Abstract
Pulmonary edema and the associated increases in vascular permeability continue to represent a significant clinical problem in the intensive care setting, with no current treatment modality other than supportive care and mechanical ventilation. Therapeutic compound(s) capable of attenuating changes in vascular barrier function would represent a significant advance in critical care medicine. We have previously reported the development of HPMA-based copolymers, targeted to endothelial glycocalyx that are able to enhance barrier function. In this work, we report the refinement of copolymer design and extend our physiological studies todemonstrate that the polymers: 1) reduce both shear stress and pressure-mediated increase in hydraulic conductivity, 2) reduce nitric oxide production in response to elevated hydrostatic pressure and, 3) reduce the capillary filtration coefficient (Kfc) in an isolated perfused mouse lung model. These copolymers represent an important tool for use in mechanotransduction research and a novel strategy for developing clinically useful copolymers for the treatment of vascular permeability.
Keywords: Endothelium, Acute respiratory distress syndrome, Mechanotransduction, Nitric oxide, Vascular tone and reactivity
1. Introduction
Pulmonary edema is the hallmark of acute lung injury (ALI) and adult respiratory distress syndrome (ARDS), which can arise as a result of sepsis, pneumonia, blunt trauma, ischemia reperfusion injury and/or organ transplantation. Currently, there is no direct treatment for excessive vascular permeability and subsequent edema beyond supportive care and mechanical ventilation, which carries its own risk of ventilator-associated pneumonia and ventilator-induced lung injury. The endothelial glycocalyx, a meshwork of cell surface proteins, proteoglycans, and glycolipids has an important role in regulating vascular barrier function by passively acting as a molecular filter for fluid and macromolecular flux across the endothelial layer and via active signaling mechanism(s) that directly regulate cell–cell junctions.
It has been long appreciated that endothelial cells are able to sense mechanical forces imparted by flowing blood and respond accordingly. The glycocalyx is exposed to shear stress as blood flow distorts surface proteoglycans parallel to the capillary wall and increased pressure exerted perpendicularly drives flow and shear stress through inter-endothelial junctions containing glycocalyx [1]; these forces can, in excess, have a deleterious effect on the integrity of the capillary barrier. In their seminal work, Ingber et al. demonstrated that an imposed force on specific components of the glycocalyx, in this case iron beads targeted to the glycocalyx via an RGD sequence in the presence of an applied magnetic torque, could produce a stiffening of the cell and a change in the actin cytoskeleton in direct proportion to the applied stress [2]. Likewise, it has been shown that fluid shear stress increases the production of nitric oxide, the cell permeable reporter molecule for mechanotransduction, and that the removal of specific components of the glycocalyx inhibits this response [3], implicating the endothelial glycocalyx as a mechanosensor. Nitric oxide (NO) is produced in response to shear through calcium dependent and calcium independent pathways culminating in the activation of nitric oxide synthase (eNOS) [4]. Low levels of constitutively expressed NO are responsible for vascular homeostasis in normal tissues whereas increased levels of NO are capable of reaction with endogenous superoxide to form the powerful oxidant peroxynitrite and thereby instigate barrier dysfunction via deleterious pathways involving reactive oxygen species [5].
The endothelial glycocalyx carries a net negative charge due to sulfate and carboxyl groups and sialic acids found on proteoglycans that decorate the cell surface and cell junctions. In vitro studies have shown that the administration of a positively charged copolymer that interacts with the negatively charged glycocalyx is able to enhance the passive barrier function of the glycocalyx and dampen the role of the active barrier regulation component, namely mechanotransduction [6]. In this work, the net negative charge of the endothelial cell surface is exploited to deliver a water-soluble cationic copolymer that interacts with the glycocalyx in order to enhance the physical barrier and reduce mechanical perturbations to the glycocalyx and thereby attenuate mechanotransduction.
The goal of these copolymers, clinically, is to lay the foundation for the development of a treatment for acute lung injury associated with inflammation and altered pulmonary vascular pressures, and to be used in the laboratory as a tool toward a better understanding of mechanotransduction pathways. Some work has been done in the realm of therapeutic polymers delivered to lung endothelium to treat acute lung injury [7] or as a free radical scavenger [8], however, to date the effort is preliminary and the mechanism is largely unexplored. This work explores the relationship between mechanotransduction and polymer molecular weight and charge distribution in order to begin to optimize future endeavors in the realm of polymer delivery to enhance barrier function.
2. Materials and methods
2.1. Materials
The following chemicals were obtained from Sigma–Aldrich Chemical Company (St. Louis, MO): bovine serum albumin (BSA 30% solution, cat. no. A7284), Minimum Essential Medium Eagle (MEM), penicillin–streptomycin solution, L-glutamine, nitro-L-arginine methyl ester hydrochloride (L-NAME), trypsin-EDTA solution (cat. no. T4049), HEPES, sodium bicarbonate, heparin (sodium salt, grade I-A, 181 USP units/mg), and fibronectin. Fetal bovine serum (FBS) was purchased from Hyclone Laboratories (Logan, UT). Transwell and Snapwell polyester filters were purchased from Costar (Cambridge, MA). Heparinase III was obtained from Ibex (Montreal, Quebec, Canada). N-2-hydroxypropyl methacrylamide (HPMA) and methacrylamidopropyltrimethylammonium chloride (MA-TMA Cl) were from Polysciences (Warrington, PA), and 2,2-azobisisobutryonitrile (AIBN) was from Alfa (Danvers, MA). Polyethylene oxide standards (PEO) were purchased from Varian (Palo Alto, CA). All other chemicals were obtained through Sigma unless otherwise specified.
2.2. Methods
2.2.1. Polymer synthesis/characterization
Polymers are synthesized by radical polymerization of HPMA and MA-TMA Cl monomers in varying feed compositions using methanol as the solvent and AIBN as initiator. The concentration of comonomers in polymerization mixture was 12.5 wt% and the AIBN concentration was 0.6 wt%. Radical polymerization was allowed to proceed under nitrogen atmosphere at 55 °C for 24 h. Molecular weight and molecular distribution were determined using AKTA FPLC (GE Healthcare, Piscataway, NJ) equipped with UV and RI detectors using a Superose 6 column in PBS pH 7.4. A Superose 6 preparative column under the same conditions was used in conjunction with this system for polymer fractionation. Quaternary amine content was determined by titration of chloride counter ion using 0.01 M AgNO3 [9].
2.2.2. Cell culture
Bovine aortic endothelial cells (BAEC) and rat lung microvascular endothelial cells (RLMVEC) were purchased from Vec Technologies (Rensselaer, NY) and grown in T-75 flasks with 10% FBS in complete medium, purchased from Vec Technologies, at 37 °C in 5% CO2. Upon reaching confluency (3–4 days) the cells were split for continuing maintenance of the cell line. BAEC were plated at a density of 1.25 × 105 cells/cm2 on polycarbonate Transwells previously coated with 1% fibronectin. RLMVEC were plated on 1% fibronectin and 0.05% gelatin or 10% FBS-MEM for Snapwells or Transwells, respectively, and incubated in 5% CO2 at 37 °C. The shear-permeability experiments were run 4–6 days after plating and pressure-permeability experiments were run on days 9 and 10 post-plating. Cells were used from passages 4–7.
2.2.3. Shear-induced hydraulic conductivity (Lp)
Prior to Lp measurements, BAEC were cultured on Transwell membrane supports (12 mm diameter, 0.4 μm pore size) and grown to confluence. On the day of the experiment, monolayers were incubated with either 20P(TMA)Cl, 20P(TMA)-F3, or 20P(TMA)-F5 (1 mg/mL) in 1% BSA for 30 min at 37 °C and then were rinsed twice with experimental media (MEM supplemented with 1% BSA) and placed in the transport apparatus to determine shear-induced hydraulic conductivity as previously described [10–12]. The measurement of fluid flow across the endothelial monolayer was performed inside a Plexiglas box maintained at 37 °C. The seeded filters were placed inside a chamber to form a luminal (top) compartment and an abluminal (bottom) compartment separated only by the BAEC monolayer. The abluminal compartment was connected to a reservoir via Tygon and borosilicate glass tubing. The vertical displacement of the reservoir with respect to the liquid covering the cells applied a hydrostatic pressure differential across the monolayer. At 10 cm H2O differential pressure, the volumetric flow rate (Jv) was measured by tracking the position of an air bubble that was placed into the calibrated borosilicate glass tube. The hydraulic conductivity was calculated from the relationship
where A is the area of the BAEC monolayer and ΔP is the pressure differential across the monolayer. During each experiment, one untreated endothelial monolayer was tested as control while a copolymer solution was applied to a separate monolayer and both experiments were run concurrently. After 60 min of applied pressure differential, a baseline Lp was established for each respective monolayer and a defined shear stress was applied to each endothelial monolayer. Lp values were collected for 4 h. Shear was applied to the surface using a rotating disk separated by a distance h (500 μm) from the monolayer surface. The rotating disk generated a fluid shear stress distribution on the monolayer surface defined by
where μ is the viscosity of the media, ω is the rotational speed, and r is the radial distance from the center of the disk. The parameters were adjusted in order to achieve a maximum steady shear stress of 20 dyn/cm2 at the edge of the disk. The average shear stress over the entire filter area is 2/3 of the maximum. The luminal compartment and the reservoir were supplied with 5% CO2 throughout the experiment to maintain the media at pH 7.4.
2.2.4. Pressure-induced hydraulic conductivity
The system used to measure the volumetric flux rate (Jv) and hydraulic conductivity (Lp) has been described previously [13]. Briefly, Snapwell filters (1.12 cm2, 0.4 μm pore size) supporting confluent monolayers of RLMVEC were inserted into custom permeability chambers. To measure Lp, the inflow port of the cell culture chamber was connected by silastic tubing to a pressure manifold filled with phenol red-free DMEM [with 25 mM HEPES, 1% BSA (Proliant, Ankeny, IA), and 0.1% penicillin/streptomycin at pH 7.4, hereafter denoted media II]. Fluid flow was tracked by the location of an air bubble as it passes through an 18 cm length of clear borosilicate capillary tubing (Wilmad Glass, Buena, NJ) with an internal diameter of 1.9913 ± 0.0004 mm. A digital video camera was mounted above the tubing and streamed continuous digital images of the capillary tubes and air bubbles to the virtual instrument written in LabView (National Instruments, Austin, TX). Cell monolayers were allowed to equilibrate for 1 h under a hydrostatic pressure of 1 cm H2O in media II. For experiments involving L-NAME, cells were incubated at 37 °C for 1 h prior to experiment with 10 μM L-NAME and the NO blocker was present in media for the duration of the experiment. For polymer-treated experimental groups, after the 1 h equilibration period, a solution of 20P(TMA)Cl (1 mg/mL) in media II was added to the luminal cell chamber and the pressure was increased to 10 cm H2O. After 30 min, polymer was washed from cells with a 6-fold volume of media II and the experiment was allowed to proceed under 10 cm H2O pressure for another half hour. The water pressure was subsequently increased by 10 cm H2O every hour; Lp data were constitutively collected every 5 s. At the end of the experiment, Snapwells were removed from permeability chambers and monolayers were fixed in formalin and stained with Ladd Multiple Stain (Ladd Research, Williston, VT) to confirm monolayer integrity. An aliquot (500 μL) of the media above the cells was collected, centrifuged and frozen for later analysis.
2.2.5. Nitric oxide detection
The nitric oxide content was evaluated using an NO Quantification Kit (Active Motif, Carlsbad, CA). Nitrates in the sample were reduced to nitrites using nitrate reductase and the Greiss reaction was utilized to colorimetrically visualize nitric oxide products in the samples. Assays were performed in triplicate and evaluated using a microplate reader (Tecan, Mannedorf, Switzerland) at absorbance wavelength 540 nm with reference wavelength 612 nm.
2.2.6. Ex vivo lung preparation
All animal experiments were approved by the University of Utah’s Institutional Animal Care and Use Committee (IACUC) and are in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council). C57/BL6 mice (20–25 g) were anesthetized with ketamine/xylazine (80/10 mg/kg), a tracheotomy was performed and they were mechanically ventilated with a pressure-controlled ventilator (Kent Scientific, Torrington, CT). Respiratory rate was 50/minute, and peak inspiratory pressure (PIP) was 7–10 cm H2O. The chest and pericardial sac were sequentially opened and ligatures were placed around the aorta and pulmonary artery. Heparin (200 U) was injected into the pulmonary artery and allowed to circulate for 2 min. The mouse was exsanguinated via a left ventriculostomy and the left atrium was cannulated via the left ventriculostomy. The pulmonary artery was cannulated via the right ventricle and lungs were perfused with Krebs-Ringers-bicarbonate solution (Sigma Chemical, St. Louis, MO) containing 3% bovine serum albumin (BSA; Fraction V, Proliant, Ankeny, IA). Pulmonary artery (PPA) and left atrial pressures (PLA) were measured continuously via in-line pressure transducers (P-75, Harvard Apparatus, Natick, MA) connected to an A/D board. Solenoids (20PSI, 12 V DC, Cole Parmer, Mount Vernon, IL) were placed in-line on both the arterial and venous tubing and could be closed simultaneously for measuring double occlusion pressures (PDO). An in-line ultrasonic flow probe (Transonic, Ithaca, NY) was placed in the pulmonary artery cannula and flow data was recorded in real-time. Lungs were suspended from a force transducer (Radnoti, Minrovia, CA) and lung weight was zeroed; lung weight, vascular pressures and flow were recorded using a custom-written program (Labview, National Instruments, Austin, TX).
2.2.7. Calculation of Kfc
Lungs were perfused at 2 ml/min, left atrial pressure = 2 cm H2O during a 20 min isogravimetric period. To measure Kfc, PLA was increased to 7 cm H2O for 20 min and the slope of weight gain/time during 18–20 min was used to derive Kfc according to the formula:
where Δwt/t is the slope of the line depicting weight gain over time and Pc is the capillary pressure calculated according to
which gives units of mL/min/cm H2O [13]; this value was normalized to 100 g of predicted lung weight. After the 20 min increase in PLA, the pressure is reduced to 2.0 cm H2O for 20 min and the polymer was perfused into the lungs via the pulmonary artery catheter at a rate of 1 mg/mL/min. PLA was then increased to 7 cm H2O and Kfc was measured from 18 to 20 min. The ratio of Kfc2/Kfc1 was used to assess the effect of polymers on whole lung permeability.
2.2.8. Statistical analysis
Lp values are presented as mean ± standard deviation. A P value less than 0.05 is considered significant. Statistics were performed with ANOVA and Tukey’s post hoc comparison where appropriate. All other statistical tests are specified in the figure caption.
3. Results
3.1. Copolymer synthesis and characterization
Copolymers of HPMA and MA-TMACl were synthesized by radical polymerization in various feed ratios to create polymers of either 10 or 20 mol% quaternary ammonium content. Table 1 gives the nomenclature and characterization data for each copolymer used and Fig. 1 shows the molecular structure for TMA-HPMA copolymers. The molecular weight of each polymer was determined by AKTA FPLC (GE Healthcare) on an analytical Superose 6 column in tetramethylammonium chloride buffer (TMAC, pH 6.0) and subsequently fractionated by the same instrument on a Superose 6 preparative column if a low polydispersity was necessary to the experiment. The actual percent composition of quaternary ammonium per macromolecule was determined by the titration of chloride, the ammonium counterion, with the stoichiometric addition of AgNO3 using fluorescein as the endpoint indicator.
Table 1.
Polymer nomenclature and characterization data. Approximate percent composition of TMA Cl is denoted as the number, in percent, before TMA Cl and fractionated polymers have either F2, F3 or F5 following the name.
| Name | Feed composition (mol %) | Polymer composition [mol % (SD)] | Molecular weight [kDa (PD)] | Charges per macromolecule (approx.) |
|---|---|---|---|---|
| 20P(TMA)Cl | 20 | 19.9 (1.4) | 70.2 (1.8) | 95 |
| 20P(TMA)Cl-F3 | 20 | 19.9 (1.4) | 135 (1.1) | 182 |
| 20P(TMA)Cl-F5 | 20 | 19.9 (1.4) | 24 (1.2) | 32 |
| 10P(TMA)Cl-F2 | 10 | 10.7 (0.7) | 90 (1.2) | 65 |
| PEO | – | – | 82 (1.1) | – |
Fig. 1.

Secondary structure of a P(TMA)Cl copolymer. All copolymers used in this study possess this basic structure. Alterations are made in percent composition of TMA Cl and molecular weight.
3.2. Shear-induced hydraulic conductivity
BAEC monolayers that were subjected to polymer treatment were incubated for 30 min with a 1 mg/mL solution of copolymer in test media (nomenclature and characterization data are shown in Table 1). Following the incubation period, monolayers were washed with test media then subjected to a shear stress of 20 dyn/cm2. When compared to controls run side-by-side that received no treatment, all polymers tested significantly decreased the hydraulic conductivity response to shear (Fig. 2). In order to test whether the molecular weight of the polymer would have an effect on the degree to which the polymer reduced the cellular response to shear, a polydisperse polymer (20P(TMA)Cl, Fig. 2A), and both a high- and low-molecular weight fraction of a polymer with similar charge density (20P(TMA)Cl-F3, Figs. 2B and 20P(TMA)Cl-F5, Fig. 2C, respectively) were tested. All variations of the polymer significantly reduced shear-induced hydraulic conductivity. The polymer data were normalized to represent percent decrease from respective control Lp data (Fig. 3) according to the following equation
where control(t) is the value of the normalized Jv/A response without polymer at time t, and polymer(t) is the value of the normalized Jv/A response in the presence of polymer at time t and 1 represents the baseline value that is obtained in the absence of shear stress. As defined, the higher the value of M, the greater suppression of mechanotransduction. Monolayers treated with 20P (TMA)Cl-F3 demonstrate a significantly higher value for M than do monolayers pre-incubated with either 20P(TMA)Cl or 20P(TMA)Cl-F5. We are able to show with these data that cationic copolymer incubation protects the endothelium against shear-induced increases in hydraulic conductivity and that this protective effect augmented in the presence of a narrowly distributed high molecular weight copolymer.
Fig. 2.

Normalized Lp values of polymer-treated monolayers and their companion controls. BAEC monolayers were incubated for 30 min with 1 mg/mL of A) 20P(TMA)Cl (○), B) 20P(TMA)-F3 (□), or C) 20P(TMA)-F5 (●). After 1 h, a shear stress was imposed on monolayers. Monolayers pre-incubated with polymer show a 50–75% decrease in Lp response to shear compared to control monolayers. *P < 0.05 ANOVA, N = 3.
Fig. 3.
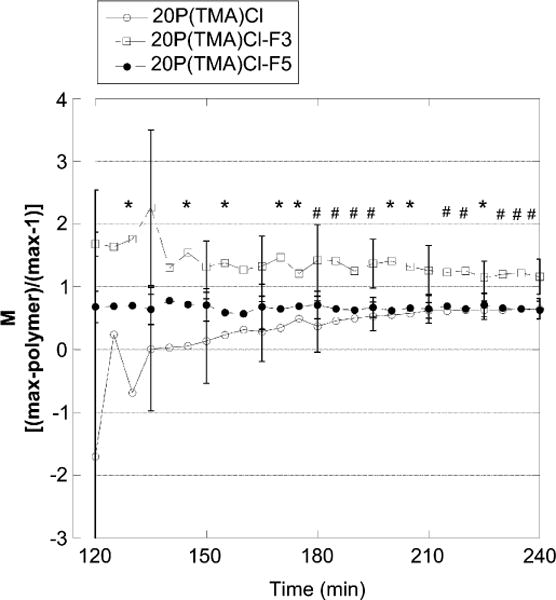
Shear-induced hydraulic conductivity of monolayers treated with 20P(TMA)Cl (○), 20P(TMA)-F3 (□), or 20P(TMA)-F5 (●) and normalized to their respective controls. Normalized values for 20P(TMA)Cl-F3 are significantly higher than for other groups indicating a greater reduction in shear-mediated hydraulic conductivity with high molecular weight copolymers. *P < 0.05 difference from 20P(TMA)Cl. #P < 0.05 difference from all groups. N = 3.
3.3. Pressure-induced hydraulic conductivity
Previously, we reported a decrease in pressure-induced hydraulic conductivity using 40P(TMA)Cl, a cationic copolymer composed of approximately 40 mol% positive charges per macromolecule [6]. Fig. 4A shows that a further reduction of the charge density to 20 mol % significantly lowers the pressure-induced hydraulic conductivity of cell monolayers in response to 30 cm H2O fluid pressure. The administration of L-NAME, an inhibitor of nitric oxide synthase (NOS), is also able to significantly reduce the hydraulic conductivity from control cells receiving no treatment. We found no significant difference between cell monolayers treated with L-NAME and monolayers transiently incubated with a 1 mg/mL dose of 20P(TMA) Cl. It is shown here that the administration of a copolymer that interacts with the endothelial glycocalyx reduces the fluid conductance of the cell monolayer in response to hydrostatic pressure to a similar degree as monolayers whose mechanotransductive signal pathway is attenuated intercellularly.
Fig. 4.
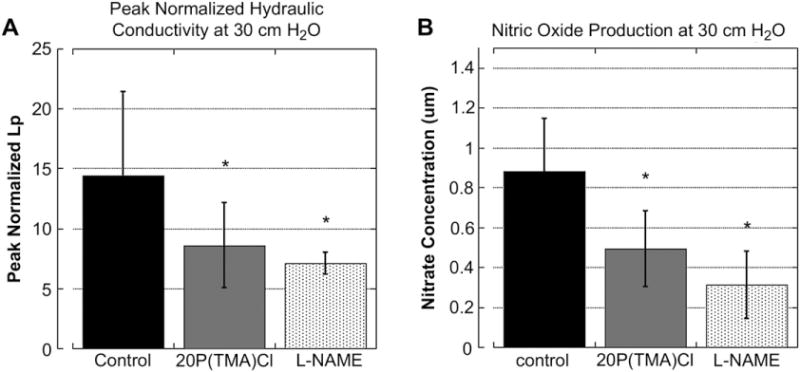
Peak normalized Lp values and nitrate concentration in cell media above tested monolayers. A) Peak normalized pressure-induced hydraulic conductivity values obtained after 30 min of 30 cm H2O pressure. Addition of 20P(TMA)Cl and L-NAME, respectively, significantly decreased the pressure-induced increase in hydraulic conductivity through monolayers. No statistical difference exists between polymer-treated and nitric oxide inhibited monolayers. *P ≤ 0.05, ANOVA/Tukey’s post hoc. N = 8 per group. B) Nitrate concentration of media above monolayers exposed to pressure of 30 cm H2O for 60 min. Incubation with polymer significantly reduces the nitric oxide concentration to that of cells incubated with the NO inhibitor L-NAME indicating an intracellular mechanism for barrier dysfunction attenuation. *P > 0.01, N = 8 per group.
3.4. Nitric oxide production in response to hydrostatic pressure
In order to determine whether the barrier-enhancing effect of polymer administration was a consequence of a physical barrier against fluid flux or whether they altered mechanotransduction pathways, the media directly above cell monolayers exposed to high pressures in hydraulic conductivity experiments outlined in Section 3.3 was sampled and assayed for nitrate and nitrite content. Because nitric oxide is unstable in aqueous media and rapidly produces both nitrate and nitrite as end-products, the supernatant was incubated with nitrate reductase and the resulting nitrite was assayed according to Greiss reaction protocol. The concentration of nitric oxide products is reported in Fig. 4B. The administration of either L-NAME (10 μM) or 20P(TMA)Cl (1 mg/mL) copolymer significantly reduced the nitric oxide production of cell monolayers when compared to control. There was no significant difference between groups treated with either L-NAME or copolymer, therefore, one may conclude that 20P(TMA)Cl attenuates nitric oxide production via an intercellular mechanism and that the polymerassisted attenuation of mechanotransduction is not solely the enhancement of a physical barrier alone.
3.5. Isolated perfused lung preparation
To test the therapeutic benefit of copolymers in a whole-organ system, we exposed isolated perfused mouse lungs to a 7 cm H2O pressure differential and measured the capillary filtration coefficient (Kfc) at the end of a 20 min pressure pulse. Lungs were then perfused with a buffered 1% solution of either 10P(TMA)Cl or PEO and a second Kfc value was recorded. The ratio of the Kfc2 value to Kfc1 is indicative of the change in whole lung permeability in the absence or presence of polymer, respectively, and is reported in Fig. 5 for all groups. 10P(TMA)Cl or PEO infusions at 1 mg/mL/min significantly reduced the ratio of Kfc2/Kfc1 when compared to lungs receiving no treatment.
Fig. 5.
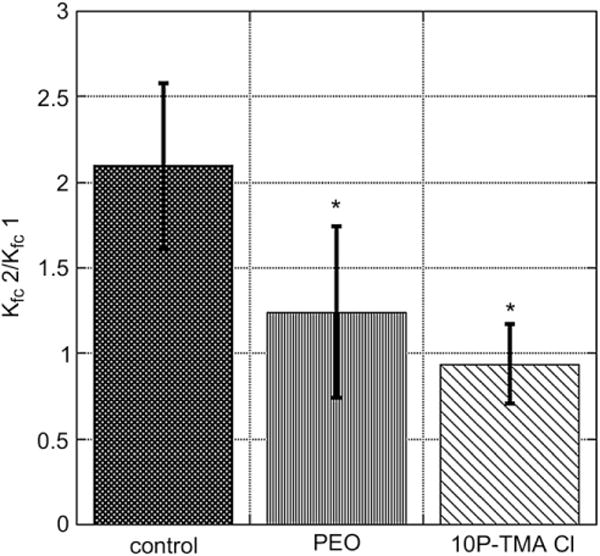
Mouse whole lung capillary filtration coefficient (Kfc) for lungs perfused with buffer alone (control, black bar), 1% PEO (grey bar), or 1% 10P(TMA)Cl (hatched bar). A significant reduction in Kfc occurs when lungs are perfused with either PEO or 10P (TMA)Cl with a lower mean Kfc observed in the presence of targeted copolymer. *P < 0.05. ANOVA Dunnett’s post hoc test, N = 4 per group.
4. Discussion
The development of water-soluble biomimetic copolymers that target the endothelial glycocalyx may be useful both as a tool to characterize mechanotransduction pathway(s) in vitro and as a prototype for developing clinical compounds to improve capillary barrier function during vascular inflammation. Cationic copolymers have historically been used as gene delivery platforms. Typically, polymers used in gene delivery are decorated with primary amine pendant groups [14–16] since these polymers are more likely to be taken up into cells [16]. We choose quaternary ammonium structures in order to minimize cellular activation and uptake by eliminating the ability for covalent bond formation as quaternary ammonium does not possess a lone pair of electrons. We have previously shown the ability of HPMA-TMA Cl copolymers to bind to the endothelial surface and demonstrated an enhancement of barrier function and a lack of cellular toxicity [6]. The lowest mol% of quaternary ammonium in copolymers used in previous studies was 40 mol%, however, in present studies we are able to show the therapeutic benefit of this copolymer with a lower overall charge density (e.g. 10–20 mol% TMA Cl composition). The benefit to lowering charge density is that there is a subsequent increase in cell viability as we have shown in previous LDH-release cytotoxicity assays [6] and as has been shown recently by Ma et al. [10].
In shear-induced hydraulic conductivity assays, a significant decrease in the shear-induced cell response is seen in cells pretreated with 20P(TMA)Cl (Fig. 2). All of the Lp experiments display the characteristic “sealing” behavior in which Lp decreases continuously to its baseline value during the first 60 min of exposure to the pressure differential. The sealing effect was not affected by any of the polymer treatments since the baseline control and treatment Lp values are not significantly different during the first 60 min (Fig. 2A–C). Three polymers were tested on endothelial monolayers, each polymer composed of the same overall charge density (20 mol%) but differing molecular weights and distributions: 20P(TMA)Cl is a polydisperse copolymer (PD 1.8), 20P(TMA) Cl-F3 is a high molecular weight fraction of 20P(TMA)Cl (135 kDa) and 20P(TMA)Cl-F5 is a low molecular weight fraction (24 kDa, characterization data given in Table 1). Both fractions have a lower polydispersity than 20P(TMA)Cl. Fig. 2 A–C shows that shear-mediated hydraulic conductivity is significantly reduced in endothelial monolayers treated with each copolymer. In Fig. 3, the hydraulic conductivity output data for each polymer-treated monolayer was normalized to their respective control monolayers and compared. We show a significantly higher values for M generated from monolayers treated with 20P(TMA)Cl-F3, the 135 kDa copolymer. The molecular weight of linear polymers is often an indicator of the hydrodynamic radius of a macromolecule, whose influence on mechanotransduction has not been well defined. We conclude that larger macromolecules have a greater protective effect against shear-mediated barrier damage.
Previously, we reported a significant decrease in pressure-mediated hydraulic conductivity in the presence of 1 mg/mL 40P(TMA)Cl, a copolymer with two times the charge density of polymers used in this study [6]. In Fig. 4A, the use of 20P(TMA)Cl is shown to reduce pressure-mediated hydraulic conductivity. We have previously demonstrated that pressure-induced increase in hydraulic conductivity is mediated in large part via an increase in nitric oxide production [17,18]. 20P(TMA)Cl significantly reduces peak normalized Lp levels, generated after 30 min at 30 cm H2O pressure, to the levels generated when cells are incubated with the nitric oxide synthase inhibitor, L-NAME. When the media above the cells in these experiments was assayed for nitric oxide metabolites, it was found that cells generated significantly less nitric oxide in the presence of 20P(TMA)Cl than in untreated cell monolayers and that nitric oxide levels in cells treated with 20P(TMA)Cl were not significantly higher than in cells pre-incubated with L-NAME. Collectively, these data suggest that glycocalyx-associated cationic copolymers attenuate mechanotransduction by inhibiting the nitric oxide signaling pathway.
In order to determine whether polymers could reduce pulmonary edema in a whole lung model, high molecular weight fractions of 10P(TMA)Cl and polyethylene oxide (PEO) were perfused through an isolated perfused mouse lung model and the whole lung filtration coefficient was measured before and after polymer treatment. Perfusions of mouse lungs with 10P(TMA)Cl significantly decreased Kfc2/Kfc1 and thus reduce the pressure-induced permeability response in comparison to lungs receiving no treatment. We compared 10P(TMA)Cl to polyethylene oxide (PEO), which carries no formal charge and is comparable to the HPMA backbone in water solubility and biocompatibility. Both polymers are high molecular weight, low polydispersity, and are able to significantly reduce the capillary filtration coefficient when compared to controls receiving no polymer perfusion. The protective effect of PEO in endothelial barrier regulation has been shown in transendothelial resistance studies and is reported in literature [7]. However, in an in vivo situation, a targeting moiety is necessary to anchor a therapeutic copolymer to the endothelium for any length of time that extends beyond transient contact.
The second optimization step of 10P(TMA)Cl was to reduce its polydispersity since in previous experiments we observed an increase in pulmonary artery pressure after perfusion with a polydisperse formulation of 40P(TMA)Cl (PD 1.8, data not shown). We hypothesized that the cause of the increase in pulmonary artery pressure was that low molecular weight polymer chains passed through the cell–cell junctions and stimulated contraction of underlying smooth muscle cells. The conclusion is that increasing molecular weight and reducing polydispersity improves vascular retentions, maintains normal pulmonary artery pressure (PPA2/PPA1 = 1.1, data not shown) and is able to reduce Kfc2/Kfc1 in whole lung studies.
5. Conclusion
In this study we demonstrate the ability of HPMA-based cationic copolymers to attenuate both shear- and pressure-mediated increases in hydraulic conductivity across endothelial monolayers and that higher molecular weight copolymers have greater barrier protection properties. We demonstrate a reduction in nitric oxide production in the presence of hydrostatic pressure in cells pretreated with 20P(TMA)Cl, suggesting a mechanism for the reduction in cellular response that impedes mechanotransduction. Here we lay the ground work for the use of copolymers in research to gain greater understanding of the mechanism involved in endothelial mechanotransduction and show the potential for biomimetic targeted polymers to be used clinically in the treatment of states of enhanced permeability. To this end, we show that 10P (TMA)Cl reduces the capillary filtration coefficient in whole lung model.
Acknowledgments
The authors would like to thank Monika Sima for assistance in polymer characterization and Mark Cluff for technical assistance with isolated perfused mouse lung work. Funding for this research is provided in part through NIH grants #53906690 and HL 57093.
References
- 1.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;89:320–30. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–7. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 3.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355:228–33. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide, and vascular inflammation. J Intern Med. 2006;259:351–63. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrate: the good, the bad, and the ugly. AJP Cell. 1996;271(5 Pt 1):C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 6.Giantsos KM, Kopeckova P, Dull RO. The use of an endothelium-targeted cationic copolymer to enhance the barrier function of lung capillary endothelial monolayers. Biomaterials. 2009;30:5885–91. doi: 10.1016/j.biomaterials.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 7.Chiang ET, Camp SM, Dudek SM, Brown ME, Usatyuk PV, Zaborina O, et al. Protective effects of high molecular weight polyethylene glycol (PEG) in human lung endothelial cell barrier regulation: role of actin cytoskeletal rearrangement. Microvasc Res. 2009;77:174–86. doi: 10.1016/j.mvr.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertuglia S, Veronese FM, Pasut G. Polyethylene glycol and a novel developed polyethylene glycol-nitric oxide normalize arteriolar response and oxidative stress in ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H1536–44. doi: 10.1152/ajpheart.01114.2005. [DOI] [PubMed] [Google Scholar]
- 9.Fritz JS, Schenk GH. Quantitative analytical chemistry. 5th. Englewood Cliffs, NJ: Prentice Hall; 1987. [Google Scholar]
- 10.Ma M, Li F, Yuan ZF, Zhuo RX. Influence of hydroxyl groups on the biological properties of cationic polymethacrylates as gene vectors. Acta Biomaterialia. 2010;6(7):2658–65. doi: 10.1016/j.actbio.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Quintero SV, Amaya R, Pahakis M, Tarbell JM. The endothelial glycocalyx mediates shear-induced changes in hydraulic conductivity. Am J Physiol Heart Circ Physiol. 2009;296:1451–6. doi: 10.1152/ajpheart.00894.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–42. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 13.Hubert CG, McJames SW, Mecham I, Dull RO. Digital imaging system and virtual platform for measuring hydraulic conductivity of vascular endothelial monolayers. Microvasc Res. 2006;71:135–40. doi: 10.1016/j.mvr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Luten J, Akeroyd N, Funhoff A, Lok MC, Talsma H, Hennik WE. Methacrylamide polymers with hydrolysis-sensitive cationic side groups as degradable gene carriers. Bioconjug Chem. 2006;17:1077–84. doi: 10.1021/bc060068p. [DOI] [PubMed] [Google Scholar]
- 15.vd Wetering P, Moret EE, Shuurmans-Nieuwenbroek NM, van Steenbergen MJ, Hennik WE. Structure–activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery. Bioconjug Chem. 1999;10(4):589–97. doi: 10.1021/bc980148w. [DOI] [PubMed] [Google Scholar]
- 16.Eldred SE, Pancost MR, Otte KM, Rozema D, Stahl SS, Gellman SH. Effects of side chain configuration and backbone spacing on the gene delivery properties of lysine-derived cationic polymers. Bioconjug Chem. 2005;16:694–9. doi: 10.1021/bc050017c. [DOI] [PubMed] [Google Scholar]
- 17.Garanich JS, Pahakis M, Tarbell JM. Shear stress inhibits smooth muscle cell migration via nitric oxide-mediated downregulation of matrix metalloproteinase-2 activity. Am J Physiol Heart Circ Physiol. 2003;288:H2244–52. doi: 10.1152/ajpheart.00428.2003. [DOI] [PubMed] [Google Scholar]
- 18.Dull RO, Mecham I, McJames S. Heparan sulfates mediate pressure-induced increase in lung endothelial hydraulic conductivity via nitric oxide/reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1452–8. doi: 10.1152/ajplung.00376.2006. [DOI] [PubMed] [Google Scholar]


