Abstract
We report that EF24, a synthetic compound 3,5-bis(2-flurobenzylidene)piperidin-4-one, greatly inhibits cisplatin-resistant (CR) human ovarian cancer cell proliferation. The inhibitory effect of EF24 on cell proliferation is associated with G2/M phase cell cycle arrest and increased G2/M checkpoint protein (pp53, p53, and p21) levels. Within 24 h following treatment, EF24 induced apoptosis in CR cells. The apoptosis was partially blocked by the general caspase inhibitor z-VAD. Within 12 h, EF24 induced a membranous FasL expression, consistent with a substantial decrease in the Ser473 and Thr308 phosphorylation of Akt, a known negative regulator of FasL transcription. Also, EF24 activated the phosphorylated PTEN and marginally upregulated total PTEN expression through the inhibition of ubiquitin-mediated PTEN degradation. Suppression of PTEN expression with siRNA significantly reduced the p53 and p21 levels and activated Akt phosphorylation at Ser473 and Thr308, resulting in decreased apoptosis and increased cell survival. On the other hand, overexpression of PTEN markedly induced apoptosis. Our results clearly suggested that EF24 induced significant increase in PTEN expression. The up-regulation of PTEN inhibited Akt and MDM2, which enhanced the level of p53, thereby inducing G2/M arrest and apoptosis. Therefore, EF24 appears to have a potential therapeutic role in human ovarian cancer through the activation of PTEN.
Drug resistance remains a major challenge in clinical cancer treatment (1). Patients suffering from advanced ovarian carcinoma are, in most cases, initially responsive to chemotherapy; however, they later experience a relapse of the disease because of the eventual recurrence of the tumor and emergence of drug-resistant tumor cells. Cisplatin is an effective and widely used chemotherapeutic agent against various human cancers, including ovarian cancer (2). Despite its great efficacy at treating ovarian cancer, a major limitation of cisplatin treatment is the development of drug resistance. Multiple mechanisms have been proposed for the cisplatin resistance, including reduced intracellular accumulation of the drug, increased levels of glutathione, up-regulation of anti-apoptotic proteins, and downregulation of pro-apoptotic proteins (3, 4). Cisplatin resistance is also associated with the altered activation of signaling pathways which include phosphatidylinositol 3-kinase (PI3K)2/Akt and MAPK, or the suppression of tumor suppressor genes, p53 and PTEN (1, 5).
The tumor suppressor gene PTEN encodes a multifunctional phosphatase that is mutated in a variety of human cancers (6, 7). PTEN inhibits the PI3K pathway and downstream functions, including activation of Akt and protein kinase B, cell survival, and cell proliferation in tumor cells carrying mutant or deletion type PTEN (6, 8, 9). Inactivation of PTEN because of a genetic mutation resulted in increased Akt activity in many types of tumor (10). The overexpression of PTEN in cancer cells carrying mutant or deletion type PTEN can inhibit cell proliferation and tumorigenicity via induction of cell cycle arrest at the G1 phase and apoptosis (11). PTEN can bind directly to p53 and prevent its degradation in a manner independent of its effect on Akt activation (12). Recently published evidence indicates the existence of a mechanistic link between PTEN and p53 functions through the control of the phosphorylation state of MDM2, which modulates the nuclear localization of MDM2 and the ubiquitination of p53 by the activation of the PI3K/Akt signal transduction pathway (13–15). In addition, an important function of p53 is to act as a transcription factor by binding to a p53-specific DNA consensus sequence in responsive genes, which would be expected to increase the synthesis of p21 (waf1) (16). The p21 is the most important protein involved in cell cycle arrest at both G1 and G2/M checkpoints.
Activation of the PI3K/Akt pathway is one of the critical steps in cell survival which occurs through suppression of apoptosis. The anti-apoptotic function of Akt is reported to be mediated by its ability to phosphorylate apoptosis regulatory molecules including BAD, caspase 9, IKK, and the forkhead transcriptional factor FKHRL1 (17, 18). Recent reports show that Akt inhibition leads to the up-regulation of FasL expression and DNA fragmentation in T-lymphocytes and glioma cells (19, 20). The study by Suhara et al. (21) examined the role of PI3K/Akt signaling in the regulation of FasL expression and cellular survival in vascular smooth muscle cells. It is shown that serum deprivation, incubation with the PI3K inhibitor wortmannin, or transduction of an inactivating Akt mutant will lead to increases in FasL mRNA and cell surface expression of the FasL protein.
In our efforts to study the efficacy and mechanisms of novel anticancer agents for ovarian carcinoma, we recently reported that curcumin, a major active component of Curcuma longa, induced cell cycle arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt/p38 pathways (22). Recently, EF24 (Fig. 1A), a new compound having close structural similarity to curcumin, has been reported to inhibit the proliferation of a variety of cancer cells in vitro (23). The anticancer effect of EF24 in breast cancer cells has been attributed, in part, to redox-mediated induction of apoptosis (24). This motivated us to investigate the anti-cancer efficacy and molecular mechanism action of EF24 on cisplatin-resistant (CR) ovarian cancer cells. We observed that EF24 greatly inhibited the proliferation of CR cells by inducing G2/M arrest and apoptosis. We further observed that EF24 significantly increased PTEN expression resulting in the down-regulation of Akt and MDM2 and activation of p53.
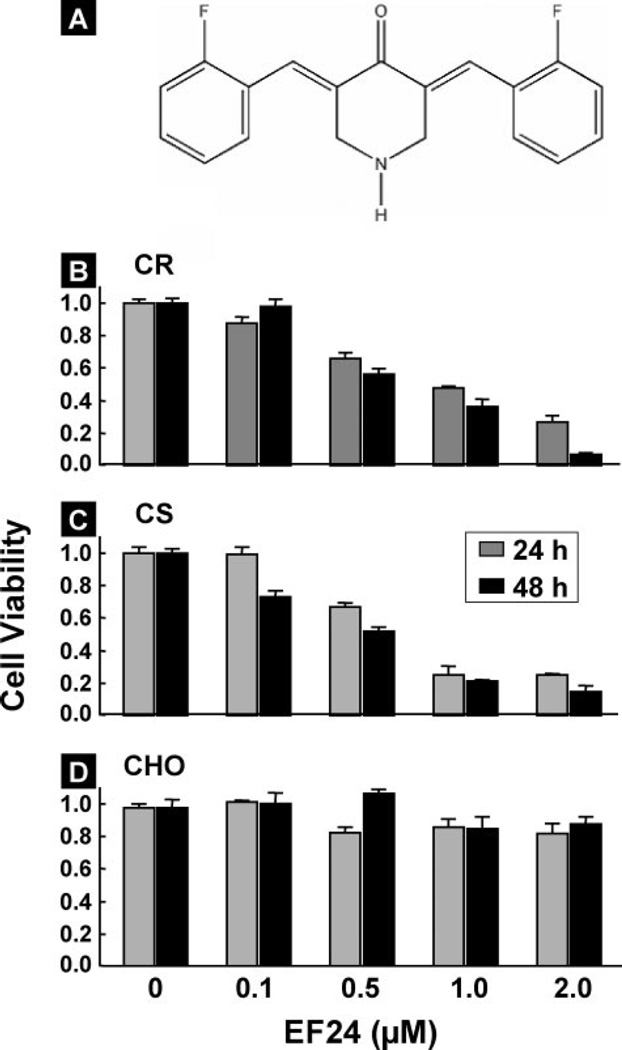
FIGURE 1. Effect of EF24 on cell viability.
A, molecular structure of EF24 (3,5-bis(2-flurobenzylidene) piperidin-4-one). Effects of EF24 dose and incubation time on the cell viability, expressed as a fraction of untreated control are shown for CR and CS ovarian cancer cells (B and C) and CHO cells (D). Cell viability assays were performed using MTT as described under “Experimental Procedures.” The graph displays the mean ± S.E. of three independent experiments. The data indicate that EF24 is more effective in inhibiting the growth of ovarian cancer cells compared with normal cells.
EXPERIMENTAL PROCEDURES
Materials
EF24 was synthesized as reported by Adams et al. (23). Dimethyl sulfoxide (Me2SO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and antibodies against actin and FLAG were obtained from Sigma. Cell culture medium (RPMI 1640), fetal bovine serum, antibiotics, sodium pyruvate, trypsin, and phosphate-buffered saline (PBS) were purchased from Invitrogen. Polyvinylidene fluoride membrane and molecular weight marker were obtained from Bio-Rad. Antibodies against Akt, pAkt (Ser473 and Thr308), ERK1/2, pERK1/2, PTEN, pPTEN (Ser380 and Thr381/382), polyadenosine diphosphate ribose polymerase (PARP), cleaved caspases-3, -7, and -8 and PTEN siRNA kit were purchased from Cell Signaling Technology (Beverly, MA). PTEN plasmid (plasmid 10786: 1437 pSG5L FLAG HA PTEN) was obtained from AddGene (Cambridge, MA). CycB1, CycA, Cyc D1, Wee1, Cdc2, Cdc25c, p-p53 Ser15, p53, p21, and p27, MDM2, Fas/CD95, FasL, TNFR1, and ubiquitin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). MG-132 and CK2 were from Calbiochem. Recombinant protein G-agarose and Lipofectamine kits were purchased from Invitrogen. The caspase inhibitor Z-VAD-FMK and RNase were from Promega (Madison, WI). Enhanced chemiluminescence (ECL) reagents were obtained from Amersham Biosciences (Buckinghamshire, UK). All other reagents and compounds were analytical grades.
Cell Lines and Cultures
Human ovarian carcinoma cell line, A2780, (cisplatin-sensitive, hereafter abbreviated as CS) and a derived cisplatin-resistant subline, A2780 cDDP (cisplatin-resistant, hereafter abbreviated as CR), were used. These cell lines were developed as in vivo s.c. models in athymic mice. The cisplatin-resistant cell line was originally developed from an in vivo tumor model by treating with cisplatin (25). Chinese hamster ovary (CHO) cells were used as controls. Cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2% sodium pyruvate, 1% penicillin, and streptomycin. Cells were grown in a 100-mm dish to 70% confluence at 37 °C in an atmosphere of 5% CO2 and 95% air. Cells were routinely trypsinized (0.05% trypsin/EDTA) and counted using a Nucleo-Counter (New Brunswick Scientific, Edison, NJ).
Cell Viability Assay
Cell viability was determined using the conversion of MTT to formazan via mitochondrial oxidation. Cells were grown in T-75 flasks to >80% confluence. They were then trypsinized, counted, and seeded in 96-well plates with an average of 7,000 cells/well. Cells were incubated overnight and then treated in triplicate with 2 µm EF24 for 24 or 48 h. All experiments were repeated at least three times.
Cell Cycle Analysis
CR cells were treated with EF24 (2 µm) for 3, 6, 12, and 24 h and then trypsinized, washed in PBS, and fixed in ice-cold 75% ethanol/PBS. The DNA was labeled with propidium iodide. Cells were sorted by flow cytometry analysis, and cell cycle profiles were determined using ModFit LT software (Becton Dickinson, San Diego, CA).
Immunoblot Analysis
CR cells were grown in RPMI 1640 medium and treated with Me2SO (control) or EF24 (2 µm) for the desired times. Equal volumes of Me2SO (0.1% v/v) were present in each treatment. Following EF24 treatments, the cell lysates were prepared in nondenaturing lysis buffer (10 mm Tris-HCl, pH 7.4, 150mm NaCl, 1% Triton X-100, 1mm EDTA, 1 mm EGTA, 0.3 mm phenylmethylsulfonyl fluoride, 0.2 mm sodium orthovanadate, 0.5% Nonidet P-40, aprotinin (1 µg/ml), and leupeptin. Cell lysates were centrifuged at 10,000 × g for 20 min at 4 °C, and the supernatant was separated. The protein concentration in the lysates was determined using a Pierce detergent-compatible protein assay kit. For Western blotting, 25 to 50 µg of protein lysate per sample was denatured in 2× SDS-PAGE sample buffer and subjected to SDS-PAGE on a 10% or 12% Tris-glycine gel. The separated proteins were transferred to a polyvinylidene difluoride membrane and then the membrane was blocked with 5% nonfat milk powder (w/v) in TBST (10 mm Tris, 100 mm NaCl, 0.1% Tween 20) for 1 h at room temperature or overnight at 4 °C. The membranes were incubated with the primary antibodies described above. The bound antibodies were detected with horseradish peroxidase-labeled sheep anti-mouse IgG or horseradish peroxidase-labeled donkey anti-rabbit IgG (Amersham Biosciences) using an enhanced chemiluminescence detection system (ECL Advanced kit). Protein expressions were determined using an Image Gauge version 3.45.
Immunoprecipitation
For immunoprecipitation, cells were lysed in lysis buffer (50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 0.5% Nonidet P-40, 1mm EDTA (pH 8.0), 1mm EGTA (pH 8.0), 0.1 mm sodium fluoride, 0.1 mm sodium orthovanadate, 1 mm dithiothreitol, 2 µg/ml aprotinin, and leupeptin (2 µg/ml). Cell lysates were centrifuged at 10,000 × g for 20 min at 4 °C, and the supernatant was separated. A 500-µg sample of the total protein was used for immunoprecipitation. Either PTEN, p53, or ubiquitin antibody was incubated with cell lysates for 2 h followed by addition of 20 µl of recombinant agarose G-protein was added in the samples, and then samples were incubated for an additional 1 h. The matrices were washed four times with the same lysis buffer. After being boiled for 8 min in the presence of 2-mercaptoethanol, samples containing cell lysate protein were separated on a 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE gel) and then transferred onto equilibrated polyvinylidene difluoride membranes. After blocking with skimmed milk, the membranes were incubated with the primary antibodies described above. The bound antibodies were detected with horseradish peroxidase-labeled sheep anti-mouse IgG or horseradish peroxidase-labeled donkey anti-rabbit IgG (Amersham Biosciences) using an ECL kit.
PTEN siRNAs Study
CR cells were transfected with PTEN siRNA and negative control siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. One day after transfection, the transfection complexes were removed and replaced with culture medium. After incubating with 2 µm EF24 in culture medium for 6 or 24 h, cell cycle arrest and sub-G1 levels were analyzed by flow cytometry. The cell lysates were subjected to immunoblot for PTEN, pAkt (Ser473 and Thr308), Akt, p53, cleaved caspases 3, 7, and PARP.
Transfection of Wild-type PTEN cDNA
The PTEN overexpression experiments were performed using a wild-type PTEN cDNA. The FLAG-tagged gene was transfected into CR and CHO cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. At 24 h after the transfection of the PTEN gene, EF24 (2 µm) was added and incubated for 24 h. The cells were then subjected to viability and immunoblot assays for PTEN, pAkt Ser473, p53, p21, cleaved caspase-3, PARP, and FLAG.
Statistical Analysis
Results were expressed as mean ± S.E. Comparisons between groups were made by a Student’s t test. The significance level was set at p < 0.05.
RESULTS
EF24 Causes Decrease in Cell Viability in Ovarian Cancer Cells
To determine the effect of EF24 on ovarian cancer cell viability, we treated cells with different concentrations (0.1, 0.5, 1, and 2 µm) of EF24 at time points (24 and 48 h). Cell viability was measured by MTT assay. The proliferation of CR cells was inhibited by EF24 in a dose-dependent manner (Fig. 1B). We also observed a similar effect of EF24 on cisplatin-sensitive (CS) human ovarian cancer cells (Fig. 1C). On the other hand, EF24 did not induce any significant cell death in CHO cells (Fig. 1D). The growth inhibition constants (IC50) of the CR, CS, and CHO cells were 0.65 µm, 0.50 µm, and 4.60 µm, respectively. These results indicated that EF24 was more effective in inhibiting the growth of the cancer cells as compared with normal cells. Further, the cell viability in a 48-h incubation period was not significantly different from a 24-h incubation period.
EF24 Induces G2/M Cell Cycle Arrest in CR Ovarian Cancer Cells
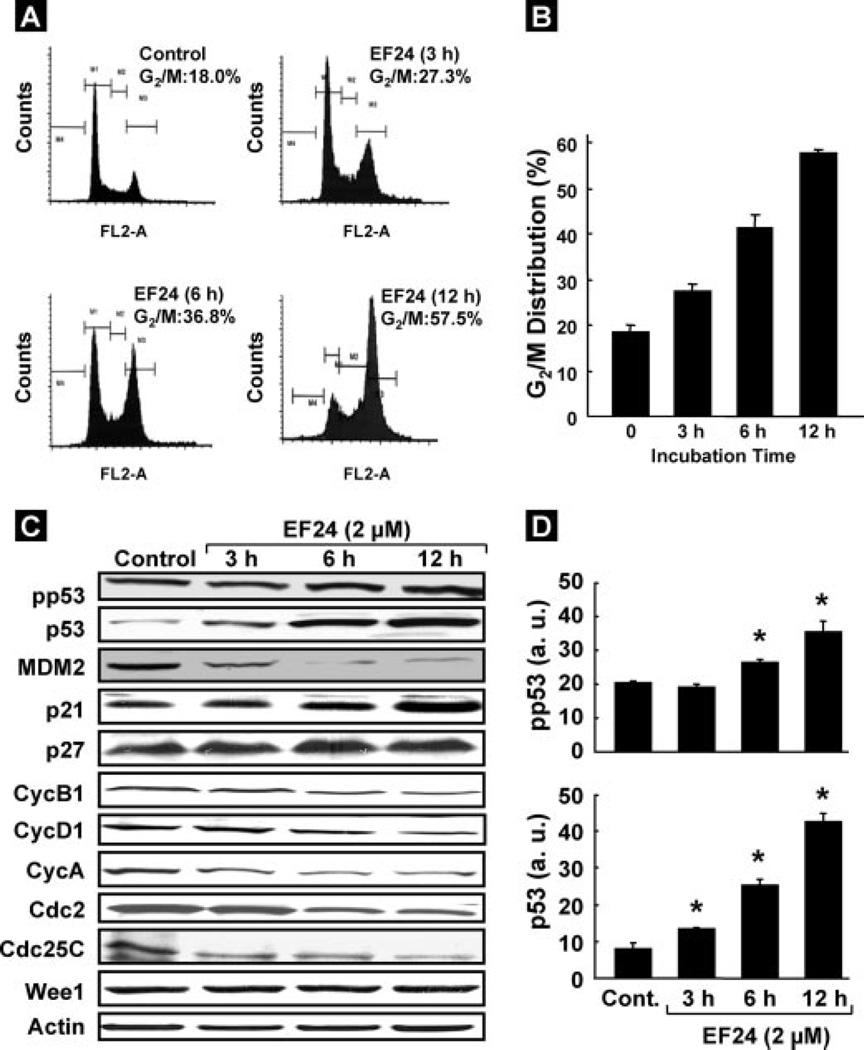
To determine whether the growth inhibition of CR cancer cells by EF24 was caused by cell cycle arrest or apoptosis, the cells were treated with 2 µm EF24 for 3, 6, or 12 h. The cells were then fixed, and cell cycle populations were determined by flow cytometry. The results showed that EF24 induced G2/M arrest in a time-dependent manner (Fig. 2A). The increase in G2/M cell population was greatest at 12 h (Fig. 2B). We next examined the effect of EF24 on cell cycle regulatory molecules, including p53, p21, p27, CycB1, CycA, CycD1, Cdc2, and Cdc25C. Exposure of cells to 2-µm EF24 enhanced the phosphorylation of p53 on Ser15 as well as the levels of p53 and p21 without any change in p27 (Fig. 2C). In addition, the level of MDM2, one of the negative regulators of p53, was decreased in a time-dependent manner. We also observed that the expression of G2/M cell-cycle regulating factors CycB1, CycA, CycD1, Cdc2, and Cdc25C showed a time-dependent decrease. However, no change was observed in the Wee1 levels. Fig. 2D shows the densitometry results of the phosphorylated p53 and p53 bands.
FIGURE 2. Effect of EF24 on cell cycle progression and cell cycle regulatory molecules in CR cells.
Cells were cultured in complete medium, and treated with either Me2SO vehicle control or 2 µmEF24. After 3, 6, or 12 h of treatment, cells were collected, washed with PBS, digested with RNase, and then the cellular DNA was stained with propidium iodide. Flow cytometric analysis was then performed for cell cycle distribution. A, representative flow cytometry graph for each treatment group. B, G2/M cell cycle distribution as a function of treatment period. C, immunoblot images of cell cycle regulatory molecules pp53, p53, MDM2, p21, p27, CycB1, CycD1, CycA, Cdc2, Cdc25C, and Wee1in CR cells treated with 2 µm EF24. D, quantitative results of phosphorylated p53 and p53 bands by densitometric analysis. Data represent mean ± S.E. of three independent experiments. *, p < 0.05 as compared with control group.
EF24 Induces Apoptosis in CR Cells
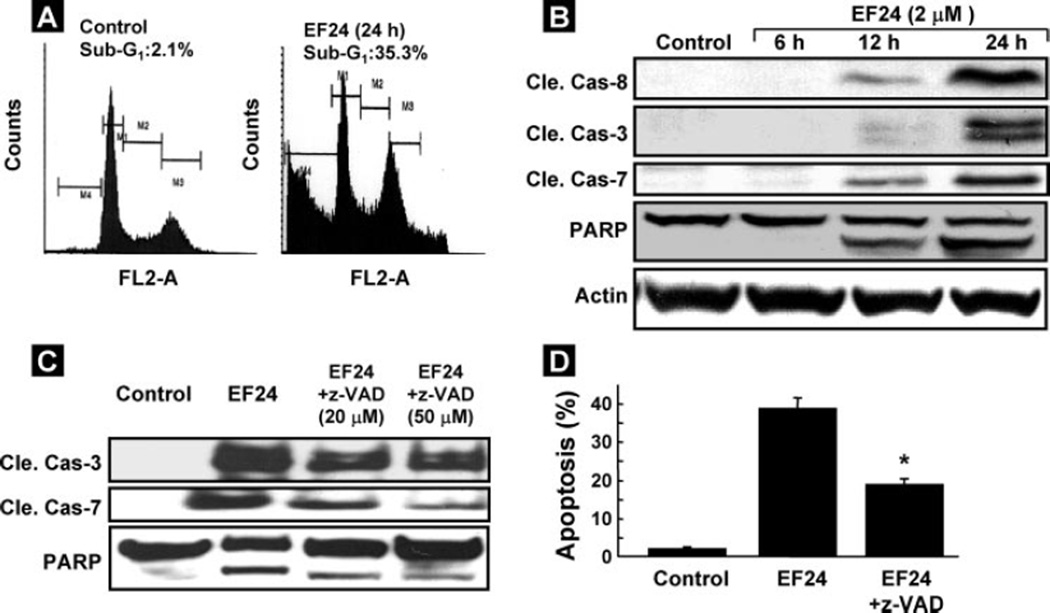
The arrest of cell cycle progression in tumor cells is usually associated with concomitant activation of cell death pathways. We, therefore, examined the contribution of apoptosis in EF24-treated CR cells. The number of cells with sub-G1 DNA content after 24 h of EF24 treatment was substantially higher as compared with control (Fig. 3A). Consistent with this finding, the increased activation of caspase was examined by Western blot analysis. The analysis demonstrated activation of the caspase cascade, including cleaved caspase-8, cleaved caspase-3, cleaved caspase-7, and PARP after treatment with EF24 (Fig. 3B). These results showed that EF24 induced apoptosis via a caspase cascade in CR cancer cells. We next investigated whether inhibition of caspase activation would affect the EF24-induced apoptosis. CR cancer cells were pretreated with the general caspase inhibitor z-VAD-fmk at 20 or 50 µm for 1 h and then treated with EF24 for another 24 h. The cells were analyzed for cleaved caspase-3, -7, and PARP by immunoblot assay and apoptosis by flow cytometry. Pretreatment with 20 or 50 µm z-VAD-fmk partially blocked the EF24-mediated activation of caspase (Fig. 3C). Consistently, pretreatment of the cells with 50 µm z-VAD-fmk significantly diminished the EF24-mediated apoptotic (sub-G1) cells (Fig. 3D). These results indicated that the EF24-induced apoptosis in CR cells was related to caspase activation, although z-VAD-fmk did not completely block apoptosis.
FIGURE 3. EF24-induced apoptosis in CR ovarian cancer cells.
Cells were treated with 2 µm EF24 for 24 h, harvested, and the sub-G1 population for 20,000 events within a fixed gate was analyzed. A, representative flow-cytometry profile for control (vehicle-treated) and EF24-treated group. B, immunoblot analysis for apoptosis. Cleavages in caspase-8, caspase-3, caspase-7, and PARP are shown in cells treated with 2 µm EF24 for 6, 12, or 24 h. C, CR cells were pretreated with 20 or 50 µm z-VAD-fmk for 1 h and then treated with EF24 for 24 h. Cell lysates were used for immunoblot assay for cleaved caspase-3, -7, and PARP. D, percentage of cells in sub-G1 phase as determined by flow cytometry. Data represent mean ± S.E. of three independent experiments. *, p < 0.05 compared with EF24 group.
EF24 Activates FasL
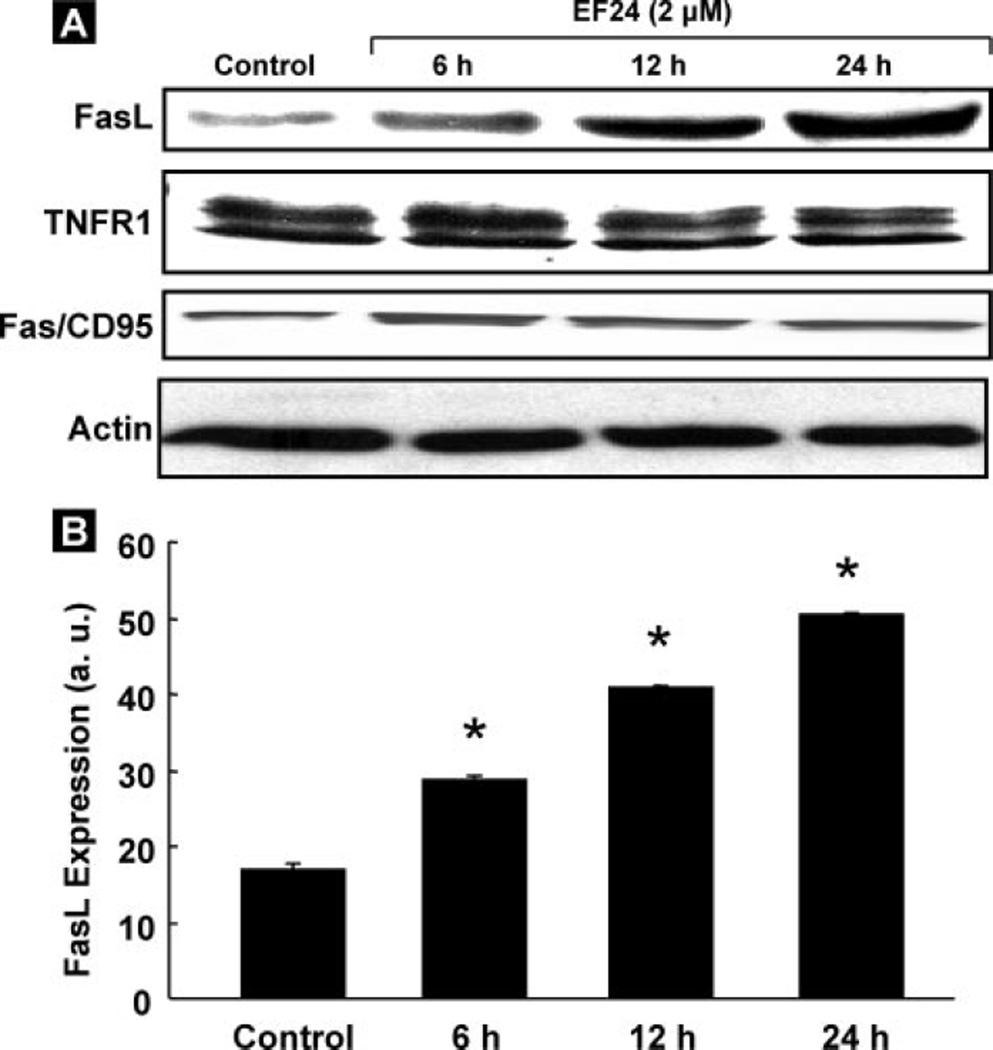
After we observed the caspase-8 activation by EF24, we analyzed the expression level of caspase-8-associated cell death receptors, such as FasL, Fas/CD95, or TNFR1. A clear increase in FasL expression in a time-dependent manner, but not in Fas/CD95 or TNFR1, was observed in the EF24-treated CR ovarian cancer cells (Fig. 4A). Fig. 4B shows the quantitative results of the bands by densitometric analyses. The results suggest a significant involvement of FasL in the EF24-induced apoptosis in CR cells.
FIGURE 4. FasL-induced apoptosis.
A, immunoblot analysis for the expression levels of caspase-8 associated death receptors, FasL, TNFR1, and Fas/ CD95 in CR cells treated with EF24 for 6, 12, or 24 h. A clear increase in the FasL expression, but not in the TNFR1 or Fas/CD95 expression, is observed. B, densitometric analyses of visualized bands for FasL expression. Data represent mean ± S.E. of three independent experiments. *, p < 0.05 as compared with control group.
EF24 Causes a Decrease in Phosphorylation of Akt and ERK1/2
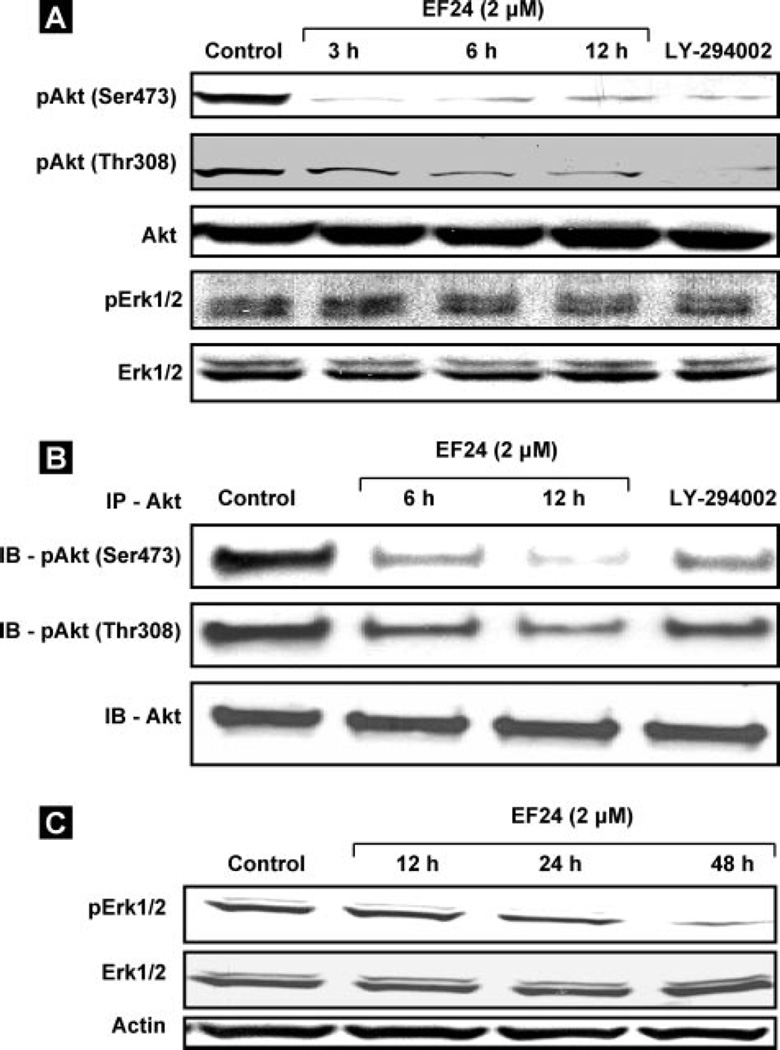
Recently, inhibition of Akt by both a PI3 kinase inhibitor as well as a selective Akt inhibitor was shown to upregulate the FasL mRNA expression and protein levels in vascular smooth muscle cells (21). The up-regulation in FasL mRNA and protein expression could be due to the effect of Akt-mediated phosphorylation at Ser473 and Thr308 (26, 27). Thus, the increase in the expression level of FasL by the EF24 treatment in this study prompted us to examine a possible decrease in the expression level of the phosphorylated Akt. Hence, we determined the effect of EF24 on the levels of phospho-Akt (Ser473 and Thr308) and total Akt levels by immunoblotting and immunoprecipitation (Fig. 5, A and B). The expression levels of Ser473- and Thr308-phosphorylated Akt were substantially decreased within 12 h in the EF24-treated cells, without any changes in the total Akt protein level. The Ser-phosphorylated Akt and Thr-phosphorylated Akt levels were also decreased by the PI3K inhibitor, LY-294002. The expression levels of pERK1/2 and ERK1/2 were unchanged at 12 h of treatment. However, at 24 and 48 h, the pERK1/2 levels were clearly downregulated while the total EKR1/2 levels were unchanged (Fig. 5C).
FIGURE 5. Inhibition of pAkt and pERK1/2 by EF24.
A, expression levels of Akt and ERK1/2 and their phosphorylated counterparts by immunoblot assay. The CR cells were treated with 2 µm EF24 for 3, 6, or 12 h. The data show a decrease in the expression of pAkt (Ser473 and Thr308) while the expressions of ERK1/2 and pERK1/2 are unchanged. The expression levels of both Ser- and Thr-phosphorylated Akt are decreased by the PI3K inhibitor, LY-294002 (10 µm). B, cell lysates were subjected to immunoprecipitation with anti-Akt, followed by immunoblotting analysis. C, expression of ERK1/2 and pERK1/2 at 12, 24, and 48 h. The pERK1/2 levels are clearly down-regulated while the total ERK levels are unchanged.
EF24 Up-regulates PTEN Activity and Expression
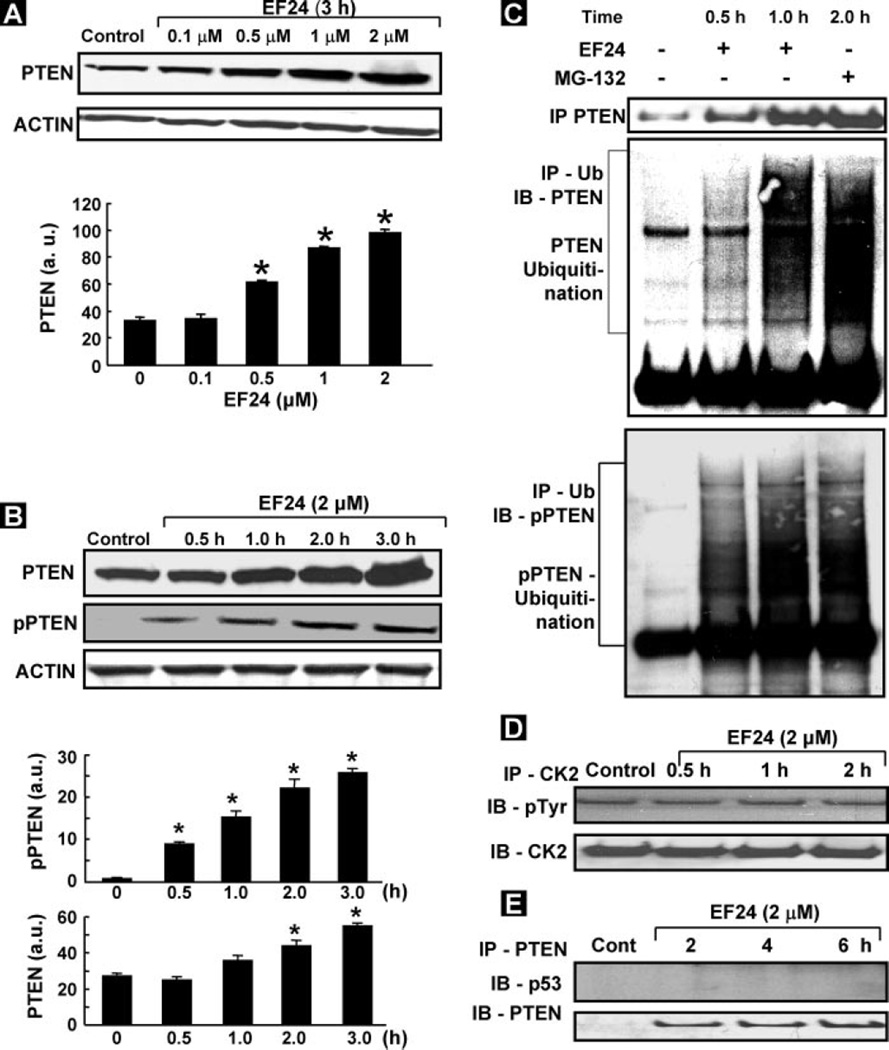
Because the PI3K/Akt pathway is negatively regulated by PTEN (8), we presumed that the inhibition of Akt by EF24 might correspond to an activation of PTEN. Hence, we determined the changes in total PTEN expression as well as the activation of PTEN (pPTEN) in the CR cells treated with EF24. We observed an increase in PTEN and pPTEN levels in a dose- and time-dependent manner (Fig. 6, A and B). We next wanted to know whether the increase in the levels of PTEN/pPTEN was caused by EF24-mediated inhibition of proteasomal degradation in CR cells. A previous report by Torres et al. (28) indicated that the turnover of PTEN protein was easily degraded in COS-7 cells, and it was inhibited by the proteasome inhibitor MG-132. We treated CR cells with EF24 for 0.5, 1, or 2 h and then measured the inhibition of PTEN proteasomal degradation. As shown in Fig. 6C, incubation CR cells with EF24 caused an accumulation of polyubiquitin-tagged proteins on pPTEN and PTEN, but not on p53. The results revealed that the degradation occurred in both PTEN and pPTEN, and that EF24 inhibited the degradation of both. We, in fact, observed the inhibition of pPTEN degradation as early as 0.5 h after treatment with EF24 compared with PTEN, which took about 2 h. Furthermore, increased levels of polyubiquitinated proteins were observed in pPTEN and PTEN blots, suggesting that the polyubiquitinated proteins might be related to PTEN. Because most of the proteasome-mediated protein degradation pathways require ubiquitination (29), the inhibition of proteasome activity by EF24 should increase the level of PTEN protein expression in CR cells. The positive control of specific proteasome inhibitor MG-132 also inhibited the PTEN-degradation in CR cancer cells. These results strongly suggested that PTEN was degraded via a proteasome-mediated mechanism in CR cells, and such a degradation of PTEN was inhibited by EF24.
FIGURE 6. Up-regulation of PTEN expression by EF24.
PTEN and pPTEN were determined using PTEN and pPTEN antibodies. Densitometric analysis of visualized bands was performed to quantify PTEN and pPTEN as a function of EF24 dose (A) and treatment period (B). The data show mean ± S.E. of three independent experiments. *, p < 0.05 as compared with respective controls. C, IP-IB of PTEN ubiquitination. CR cells were incubated with EF24 for 0.5, 1, or 2 h prior to protein extraction. Cell lysates were immunoprecipitated with agarose-conjugated ubiquitin antibody. The precipitates were subjected to SDS-PAGE and immunoblotting using PTEN, pPTEN, and p53 antibodies. Ubiquitinated PTEN bands were detected as described under “Experimental Procedures.” MG-132 was used as a positive control. PTEN, but not p53, shows a clear increase in ubiquitination. D, CK2 activity in CR cells treated with EF24 showing no change in the pCK2 levels. E, IP-IB of PTEN and p53 interaction showing no significant interaction between PTEN and p53 in EF24-treated CR cells.
Because casein kinase2 (CK2) is known to regulate PTEN phosphorylation, we next checked the activity of CK2 in EF24-treated CR cells. As shown in Fig. 6D, we did not observe any change in the activity of CK2 suggesting that phosphorylation of PTEN was not affected by the treatment. This further suggested that the increase in the levels of pPTEN in EF24-treated cells was due to the inhibition of the proteasomal degradation of pPTEN. Many reports have shown an interaction of PTEN with p53 (8, 12). It has been shown that PTEN stabilizes p53, however, this interaction results in the degradation of PTEN protein levels. We analyzed the PTEN and p53 interaction by IP. As shown in Fig. 6E, the results did not indicate any interaction of PTEN with p53. Taken together, the results suggested that EF24 up-regulated PTEN expression and inhibited pAkt (Ser473 and Thr308) and MDM2, a known negative regulator of p53.
PTEN siRNA Blocks Apoptosis Induced by EF24
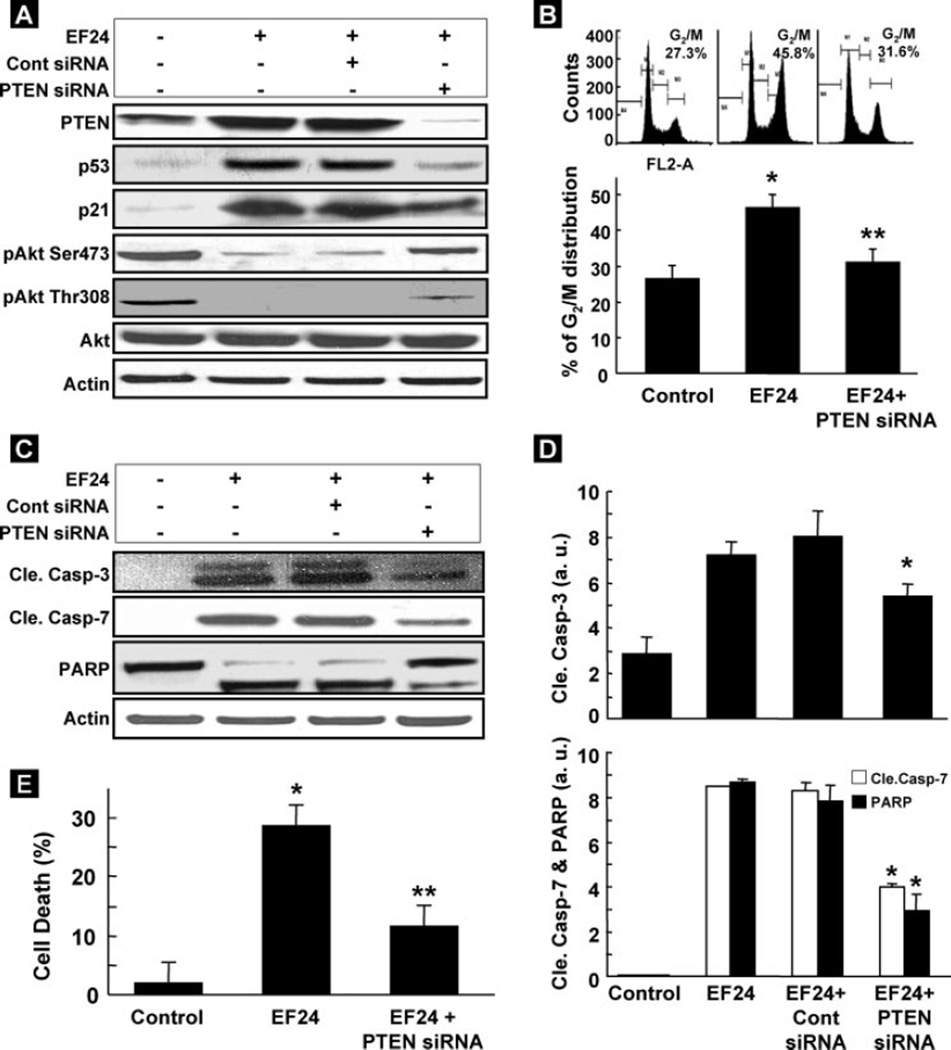
We next examined the effect of the inhibition of PTEN expression on cell proliferation and apoptosis in EF24-treated CR cancer cells. As shown in Fig. 7A, the level of PTEN, induced by EF24, was repressed in cells transfected with siRNA targeting PTEN, compared with EF24-treated cells without PTEN siRNA, or EF24-treated cells transfected with negative control siRNA. Further-more, the suppression of PTEN by PTEN siRNA in EF24-treated cells resulted in the increase of Akt phosphorylation (Ser473 and Thr308) and decreased p53 and p21 levels. In addition, the suppression of PTEN decreased the G2/M cell cycle arrest by more than 30% as compared with EF24-treated groups (Fig. 7B) and prevented the activation of cleaved caspase-3, -7, and PARP and reduced apoptosis induced by EF24 (Fig. 7, C–E). These data indicated that up-regulation of PTEN accounted, at least in part, for the G2/M cell-cycle arrest and apoptosis caused by EF24.
FIGURE 7. PTEN siRNA inhibits EF24-induced cell death.
A, effect of PTEN siRNA on EF24-induced overexpression of PTEN. Control siRNA was used a negative control. B, PTEN siRNA-transfected cells treated with EF24 for 6 h resulted in a significant decrease in G2/M cell-cycle arrest as compared with non-transfected cells treated with EF24 (**, p < 0.05). *, p < 0.05 versus untreated control cells. Data represent mean ± S.E. of three independent experiments. C, Western blots of cleaved caspases-3, -7, and PARP in PTEN siRNA- or control siRNA-transfected cells treated with EF24 for 24 h. Control represents cells untreated with EF24. D, densitometry analysis of visualized bands for cleaved caspase-3, -7, and PARP. Suppression of PTEN shows significant inhibition of cleaved caspase-3, -7, and PARP activation as compared with non-transfected or control siRNA-transfected cells treated with EF24 (*, p < 0.05; n = 3). E, quantification of apoptotic cells (cell death) determined by flow cytometry. Data represent mean ± S.E. of three independent experiments. *, p < 0.05 versus untreated control; **, p < 0.05 versus non-transfected cells treated with EF24.
Overexpression of PTEN Selectively Enhances Apoptosis in CR Cell
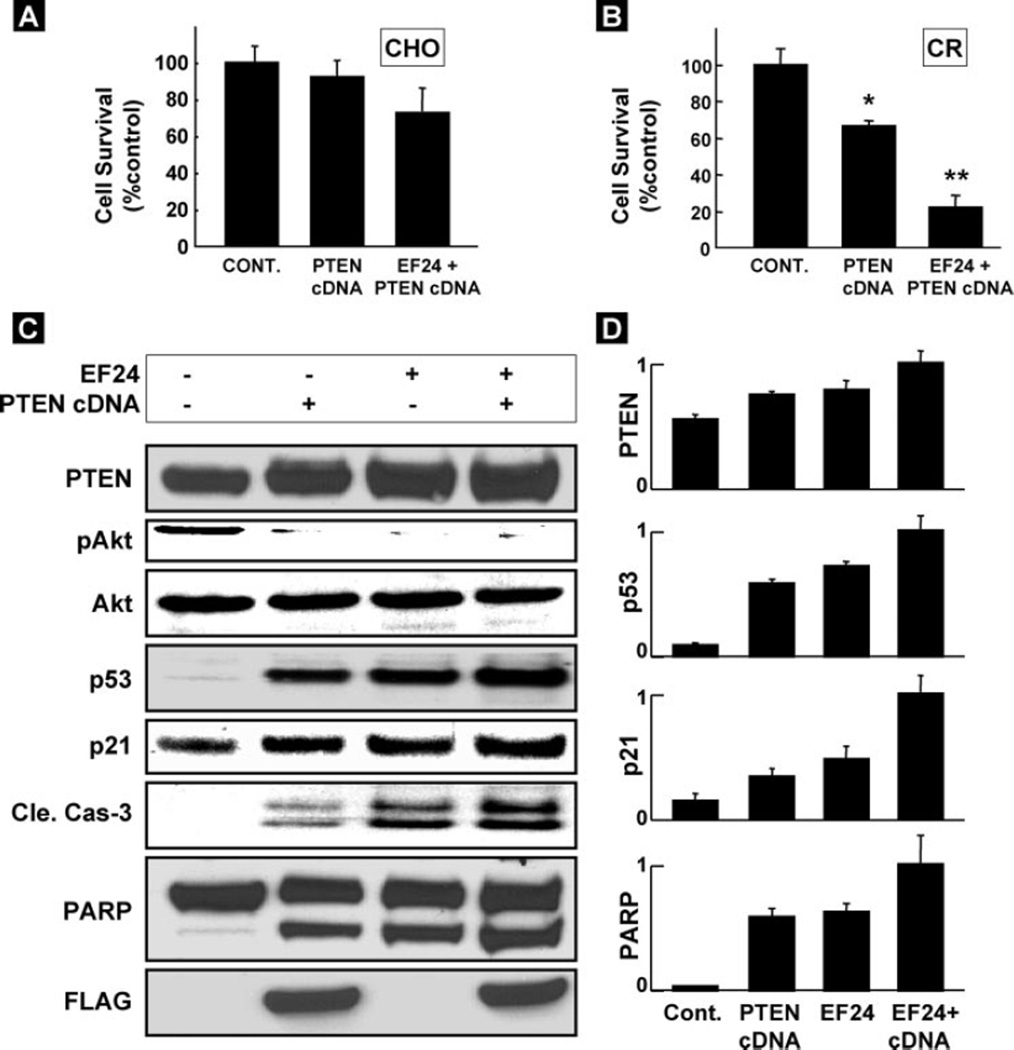
To check the effect of overexpression of PTEN on inducing apoptosis, we used wild-type PTEN cDNA transfected into cells. Twenty-four hours after the transfection of the PTEN gene, the cells were treated with EF24 (2 µm) for 24 h and then their viability checked. As seen in Fig. 8A, overexpression of PTEN did not induce any significant cell death in CHO cells. However, cell death in PTEN cDNA-transfected CR cell was significantly higher as compared with control cells (Fig. 8B). The effect was further enhanced by EF24 treatment. EF24 induced inhibition of pAkt and enhancement of p53, p21, cleaved caspase-3, and PARP levels in CR cells overexpressed with wild-type cDNA as compared with untransfected group (EF24) or transfected, but EF24-untreated (cDNA) groups (Fig. 8, C and D). The EF24-treated PTEN-overexpressing cells showed increased cleavages in caspase-3 and PARP, in comparison with the control and cDNA alone transfected cells, suggesting that EF24 might target, at least in part, activation of PTEN in the CR cells.
FIGURE 8. Effect of PTEN overexpression on EF24-induced apoptosis.
The PTEN/FLAG gene was transfected into cells and then treated with 2 µm EF24 for 24 h. The effect of PTEN overexpression and EF24 on the viability of (A) CHO and (B) CR cells. Cell viability assays were performed using MTT assay. Data represent mean ± S.E. of three independent experiments. *, p < 0.05 versus Control; **, p < 0.05 versus PTEN cDNA. C, effect of overexpression of PTEN and EF24 treatment on the apoptotic markers in CR cells. Expression of PTEN, p53, p21, pAkt, Akt, cleaved caspase-3, PARP, and FLAG expression in untransfected cells treated without (Control) and with EF24 treatment (EF24), and cDNA-transfected cells without (cDNA) and with EF24 treatment (EF24 + cDNA) were determined by Western blot. D, densitometry analysis of the bands shown in C. Data represent mean ± S.E. of three independent experiments.
DISCUSSION
We have demonstrated that EF24, a synthetic compound, effectively inhibits the growth of cisplatin-resistant human ovarian cancer cells in vitro. Particularly, we have shown that EF24 induces G2/M cell-cycle arrest, increases the expression of FasL, inhibits the PI3K/Akt pathway, and up-regulates PTEN expression by inhibiting its ubiquitin-mediated degradation.
Tumor suppressor protein p53 controls the G1 and G2/M cell-cycle checkpoints that mediate growth arrest (30). MDM2 is a major regulator of p53. It regulates the activity of p53 protein by blocking its transcriptional activity, exporting of nuclear p53 protein into the cytoplasm, and/or by promoting the degradation of the p53 protein. PTEN inhibits PI3K/Akt signaling that promotes translocation of MDM2 into the nucleus. When restricted to the cytoplasm, MDM2 is degraded. The ability of PTEN to inhibit the nuclear entry of MDM2 increases the cellular content and transactivation of the p53 tumor suppressor protein to promote the induction of p21 (13, 31). The induction of p21 causes subsequent arrest in the G1/G0 or G2/M phase of the cell cycle by binding of the cyclin-cdk complex (32). In this study, we have shown that treatment of CR ovarian cancer cells with EF24 resulted in the accumulation of p53 and p21 in a time-dependent manner, without any changes in p27 level. The results further indicated that the early (within 1 h) activation of PTEN by EF24 is effective with respect to p53 and p21 accumulation for cell cycle arrest by inactivating Akt and MDM2-signaling in CR cells. We observed that EF24-induced up-regulation of PTEN resulted in the induction of p53 and p21 protein expression and G2/M cell cycle arrest. These results are consistent with previous reports that showed up-regulation of PTEN expression induced cell cycle arrest through the inhibition of the Akt pathway (33, 34). Recent reports showed that both PTEN protein and lipid phosphatase activities are required for cell cycle arrest and apoptosis (8). We observed that EF24 did not alter the FAK and Shc levels in CR cells (data not shown). However, it down-regulated the cyclin D1 and phosphorylated MAPK levels, which might be involved in the EF24-induced cell cycle arrest in CR cells. A recent study also showed that activation of PTEN downregulated cyclin D1 and MAPK expression via its protein phosphatase activity and was involved in cell cycle arrest (11, 35).
Although most antineoplastic agents induce apoptosis in cancer cells, the mechanism by which they do so remains unclear. In this study, we showed that caspase activation was involved in the EF24-induced apoptosis, as in the case with numerous chemotherapeutic agents (36, 37). Cleaved caspase-8, caspase-3, and caspase-7, and PARP were activated by EF24 in CR cells, and the general caspase inhibitor z-VAD-fmk partially blocked the EF24-induced apoptosis. However, even treatment with up to 50 µm z-VAD-fmk did not completely block the EF24-induced apoptosis, indicating that there were multiple mechanisms involving both caspase-dependent and caspase-independent pathways.
Several reports have shown that some anticancer agents induced apoptosis, in part, by blocking the activation of Akt (38, 39). Akt prevents cells from undergoing apoptosis by inhibiting the pro-apoptotic factors and suppression of death receptors signals such as Fas and FasL (21). The role of Akt signaling in regulating death receptor signaling is not fully understood. In a recent study using prostate cancer cells and T lymphocytes, blocking Akt signaling has been shown to increase caspase-8 activity, resulting ultimately in FasL-dependent apoptosis (20, 40). Inhibition of Akt activation by canstatin has been shown to induce Fas-mediated apoptosis in endothelial cells (41). Taken together, our results strongly support Akt signaling as a critical downstream target of the death receptor-mediated apoptotic pathway.
PTEN is a tumor suppressor gene that is frequently mutated in human tumors (11). It acts as an inhibitor of the phosphatidylinositol-3-kinase (PI3K)-Akt pathway, which is considered to be a central regulator of cell proliferation and apoptosis (8, 11, 42). This study confirmed that EF24 down-regulated the pAkt Ser473 and Thr308 through the up-regulation of PTEN expression in CR cells. It has been found that COX-2 inhibitors enhance Fas-mediated apoptosis via modulation of the PTEN-Akt pathway in human gastric carcinoma cell lines (43). Thus, the results of the present study confirm our hypothesis that EF24 induced PTEN expression, which inhibited the Akt pathways and induced FasL-mediated apoptosis in CR ovarian cancer cells. Our PTEN overexpression study showed inhibition of Akt activation, further supporting the idea that PTEN up-regulates p53 through the Akt pathway, and it might be involved the cell cycle arrest and apoptosis in CR cells.
The ubiquitin-proteasome pathway plays an important role in the regulation of cell cycle, neoplastic growth, and metastasis, and may also be critical in the regulation of the amount of activated signal transduction proteins and activators of transcription in various cell lines (29). The involvement of the proteasome in the degradation and regulation of the function of short-lived proteins, including oncoproteins, tumor suppressors, and cell cycle proteins, has been extensively investigated (37, 44). In the case of the regulation of PTEN expression, a previous report indicated that turnover of PTEN depends mainly on proteasome, not lysosome-mediated degeneration in COS-7 cell lines (28). Therefore, we tested whether EF24 might inhibit PTEN proteasome degradation, leading to the accumulation of polyubiquitinated proteins in the cells. EF24 clearly inhibited the proteasome degradation of pPTEN and PTEN in CR cancer cells. These results suggest that EF24 may exert its cytotoxic effect on cancer cells through inhibition of the pPTEN and PTEN proteasome degradation, leading to the accumulation of polyubiquitinated PTEN proteins. This is consistent with previous studies, which showed that the turnover of PTEN in cultured COS-7 cells was dependent mainly on proteasomal degradation (28). The enhanced PTEN degradation was preceded by the ubiquitination of PTEN in 293T cells (45). The notion that PTEN ubiquitination played a critical role in its degradation was strongly supported by a recent study which showed that BMP-2 stimulation inhibited PTEN degradation by decreasing the association of PTEN with two proteins in the degradative pathway, UbCH7 and UbC9 (46). Previous work has implicated casein kinase 2 (CK2) as the kinase responsible for this phosphorylation of PTEN (28). The absence of any significant changes in the CK2 activity suggested the inhibition of proteasomal degradation of pPTEN as the major contributing factor to the enhanced levels of PTEN activity in the EF24-treated CR cells.
The up-regulation of PTEN expression and its role in cell cycle arrest and apoptotic signaling is not completely understood. Our siRNA studies clearly established a link between PTEN expression and induction of cell cycle arrest and apoptosis. Upon inhibition of PTEN expression by PTEN-siRNA, the pAkt (Ser473 and Thr308) was significantly increased, and p53 and p21 levels significantly decreased, resulting in increased cell survival and decreased apoptosis in EF24-treated CR cells. However, the complete inhibition of PTEN did not totally block the apoptosis in CR cancer cells, so the actual mechanism by which EF24 contributes to the outcome of PTEN activation and apoptosis in cancer cells requires further investigation. This result is consistent with previous reports that up-regulation of PTEN expression blocked cancer cell proliferation and induced apoptosis (34, 47, 48). On the basis of these results, we hypothesized that the inhibition of cell proliferation and induction of apoptosis in CR human ovarian cancer cells are associated with PTEN expression caused by EF24. Further work is required to determine the feasibility of PTEN activation as an adjuvant therapy for human ovarian can-the effects of PTEN activation by anticancer drugs, some studies using nude mouse xenografts of human tumors have established that PTEN induction results in reduced tumor metastases, tumor regression, and most importantly, the induction of apoptosis (33, 47, 49).
In summary, we have shown, for the first time that EF24 increased PTEN expression in human ovarian cancer cells. The up-regulation of PTEN resulted in a decrease in Akt and MDM2and an increase in the expression of p53, thereby inducing G2/M arrest and apoptosis (Fig. 9). Our study provided a basic mechanism for the anticancer effect of EF24 in the CR cells. Future in vivo studies using animal models and other cancer cell lines could ascertain whether this pro-apoptotic effect of EF24 might contribute to its overall effect in the fight against cancer and possibly show novel therapeutic applications.
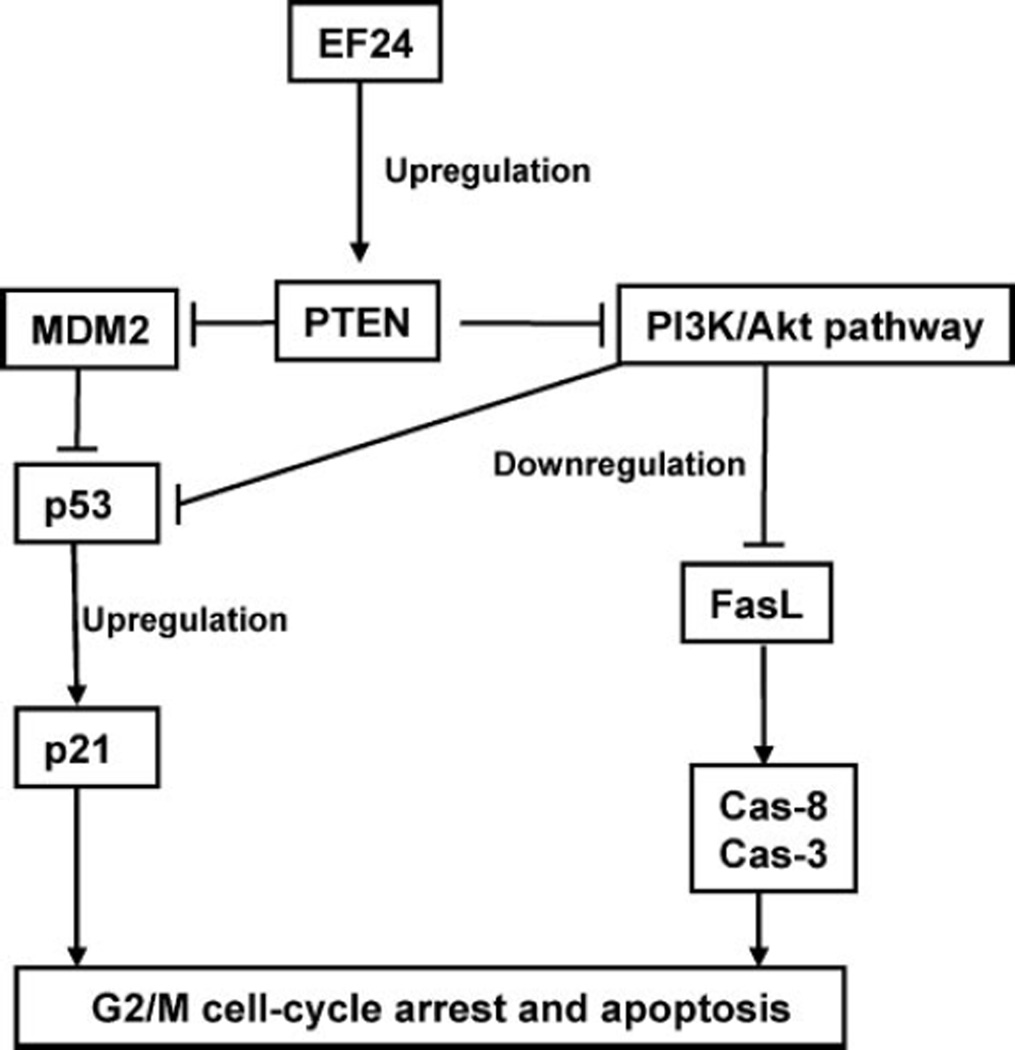
FIGURE 9. A schematic presentation for the anticancer mechanisms of EF24 shown in the present study.
EF24 up-regulates PTEN expression through the inhibition of proteasomal degradation of PTEN. Activation of PTEN down-regulates pAkt and MDM2, which enhances the level of p53. The up-regulation of PTEN by EF24 leads to the PTEN-mediated cell cycle progression, and increase in sensitivity to apoptosis through the activation of FasL.
Acknowledgments
We thank Dr. William Sellers (Novartis Institute of Biomedical Research) for providing the PTEN plasmid and Martina Matulova and Dr. Michael Ostrowski for help with the PTEN plasmid experiments.
Footnotes
This work was supported by National Institutes of Health Grant CA102264. The synthesis of EF24 was supported by Hungarian Research Fund Grant OTKA T048334. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: PI3K, phosphatidylinositol 3-kinase; MAPK, mitogen-activated protein kinase; PBS, phosphate-buffered saline; HA, hemagglutinin; Z, benzyloxycarbonyl; fmk, fluoromethylketone; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; CR, cisplatin-resistant; PARP, polyadenosine diphosphate ribose polymerase.
REFERENCES
- 1.Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 2.Markman M, Howell SB, Lucas WE, Pfeifle CE, Green MR. J. Clin. Oncol. 1984;2:1321–1326. doi: 10.1200/JCO.1984.2.12.1321. [DOI] [PubMed] [Google Scholar]
- 3.Johnson SW, Ozols RF, Hamilton TC. Cancer. 1993;71(Suppl. 2):644–649. doi: 10.1002/cncr.2820710224. [DOI] [PubMed] [Google Scholar]
- 4.Bratasz A, Weir NM, Parinandi NL, Zweier JL, Sridhar R, Ignarro LJ, Kuppusamy P. Proc. Natl. Acad. Sci U. S. A. 2006;103:3914–3919. doi: 10.1073/pnas.0511250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Choi EJ, Jin C, Kim DH. Gynecol. Oncol. 2005;97:26–34. doi: 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Simpson L, Takahashi M, Miliaresis C, Myers MP, Tonks N, Parsons R. Cancer Res. 1998;58:5667–5672. [PubMed] [Google Scholar]
- 7.Eng C. Hum. Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 8.Di Cristofano A, Pandolfi PP. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 9.Parsons R. Semin. Cell Dev. Biol. 2004;15:171–176. doi: 10.1016/j.semcdb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Kurose K, Zhou XP, Araki T, Cannistra SA, Maher ER, Eng C. Am. J. Pathol. 2001;158:2097–2106. doi: 10.1016/S0002-9440(10)64681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung JH, Eng C. Cancer Res. 2005;65:8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M, Gu L, Findley HW, Jiang R, Woods WG. Cancer Res. 2003;63:6357–6362. [PubMed] [Google Scholar]
- 13.Mayo LD, Dixon JE, Durden DL, Tonks NK, Donner DB. J. Biol. Chem. 2002;277:5484–5489. doi: 10.1074/jbc.M108302200. [DOI] [PubMed] [Google Scholar]
- 14.Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H. Cancer Cell. 2003;3:117–130. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y, Eng C. Cancer Res. 2006;66:736–742. doi: 10.1158/0008-5472.CAN-05-1557. [DOI] [PubMed] [Google Scholar]
- 16.Facchinetti MM, De Siervi A, Toskos D, Senderowicz AM. Cancer Res. 2004;64:3629–3637. doi: 10.1158/0008-5472.CAN-03-3741. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson KM, Anderson NG. Cell. Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 18.Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, Cocco L. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 19.Ciechomska I, Pyrzynska B, Kazmierczak P, Kaminska B. Oncogene. 2003;22:7617–7627. doi: 10.1038/sj.onc.1207137. [DOI] [PubMed] [Google Scholar]
- 20.Uriarte SM, Joshi-Barve S, Song Z, Sahoo R, Gobejishvili L, Jala VR, Haribabu B, McClain C, Barve S. Cell Death Differ. 2005;12:233–242. doi: 10.1038/sj.cdd.4401549. [DOI] [PubMed] [Google Scholar]
- 21.Suhara T, Kim HS, Kirshenbaum LA, Walsh K. Mol. Cell. Biol. 2002;22:680–691. doi: 10.1128/MCB.22.2.680-691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridantapani S, Anant S, Kuppusamy P. Cancer Biol. Ther. 2007;6:178–184. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Bioorg. Med. Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, Snyder JP, Liotta DC, Jones DP, Shoji M. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Andrews PA, Jones JA, Varki NM, Howell SB. Cancer Commun. 1990;2:93–100. doi: 10.3727/095535490820874641. [DOI] [PubMed] [Google Scholar]
- 26.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 27.Sata M, Suhara T, Walsh K. Arterioscler. Thromb. Vasc. Biol. 2000;20:309–316. doi: 10.1161/01.atv.20.2.309. [DOI] [PubMed] [Google Scholar]
- 28.Torres J, Pulido R. J. Biol. Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 29.Orlowski RZ, Dees EC. Breast Cancer Res. 2003;5:1–7. doi: 10.1186/bcr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal ML, Agarwal A, Taylor WR, Stark GR. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 32.Coqueret O. Trends Cell Biol. 2003;13:65–70. doi: 10.1016/s0962-8924(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 33.Choi HJ, Chung TW, Kang SK, Lee YC, Ko JH, Kim JG, Kim CH. Glycobiology. 2006;16:573–583. doi: 10.1093/glycob/cwj105. [DOI] [PubMed] [Google Scholar]
- 34.Weng LP, Smith WM, Dahia PL, Ziebold U, Gil E, Lees JA, Eng C. Cancer Res. 1999;59:5808–5814. [PubMed] [Google Scholar]
- 35.Chung JH, Ostrowski MC, Romigh T, Minaguchi T, Waite KA, Eng C. Hum. Mol. Genet. 2006;15:2553–2559. doi: 10.1093/hmg/ddl177. [DOI] [PubMed] [Google Scholar]
- 36.Oyaizu H, Adachi Y, Taketani S, Tokunaga R, Fukuhara S, Ikehara S. Mol. Cell. Biol. Res Commun. 1999;2:36–41. doi: 10.1006/mcbr.1999.0146. [DOI] [PubMed] [Google Scholar]
- 37.Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, Yano H, Kojiro M, Sata M. Cancer Res. 2006;66:4826–4834. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- 38.Krystal GW, Sulanke G, Litz J. Mol. Cancer Ther. 2002;1:913–922. [PubMed] [Google Scholar]
- 39.Liu X, Shi Y, Giranda VL, Luo Y. Mol. Cancer Ther. 2006;5:494–501. doi: 10.1158/1535-7163.MCT-05-0049. [DOI] [PubMed] [Google Scholar]
- 40.Garrison JB, Kyprianou N. Cancer Res. 2006;66:464–472. doi: 10.1158/0008-5472.CAN-05-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panka DJ, Mier JW. J. Biol. Chem. 2003;278:37632–37636. doi: 10.1074/jbc.M307339200. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez F, Sellers WR. Biochim. Biophys. Acta. 2000;1470:M21–M35. doi: 10.1016/s0304-419x(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 43.Honjo S, Osaki M, Ardyanto TD, Hiramatsu T, Maeta N, Ito H. DNA Cell Biol. 2005;24:141–147. doi: 10.1089/dna.2005.24.141. [DOI] [PubMed] [Google Scholar]
- 44.Lee DH, Goldberg AL. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 45.Tolkacheva T, Boddapati M, Sanfiz A, Tsuchida K, Kimmelman AC, Chan AM. Cancer Res. 2001;61:4985–4989. [PubMed] [Google Scholar]
- 46.Waite KA, Eng C. Hum. Mol. Genet. 2003;12:679–684. [PubMed] [Google Scholar]
- 47.Saito Y, Gopalan B, Mhashilkar AM, Roth JA, Chada S, Zumstein L, Ramesh R. Cancer Gene Ther. 2003;10:803–813. doi: 10.1038/sj.cgt.7700644. [DOI] [PubMed] [Google Scholar]
- 48.Yan X, Fraser M, Qiu Q, Tsang BK. Gynecol Oncol. 2006;102:348–355. doi: 10.1016/j.ygyno.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 49.McNeish IA, Bell SJ, Lemoine NR. Gene Ther. 2004;11:497–503. doi: 10.1038/sj.gt.3302238. [DOI] [PubMed] [Google Scholar]