Results
Over 9.5 years, ESVi decreased by 1.3 ml/m2, EDVi decreased by 5.1 ml/m2 and EF decreased by 0.6% (p<0.0001). There were no clinically relevant differences in LV volumes and systolic function change between the quintiles of trabeculation extent, even in subjects with the excessive trabeculation.
Conclusions
Greater extent of and even excessive LV trabeculations measured in end-diastole in asymptomatic population representative individuals appears benign and is not associated with deterioration in left ventricular volumes or function over an almost 10 year period.
Introduction
Human left ventricular (LV) cardiac trabeculation is highly variable between individuals. Although some of these differences may be related to ethnicity (1), there have been concerns that extreme trabeculation may be either pathologic or a marker of underlying heart muscle disease. Left ventricular non-compaction (LVNC) is considered to be a distinct form of cardiomyopathy (2, 3) where the hallmark phenotypic feature is extensive LV trabeculation. The disease may lead to cardiac failure, thromboembolism, and malignant arrhythmias. To date, only small studies using cardiovascular magnetic resonance (CMR) have described patterns and extent of LV trabeculations in cohorts with a probable diagnosis of LVNC based on symptoms, family history or impaired cardiac function. (4, 5) However, increased LV trabeculation is also associated with other cardiac conditions such as cardiomyopathies (6) and congenital heart diseases (7), but it has also been frequently observed in healthy individuals. (8) Extensive LV trabeculation is commonly detected following CMR imaging. When LVNC imaging diagnostic criteria are met as an incidental finding, a diagnosis of LVNC remains controversial. The natural history and the outcomes in people with pronounced LV trabeculation in the absence of any other structural heart abnormalities is unknown.
This background, combined with difficulties in measuring trabeculae have raised concerns that extensive trabeculation in apparently normal individuals may be either a pre-phenotypic marker of underlying disease, a marker of adverse outcome or just a normal phenotypic variant.
Accordingly, individuals with extensive trabeculation may be offered costly long-term follow-up and are subject to the emotional and financial implications of a cardiomyopathy diagnosis.
The purpose of this study was to determine the relationship between the extent of LV trabeculation (using CMR) and myocardial structure and function in a large population based cohort study. Specifically, our primary aim was to evaluate if excessive LV trabeculation, measured as the maximal non-compaction to compaction (NC/C) ratio, was associated with preceding changes in end-systolic volume indexed to the body surface area. In secondary analysis we evaluated associations between maximal NC/C and preceding changes in end-diastolic volume indexed to the body surface area and development of LV dysfunction expressed by deterioration in LV ejection fraction.
Methods
Study population
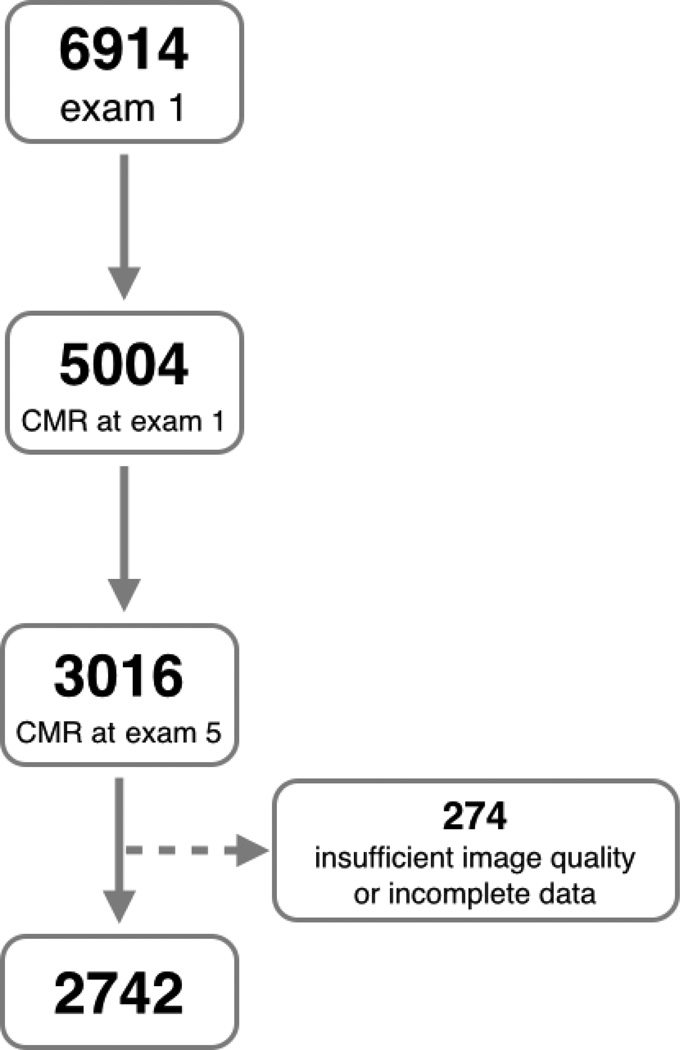
The Multi-Ethnic Study of Atherosclerosis (MESA) is a population-based prospective cohort study. At enrollment between 2000 and 2002 (exam 1), 5004 of the 6814 study participants free of clinically recognized cardiovascular disease from four different ethnicities underwent CMR imaging. (9) Of these, 3016 participants underwent CMR imaging between 2010 and 2011 (exam 5). Study subjects were excluded due to insufficient image quality (n=241) and incomplete CMR data sets (n=33), leaving 2742 participants (Figure 1). Clinical data, including the incidence of heart failure, atrial fibrillation, myocardial infarction, stroke and transient ischemic attacks were available for all participants. MESA criteria for clinical events and follow-up procedures have been previously described. (10) Institutional Review Boards of each of the 6 participating field sites in the United States approved the study, and all participants provided written informed consent at the time of enrollment into MESA.
Figure 1. Flow diagram of exclusion process.
Exclusion criteria of subjects from the Multi-Ethnic Study of Atherosclerosis (MESA) for this study. CMR – cardiovascular magnetic resonance.
Magnetic resonance imaging
CMR examinations were performed at 6 centers (in Baltimore, Winston-Salem, New York, Minneapolis, Los Angeles, Chicago) using either a Signa Excite (General Electric Medical Systems, Waukesha, WI) or an Avanto/Espree (Siemens, Erlangen, Germany) 1.5-Tesla MR scanners for exams 1 and 5. Planning of the cardiac cine images for both exams was standardized in order to minimize variation between centers. Cine images were obtained with a temporal resolution of approximately 50 milliseconds or less using segmented k-space, electrocardiogram-gated fast spoiled gradient recalled echo (GRE) pulse sequence during MESA exam 1 (11) and retrospectively electrocardiogram-gated long- and short-axis cine images acquired using a steady-state free precession (SSFP) sequence at MESA exam 5. (12)
Image Evaluation
LV volumes and function
All MESA exam 1 and exam 5 CMR images (Figure 2) were analyzed for LV volumes and function in a core laboratory and analyzed at a single image analysis center by readers blinded to clinical outcomes as previously described. (11, 12) Calibration between the two CMR examinations was performed in 498 participants who had both image sequences acquired at MESA exam 5. The calibration group was selected to be representative of all body sizes. Calibration was performed for both technologist readers (by re-reading 498 MESA exam 1 images) as well as pulse sequence (same technologist reader, analyzing both pulse sequences using identical software). All calibration curves were found to be linear and were fitted with ordinary regression methods.
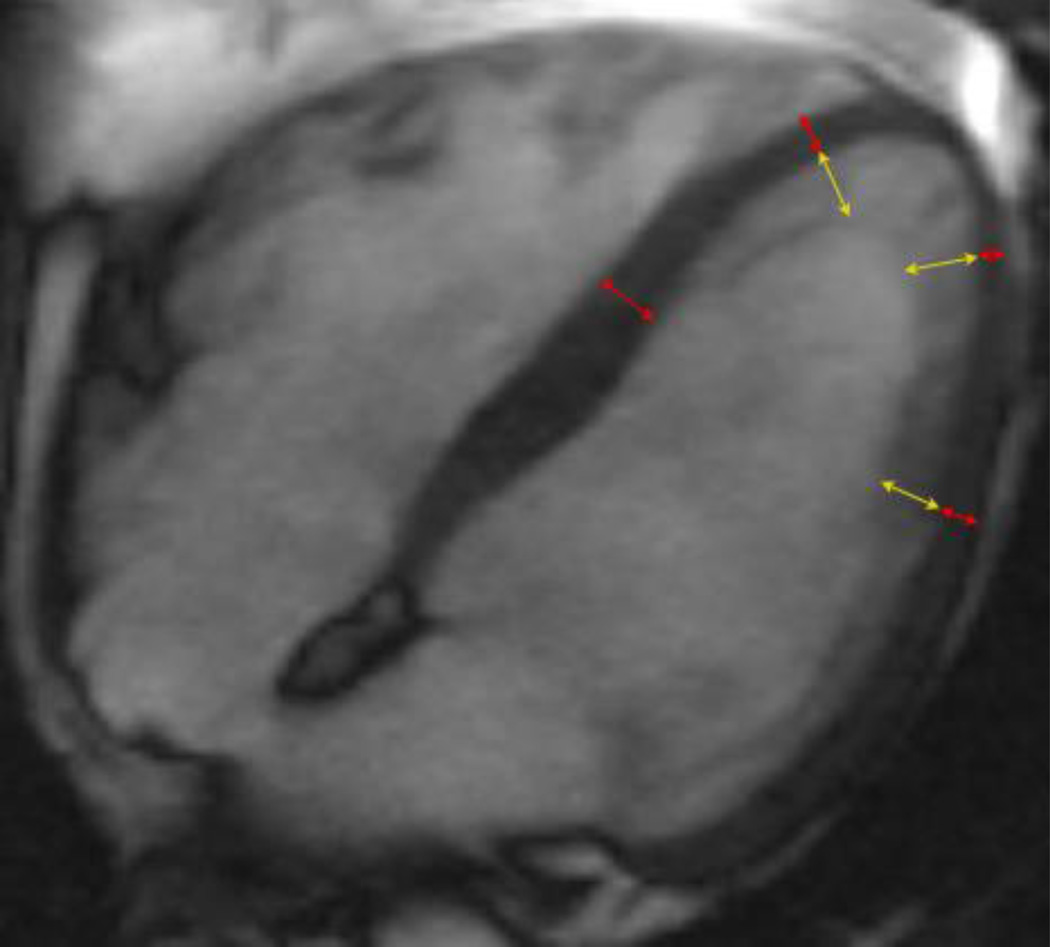
Figure 2. Measurement of NC/C ratios.
Example of end-diastolic four chamber steady state free precession image of a participant with very high maximal NC/C ratio (=4.2) in the Multi-Ethnic Study of Atherosclerosis exam 5. Red arrows show measurements of compacted myocardium, yellow arrows represent measurements of the non-compacted (trabeculated) layer.
For quality control purposes, all readers independently analyzed every tenth consecutive CMR exam. The overall interobserver intraclass correlation coefficients for LV mass and LV end-diastolic volume were 0.95 and 0.96, respectively, and technical errors of measurement were 6.1% and 5.4%, respectively. The inter-observer agreement was similar to those of the baseline study. (11)
LV trabeculation
CMR examinations were evaluated for NC/C ratio using the post-processing software tool CVI42 (Circle Cardiovascular Imaging Inc, Calgary, Canada). NC/C ratio could only be determined for the MESA exam 5 CMR data, as the CMR sequence used at MESA exam 1 does not provide the level of detail and contrast required. There are different approaches to assessing the extent of LV trabeculation in both echocardiography and CMR. We restricted our analysis to the NC/C ratio as proposed by Petersen et al. (4)
Horizontal and vertical long axis cine SSFP images were used for measuring the thickness of the compacted myocardium and of the trabeculations at the center of 8 LV regions: anterior, inferior, septal, and lateral at mid-ventricular and apical levels at end-diastole as previously described by Kawel. (8) (Figure 2) The NC/C ratio was calculated for each segment. Measurements were not performed at the LV base, as trabeculations are typically not observed in this region. In normal subjects, the true apex is usually very thin and has prominent trabeculations, therefore, it was also excluded. (4, 13) Compacted myocardium was defined as a myocardial layer of homogeneous moderate signal intensity on SSFP images without inclusion of blood of higher signal intensity. Trabeculations were defined as a meshwork of the trabeculae carneae of moderate signal intensity adjacent to compacted myocardium interspersed with blood of higher signal intensity. Measurements of the thickness of the compacted myocardium as well as of the adjacent trabeculations were obtained perpendicular to the compacted myocardium. Fifty percent of the thickness of chemical shift artifact (appearing as a black line) on the epicardial surface was included in the compact myocardium. Papillary muscles that were clearly observed as compact tubular structures were not included in the measurements. Short-axis views and cine mode were used additionally to separate papillary muscles from trabeculation. The orientation of long axis images was cross-referenced with short axis views allowing for exclusion of off-axis images. Measurements of 60 randomly selected studies were repeated by the first reader and by a second reader to quantify intra- and inter-observer variability. The NC/C ratio > 2.3 was considered a current diagnostic criterion for LVNC (Petersen’s criterion). (4)
Statistical Analysis
Unless otherwise stated -, descriptive statistics for continuous variables are presented as mean and the standard deviation (SD) if normally distributed. Categorical variables are presented as a percentage. Differences between quintiles were evaluated by the analysis of variance (ANOVA) with post-hoc Tukey test for continuous variables and with chi-squared test for categorical variables.
Simple and multivariate linear regression models were developed to examine the relationship of the independent variables (maximal NC/C ratio, NC/C ratio >2.3 in one segment and NC/C ratio >2.3 in more than one segment) to functional continuous dependent variables related to heart failure, which included changes in LV volumes and ejection fraction between exam 1 and exam 5. Covariates used for multivariable regression models are listed in table 2.
Table 2. Regression models with maximal NC/C ratio, demographic data, CMR data and classic risk factors.
Univariate and multivariate linear regression analysis models for the changes in the end-diastolic volume, end-diastolic volume index and ejection fraction between MESA exam 1 and 5 as dependent variables in models incorporating maximal NC/C ratio, demographic data, baseline CMR parameters at MESA exam 1 and traditional risk factors as predictor variables.
| Exposure Variable |
Outcome variables (change between exam 1 and exam 5) |
Univariable regression | Multivariable regression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| Beta (95%CI) | R2 | Beta (95%CI) | R2 | Beta (95%CI) | R2 | Beta (95%CI) | R2 | ||
| Maximal NC/C ratio |
End-diastolic volume index (ml/m2) |
1.6*** (0.9 to 2.3) |
0.008 | 1.9*** (1.2 to 2.6) |
0.034 | 2.7*** (2.1 to 3.3) |
0.261 | 2.6*** (2.0 to 3.3) |
0.269 |
| End-systolic volume index (ml/m2) |
0.3 (−0.1 to 0.8) |
0.0009 | 0.5* (0.1 to 0.9) |
0.026 | 1.0*** (0.6 to 1.4) |
0.195 | 1.0*** (0.6 to 1.4) |
0.200 | |
| Ejection fraction (%) | 0.4 (−0.1 to 0.8) |
0.0009 | 0.3 (−0.1 to 0.7) |
0.013 | −0.2 (−0.6 to 0.2) |
0.324 | −0.2 (−0.6 to 0.1) |
0.320 | |
Model 1: adjusted for age, gender, ethnicity
Model 2: adjusted for model1 parameters plus baseline EDVi (for change in EDVi), ESVi (for change in ESVi) and EF (for change in EF)
Model 3: adjusted for model 2 parameters plus diabetes, smoking history, total cholesterol, systolic blood pressure and BMI.
p<0.05,
p<0.01,
p<0.001,
95%CI – 95% confidence intervals, EDVi – end-diastolic volume index, EF – ejection fraction, ESVi – end-systolic volume index, m – meter, ml – millilitre, NC/C – non-compaction to compaction
All statistical analyses were performed using R software version 3.0.1 (R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/). Intra-class correlation coefficients were used to evaluate intra- and inter-observer agreement. In all cases statistical significance was set for a p<0.05 (two-tailed).
Results
Demographics
Detailed demographic and CMR data are presented in Table 1. The mean age of study subjects at exam 5 was 68.7 years (52.3% women). Ethnicity was self-reported as Caucasian/white in 42.1%, Chinese American in 12.5%, African-American/black in 24.9% and Hispanic in 20.5%. Hypertension was present in 56.4% of participants. At exam 5, 536 (19.5%) of the study subjects were treated with angiotensin converting enzyme inhibitors, 248 (9.0%) with angiotensin II antagonists, 475 (17.3%) with beta-blockers and 1035 (37.7%) subjects were treated with one or more of the above agents. Four hundred and twenty three participants (15.5%) had treated diabetes and 1456 (53.3%) were current or former smokers. Impaired systolic function at baseline exam 1 (ejection fraction <50%) was present in 111 (4.0%) subjects, but they were free from symptoms and there were no differences between quintiles of maximal NC/C ratio (p=0.62).
Table 1. Demographic and Cardiovascular Magnetic Resonance data at MESA exam 5.
Subjects with the highest trabelulation (Quintile 5) had larger left ventricular volumes, end-diastolic mass index, blood pressure values and lower incidence of treated diabetes, weight and body mass index.
| All | Quintile 1 NC/C: 0 – 1.41 |
Quintile 2 NC/C: 1.42 – 1.71 |
Quintile 3 NC/C: 1.72 – 2.00 |
Quintile 4 2.01 – 2.45 |
Quintile 5 2.46 – 5.41 |
p | |
|---|---|---|---|---|---|---|---|
| n= | 2742 | 549 | 548 | 548 | 548 | 549 | |
| Age | 68.7 ± 9.1 | 69.1 ± 9.2 | 69.1 ± 9.3 | 68.6 ± 8.9 | 68.6 ± 9.0 | 68.0 ± 9.2 | 0.29 |
| Females | 1435 (52.3%) | 266 (48.5%) | 286 (52.2%) | 261 (47.6%) | 304 (55.5%) | 318 (57.9%) | <0.01 |
| Race | |||||||
| Caucasian | 1154 (42.1%) | 230 (41.9%) | 242 (44.2%) | 207 37.8%) | 238 (43.4%) | 237 (43.2%) | 0.49 |
| Chinese American | 343 (12.5%) | 60 (10.9%) | 75 (13.7%) | 82 (15.0%) | 69 (12.6%) | 57 (10.4%) | 0.18 |
|
Black, African- American |
682 (24.9%) | 148 (27.0%) | 141 (25.7%) | 139 (25.4%) | 131 (23.9%) | 123 (22.4%) | 0.61 |
| Hispanic | 563 (20.5%) | 111 (20.2%) | 90 (16.4%) | 120 (21.9%) | 110 (20.1%) | 132 (24.0%) | 0.07 |
| Body mass index | 27.9 ± 5.1 | 28.3 ± 5.2 | 28.1 ± 5.2 | 28.1 ± 5.3 | 28.0 ± 5.2 | 27.2 ± 4.7 | <0.01 |
| Height (cm) | 165.7 ± 9.8 | 166.0 ± 9.8 | 165.5 ± 9.5 | 165.7 ± 10.1 | 165.7 ± 10.0 | 165.5 ± 9.7 | 0.91 |
| Weight (kg) | 76.9 ± 16.5 | 78.0 ± 16.0 | 77.3 ± 17.1 | 77.5 ± 17.5 | 77.0 ± 16.5 | 74.8 ± 15.4 | <0.05 |
| Hypertension | 1546 (56.4%) | 323 (58.8%) | 326 (59.5%) | 314 (57.3%) | 305 (55.8%) | 278 (50.6%) | <0.05 |
| Systolic blood pressure (mmHg) |
122.6 ± 20.0 | 123.7 ± 21.1 | 125.7 ± 20.8 | 122.9 ± 20.3 | 121.4 ± 19.0 | 119.4 ± 18.1 | <0.0001 |
| Diastolic blood pressure (mmHg) |
68.3 ± 9.9 | 69.0 ± 9.8 | 69.0 ± 10.4 | 68.8 ± 9.8 | 67.6 ± 9.7 | 67.0 ± 9.7 | <0.001 |
|
Diabetes by 2003 ADA (n=2613) |
|||||||
| Normal | 1702 (62.5%) | 337 (61.7%) | 319 (58.5%) | 329 (60.5%) | 341 (62.5%) | 376 (69.2%) | 0.24 |
| Impaired Fasting Glucose |
564 (20.7%) | 104 (19.0%) | 122 (22.4%) | 118 (21.7%) | 118 (21.6%) | 102 (18.8%) | 0.57 |
| Untreated Diabetes | 35 (1.3%) | 8 (1.5%) | 7 (1.3%) | 8 (1.5%) | 5 (0.9%) | 7 (1.3%) | 0.93 |
| Treated Diabetes | 423 (15.5%) | 97 (17.8%) | 97 (17.8%) | 89 (16.4%) | 82 (15.0%) | 58 (10.7%) | <0.05 |
| Family history of a heart attack |
1127 (43.5%) | 240 (46.2%) | 226 (44.1%) | 206 (39.9%) | 243 (46.6%) | 212 (40.8%) | 0.1 |
| Coronary disease | 96 (3.5%) | 27 (4.9%) | 14 (2.6%) | 15 (2.7%) | 19 (3.5%) | 21 (3.8%) | 0.21 |
| Smoking | |||||||
| Never | 1275 (46.7%) | 242 (44.2%) | 260 (47.7%) | 262 (48.1%) | 247 (45.2%) | 264 (48.4%) | 0.82 |
| Former | 1249 (45.7%) | 260 (47.4%) | 240 (44.0%) | 245 (45.0%) | 262 (47.9%) | 242 (44.3%) | 0.78 |
| Current | 207 (7.6%) | 46 (8.4%) | 45 (8.3%) | 38 (7.0%) | 38 (6.9%) | 40 (7.3%) | 0.84 |
| Education | |||||||
| No school | 9 (0.3%) | 3 (0.5%) | 0 | 0 | 3 (0.5%) | 3 (0.5%) | - |
| Grades 1–8 | 196 (7.2%) | 39 (7.1%) | 45 (8.2%) | 38 (7.0%) | 32 (5.9%) | 42 (7.7%) | 0.66 |
| Grades 9–11 | 119 (4.3%) | 26 (4.7%) | 22 (4.0%) | 31 (5.7%) | 17 (3.1%) | 23 (4.2%) | 0.34 |
|
Completed High School |
445 (16.3%) | 87 (15.8%) | 73 (13.4%) | 98 (17.9%) | 101 (18.5%) | 86 (15.7%) | 0.24 |
|
Some College but no degree |
435 (15.9%) | 84 (15.3%) | 90 (16.5%) | 92 (16.8%) | 98 (17.9%) | 71 (12.9%) | 0.31 |
|
Technical School Certificate |
203 (7.4%) | 39 (7.1%) | 44 (8.1%) | 44 (8.1%) | 40 (7.3%) | 36 (6.6%) | 0.88 |
| Associate Degree | 145 (5.3%) | 26 (4.7%) | 36 (6.6%) | 26 (4.8%) | 25 (4.6%) | 32 (5.8%) | 0.53 |
| Bachelor’s Degree | 555 (20.3%) | 116 (21.1%) | 110 (21.1%) | 95 (17.4%) | 109 (19.9%) | 125 (22.8%) | 0.36 |
|
Graduate or Professional School |
630 (23.0%) | 129 (23.5%) | 126 (23.1%) | 122 (22.3%) | 122 (22.3%) | 131 (23.9%) | 0.97 |
| LV end-diastolic volume index (ml/m2) |
65.2 ± 13.6 | 62.4 ± 14.3 | 63.5 ± 13.4 | 65.7 ± 13.1 | 66.0 ± 13.3 | 68.3 ± 13.2 | <0.0001 |
| LV end-systolic volume index (ml/m2) |
25.0 ± 8.5 | 24.1 ± 9.3 | 23.9 ± 8.0 | 25.4 ± 8.6 | 25.5 ± 8.4 | 26.3 ± 8.0 | <0.0001 |
| LV ejection fraction (%) |
62.0 ± 7.3 | 62 ± 7.4 | 62.8 ± 7.3 | 61.7 ± 7.8 | 61.7 ± 6.8 | 61.9 ± 7.0 | 0.1 |
| LV mass index (g/m2) | 66.4 ± 13.8 | 68.1 ± 14.3 | 68.2 ± 14.9 | 67.3 ± 14.0 | 65.1 ± 12.8 | 63.1 ± 12.4 | <0.0001 |
ADA – American Diabetes Association, cm – centimetre, g – gram, kg – kilogram, LV – left ventricle, m – meter, mmHg – millimetres Mercury, NC/C – non-compaction to compaction ratio
Extent of LV trabeculation
The NC/C ratio was calculated in 19,320 (88.1%) segments; 2,616 (11.9%) segments were excluded because of insufficient contrast between blood pool and myocardium to confidently measure the NC/C ratios. The intra-class correlation coefficient for intra-observer NC/C ratio measurements was 0.83 (p<0.0001) and for inter-observer measurements was 0.82 (p<0.0001). Petersen’s LVNC criterion (NC/C >2.3) was fulfilled in 706 (25.7%) of participants for at least 1 cardiac segment and in 218 (8.0%) for at least 2 segments.
The mean of the maximal NC/C ratio of each subject’s analyzed segments was 1.96 ± 0.66. This was higher in women: 2.0 ± 0.68 vs. 1.92 ± 0.64 (p<0.001), but was independent of age (p=0.051). There were no differences in the maximal NC/C ratio between the four ethnicities studied in MESA: 1.98 ± 0.69 in Caucasians, 1.91 ± 0.56 in Chinese Americans, 1.93 ± 0.66 in African-Americans and 2.00 ± 0.65 in Hispanics (p=0.08).
The maximal NC/C ratio correlated with the number of segments fulfilling LVNC criteria (r=0.76, p<0.0001) and was greater in larger LV cavities: the ratio increased by 0.2 ± 2.1 for each 100 ml larger end-diastolic volume (p<0.0001) and by 0.3 ± 3.6 for each 100 ml larger end-systolic volume (p<0.0001). There was no association of maximal NC/C ratio with LV ejection fraction (p=0.16).
Relationship between extent of trabeculation and precedent changes in LV volumes and ejection fraction
We divided the cohort into quintiles by the extent of maximal NC/C ratio. Detailed demographic data and CMR parameters are presented in table 1.
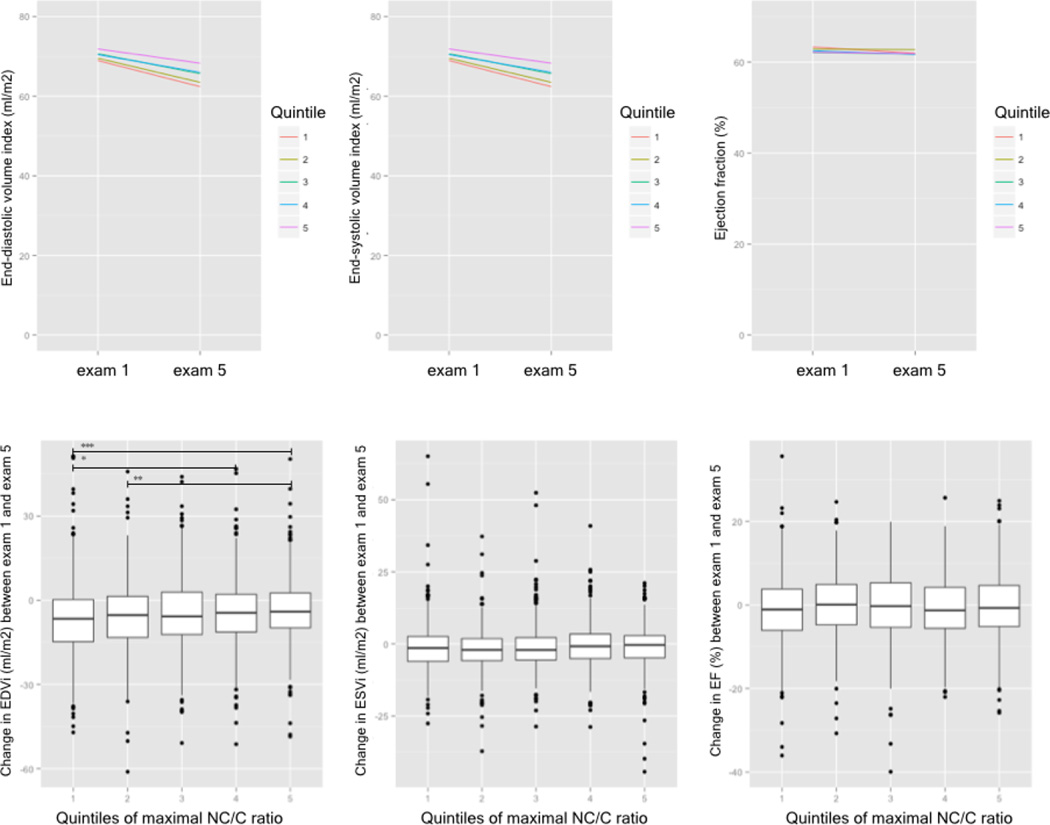
In the 9.5 years interval between exam 1 and 5, end-systolic volume index decreased on average by 1.3 ± 7.3 ml/m2 (p<0.0001), and there were no differences between quintiles of maximal NC/C ratio (Figure 3).
Figure 3. Changes in cardiac volumes and function.
Changes in left ventricular volumes and function between MESA exam 1 and exam 5 and the relationship to the extent of LV trabeculation (in quintiles) at exam 5 presented in two different ways: The bottom panel shows box and whisker plots and the lack of clinically relevant change in volume or function; the top panel shows similar slopes for the quintiles of trabeculation extent with regards to volumes and function between both exams. Boxes represent the interquartile range (IQR) and whiskers are within 1.5 *IQR, outliers are plotted as points. The line within the box represents the median. NC/C non-compaction to compaction. * - p<0.05; ** - p<0.01; *** - p<0.001
Similarly, end-diastolic volume index decreased on average by 5.1 ± 12.3 ml/m2 (p<0.0001). The decrease was smaller with increasing extent of trabeculation; significant differences were seen between quintile 1 and 4 (−6.5 ± 13.1 ml/m2 vs. −4.4 ± 12.3 ml/m2, p<0.05), between quintile 1 and 5 (−6.5 ± 13.1 ml/m2 vs. −3.6 ± 11.3 ml/m2, p<0.001) and also between quintile 2 and 5 (−6.0 ± 12.4 ml/m2 vs. −4.4 ± 12.3 ml/m2, p<0.01). (Figure 3)
The ejection fraction decreased by 0.6 ± 7.8% (p<0.0001) over 9.5 years, however there were no differences between the quintiles of maximal NC/C ratio (Figure 3). Several demographic parameters and clinical characteristics differed by small amounts but reached statistical significance in univariate analyses with regards to the extent or distribution between quintiles of maximal NC/C ratio (Table 1).
After adjustment for age, gender, ethnicity and baseline CMR parameters from exam 1 (model 2), one unit greater maximal NC/C ratio (from 1 to 2, from 2 to 3, etc.) was associated with 2.7 ± 16.1 ml/m2 (p<0.0001) smaller decrease in end-diastolic volume index and 1.0 ± 10.2 ml/m2 (p<0.0001) smaller decrease in end-systolic volume index. These models accounted for 26.1% and 19.5% of the variances in the volume changes respectively (Table 2). Multivariate regression models with conventional risk factors (body mass index, systolic blood pressure, diabetes, smoking, total cholesterol to high density lipoprotein ratio) (Table 2) as well as exercise, education and the family history of heart attack showed similar findings to model 2 (data not presented). Overall, the observed changes in end-diastolic volume index and end-systolic volume index in relationship to NC/C were not clinically relevant despite statistical significance. The maximal NC/C ratio was not associated with a change in LV ejection fraction.
Sensitivity analysis
The relationships described above for maximal NC/C ratio as a continuous variable were repeated using NC/C ratio >2.3 and number of segments with NC/C ratio >2.3. All results showed the same trends as for the continuous NC/C ratio variable.
Extent of LV trabeculation and adverse clinical events
The incidence of atrial fibrillation, congestive cardiac failure, stroke, transient ischaemic attack, history of myocardial infarction, composite cardiovascular endpoints and all cardiovascular endpoints in quintiles of maximal NC/C ratio are presented in table 3. In view of low event incidence no formal statistical analysis was performed.
Table 3. Cardiovascular adverse outcomes in relation to the extent LV trabeculations.
The incidence of cardiovascular adverse outcomes in the whole studied cohort and quintiles of maximal NC/C ratio.
| All | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
|---|---|---|---|---|---|---|
| Atrial fibrillation | 24 | 10 (1.9%) | 7 (1.3%) | 1 (0.2%) | 3 (0.6%) | 3 (0.6%) |
| Congestive heart failure | 22 | 4 (0.7%) | 5 (0.9%) | 5 (0.9%) | 4 (0.7%) | 4 (0.7%) |
| Stroke | 28 | 4 (0.7%) | 8 (1.5%) | 10 (1.9%) | 2 (0.4%) | 4 (0.7%) |
| Transient ischaemic attack | 17 | 4 (0.7%) | 3 (0.6%) | 4 (0.7%) | 4 (0.7%) | 2 (0.4%) |
| Myocardial infarction | 41 | 15 (2.8%) | 2 (0.4%) | 8 (1.5%) | 9 (1.7%) | 7 (1.3%) |
| Hard cardiovascular endpoints |
68 | 19 (3.6%) | 10 (1.9%) | 18 (3.4%) | 11 (2.0%) | 10 (1.9%) |
| All cardiovascular endpoints | 121 | 31 (6.0%) | 21 (4.0%) | 25 (4.8%) | 20 (3.8%) | 24 (4.6%) |
Hard cardiovascular endpoints include: myocardial infarction, resuscitated cardiac arrest, stroke
All cardiovascular endpoints include hard cardiovascular endpoints plus: definite angina, probable angina followed by coronary revascularisation
Discussion
The long-term relationship between excess trabeculation of the left ventricle and change in myocardial function and structure was not previously known. This is the first study to show that the greater extent of LV trabeculation is not associated with an absolute increase in end-systolic and end-diastolic volumes over almost ten years of the MESA study. Greater NC/C ratio was not associated with a decline in systolic function.
These results advance our understanding of ventricular morphology in regards to “asymptomatic trabeculation” in relatively healthy individuals in the community. In particular, MESA subjects with greater trabeculation had only minor relative changes in left ventricular end-diastolic volume index compared to subjects with lesser trabeculation that, although statistically significant, were unlikely to have clinical implications.
Prevalence of imaging diagnosis LVNC
The prevalence of the disease LVNC has been estimated between 0.014 and 1.3% in the general population. (2, 14, 15) However, all echocardiographic or CMR-based imaging criteria for LVNC were established on preselected and symptomatic individuals with heart failure or cardiovascular complications. It is important to emphasize here that currently there is no diagnostic tool, neither genetic nor imaging, that can identify patients affected by LVNC with absolute certainty. The absence of a definitive gold standard is the main reason why cardiac imaging studies have limitations when attempting to determine their diagnostic accuracies on the basis of a likely rather than definitive diagnosis of LVNC.
Recent studies using high-resolution imaging techniques, such as multi-detector computed tomography and CMR, have shown the frequent presence of pronounced trabeculation reaching diagnostic thresholds for LVNC in healthy volunteers. (8, 16) This study, which extended Kawel’s analysis of 323 MESA participants free from cardiovascular disease to the whole MESA cohort, showed that only 25.7% fulfilled current diagnostic criteria for LVNC compared to the 43% described by Kawel. This discrepancy is likely to be partly related to software differences. CVI42 software used in this analysis allowed to identify “off-axis acquisitions” while the software used by Kawel did not provide a cross-reference tool.
Association of extent of LV trabeculation on adverse cardiac remodelling and clinical outcomes
Baseline LV dimensions and ejection fraction have been shown to be some of the most powerful predictors of survival in heart failure and in people without cardiovascular disease and are now well-established surrogate markers in heart failure trials. (17–20) In this study we evaluated the change in end-systolic volume index, end-diastolic volume index and ejection fraction. Our main findings were that maximal NC/C ratio and other measures of extent of LV trabeculation were associated with small changes in LV parameters over an almost 10 year of MESA study period, but these associations were clinically negligible. The fact that the multivariate regression model incorporating maximal NC/C ratio, demographic data and the baseline CMR data (Model 2, Table 2) increased the explained variance almost ten-fold when compared with the model incorporating only the extent of trabeculations and demographic factors (Model 1, Table 2) suggests that the maximal NC/C ratio plays a very small clinical role in cardiac remodelling. End-diastolic and end-systolic volume indices decreased over the 9.5 years of MESA study period, even in participants with the most pronounced trabeculation (by 3.6 ml/m2 and 1.0 ml/m2 in the highest quintile of maximal NC/C ratio).
In comparison, a previous study by Doughty and colleagues in subjects with congestive heart failure due to ischaemic heart disease described an increase in end-diastolic volume index by 10.5 ml/m2 over only 12 months in the placebo group. (21) Similarly, measures of the degree of LV trabeculation were not associated with adverse clinical outcomes known to be associated with a clinical diagnosis of LVNC. These data may seem contrary to the many reports of embolic events in patients with LVNC, however the studied population was considered to be healthy and the probability of LVNC (or other cardiac diseases) was extremely low in this group.
Importance of clinical information when interpreting imaging LVNC criteria
Our study demonstrates again that Petersen’s criteria (NC/C > 2.3) are frequent findings in a “healthy” population representative cohort. Importantly, our study underlines the importance of interpreting such an imaging diagnosis in the context of clinical information available. (22) Our findings suggest that in subjects or patients with a low pre-test probability for cardiomyopathy or LVNC and marked trabeculation regular and frequent imaging and clinical follow up may not be necessary.
Petersen’s criteria were derived from a group of patients with high pre-test probability imaged at a tertiary cardiomyopathy centre and showed high sensitivity and specificity for diagnosing LVNC. (4) Current diagnostic criteria have application as a rule-in test if the suspicion (pre-test probability) of LVNC is over 10% and could theoretically be used as a rule-out test if applied in patients with low pre-test probability for LVNC. (22)
Study limitations
The data of the current study must be interpreted in the context of the study design. As described in the methods section, we were only able to analyse trabeculation on the most recent SSFP cine images from the MESA exam 5 data, but not on the gradient-recalled echo cine images acquired during the MESA exam 1; although, this makes the data more applicable to current clinical practice, we imply that trabeculation has not changed over the approximately 10 years beforehand. This implication may be rationalised through the evidence that the development and extent of trabeculations is determined during the cardiac development in utero. (23) Some case reports describe an undulating phenotype of left ventricular trabeculations, but this is unlikely to be common enough to influence the results of this study. Despite this, survivor bias may be present and firm conclusions on causality cannot be drawn. This question cannot be addressed without this limitation for another fifteen years, until MESA or other large-scale population based cohort studies, such as UK Biobank, may have sufficient serial CMR studies that used SSFP cines.
Adverse clinical events were rare and were therefore not treated as primary outcomes in this paper. Measurement of NC/C ratio is operator dependent; however, there was a good intra- and interobserver agreement. We have only used one of the approaches to measure the extent of LV trabeculations. Other reported strategies differ with regards to measuring in the short axis or long axis views, with regards to measuring at end-diastole or end-systole, with regards to calculating ratios based on the thicknesses of trabeculated and compacted myocardial layers or trabecular mass as a percentage of total LV mass. Currently, there is no consensus to the best approach as each one has advantages and disadvantages regarding the reported diagnostic accuracy, reproducibility, observer variability and ability to avoid inadvertent inclusion of papillary muscle in the measurements.
Conclusions
Although there is the potential for confounding and survivor bias, this study does not find a clinically relevant impact of trabeculae on LV function measured in end-diastole over an almost 10 year period in population representative adult individuals. This information should guide clinical decision-making in the common scenario of identifying patients with marked LV trabeculation and low pre-test probability of LV non-compaction that there is no clear need for follow-up imaging or pharmacotherapy.
Perspectives.
Competency in medical knowledge: Greater extent of left ventricular trabeculation is not associated with an absolute increase in end-systolic and end-diastolic volumes over almost ten years of the MESA study. Greater NC/C ratio was not associated with a decline in systolic function.
Competency in Patient Care: Asymptomatic individuals who meet Petersen’s criteria for left ventricular non-compaction should not be labeled as having potential left ventricular non-compaction cardiomyopathy unless the clinical situation mandates.
Acknowledgements
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The authors (SEP, FZ, SAM) gratefully acknowledge the funding from the National Institute for Health Research Cardiovascular Biomedical Research Unit at Barts. This work (SEP) is supported by awards establishing the Farr Institute of Health Informatics Research at UCLP Partners from the MRC, in partnership with Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Sciences Research Council, the National Institute of Health Research, the National Institute for Social Care and Health Research (Welsh Assembly Government), the Chief Scientist Office (Scottish Government Health Directorates) and the Wellcome Trust (MR/K006584/1). SEP provides Consultancy to Circle Cardiovascular Imaging Inc., Calgary, Canada.
Abbreviations
- CMR
cardiovascular magnetic resonance
- EDVi
end-diastolic volume index
- EF
ejection fraction
- ESVi
end-systolic volume index
- GRE
gradient recalled echo
- LV
left ventricle
- LVNC
left ventricular non-compaction
- MESA
Multi-Ethnic Study of Atherosclerosis
- NC/C
non-compaction to compaction
- SD
standard deviation
- SSFP
steady-state free precession
Contributor Information
Filip Zemrak, Centre for Advanced Cardiovascular Imaging, Queen Mary University of London, The London Chest Hospital, United Kingdom.
Mark A. Ahlman, Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, United States.
Gabriella Captur, Institute of Cardiovascular Science, University College London and The Heart Hospital, United Kingdom.
Saidi A Mohiddin, Centre for Advanced Cardiovascular Imaging, Queen Mary University of London, The London Chest Hospital, United Kingdom.
Nadine Kawel-Boehm, Department of Radiology, Kantonsspital Graubuenden, Switzerland.
Martin R. Prince, Weill Cornell Medical Center, New York, United States.
James C. Moon, Institute of Cardiovascular Science, University College London and The Heart Hospital, United Kingdom.
William G. Hundley, Department of Internal Medicine, Wake Forest University, Winston-Salem, North Carolina, United States.
João A.C. Lima, Cardiology Division, Department of Medicine, Johns Hopkins Hospital, Baltimore, Maryland, United States.
David A Bluemke, Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, United States.
Steffen E Petersen, Centre for Advanced Cardiovascular Imaging, Queen Mary University of London, The London Chest Hospital, United Kingdom.
References
- 1.Kohli SK, Pantazis AA, Shah JS, et al. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J. 2008;29:89–95. doi: 10.1093/eurheartj/ehm481. [DOI] [PubMed] [Google Scholar]
- 2.Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36:493–500. doi: 10.1016/s0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- 3.Ritter M, Oechslin E, Sütsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72:26–31. doi: 10.4065/72.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–105. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Jacquier A, Thuny F, Jop B, et al. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J. 2010;31:1098–1104. doi: 10.1093/eurheartj/ehp595. [DOI] [PubMed] [Google Scholar]
- 6.Captur G, Nihoyannopoulos P. Left ventricular non-compaction: genetic heterogeneity, diagnosis and clinical course. Int J Cardiol. 2010;140:145–153. doi: 10.1016/j.ijcard.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Stähli BE, Gebhard C, Biaggi P, et al. Left ventricular non-compaction: prevalence in congenital heart disease. Int J Cardiol. 2013;167:2477–2481. doi: 10.1016/j.ijcard.2012.05.095. [DOI] [PubMed] [Google Scholar]
- 8.Kawel N, Nacif M, Arai AE, et al. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2012;5:357–366. doi: 10.1161/CIRCIMAGING.111.971713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 12.Liu CY, Liu YC, Wu C, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;62:1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson DK, Maceira AM, Raj VJ, Graham C, Pennell DJ, Kilner PJ. Regional thicknesses and thickening of compacted and trabeculated myocardial layers of the normal left ventricle studied by cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2011;4:139–146. doi: 10.1161/CIRCIMAGING.110.960229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanton C, Bruce C, Connolly H, et al. Isolated left ventricular noncompaction syndrome. Am J Cardiol. 2009;104:1135–1138. doi: 10.1016/j.amjcard.2009.05.062. [DOI] [PubMed] [Google Scholar]
- 15.Aras D, Tufekcioglu O, Ergun K, et al. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail. 2006;12:726–733. doi: 10.1016/j.cardfail.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Axel L. Papillary muscles do not attach directly to the solid heart wall. Circulation. 2004;109:3145–3148. doi: 10.1161/01.CIR.0000134276.06719.F3. [DOI] [PubMed] [Google Scholar]
- 17.Bettari L, Borges-Neto S. Imaging surrogate end points in heart failure trials. Heart Fail Clin. 2011;7:509–518. doi: 10.1016/j.hfc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Lipicky RJ, Packer M. Role of surrogate end points in the evaluation of drugs for heart failure. J Am Coll Cardiol. 1993;22:179A–184A. doi: 10.1016/0735-1097(93)90487-l. [DOI] [PubMed] [Google Scholar]
- 19.Anand IS, Florea VG, Fisher L. Surrogate end points in heart failure. J Am Coll Cardiol. 2002;39:1414–1421. doi: 10.1016/s0735-1097(02)01773-4. [DOI] [PubMed] [Google Scholar]
- 20.Wong M, Johnson G, Shabetai R, et al. Echocardiographic variables as prognostic indicators and therapeutic monitors in chronic congestive heart failure. Veterans Affairs cooperative studies V-HeFT I and II. V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI65–VI70. [PubMed] [Google Scholar]
- 21.Doughty RN, Whalley GA, Gamble G, MacMahon S, Sharpe N. Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia-New Zealand Heart Failure Research Collaborative Group. J Am Coll Cardiol. 1997;29:1060–1066. doi: 10.1016/s0735-1097(97)00012-0. [DOI] [PubMed] [Google Scholar]
- 22.Petersen SE. CMR and LV noncompaction: does it matter how we measure trabeculations? JACC Cardiovasc Imaging. 2013;6:941–943. doi: 10.1016/j.jcmg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Freedom RM, Yoo SJ, Perrin D, Taylor G, Petersen S, Anderson RH. The morphological spectrum of ventricular noncompaction. Cardiol Young. 2005;15:345–364. doi: 10.1017/S1047951105000752. [DOI] [PubMed] [Google Scholar]