Abstract
The advent of major histocompatibility complex (MHC) tetramer technology has been a major contribution to T cell immunology, because tetramer reagents permit detection of antigen-specific T cells at the single-cell level in heterogeneous populations by flow cytometry. However, unlike MHC class I tetramers, the utility of MHC class II tetramers has been less frequently reported. MHC class II tetramers can be used successfully to enumerate the frequencies of antigen-specific CD4 T cells in cells activated in vitro, but their use for ex vivo analyses continues to be a problem, due in part to their activation dependency for binding with T cells. To circumvent this problem, we recently reported the creation of a new generation of reagents called MHC class II dextramers, which were found to be superior to their counterparts. In this review, we discuss the utility of class II dextramers vis-a-vis tetramers, with respect to their specificity and sensitivity, including potential applications and limitations.
Introduction
Historically, studies related to the detection and functionalities of antigen-specific T cells at the single-cell level have been limited because the appropriate reagents and tools were not available. Commonly employed readouts included T cell proliferation assays based on incorporation of tritiated 3[H]-thymidine or 5-bromo-2'-deoxyuridine (BrdU); Carboxyfluorescein succinimidyl ester (CFSE)-labelling; enzyme-linked immunospot (ELISPOT) assays; limiting-dilution analysis (LDA); and intracellular cytokine analysis [1–5]. Although most of these assays are helpful in ascertaining antigen-specific T cell responses in mixed cell cultures at the population level, accurate enumeration of the frequencies of antigen-specific T cells at the single-cell level has been a major limitation. Even assays like ELISPOT or cytokine-analysis can be prone to errors because it is difficult to eliminate the contribution of bystander T cells that can be non-antigen-specifically activated, leading to the possibility of overestimating the antigen-specific T cells [1]. Similarly, although LDA permits analysis of antigen-specific T cells at the single cell-level, this assay can never be routinely practical due to both the need to repeatedly activate the cells and the laborious nature of the assay [1, 6].
These circumstances changed, however, with the publication of a 1996 landmark paper by Altman et al. describing the creation of major histocompatibility complex (MHC) class I tetramer technology; the tetramer reagents were found to be valuable in enumerating the frequencies of antigen-specific CD8 T cells by flow cytometry [7]. In 1998, Kappler’s group provided a similar platform for CD4 T cells by generating peptide-tethered MHC class II tetramers [8]. These discoveries enabled researchers to determine the specificity of antigen-responsive T cells, particularly with respect to their appearance, disappearance and/or persistence in both basic and clinical research investigations (Table 1). MHC tetramers can be defined as artificially created soluble fluorochrome-conjugated MHC molecules assembled with peptides of interest. Their binding to antigen-specific T cells is captured by flow cytometry using the signals emitted by fluorochromes as readouts. However, some issues have continued to persist related to the inherent inability of MHC class II tetramers to bind CD4 T cells, especially low-affinity T cell receptor (TCR)-bearing autoreactive T cells, in spite of the fact that they are antigen-specific [9, 10]. To alleviate this problem, we created a newer version of tetramers called MHC class II dextramers for various autoantigens and successfully tested their utility in several experimental autoimmune and infectious disease models [11–15]. In this review, we discuss the utility of MHC class II dextramer reagents, most importantly their advantages over tetramers, as well as potential applications and limitations (Table 2). Nonetheless, for extensive details on the derivation and use of MHC class II tetramers, readers are encouraged to consult other excellent reviews published by various groups [9, 10, 16, 17].
Table 1.
List of MHC class II tetramers and dextramers, and their use for the determination of antigen-specific T cell responses in mice and humans
| MHC class II alleles/epitopes | Application |
|---|---|
| (a) Tetramers | |
| Mice | |
| IAb | |
| IRBP271–290 and IRBP771–790 | Thymic selection of T cells [71] |
| LCMV GP66–77, GP126–140, GP6–20 and CLIP | Detection of LCMV-specific T cells in infected mice [72] |
| MOG 35–55, MOG38–49 and hCLIP103–117 | Detection of MOG-specific T cells in EAE mice [40, 73] |
| OVA323–339, CLIP and LCMV GP61 | Detection of OVA-specific T cells in immunized mice[74] |
| FliC427–441, 2W1S, OVA323–339 | Determination of the frequencies of antigen- specific T cells in naïve repertoires [43] |
| HY | Detection of antigen-specific T cells in naïve and immunized mice [75] |
| IAd | |
| OVA323–339 and influenza HA126–138 | Peptide/MHC display and antigen-specificity studies [8, 76, 77] |
| IAg7 | |
| p8G p6-α62, p8G p11-α72, p8E p6-α62, p8E p11-α72, insulin, CHGA and HEL |
Detection of insulin-specific T cells in NOD mice [78] |
| GAD524–543, GAD1040–79, GAD1136–17, p206, p524, GAD206–220 and GAD471–490 |
Detection of GAD-specific T cells in NOD mice [22, 47, 79] |
| IAk | |
| Myhc-α334–352 and RNase43–56 | Detection of Myhc-α-specific T cells in EAM mice [31] |
| IRBP201–216, and EHC44–59 | Detection of IRBP, and IRBP- and EHC-cross- reactive T cells in EAU mice [36] |
| IAs | |
| PLP139–151 and TMEV70–86 | Detection of PLP-specific T cells in EAE mice [33] |
| PLP139–151 and ACA83–95 | Detection of PLP- and ACA-cross-reactive T cells in EAE mice [14] |
| IEk | |
| MCC88–103 | First report to demonstrate that MHC class II tetramers detect antigen-specific CD4 T cells [78] |
| U1–70131–150, U1–70131–150, P(140) and MCC88–103 |
TCR-signaling studies, and antigen-specific T cell responses [58, 80, 81] |
| Humans | |
| DP4 | |
| MAGE-3 | Detection of MAGE-3-specific T cells in vaccinated patients [70] |
| DQ2 | |
| αI- and αII-peptides of gliadin | Detection of gliadin-specific T cells in patients with celiac disease [82] |
| DR*1101 | |
| MAGE-A3191–205, MAGE-A3281–295 and | Epitope-binding studies [83] |
| MAGE-A3286–300 | |
| DR0301 | |
| IGRP peptides and Flu MP169–181 | Tetramer-guided epitope mapping [84] |
| DR0401 | |
| IGRP peptides and Flu HA306–318 | Tetramer-guided epitope mapping [84] |
| DRB1 | |
| Bet v 1141–155, Der p 1 16–30, Der p 1 171–185 and |
T cell responses in allergy [85] |
| Phl p 1 194–208 | |
| DRB1*0101 | |
| HIV Gag p24294–313, Gag p24264–283, TT830–843, influenza HA307–319 and CMV pp65108–127 |
Detection of HIV-, tetanus toxoid-, influenza-, or CMV-specific T cells by magnetic bead enrichment [86] |
| HA307–319 and HA306–318 | Detection of influenza-specific T cells [87, 88] |
| Fel d 1 | Detection of Fel d 1-specific T cells in atopic dermatitis patients [64] |
| DRB1*0401 | |
| HA306–318 and GAD | Detection of influenza-specific T cells in vaccine- recipients [88] |
| MOG97–109 | Detection of MOG-specific T cells [89] |
| OspA164–175 | Detection of OspA-specific T cells in lyme arthritis patients [62] |
| Human CII, HCgp39 and Osp-A | Detection of cartilage antigen-specific T cells in rheumatoid arthritis patients [90] |
| HCV 1248, HCV 1579 and HCV 1770 | Detection of HCV-specific T cells in viral hepatitis [21, 63] |
| MART 151–173 | Detection of MART-1-specific T cells in vaccine- recipients [69] |
| Lol P1 | Detection of allergen-specific T cells in allergic individuals [65] |
| DRB1*0402 | |
| Dsg3 | Detection of Dsg3-specific T cells in pemphigus vulgaris [91] |
| DRB1*0404 | |
| PA112–127 | Detection of antigen-specific T cells in recipients of Anthrax vaccine [67] |
| HIV Gag p24164–183 and HCV NS31248–1261 | Detection of HIV- or HCV-specific T cells by magnetic bead enrichment [86] |
| DRB1*1302 | |
| PA373–393 and PA381–392 | Detection of antigen-specific T cells in recipients of Anthrax vaccine [67] |
| DR3 | |
| MTB 1–13 and CMV pp65510–522 | Detection of MTB- and CMV-specific T cells in patients/vaccine-recipients [92] |
| DR4 | |
| HA p306–318 and NY-ESO-1 p119–130 | Detection of influenza- or NY-ESO-1-specific T cells by immunoscope [25] |
| Ag85B and ESAT-6 | Detection of MTB-specific T cells in patients with pulmonary tuberculosis [61] |
| DR401 | |
| GAD65555–567 and HSV-p61 | Detection of GAD-specific T cells in type 1 diabetes [47] |
| DR404 | |
| GAD65555–567 and HSV-p61 | Detection of GAD-specific T cells in type 1 diabetes [47] |
| (b) Dextramers | |
| Mice | |
| IAb | |
| MOG 35–55 | Detection of MOG-specific T cells in EAE mice [59] |
| IAk | |
| Myhc-α 334–352 and RNase 43–56 | Detection of Myhc-α-specific T cells in EAM mice and CVB3-infected mice [11, 15] |
| IAs | |
| PLP139–151 and TMEV70–86 | Detection of PLP-specific T cells ex vivo, in vitro and in situ in EAE mice [12, 13, 15] |
| PLP139–151 and ACA83–95 | Detection of PLP- and ACA-cross-reactive T cells in ACA infected mice [14] |
| Humans | |
| HLA-DR1 | |
| Influenza A HA307–319 and HIV-1 p24 Gag299–312 |
Detection of influenza-specific T cells [49] |
| HLA-DP2 | |
| Mim2, Mim2 and Be, and PLXNA4 and Be | Detection of Be-specific T cells [93, 94] |
This list is intended to capture broad applications of tetramer and dextramer reagents in basic and clinical research, and by no means is a comprehensive list. As such, some reagents may not have been listed, and the authors wish to acknowledge this limitation.
IRBP, interphotoreceptor retinoid-binding protein; LCMV, Lymphocytic choriomeningitis virus; GP, glycoprotein; CLIP, Class II-associated invariant chain peptide; MOG, myelin oligodendrocyte glycoprotein; EAE, experimental autoimmune encephalomyelitis; OVA, ovalbumin; HA, hemagglutinin; FLiC, flagellar filament structural protein; HY, Y chromosome-encoded transplantation antigens; NOD, non-obese diabetic; GAD, glutamic acid decarboxylase; CHGA, chromogranin A; HEL, hen egg lysozyme; Myhc-α, cardiac myosin heavy chain-α; RNase, Ribonuclease; EAM, experimental autoimmune myocarditis; EHC, Ehrlichia canis; EAU, experimental autoimmune uveitis; PLP, myelin proteolipid protein; TMEV, Theiler’s murine encephalomyelitis virus; ACA, Acanthamoeba castellanii; MCC, moth cytochrome C; MAGE, melanoma-associated antigen; IRGP, islet-specific glucose-6-phosphatase catalytic subunit-related protein; MP, Matrix protein; Bet v 1, Betula verrucosa; Der p 1, Dermatophagoides pteronyssinus; Phl p 1, pollen allergen; HIV, Human immunodeficiency virus; Gag, group-specific antigen; TT, Tetanus toxoid; CMV, Cytomegalovirus; OspA, Borrelia burgdorferi outer surface protein-A; CII, type 2 collagen; HCV, Hepatitis C virus; MART, from Melan-Ae/melanoma antigen recognized by T cells; Lol P1, rye grass allergen; HCgp, Human cartilage glycoprotein; Dsg3, desmoglein3; PA, protective antigen of Bacillus anthracis; NS, nonstructural protein; MTB, Mycobacterium tuberculosis; NY-ESO-1, New York esophageal squamous cell carcinoma; Ag85B, Antigen 85; ESAT-6, Early Secretory Antigenic Target; CVB3, coxsackie virus B3; Mim2, mimotope-2; Be, beryllium
Table 2.
Advantages of using MHC class II dextramers over tetramers for detecting antigen-specific, autoreactive CD4 T cells.
| Parameters | Tetramers | Dextramers |
|---|---|---|
| a. Generation of reagents | ||
| • Creation of MHC constructs, expression of soluble MHC monomers and biotinylation |
Steps are similar for both the reagents | |
| • Multimerization | Require fluorophore/streptavidin- conjugated molecules |
Require fluorophore/streptavidin- conjugated dextran molecules |
| b. Staining characteristics based on flow cytometry | ||
| • Number of MHC monomers per reaction |
12.6 × 10−12 moles | 2.78 × 10−12 moles |
| • Specificity | Yes | Yes, but enhanced |
| • Sensitivity | Low | High |
| • Duration | Variable (usually 2 to 3 hours in our hands) |
Relatively short (0.5 to 2 hours) |
| c. Applications | ||
| • Detection of T cells ex vivo | May or may not work | Reliable and consistent |
| • Detection of T cells activated in vitro |
Useful | Useful |
| • Detection of T cells in situ | May work | Reliable and consistent |
| • Cytokine-phenotyping by flow cytometry |
Yes | Yes, but with enhanced sensitivity |
| • Bioassay | Yes | Yes |
What are MHC class II tetramers and dextramers and how are they created?
To understand the derivation of MHC class II tetramers and dextramers, it is useful to understand how MHC molecules display peptides for recognition by T cells. In contrast to MHC class I molecules that are composed of a single alpha chain supported by β2-microglobulin as a scaffolding molecule, MHC class II molecules are made up of two chains, α and β. While the peptide-binding groove in the MHC class I molecule is formed by the participation of α1 and α2 domains, the α1 and β1 domains from their corresponding chains within the MHC class II molecules take part in the formation of grooves to which the peptides can anchor. The peptides thus displayed by MHC class I and MHC class II molecules are recognized by CD8 or CD4 T cells, respectively, and CD8 and CD4 molecules act as co-receptors by binding to the corresponding MHC molecules [18]. Nonetheless, it should be noted that the affinity of T cell recognition of MHC-displayed peptides is weak (KD~ 0.1–500 µM), and engagement of multiple TCRs with as many multiple MHC-peptide complexes favors stability of their interactions, leading to enhanced avidity [19, 20].
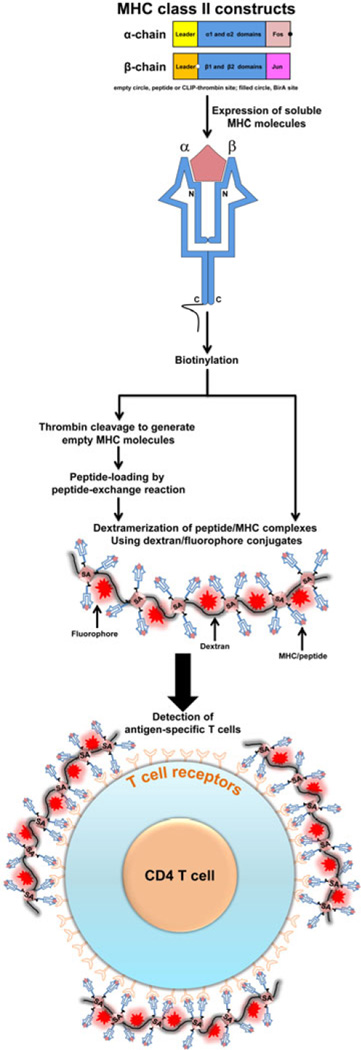
The creation of tetrameric reagents requires expression of soluble MHC molecules that represent the extracellular portions in each class of MHC molecule [7, 8]. The presence of a single α chain may offer some advantage in allowing MHC class I molecules to be expressed more easily than MHC class II molecules in which both α and β chains must stay together so peptides can be displayed in the MHC clefts. Several strategies have been successfully employed for the expression of MHC class II molecules for various alleles in both mice and humans [8, 21–24]. Expression of soluble MHC class II molecules requires the creation of two constructs, one each for α and β chains. A BirA site for biotinylation is introduced into one of the two chains, usually the α chain (Fig 1).
Figure 1. Derivation of MHC class II dextramers.
To produce soluble MHC class II monomers, two constructs, each representing MHC class II α and MHC class II β chains, are first created. Generally, we insert the nucleotide sequence for specific peptides of interest or thrombin-cleavage site-tagged/CLIP into the N-terminal end of the β chain construct. Likewise, Fos-and Jun-sequences are attached to the C-terminal ends of α and β constructs, respectively, to facilitate stability of α and β chains during protein expression, whereas the BirA site for biotinylation is introduced in the α-construct. The constructs are used to express soluble MHC molecules using Baculovirus in Sf9 insect cells. After biotinylation, the MHC monomers obtained from the constructs containing covalently tethered, specific peptide sequences are assembled with dextran molecules to obtain dextramers directly. In contrast, the biotinylated proteins generated from the CLIP-tethered constructs are first treated with thrombin to release the CLIP peptide, and the resulting empty MHC monomers are then used to load peptides of interest exogenously by peptide-exchange reaction. For dextramerization, the biotinylated MHC monomers generated from any of the above two approaches are mixed with dextran-SA-fluorochrome at molar ratios ranging from ~9:1 or 20:1, and the dextramers are then used to stain T cells to analyze their frequencies by flow cytometry.
To express soluble molecules, various mammalian and non-mammalian expression systems have been used [9, 25–27]. However, it is to be noted that creation of MHC constructs depends on the intended use of the soluble molecules. Three major approaches are commonly used. (1) Derivation of peptide-tethered soluble MHC molecules. In this approach, the nucleotide sequence for the desired peptide is covalently tethered to the N-terminus of the β chain such that the peptide-containing soluble MHC molecules are expressed directly [8, 23]. Two advantages have been noted with this approach: (i) the peptide-bound MHC molecules are readily available for downstream applications, so the loss/instability of peptides is not an issue [8, 27]; and (ii) the covalently assembled peptides may provide stability for MHC monomers during protein expression [8, 9]. The major disadvantage is the need to generate constructs individually for each peptide of interest. (2) Derivation of soluble, empty MHC molecules. Here, α and β chains without peptide sequences are expressed to obtain empty molecules, and the desired peptides are then loaded exogenously to generate peptide-bound MHC complexes. Technically, this approach is versatile and desirable, but the stability of peptides within the groove can be an issue [28–30]. (3) Derivation of soluble MHC molecules containing class II-associated invariant chain peptide (CLIP). This approach is similar to approach 1 as described above, but instead of tethering the specific peptide of interest to the N-terminus of the β chain, the CLIP-conjugated sequence with a site for thrombin cleavage is inserted covalently to the N-terminus of the β chain. As a result, upon protein expression, the CLIP is cleaved by thrombin to obtain empty MHC molecules, into which peptides of interest can be loaded exogenously by a peptide-exchange reaction similar to that employed in approach 2 [21, 22, 31].
In all of these approaches, however, because it is possible for α and β chains to separate during expression, it is a common practice to insert leucine-zipper dimerization motifs from the transcription factors Fos (3.98 KDa) and Jun (3.92 KDa) to the α and β chains, respectively, of MHC class II molecules [23, 32, 33]. By virtue of high-affinity binding between Fos and Jun proteins (KD 5.4 × 10−8 M; [34]), the α and β chains are expected to stay together as single monomers during protein expression, as well as in the downstream steps of protein purification, multimerization and staining by flow cytometry. We commonly employ approaches 1 and 3 in our routine tetramer and dextramer preparation.
The purified soluble MHC class II monomers are first biotinylated using biotin ligase (BirA) (Fig 1). While the peptide-tethered biotinylated proteins are directly multimerized using streptavidin (SA) conjugated with fluorochromes, such as phycoerythrin (PE) or allophycocyanin (APC), the CLIP-tethered proteins are first subjected to thrombin cleavage to release the CLIP, and the empty molecules are then assembled with peptides in the peptide-exchange reaction, followed by multimerization with SA-fluorochromes [21–23]. Although the latter approach is versatile in that we can generate reagents for multiple antigens by loading the peptides exogenously into empty MHC molecules, we noted a few limitations. First, peptides that are readily soluble in water or PBS can be reliably used to prepare the reagents, and addition of dimethyl sulfoxide to enhance the solubility of peptides did not yield better results. Second, the sensitivity of tetramers and dextramers generated for some peptides was lower than that could be achieved with the reagents generated from the use of covalently tethered MHC molecules (unpublished observations).
To prepare multimeric MHC-peptide complexes using SA-fluorochrome conjugates, it is important to note that each SA can assemble up to four biotinylated MHC molecules. Hence, tetramers typically are expected to possess up to four peptide-bound biotinylated MHC molecules. In contrast to tetramers, the dextramers offer an advantage of assembling more peptide-bound MHC molecules – up to 20 per dextran molecule [15, 35]. Structurally, dextramers contain dextran backbones, which are polymers of glucose molecules attached through 1–6 and 1–3 linkages [35]. Each dextran molecule carries multiple moieties of SA to which biotinylated peptide-tethered MHC molecules can be assembled [35]. Therefore, dextramers can yield MHC-peptide complexes of large molecular weight, allowing them to engage multiple TCRs – more than could be achieved with tetramers.
To prepare the dextramers, we use dextran molecules (kind gift from Immudex, Denmark) at a working concentration of 3.2 × 10−8 M. Conjugation of dextran molecules with MHC-peptide molecules depends on the number of SA-fluorochrome-assembled moieties available in the dextran backbone. For example, a dextran-PE backbone containing 6.6 SA moieties has a molar ratio of 19.8 (6.6 × a valency of 3). Therefore, each mole of dextran-PE can be assembled with 19.8 moles of MHC class II monomers. We usually assemble 21 to 22 µg, equivalent to 3.17×10−10 moles of MHC class II monomers (~68 KDa) with 1.6 × 10−11 moles of dextran-PE [15]. On a reaction basis, we routinely use PE-dextramers containing 6.3 × 10−12 moles, equivalent to 0.45 µg of MHC protein (~3.8 × 1012 molecules), to stain 1 × 106 cells [15]. Likewise, one mole of dextran-APC with a molar ratio of ~8.7 is used to assemble 8.7 moles of MHC class II monomers. To prepare APC-dextramers, we use ~9.5 to 10 µg of MHC class II protein, equivalent to 1.39 × 10−10 moles, to assemble 1.6 × 10−11 moles of dextran-APC; and to stain 1 × 106 cells, 2.78 × 10−12 moles (0.2 µg; /~1.67 × 1012 MHC monomers) are used. Overall, the amount of MHC protein needed to prepare dextramer-APC is half the amount needed to prepare dextramer-PE, but the staining intensities obtained by the two reagents are comparable [15]. For routine use, the reaction conditions for each dextramer reagent are to be optimized. We found that staining at room temperature for a minimum of 30 minutes in growth medium (RPMI with 2.5% fetal bovine serum and interleukin-2, pH 7.63) was optimum for various MHC class II alleles assembled with a wide range of self- and foreign antigens [11, 14, 15].
Are MHC class II dextramers better reagents than MHC class II tetramers?
During the past 10 to 15 years, we have been using MHC class II tetramers in various autoimmune disease models in mice, primarily experimental autoimmune encephalomyelitis (EAE) induced with myelin proteolipid protein (PLP) 139–151 and myelin oligodendrocyte glycoprotein (MOG) 35–55; amoebic encephalitis; experimental autoimmune myocarditis (EAM) induced with cardiac myosin heavy chain (Myhc)-α 334–352; and experimental autoimmune uveoretinitis induced with interphotoreceptor retinoid-binding protein 201–216 [11, 15, 23, 33, 36–42]. Although the tetramer reagents were helpful, their use was largely limited to enumerating the frequencies of antigen-specific CD4 T cells in cultures activated in vitro, and the direct ex vivo analysis of antigen-sensitized cells was a persistent problem. Enrichment procedures involving the use of antibodies to fluorochromes like PE to enrich the tetramer-PE-bound CD4 T cells by magnetic separation followed by extrapolating their frequencies to the total number of T cells have been commonly employed in the field [16, 22, 43, 44]. In these settings, the use of enzymes like protein tyrosine kinase inhibitors (PKIs) (e.g., Dasatinib, Bristol-Myers Squibb) prior to tetramer staining and enrichment procedures appears to enhance the sensitivity of tetramers [45, 46]. These methods can be labor intensive, and time consuming. In addition, technically, there also exists a possibility of non-antigen-specific cells to be present as contaminants in the enriched populations necessitating the use of multiple surface markers to eliminate them [16]. Likewise, peripheral blood mononuclear cells (PBMCs) from humans are usually stimulated with antigen-pulsed autologous antigen-presenting cells (APCs) for up to ~7 days prior to enrichment procedures [47]. Although these analyses may yield valuable information, absolute enumeration of the frequencies of antigen-specific T cells can be prone for errors leading to inaccurate estimations of their repertoire sizes. In some instances, frequencies of antigen-specific T cells can be detected only marginally higher than the background staining with the control tetramers because of difficulties in differentiating background staining from the specific tetramer staining [48]. To overcome these limitations with tetramers, we made efforts to create dextramers utilizing the dextran reagents conjugated with various fluorochromes (kind gift from Immudex, Denmark) for IAs/PLP 139–151, IAb/MOG 35–55, and IAk/Myhc-α 334–352 [15]. We found that dextramers, but not tetramers, were helpful in enumerating the frequencies of antigen-specific CD4 T cells ex vivo in all the above models [15]. In addition, we noted that the detection sensitivity of dextramers could be increased by at least 4- to 5-fold compared with tetramers. Similarly, the specificity of staining obtained with dextramers was better than that could be achieved with tetramers, as background staining with the control dextramers was negligible [15]. One other group recently reported similar observations by generating the dextramers for human MHC class II allele, HLA-DR1 [49]. We think that dextramers consisting of multiple MHC-peptide complexes may more easily engage with multiple TCRs, leading to enhanced stability of dextramer-T cell interactions. As such, the dextramer-bound T cells are less likely to be detached during various steps in the staining reactions.
It is a common belief that tetramers bind poorly to low-affinity TCR-bearing T cells, because their binding depends on the activation status of T cells, and tetramers therefore are unlikely to bind the resting cells even if they are antigen-responsive [33, 50–52]. We and others have previously reported that the tetramer+ cells were found almost exclusively within the activated (CD4high/CD25+) population [33, 47, 50, 51]. We had proposed that the activation status of cells may result in the reorientation or change in the configuration of the TCR and cell surface molecules leading to increase in the avidity of TCR-MHC binding [33]. To overcome this limitation, we had previously shown that antigen-responsive, rested cells can be made to bind with tetramers by first exposing the cells to neuraminidase (NASE), which essentially promotes T cell activation by an unknown mechanism [15, 23, 33]. In addition, removal of sialic acid residues from the surface of T cells by NASE-treatment may increase TCR affinity as shown with an insulin-insulin receptor system [53]. We verified that the dextramers can detect rested cells without the need for NASE-treatment, thus overcoming the activation dependency problem noted with tetramers. Based on these successes, we expanded the utility of dextramers to detect antigen-specific CD4 T cells in target organs, such as brains in EAE mice and hearts in EAM mice, by flow cytometry [11, 15]. More importantly, we showed for the first time that MHC class II dextramers can be used to detect antigen-specific CD4 T cells in situ directly, both in the brain and heart in EAE and EAM models, respectively, by confocal microscopy without having to amplify the fluorochrome signals using secondary antibodies, which had been the case with MHC class I tetramers [12, 13, 54, 55]. This modality of in situ staining considerably reduced the duration of the process to less than a day (~12 hours), as compared to up to 3 days with MHC class I tetramers [54, 55]. Furthermore, the amount of reagent needed per in situ reaction is 2.5 µg/ml, which is significantly less than required with tetramers (20 µg/ml) [15, 56].
By extending the utility of dextramers to phenotype antigen-specific CD4 T cells in relation to cytokine-producing cells, we noted the unexpected finding that only a fraction of dextramer+ CD4 T cells are capable of producing Th cytokines, while the majority of cytokine-producing cells lie within the dextramer− CD4 T cell populations (unpublished preliminary observations). Using IAs/PLP 139–151 and IAb/MOG 35–55 dextramers in the EAE system and IAk/Myhc-α 334–352 dextramers in the EAM system, we are now phenotyping the cytokine-producing dextramer+ and cytokine-producing dextramer− CD4 T cells corresponding to Th1, Th2 and Th17 subsets of cytokines to determine whether common cytokine signatures exist in different autoimmune diseases. More recently, we tested whether dextramers prepared by using naked (fluorochrome-unconjugated) dextran backbones (kind gift from Immudex) can be used as APCs to stimulate T cells in bioassays. An amount of 0.07 µg was found sufficient to induce robust proliferative responses in T cell hybridomas specific to PLP 139–151 and Myhc-α 334–352 (unpublished observations). These reagents may be useful in studying T cell-specific responses when the contribution of APCs must be avoided. One such potential application is the evaluation of reactive oxygen species (ROS) in T cells, whose detection within T cells has been debated, and the argument made that the T cells derive ROS as a contaminant from APCs.
Can MHC class II dextramers solve all problems with MHC class II tetramers? If not, what improvements can be made?
As described above, we have shown the utility of MHC class II dextramers in several experimental systems, finding the dextramers to be superior to their counterparts in terms of sensitivity, specificity and applications. If the dextramers can be so much more versatile than tetramers, a critical question is whether they can be used as reliable reagents to detect CD4 T cells with varied TCR affinity. Unfortunately, the answer is no. We performed an experiment to sort the dextramer+ and dextramer− CD4 T cells from PLP 139–151-sensitized primary T cell cultures by flow cytometry, expecting that the dextramer− cells would neither respond to antigen nor bind PLP dextramers upon restimulation. Contrary to our expectations, however, the dextramer− cells did respond to PLP 139–151 and also stained with PLP dextramers [15]. We sorted CD4 T cells sensitized with PLP 139–151 that are negative for PLP 139–151 dextramers, and later stimulated them with PLP 139–151. Approximately, 4% of cells from these cultures were found positive for PLP 139–151 dextramers, and expectedly, under similar conditions, the PLP 139–151 tetramer− counterparts revealed more number of cells (7.2%) to be positive for PLP 139–151 dextramers [15]. These data suggest that a proportion of antigen-responsive T cells, possibly very low-affinity TCR-bearing cells, may not be detected by dextramers. Thus, the need for improving the detection sensitivity of dextramers still continues. Reports indicate that pre-exposure of cells to PKIs enhance the staining intensity of MHC class I tetramers/dextramers [49, 57]. Mechanistically, PKIs appear to inhibit downregulation of TCRs, including CD8 co-receptors, leading to their persistence on the surface of T cells [49, 57]. It is possible that similar strategies may work for MHC class II dextramers, although participation of CD4 co-receptor is not shown to be critical for binding with MHC class II tetramers [8, 58].
In summary, we have analyzed the importance of the utility of MHC class II dextramers, especially for detecting autoreactive CD4 T cells in relation to tetramers. While tetramers and other similar reagents (pentamers and protein-A conjugated multimers) are being made available through several academic (NIH Tetramer Core Facility, Atlanta, GA) and non-academic sources (Proimmune Inc, Beckman Coulter Inc, and MBL Inc, and TCMetrix Inc), dextramers are currently available only commercially (Immudex, Denmark). By creating dextramers for various autoantigens, we showed them to be superior to tetramers with respect to staining specificity, detection sensitivity, and various applications, but a fraction of antigen-specific CD4 T cells may still be undetected by dextramers [11–15, 59]. Nonetheless, specifically for ex vivo analysis, the dextramer reagents may offer an advantage by enumerating the frequencies of antigen-specific T cells directly, thereby avoiding the need to adopt other laborious procedures like enrichment of tetramer-bound T cells by magnetic separation [22, 43]. Finally, it should be noted that MHC class II tetramers are increasingly being used in clinical research in various areas, including, for example, studies of infectious diseases (Lyme arthritis, pulmonary tuberculosis, cytomegalovirus infection, and chronic hepatitis), immune-mediated diseases (type I diabetes and multiple sclerosis), and allergic diseases (atopic dermatitis and rye grass-associated allergy), and for monitoring T cell responses to vaccines (melanoma, influenza, and anthrax) [9, 17, 21, 47, 60–70]. In all of these evaluations, the frequencies of antigen-specific T cells have been reported to range from 0.006% to 3.5% in different body fluids, proving that tetramers are helpful in clinical research. But the real challenge is to be able to reliably detect the T cells ex vivo in a short period of time without having to stimulate the PBMCs, which is often the case with the human samples [21, 63]. Such limitations can possibly be overcome with the availability of MHC class II dextramers, since their detection sensitivity is higher than that of tetramers [15, 35]. To this end, we are now in the process of creating dextramers for use in patients with multiple sclerosis. We believe that potential exists for the use of MHC class II dextramers in both basic and clinical research as the reagents become available.
References
- 1.Buckner JH, Holzer U, Novak EJ, Reijonen H, Kwok WW, Nepom GT. Defining antigen-specific responses with human MHC class II tetramers. The Journal of allergy and clinical immunology. 2002 Aug;110:199–208. doi: 10.1067/mai.2002.125976. [DOI] [PubMed] [Google Scholar]
- 2.Carneiro J, Duarte L, Padovan E. Limiting dilution analysis of antigen-specific T cells. Methods Mol Biol. 2009;514:95–105. doi: 10.1007/978-1-60327-527-9_7. [DOI] [PubMed] [Google Scholar]
- 3.Mayer S, Scheibenbogen C, Lee KH, et al. A sensitive proliferation assay to determine the specific T cell response against HLA-A2.1-binding peptides. Journal of immunological methods. 1996 Oct 16;197:131–137. doi: 10.1016/0022-1759(96)00124-x. [DOI] [PubMed] [Google Scholar]
- 4.Mannering SI, Morris JS, Jensen KP, et al. A sensitive method for detecting proliferation of rare autoantigen-specific human T cells. Journal of immunological methods. 2003 Dec;283:173–183. doi: 10.1016/j.jim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Meierhoff G, Ott PA, Lehmann PV, Schloot NC. Cytokine detection by ELISPOT: relevance for immunological studies in type 1 diabetes. Diabetes/metabolism research and reviews. 2002 Sep-Oct;18:367–380. doi: 10.1002/dmrr.320. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann H, Lefkovits I, Quintans J. Limiting dilution analysis of helper T-cell function. Immunology. 1975 Jun;28:1135–1148. [PMC free article] [PubMed] [Google Scholar]
- 7.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996 Oct 4;274:94–96. [PubMed] [Google Scholar]
- 8.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998 Jun;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 9.Vollers SS, Stern LJ. Class II major histocompatibility complex tetramer staining: progress, problems, and prospects. Immunology. 2008 Mar;123:305–313. doi: 10.1111/j.1365-2567.2007.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wooldridge L, Lissina A, Cole DK, van den Berg HA, Price DA, Sewell AK. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology. 2009 Feb;126:147–164. doi: 10.1111/j.1365-2567.2008.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangaplara A, Massilamany C, Brown DM, et al. Coxsackievirus B3 infection leads to the generation of cardiac myosin heavy chain-alpha-reactive CD4 T cells in A/J mice. Clinical Immunology. 2012 Sep;144:237–249. doi: 10.1016/j.clim.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Massilamany C, Gangaplara A, Jia T, et al. Direct staining with major histocompatibility complex class II dextramers permits detection of antigen-specific, autoreactive CD4 T cells in situ. PLoS One. 2014;9:e87519. doi: 10.1371/journal.pone.0087519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massilamany C, Gangaplara A, Jia T, et al. In situ detection of autoreactive CD4 T cells in brain and heart using major histocompatibility complex class II dextramers. Journal of visualized experiments : JoVE. 2014:e51679. doi: 10.3791/51679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massilamany C, Marciano-Cabral F, Rocha-Azevedo B, et al. SJL mice infected with Acanthamoeba castellanii develop central nervous system autoimmunity through the generation of cross-reactive T cells for myelin antigens. PLoS One. 2014;9:e98506. doi: 10.1371/journal.pone.0098506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massilamany C, Upadhyaya B, Gangaplara A, Kuszynski C, Reddy J. Detection of autoreactive CD4 T cells using major histocompatibility complex class II dextramers. BMC Immunol. 2011;12:40. doi: 10.1186/1471-2172-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. Journal of Immunology. 2012 May 1;188:4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nepom GT. MHC class II tetramers. Journal of Immunology. 2012 Mar 15;188:2477–2482. doi: 10.4049/jimmunol.1102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbas AK, Lichtman AH, Pillai S. Cellular and molecular Immunology. 6th edition ed. Philadelphia: Saunders Elsevier; 2010. [Google Scholar]
- 19.Bridgeman JS, Sewell AK, Miles JJ, Price DA, Cole DK. Structural and biophysical determinants of alphabeta T-cell antigen recognition. Immunology. 2012 Jan;135:9–18. doi: 10.1111/j.1365-2567.2011.03515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole DK, Miles KM, Madura F, et al. T-cell receptor (TCR)-peptide specificity overrides affinity-enhancing TCR-major histocompatibility complex interactions. The Journal of biological chemistry. 2014 Jan 10;289:628–638. doi: 10.1074/jbc.M113.522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day CL, Seth NP, Lucas M, et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. Journal of Clinical Investigation. 2003;112:831. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang MH, Seth NP, Wucherpfennig KW. Ex vivo analysis of thymic CD4 T cells in nonobese diabetic mice with tetramers generated from I-A(g7)/class II-associated invariant chain peptide precursors. Journal of Immunology. 2003 Oct 15;171:4175–4186. doi: 10.4049/jimmunol.171.8.4175. [DOI] [PubMed] [Google Scholar]
- 23.Massilamany C, Gangaplara A, Chapman N, Rose N, Reddy J. Detection of cardiac myosin heavy chain-alpha-specific CD4 cells by using MHC class II/IA(k) tetramers in A/J mice. Journal of immunological methods. 2011 Sep 30;372:107–118. doi: 10.1016/j.jim.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4+ T cells proliferating in response to influenza A antigen. Journal of Clinical Investigation. 1999;104:R63. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemaitre F, Viguier M, Cho MS, et al. Detection of low-frequency human antigen-specific CD4(+) T cells using MHC class II multimer bead sorting and immunoscope analysis. European journal of Immunology. 2004 Oct;34:2941–299. doi: 10.1002/eji.200425281. [DOI] [PubMed] [Google Scholar]
- 26.Arnold PY, Vignali KM, Miller TB, et al. Reliable generation and use of MHC class II:gamma2aFc multimers for the identification of antigen-specific CD4(+) T cells. Journal of immunological methods. 2002 Dec 20;271:137–151. doi: 10.1016/s0022-1759(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 27.Kozono H, White J, Clements J, Marrack P, Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994 May 12;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- 28.Sloan VS, Cameron P, Porter G, et al. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995 Jun 29;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 29.Stern LJ, Wiley DC. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell. 1992 Feb 7;68:465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- 30.Zarutskie JA, Sato AK, Rushe MM, et al. A conformational change in the human major histocompatibility complex protein HLA-DR1 induced by peptide binding. Biochemistry. 1999 May 4;38:5878–5887. doi: 10.1021/bi983048m. [DOI] [PubMed] [Google Scholar]
- 31.Massilamany C, Gangaplara A, Chapman N, Rose N, Reddy J. Detection of cardiac myosin heavy chain-α-specific CD4 cells by using MHC class II/IAk tetramers in A/J mice. Journal of immunological methods. 2011 doi: 10.1016/j.jim.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Kalandadze A, Galleno M, Foncerrada L, Strominger JL, Wucherpfennig KW. Expression of recombinant HLA-DR2 molecules. Replacement of the hydrophobic transmembrane region by a leucine zipper dimerization motif allows the assembly and secretion of soluble DR alpha beta heterodimers. The Journal of biological chemistry. 1996 Aug 16;271:20156–20162. doi: 10.1074/jbc.271.33.20156. [DOI] [PubMed] [Google Scholar]
- 33.Reddy J, Bettelli E, Nicholson L, et al. Detection of autoreactive myelin proteolipid protein 139–151-specific T cells by using MHC II (IAs) tetramers. Journal of Immunology. 2003 Jan 15;170:870–877. doi: 10.4049/jimmunol.170.2.870. [DOI] [PubMed] [Google Scholar]
- 34.Kohler JJ, Schepartz A. Kinetic studies of Fos.Jun.DNA complex formation: DNA binding prior to dimerization. Biochemistry. 2001 Jan 9;40:130–142. doi: 10.1021/bi001881p. [DOI] [PubMed] [Google Scholar]
- 35.Batard P, Peterson DA, Devevre E, et al. Dextramers: new generation of fluorescent MHC class I/peptide multimers for visualization of antigen-specific CD8+ T cells. Journal of immunological methods. 2006 Mar 20;310:136–148. doi: 10.1016/j.jim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Gangaplara A, Massilamany C, Steffen D, Reddy J. Mimicry epitope from Ehrlichia canis for interphotoreceptor retinoid-binding protein 201–216 prevents autoimmune uveoretinitis by acting as altered peptide ligand. Journal of neuroImmunology. 2013 Oct 15;263:98–107. doi: 10.1016/j.jneuroim.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Massilamany C, Gangaplara A, Steffen D, Reddy J. Identification of novel mimicry epitopes for cardiac myosin heavy chain-alpha that induce autoimmune myocarditis in A/J mice. Cellular Immunology. 2011;271:438–449. doi: 10.1016/j.cellimm.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Massilamany C, Steffen D, Reddy J. An epitope from Acanthamoeba castellanii that cross-react with proteolipid protein 139–151-reactive T cells induces autoimmune encephalomyelitis in SJL mice. Journal of neuroImmunology. 2010 Feb 26;219:17–24. doi: 10.1016/j.jneuroim.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Massilamany C, Thulasingam S, Steffen D, Reddy J. Gender differences in CNS autoimmunity induced by mimicry epitope for PLP 139–151 in SJL mice. Journal of neuroImmunology. 2011 Jan;230:95–104. doi: 10.1016/j.jneuroim.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature medicine. 2007 Apr;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy J, Illes Z, Zhang X, et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2004 Oct 26;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy J, Waldner H, Zhang X, et al. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. Journal of Immunology. 2005 Nov 1;175:5591–5595. doi: 10.4049/jimmunol.175.9.5591. [DOI] [PubMed] [Google Scholar]
- 43.Moon JJ, Chu HH, Pepper M, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007 Aug;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013 Feb 21;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James EA, Rieck M, Pieper J, et al. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis & rheumatology. 2014 Jul;66:1712–1722. doi: 10.1002/art.38637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wambre E, DeLong JH, James EA, et al. Specific immunotherapy modifies allergen-specific CD4(+) T-cell responses in an epitope-dependent manner. The Journal of allergy and clinical Immunology. 2014 Mar;133:872 e7–879 e7. doi: 10.1016/j.jaci.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reijonen H, Novak EJ, Kochik S, et al. Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes. 2002 May;51:1375–1382. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- 48.Mallone R, Nepom GT. MHC Class II tetramers and the pursuit of antigen-specific T cells: define, deviate, delete. Clinical Immunology. 2004 Mar;110:232–242. doi: 10.1016/j.clim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Dolton G, Lissina A, Skowera A, et al. Comparison of peptide-major histocompatibility complex tetramers and dextramers for the identification of antigen-specific T cells. Clin Exp Immunol. 2014 Jul;177:47–63. doi: 10.1111/cei.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cameron TO, Cochran JR, Yassine-Diab B, Sekaly RP, Stern LJ. Cutting edge: detection of antigen-specific CD4+ T cells by HLA-DR1 oligomers is dependent on the T cell activation state. Journal of Immunology. 2001 Jan 15;166:741–745. doi: 10.4049/jimmunol.166.2.741. [DOI] [PubMed] [Google Scholar]
- 51.Novak EJ, Masewicz SA, Liu AW, Lernmark A, Kwok WW, Nepom GT. Activated human epitope-specific T cells identified by class II tetramers reside within a CD4high, proliferating subset. International Immunology. 2001 Jun;13:799–806. doi: 10.1093/intimm/13.6.799. [DOI] [PubMed] [Google Scholar]
- 52.Reijonen H, Kwok WW, Nepom GT. Detection of CD4+ autoreactive T cells in T1D using HLA class II tetramers. Ann N Y Acad Sci. 2003 Nov;1005:82–87. doi: 10.1196/annals.1288.009. [DOI] [PubMed] [Google Scholar]
- 53.Fujita-Yamaguchi Y, Sato Y, Kathuria S. Removal of sialic acids from the purified insulin receptor results in enhanced insulin-binding and kinase activities. Biochemical and biophysical research communications. 1985 Jun 28;129:739–745. doi: 10.1016/0006-291x(85)91954-0. [DOI] [PubMed] [Google Scholar]
- 54.Skinner PJ, Daniels MA, Schmidt CS, Jameson SC, Haase AT. Cutting edge: In situ tetramer staining of antigen-specific T cells in tissues. Journal of Immunology. 2000 Jul 15;165:613–617. doi: 10.4049/jimmunol.165.2.613. [DOI] [PubMed] [Google Scholar]
- 55.Skinner PJ, Haase AT. In situ tetramer staining. Journal of immunological methods. 2002 Oct 1;268:29–34. doi: 10.1016/s0022-1759(02)00197-7. [DOI] [PubMed] [Google Scholar]
- 56.Bischof F, Hofmann M, Schumacher TN, et al. Analysis of autoreactive CD4 T cells in experimental autoimmune encephalomyelitis after primary and secondary challenge using MHC class II tetramers. Journal of Immunology. 2004 Mar 1;172:2878–2884. doi: 10.4049/jimmunol.172.5.2878. [DOI] [PubMed] [Google Scholar]
- 57.Lissina A, Ladell K, Skowera A, et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. Journal of immunological methods. 2009 Jan 1;340:11–24. doi: 10.1016/j.jim.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamad AR, O'Herrin SM, Lebowitz MS, et al. Potent T cell activation with dimeric peptide-major histocompatibility complex class II ligand: the role of CD4 coreceptor. The Journal of experimental medicine. 1998 Nov 2;188:1633–1640. doi: 10.1084/jem.188.9.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massilamany C, Gangaplara A, Kim H, et al. Copper-zinc superoxide dismutase-deficient mice show increased susceptibility to experimental autoimmune encephalomyelitis induced with myelin oligodendrocyte glycoprotein 35–55. Journal of neuroImmunology. 2013 Mar 15;256:19–27. doi: 10.1016/j.jneuroim.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bronke C, Palmer NM, Westerlaken GH, et al. Direct ex vivo detection of HLA-DR3-restricted cytomegalovirus- and Mycobacterium tuberculosis-specific CD4+ T cells. Hum Immunol. 2005 Sep;66:950–961. doi: 10.1016/j.humimm.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Höhn H, Kortsik C, Zehbe I, et al. MHC class II Tetramer Guided Detection of Mycobacterium tuberculosis-specific CD4+ T Cells in Peripheral Blood from Patients with Pulmonary Tuberculosis. Scandinavian journal of Immunology. 2007;65:467–478. doi: 10.1111/j.1365-3083.2007.01924.x. [DOI] [PubMed] [Google Scholar]
- 62.Meyer AL, Trollmo C, Crawford F, et al. Direct enumeration of Borrelia-reactive CD4 T cells ex vivo by using MHC class II tetramers. Proceedings of the National Academy of Sciences of the United States of America. 2000 Oct 10;97:11433–11438. doi: 10.1073/pnas.190335897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulsenheimer A, Lucas M, Seth N, et al. Transient immunological control during acute hepatitis C virus infection: ex vivo analysis of helper T-cell responses. Journal of viral hepatitis. 2006;13:708–714. doi: 10.1111/j.1365-2893.2006.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bateman EAL, Ardern-Jones MR, Ogg GS. Persistent central memory phenotype of circulating Fel d 1 peptide/DRB1* 0101 tetramer-binding CD4+ T cells. Journal of allergy and clinical Immunology. 2006;118:1350–1356. doi: 10.1016/j.jaci.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 65.Macaubas C, Wahlstrom J, Galvao da Silva AP, et al. Allergen-specific MHC class II tetramer+ cells are detectable in allergic, but not in nonallergic, individuals. Journal of Immunology. 2006 Apr 15;176:5069–5077. doi: 10.4049/jimmunol.176.8.5069. [DOI] [PubMed] [Google Scholar]
- 66.Danke NA, Kwok WW. HLA class II-restricted CD4+ T cell responses directed against influenza viral antigens postinfluenza vaccination. The Journal of Immunology. 2003;171:3163–3169. doi: 10.4049/jimmunol.171.6.3163. [DOI] [PubMed] [Google Scholar]
- 67.Laughlin EM, Miller JD, James E, et al. Antigen-specific CD4+ T cells recognize epitopes of protective antigen following vaccination with an anthrax vaccine. Infect Immun. 2007 Apr;75:1852–1860. doi: 10.1128/IAI.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lucas M, Day CL, Wyer JR, et al. Ex vivo phenotype and frequency of influenza virus-specific CD4 memory T cells. Journal of virology. 2004;78:7284–7287. doi: 10.1128/JVI.78.13.7284-7287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong R, Lau R, Chang J, et al. Immune responses to a class II helper peptide epitope in patients with stage III/IV resected melanoma. Clinical cancer research. 2004;10:5004–5013. doi: 10.1158/1078-0432.CCR-04-0241. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Renkvist N, Sun Z, et al. Apolyclonal anti-vaccine CD4 T cell response detected with HLA-DP4 multimers in a melanoma patient vaccinated with MAGE-3. DP4-peptide-pulsed dendritic cells. European journal of Immunology. 2005;35:1066–1075. doi: 10.1002/eji.200425847. [DOI] [PubMed] [Google Scholar]
- 71.Taniguchi RT, DeVoss JJ, Moon JJ, et al. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proceedings of the National Academy of Sciences of the United States of America. 2012 May 15;109:7847–7852. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi JY, Eo SK. Detection of Foreign Antigen-specific CD4(+)Foxp3(+) Regulatory T Cells by MHC Class II Tetramer and Intracellular CD154 Staining. Immune Netw. 2013 Dec;13:264–274. doi: 10.4110/in.2013.13.6.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabatino JJ, Jr, Huang J, Zhu C, Evavold BD. High prevalence of low affinity peptide-MHC II tetramer-negative effectors during polyclonal CD4+ T cell responses. The Journal of experimental medicine. 2011 Jan 17;208:81–90. doi: 10.1084/jem.20101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mallet-Designe VI, Stratmann T, Homann D, Carbone F, Oldstone MB, Teyton L. Detection of low-avidity CD4+ T cells using recombinant artificial APC: following the antiovalbumin immune response. Journal of Immunology. 2003 Jan 1;170:123–131. doi: 10.4049/jimmunol.170.1.123. [DOI] [PubMed] [Google Scholar]
- 75.Verginis P, McLaughlin KA, Wucherpfennig KW, von Boehmer H, Apostolou I. Induction of antigen-specific regulatory T cells in wild-type mice: visualization and targets of suppression. Proceedings of the National Academy of Sciences. 2008;105:3479–3484. doi: 10.1073/pnas.0800149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Landais E, Romagnoli PA, Corper AL, et al. New design of MHC class II tetramers to accommodate fundamental principles of antigen presentation. Journal of Immunology. 2009 Dec 15;183:7949–7957. doi: 10.4049/jimmunol.0902493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scott-Browne JP, Crawford F, Young MH, Kappler JW, Marrack P, Gapin L. Evolutionarily conserved features contribute to αβ T cell receptor specificity. Immunity. 2011;35:526–535. doi: 10.1016/j.immuni.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crawford F, Stadinski B, Jin N, et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proceedings of the National Academy of Sciences of the United States of America. 2011 Oct 4;108:16729–16734. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.You S, Chen C, Lee WH, et al. Detection and characterization of T cells specific for BDC2.5 T cell-stimulating peptides. Journal of Immunology. 2003 Apr 15;170:4011–4020. doi: 10.4049/jimmunol.170.8.4011. [DOI] [PubMed] [Google Scholar]
- 80.Boniface JJ, Rabinowitz JD, Wülfing C, et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands. Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 81.Kattah NH, Newell EW, Jarrell JA, et al. Tetramers reveal IL-17-secreting CD4+ T cells that are specific for U1–70 in lupus and mixed connective tissue disease. Proceedings of the National Academy of Sciences of the United States of America. 2015 Mar 10;112:3044–3049. doi: 10.1073/pnas.1424796112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raki M, Fallang LE, Brottveit M, et al. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proceedings of the National Academy of Sciences of the United States of America. 2007 Feb 20;104:2831–2836. doi: 10.1073/pnas.0608610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cecconi V, Moro M, Del Mare S, et al. The CD4+ T-cell epitope-binding register is a critical parameter when generating functional HLA-DR tetramers with promiscuous peptides. European journal of Immunology. 2010 Jun;40:1603–1616. doi: 10.1002/eji.200940123. [DOI] [PubMed] [Google Scholar]
- 84.Yang J, Danke NA, Berger D, et al. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. Journal of Immunology. 2006 Mar 1;176:2781–2789. doi: 10.4049/jimmunol.176.5.2781. [DOI] [PubMed] [Google Scholar]
- 85.Bonvalet M, Wambre E, Moussu H, et al. Comparison between major histocompatibility complex class II tetramer staining and surface expression of activation markers for the detection of allergen-specific CD4(+) T cells. Clin Exp Allergy. 2011 Jun;41:821–829. doi: 10.1111/j.1365-2222.2011.03708.x. [DOI] [PubMed] [Google Scholar]
- 86.Scriba TJ, Purbhoo M, Day CL, et al. Ultrasensitive detection and phenotyping of CD4+ T cells with optimized HLA class II tetramer staining. Journal of Immunology. 2005 Nov 15;175:6334–6343. doi: 10.4049/jimmunol.175.10.6334. [DOI] [PubMed] [Google Scholar]
- 87.Cameron TO, Cohen GB, Islam SA, Stern LJ. Examination of the highly diverse CD4(+) T-cell repertoire directed against an influenza peptide: a step towards TCR proteomics. Immunogenetics. 2002 Dec;54:611–620. doi: 10.1007/s00251-002-0508-y. [DOI] [PubMed] [Google Scholar]
- 88.Rastogi D, Wang C, Mao X, Lendor C, Rothman PB, Miller RL. Antigen-specific immune responses to influenza vaccine in utero. J Clin Invest. 2007 Jun;117:1637–1646. doi: 10.1172/JCI29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raddassi K, Kent SC, Yang J, et al. Increased frequencies of myelin oligodendrocyte glycoprotein/MHC class II-binding CD4 cells in patients with multiple sclerosis. Journal of Immunology. 2011 Jul 15;187:1039–1046. doi: 10.4049/jimmunol.1001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kotzin BL, Falta MT, Crawford F, et al. Use of soluble peptide-DR4 tetramers to detect synovial T cells specific for cartilage antigens in patients with rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2000 Jan 4;97:291–296. doi: 10.1073/pnas.97.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Veldman C, Eming R, Wolff-Franke S, Sonderstrup G, Kwok WW, Hertl M. Detection of low avidity desmoglein 3-reactive T cells in pemphigus vulgaris using HLA-DR beta 1*0402 tetramers. Clinical Immunology. 2007 Mar;122:330–337. doi: 10.1016/j.clim.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 92.Bronke C, Palmer NM, Westerlaken GH, et al. Direct Ex Vivo Detection of HLA-DR3- Restricted Cytomegalovirus-and Mycobacterium tuberculosis-Specific CD4+ T Cells. Human Immunology. 2005;66:950–961. doi: 10.1016/j.humimm.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 93.Falta MT, Pinilla C, Mack DG, et al. Identification of beryllium-dependent peptides recognized by CD4+ T cells in chronic beryllium disease. The Journal of experimental medicine. 2013 Jul 1;210:1403–1418. doi: 10.1084/jem.20122426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mack DG, Falta MT, McKee AS, et al. Regulatory T cells modulate granulomatous inflammation in an HLA-DP2 transgenic murine model of beryllium-induced disease. Proceedings of the National Academy of Sciences of the United States of America. 2014 Jun 10;111:8553–8558. doi: 10.1073/pnas.1408048111. [DOI] [PMC free article] [PubMed] [Google Scholar]