Abstract
High-risk populations exhibit early transformation of localized prostate cancer (CaP) disease to metastasis which results in the mortality of such patients. The paucity of knowledge about the molecular mechanism involved in acquiring of metastatic behavior by primary tumor cells and non-availability of reliable phenotype-discriminating biomarkers are stumbling blocks in the management of CaP disease. Here, we determine the role and translational relevance of ROBO1 (an organogenesis-associated gene) in human CaP. Employing CaP-progression models and prostatic tissues of Caucasian and African-American patients, we show that ROBO1 expression is localized to cell-membrane and significantly lost in primary and metastatic tumors. While Caucasians exhibited similar ROBO1 levels in primary and metastatic phenotype, a significant difference was observed between tumor phenotypes in African-Americans. Epigenetic assays identified promoter methylation of ROBO1 specific to African-American metastatic CaP cells. Using African-American CaP models for further studies, we show that ROBO1 negatively regulates motility and invasiveness of primary CaP cells, and its loss causes these cells to acquire invasive trait. To understand the underlying mechanism, we employed ROBO1-expressing/ROBO1-C2C3-mutant constructs, immunoprecipitation, confocal-microscopy and luciferase-reporter techniques. We show that ROBO1 through its interaction with DOCK1 (at SH3-SH2-domain) controls the Rac-activation. However, loss of ROBO1 results in Rac1-activation which in turn causes E-Cadherin/β-catenin cytoskeleton destabilization and induction of cell migration. We suggest that ROBO1 is a predictive biomarker that has potential to discriminate among CaP types, and could be exploited as a molecular target to inhibit the progression of disease as well as treat metastasis in high-risk populations such as African-Americans.
Keywords: ROBO1, prostate cancer, indolent, metastatic, African-American
Recent studies show that organ-confined prostate cancer (CaP) constitutes approximately 90% of all newly diagnosed CaP cases.1 Most of these will in fact behave as indolent cancers.1 The principal problem arising from CaP is its propensity of local invasion and metastasis to distant organs.2 Failure to control the spread of metastases has now been established as a major cause for therapy failure in CaP patients, particularly in African-Americans who are at high-risk to develop aggressive disease and have high mortality rate.3–5 Previous reports showed a faster tumor cell turnover and an earlier transformation from latent to aggressive CaP among African-Africans.3–5 The paucity of knowledge about the molecular mechanism involved in acquiring of metastatic behavior by primary tumor cells and nonavailability of reliable phenotype-discriminating biomarkers are stumbling blocks in the management of CaP in high-risk populations. The metastatic process is a series of interrelated steps that must be accomplished before distant tumor foci can be established. Localized/primary prostatic tumor cells undergo several phenotypical and genotypical changes to become invasive, and acquire motility.5 Motile CaP cells have the capability to break the prostatic tissue barriers to travel to distant organs in the body and establish themselves as metastatic tumors.5,6 Therefore, understanding mechanism that confers motility to tumor cells is a key to develop novel therapies to inhibit the metastasis in patients diagnosed with primary CaP.
ROBO1 is a member of roundabout (ROBO) immunoglobulin superfamily of proteins.7 Recent studies have shown the importance of ROBO1 protein in the cell motility and migration during embryogenesis and organogenesis.7–9 Emerging studies indicate that loss of ROBO1 could derail the checkpoints regulating process of motility in transformed cells.10 The expression of ROBO1 has been shown in primary tumors; however, very little is known about its expression and activity in metastatic tumors.9,10 In this study, we provide evidence that ROBO1 acts as a tumor suppressor gene and its loss has a significant bearing on the emergence of invasive CaP disease in African-American men. We showed the underlying mechanism of action of ROBO1 and established its relevance as a predictive biomarker that could discriminate primary disease from metastatic phenotype in African-American CaP patients.
Material and Methods
Plasmids, siRNA, antibodies and chemicals
Human ROBO1-expressing plasmid was provided by Dr Aburatani (University of Tokyo, Japan). Mutant ROBO1 {pcDNA3-ROBO1-Δ(CC2)Δ(CC3)} was provided by Dr Greg Bashaw (University of Pennsylvania, PA). Top flash plasmids were purchased from Clontech (Mountain view, CA). ROBO1-specific and DOCK1-specific pool of siRNAs were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Anti-ROBO1 antibody was purchased from Abcam antibodies (Cambridge, MA). Anti-DOCK1 was purchased from Gen-Tex (Irvine, CA). Anti-Rac1 and anti-Rac-GTP antibodies were purchased from New East Biosciences (King of Prussia, PA). Other antibodies were purchased from Cell Signaling (Danvers, MA). Recombinant SLIT2 protein was purchased from R&D chemicals (Minneapolis, MN).
Cell lines
E006AA cell line (representing localized primary tumor) was generated from the prostatic tissue of an African-American CaP patient and characterized as described.11 Both RC77/N (representing normal prostatic epithelial cell) and RC77/T (representing localized primary tumor) cell lines were generated from the prostectomy tissue of an African-American patient and characterized as described.12 MDA-PCa2b is the metastatic cell line generated from African-American patient by Navone et al. and was the first of its kind reported for African-American CaP.13 MDA-PCa2b, LNCaP, PC-3, RWPE-1, RW-NB26, 22Rv1, LNCaP and PC-3 were purchased from ATCC (Manassas, VA).
Tissue specimens
Paraffin-embedded CaP and benign hyperplasia specimens of Caucasian and African-American men were obtained from NIH-designated Cooperative Human Tissue Network (CHTN-Midwest, Ohio State University, Columbus, OH), Imagenex tissue bank (San Diego, CA) and Roswell Park Cancer Institute (RPCI) at Buffalo, NY. Sample collection was performed after obtaining written informed consent from patients for the use of their samples for scientific purposes. All clinical samples were provided based on a unique and random alphanumerical coded system without referring to the patients by name or any other identifier term. Ethical approval was obtained from the Institutional Review Boards at the University of Minnesota and RPCI.
qRT-PCR
The reactions were carried out with an ABI Prism-7000 system as described earlier.14 Following primers were used for ROBO1: Forward, 5’-gcatcgctggaagtagccatact—3’; Reverse, 5’-ctagaaatggtgggctcaggat3’. A constitutively expressed GAPDH was used as internal control.14 PCR conditions were as follows: one cycle of initial annealing temperature of 94 °C for 3 min followed by 40 cycles of 94° C for 15 sec, 63° C for 15 sec, and an elongation phase of 72° C for 30 sec. The expression was calculated as the relative ratio of ROBO1 to GAPDH in each sample.
Immunoflorescence confocal microscopy
Immunoflorescence-confocal microscopy in tissues and cells was performed by the methods as described earlier.14–16 For in vitro assay, cells seeded in chamber slides were grown in presence of recombinant Slit-2 (rSlit-2) protein. At 48 hr post-transfection, cells were fixed and immunostained with primary antibodies (anti-ROBO1; anti-Rac1-GTP, anti-DOCK1) at 1:200 dilution by the method as described.14–16 Cy3 anti-mouse (ROBO1), Alexa-Fluor 488 anti-mouse (ROBO1& Rac-GTP) and Alexa-Fluor 488 anti-rabbit (DOCK1) were used as secondary antibodies. This was followed by confocal microscopy and image analysis as described. 14,15
ROBO1 promoter methylation assay
Genomic DNA was modified using Qiagen EpiTect kit. Detection of the regulator CpG region of the promoter was as described by Dallol et al.17. Briefly, bisulfite modified template was used to PCR amplify the DF3 promoter region of ROBO1 (forward-5′-ttttggatggtttttgaaagtgtttgagtt-3′ and reverse-5′-cctaaacaaaaacatattaatccaaacaa-3)′. CpG methylation creates a TaqI restriction enzyme site. PCR product was incubated with TaqI restriction enzyme and digestion products were visualized by separation on a 3% agarose gel.
5-Aza-2’-deoxycytidine treatment
Cells were treated with freshly prepared and filter-sterilized demethylating agent, 5-aza-2′-deoxycytidine (5-Aza-D; 10 µM) (Sigma; St. Louis, MO) for 6 days. At alternate days, 5-Aza-D in fresh media was added to the culture. After 6 days, cells were harvested and RNA, DNA and cell lysate were prepared.
Wound healing migration assay
Wild-type and transfected cells (at 12-hr post transfection) were scratch wounded and grown under 3 µg/ml rSlit-2 recombinant proteins for 36 hr. The cells were washed twice with PBS to remove cellular debris and imaged with LyCa camera attached to LEICA-DM-IRB microscope. The quantification of cell migration was performed by using Image Proplus software (Media Cybernetics, Inc., Rockville, MD).
siRNA transfections
Transient transfections were performed by using the method as described earlier.13–15 For ROBO1 suppression, we used a target-specific pool of siRNAs. Following are the sequences of siRNA’s (first set). Sense: agaguaugcuggucugaaatt; Antisense: uuucagaccagcauacucutt; (second set). Sense: agaggauauucuaccuuautt, Antisense: auaagguagaauauccucutt; (third set). Sense: gaaguaacaugcauacuuutt,; Antisense: aaaguaugcauguuacuuctt. For DOCK1 suppression, we used following sequence of siR-NA’s (first set) Sense: gagacagauuggcuuugaatt; Antisense: uucaaagccaaucugucuctt.
(second set) Sense: gagagaaccauauauacaatt; Antisense: uuguauauaugguucucuctt; (third set) Sense: cagcaaacaucaagagauatt; Antisense: uaucucuugauguuugcugtt.
Luciferase reporter activity, immunoprecipitation, immunoblot, immunohistochemistry, chemoinvasion and statistical analysis
Results
ROBO1 mRNA and protein expression is lost during progression of CaP disease
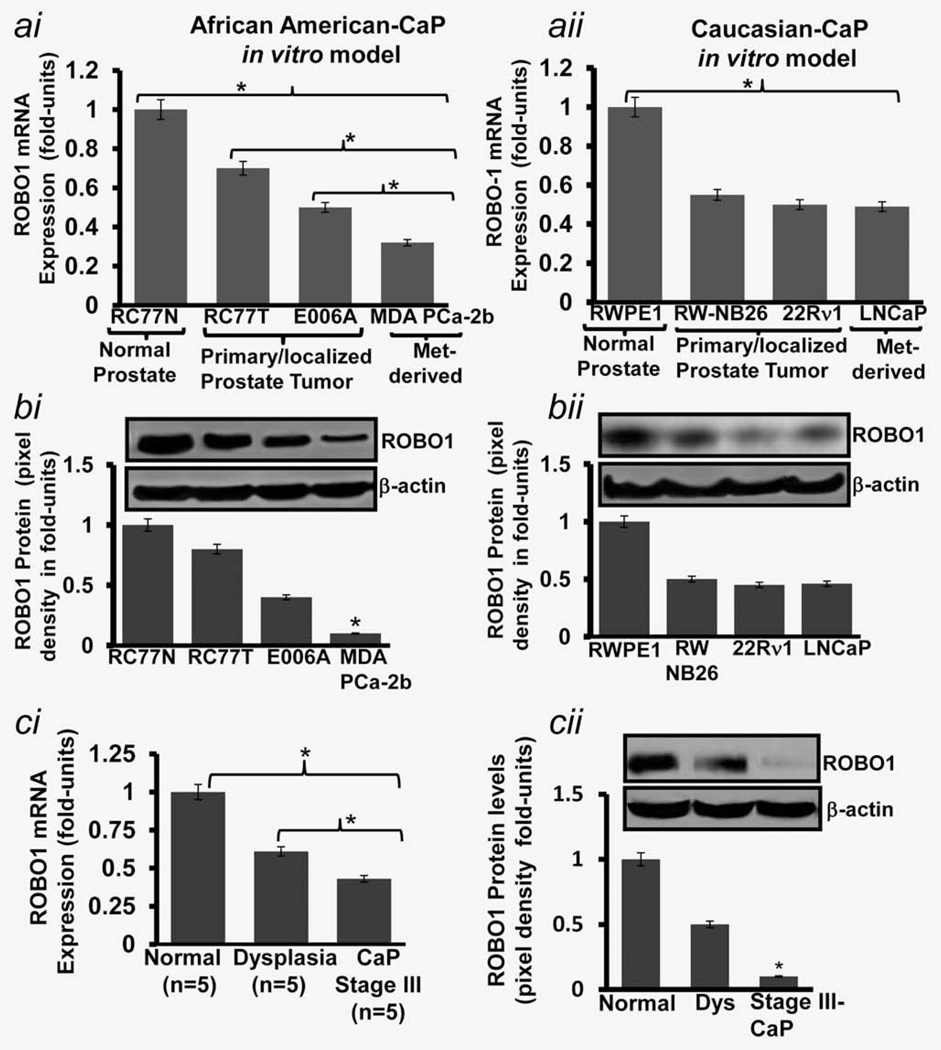
As an attempt toward identifying the status of ROBO1 during CaP progression in Caucasians and African American men, we utilized a cell-based carcinogenesis model that is comprised of an array of cells representing different stages of CaP disease. For Caucasians, this models includes RWPE1 (representing normal prostate epithelium), RWP-NB26 & 22Rν1 (representing organ-localized/primary CaP) and LNCaP & PC-3 (representing metastatic CaP) cells. The African-American model includes RC77/N (Normal prostate epithelium), RC77/T, E006AA (organ-confined/primary tumors and MDA-PCa2b (metastatic CaP) cells.11–13 We recently tested the relevance of this in vitro model.19 By employing quantitative real-time PCR (qRT-PCR technique, we observed that as compared to normal cells, the ROBO1 mRNA expression is significantly decreased in primary and metastatic CaP cells in both Caucasian and African-American models (Fig. 1ai–aii). We next compared ROBO1 expression between tumor cells representing primary (RW-NB26, 22Rν1, RC77T and E006AA) and metastatic (LNCaP, PC-3, MDA-PCa2b) stages. It is noteworthy that Caucasian primary (RW-NB26, 22Rν1) and metastatic phenotype (LNCaP, PC-3) exhibited almost similar ROBO1 mRNA-expression status, however, a distinguishable and significant (p< 0.05) difference in ROBO1 status was observed between primary (RC77T and E006AA) and metastatic (MDA-PCa2b) phenotypes in African-American models (Fig. 1ai–aii). Furthermore, a significant difference in mRNA expression levels between localized/non-invasive (RC77T) and localized/invasive (E006AA) tumor cells was also observed (Fig. 1ai).
Figure 1.
ROBO1 mRNA expression and protein levels in (a) cell-based CaP progression models representing normal (RWPE1; RC77/N), primary (RW-NB26; E006AA) and metastatic (PC-3; MDA-PCa-2b) CaP in African-American and Caucasian men, and (b) tissues representing human normal, dysplasia and malignant prostatic conditions. (a) Histogram shows the mRNA expression of ROBO1 in cell lines representing normal, primary/localized and metastatic stages of CaP disease in (ai) African-American and (aii) Caucasian men as assessed by quantitative real-time PCR (QRT-PCR technique. qRT-PCR product of GAPDH was used as an internal control. (b) Immunoblots and respective histograms shows the ROBO1 protein levels in cell lines representing normal, primary organ-confined and metastatic stages of CaP in (bi) African-Americans and (bii) Caucasians as assessed by immunoblotting and densitometry analysis. Equal loading of protein was confirmed by stripping the blots and re-probing with β-actin antibody. (ci) Histogram shows the mRNA expression of ROBO1 in human prostatic tissues of normal, dysplasia and malignant conditions as assessed by qRT-PCR. GAPDH was used as an internal control. Fold change (Delta Ct is the normalized expression in tissues. (cii) Immunoblot and histogram shows the ROBO1 protein levels in human prostatic tissues as assessed by immunoblotting and densitometry analysis. Equal loading of protein was confirmed by stripping the blots and re-probing with β-actin antibody. (bi–bii; cii) Each histogram represents mean ± SE of three independent experiments, *p < 0.05.
We next determined if mRNA expression of status ROBO1 corroborate to the translational levels by performing immunoblot analysis of cell-based models. As compared to normal cell, ROBO1 protein levels were found to be significantly decreased in cells representing Caucasian and African-American disease (Fig. 1bi). The comparative analysis (using densitometry analysis of immunoblots) showed that Caucasian primary (RW-NB26, 22Rν1) and metastatic (LNCaP, PC-3) CaP cells exhibited almost similar ROBO1 protein levels, however, a significant (p <0.001) difference between primary (RC77/T and E006AA) and metastatic (MDA-PCa2b) cells representing African-American disease was observed (Fig. 1bi–bii). MDA-PCa2b exhibit more than 2-fold (p < 0.001) decrease in expression of ROBO1 than RC77/T and E006AA cells (Fig. 1bi–bii). These data suggest that ROBO1 expression is lost in during progression of disease from localized CaP to metastatic phenotype in African-Americans.
ROBO1 expression is lost during progression of CaP in men
Proof of Principle
To establish the translational relevance of our in vitro data, we determined the ROBO1 mRNA expression and protein levels by employing qRT-PCR and immunoblot analysis in human frozen prostatic tissues of a small cohort (n = 15) of patients. Frozen prostatic tissues were procured from NCI-Cooperative human tissue network (CHTN-Midwest, Ohio State University, Columbus, OH). The qRT-PCR and immunoblot analysis showed that ROBO1 expression levels are high is normal prostatic tissues, however, significantly (p < 0.005) decreased in dysplasia and stage III CaP (Fig. 1ci–cii). When compared, stage III CaP tissues exhibited significantly (p< 0.005) lower ROBO1 expression than dysplastic prostatic tissues (Fig. 1ci–cii). These data suggested a progressive loss of ROBO1 during the advancement of CaP disease in humans.
ROBO1 expression is lost in prostatic tissues during CaP disease progression
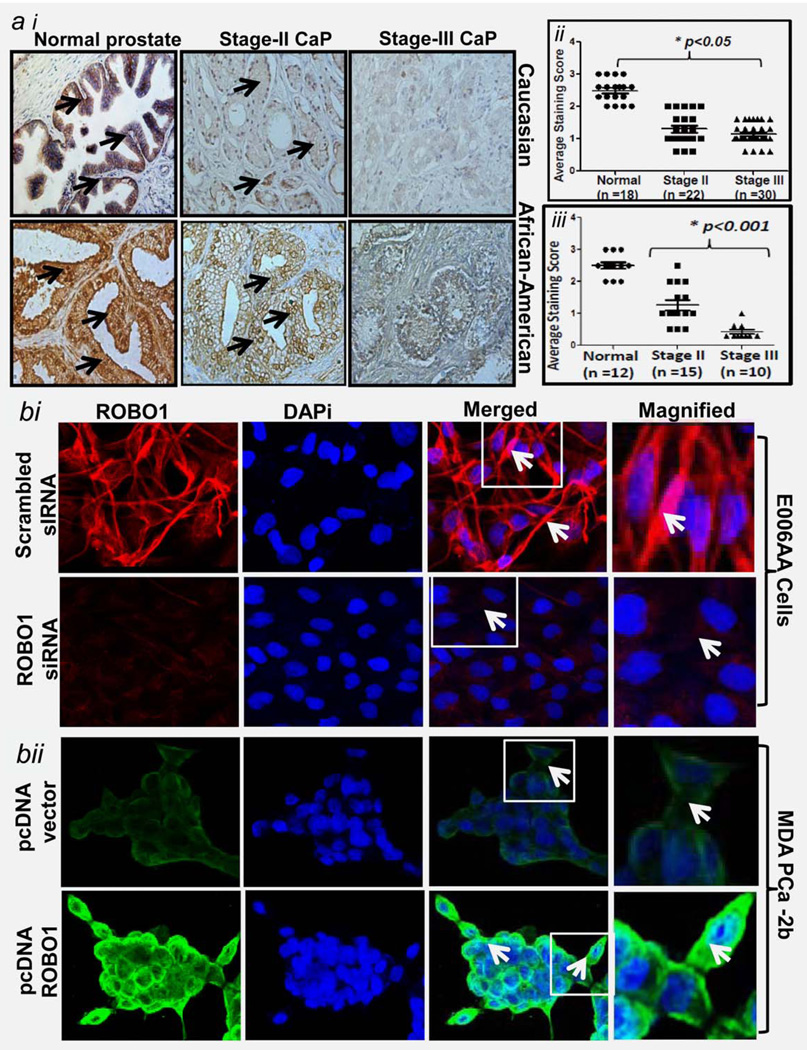
We determined ROBO1 protein status in paraffin-embedded prostatic specimens of a cohort that consisted of Caucasian (total n = 70) and African-American (total n = 37) CaP patients by performing ROBO1-specific immunohistochemical analysis. Caucasian specimens included normal (n = 18), stage II-CaP (n = 22) and stage III-CaP (n = 30). African-American specimens included normal (n = 12) stage II-CaP (n = 15) and stage III-CaP (n = 10). ROBO1-positive staining was observed to be strong in epithelial cells and weak in stromal region in prostatic tissues of both Caucasian (Fig. 2ai; upper panel) and African-American (Fig. 2ai; bottom panel). ROBO1 protein was found to be localized in membrane and cytoplasm of the epithelial cells including basal cells (Fig. 2). The staining intensity in tissue specimens were scored on a scale of 0 (none), 1 (weak), 2 (moderate) and 3 (strong) as described previously.18 Based on the visualization under microscope and scoring patterns, ROBO1 expression was observed to be highly decreased in malignant prostatic tissues than in normal tissue in both ethnic populations (Fig. 2ai– aii). We next asked if the loss of ROBO1 is progressive in nature during CaP development in humans by determining the difference of ROBO1 expression between two tumor stages. Although ROBO1 expression in Caucasian CaP tissues was significantly decreased than normal, there were no significant differences between stages II and III (Fig. 2aii). On the contrary, ROBO1 expression exhibited a significant progressive loss in its expression with the progression of CaP disease in African-American men (Fig. 2aiii). As evident from the distribution graph (for staining scores), a significantly lower expression of ROBO1 was observed in stage III than stage II-CaP in African-American men (Fig. 2aiii; p<0.05). Of 15 stage-II CaP specimens in African-Americans, ROBO1-positive staining was moderate in 3 (20%; p < 0.05) and weak in 12 (80%; p < 0.05) (Fig. 2aiii). Of 10 stage III cases, ROBO1-positive staining was moderate in two (20%; p <0.05), weak in three (30%; p < 0.05) and none in five (50%; p< 0.05) cases (Fig. 2aiii).
Figure 2.
Immunohistochemical analysis for ROBO1 in African-American and Caucasian CaP patients and subcellular localization of ROBO1 in primary and metastatic CaP cells. (ai) Photomicrographs show the expression of ROBO1 in prostatic specimens representing hyperplasia, stage II and stage-III in Caucasian (upper panel) and African-American (bottom panel) men, → in representative photomicrographs point to ROBO1-positive immunostaining in prostatic specimens. Magnification 40×. (aii–iii) Distribution plots for ROBO1 protein based on score pertaining to immunostaining pattern in prostatic specimens in epithelial region of (aii) Caucasians and (aiii) African-American men .*p<0.05; black bar represent median values. (b) Showing the ROBO1 protein subcellular localization in primary (E006AA) and metastatic (MDA-PCa-2b) CaP cells as assessed by confocal-scanning microscopy technique. DAPi was used for nuclear staining and red staining represents ROBO1-positive staining (bi; bottom panel). Showing the protein levels of ROBO1 in E006AA cells after siRNA-mediated silencing of ROBO1 (bii; bottom panel). Showing the ROBO1 protein subcellular localization in metastatic CaP cell MDA-PCa2b after ectopic expression of ROBO1 achieved by pcDNA31-ROBO1 transfection and assessed by confocal-scanning microscopy technique.
ROBO1 is localized to membrane of prostate tumor cells in African-Americans
We next determined the subcellular localization of ROBO1 protein in tumor cells by employing ROBO1-specific immunoflorescence-confocal microscopy. ROBO1 protein was observed to be primarily localized to the plasma membrane of primary E006AA and MDA-PCa-2b cells (Fig. 2b). As evident from merged and 400× magnified images (showing ROBO1-positive and DAPi-positive nuclear staining, tumor cells exhibited strong ROBO1 positive-reactiveness at cell membranes (Fig. 2b).
We asked if ROBO1 protein is localized to cellular membrane after its generation/expression from ROBO1 gene in tumor cells. We hypothesized that modulation of ROBO1 at transcription (gene level and post-transcriptional (mRNA level) should modulate the membrane-localized ROBO1 protein levels. To validate our assumption, we employed an approach of pcDNA3.1-ROBO1 transfection (inducing gene transcription) in MDA-PCa2b cells and gene-suppression by siRNA transfection (targeting ROBO1 mRNA) in E006AA cells. The immunoblotting assays validated the suppression of ROBO1 expression in E006A cells by siRNA and expression of ROBO1 in MDA-PCa2b cells by pcDNA3.1-ROBO1 (Supporting Information Fig. 1). The transfected cells were evaluated for subcellular localization of ROBO1 protein. On the basis of confocal microscopy data, we show that siRNA-mediated suppression caused a reduction in membrane localization of ROBO1 protein in E006AA cells (Fig. 2bi). However, the forced-expression of ROBO1 in MDA-PCa2b cells caused an increase in membrane localization of ROBO1 protein (Fig. 2bii). These data indicate that ROBO1 protein is primary localized within the plasma membrane of prostatic tumor cells.
Identifying molecular events which cause loss of ROBO1 expression during progression of CaP in African-American men
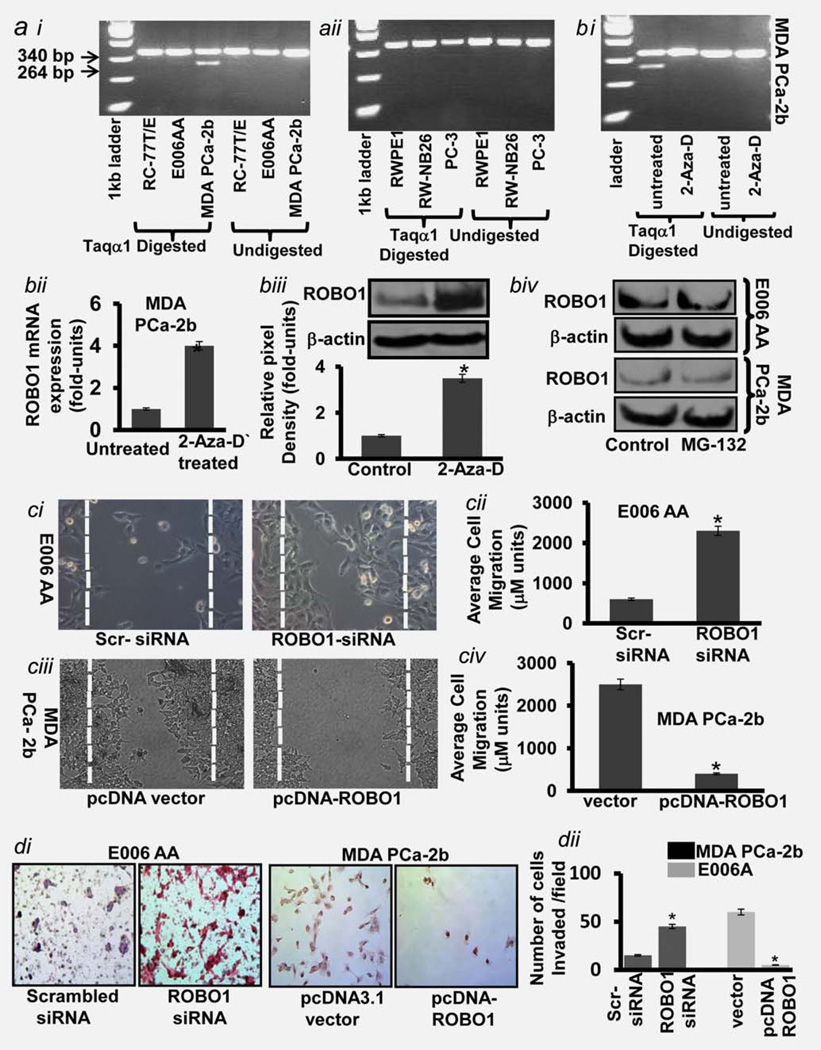
It became imperative to investigate the molecular events which cause the loss of ROBO1 during advanced metastatic stage of disease. Progression of CaP disease is the result of the accumulation of genetic, epigenetic and post-translational alterations.20 Although genetic changes are involved in the inactivation of certain antitumor genes, epigentic changes such as DNA methylation in the promoter region are reported of causing suppression of genes.20 To investigate if DNA methylation is involved in the suppression of ROBO1 expression during CaP progression in Caucasians and African-Americans, we examined normal (RWPE1 and RC77T/E) primary (RW-NB26; E006AA) and metastatic (PC-3; MDA-PCa-2b) CaP cells for ROBO1 promoter hyper-methylation. We performed restriction fragment length polymorphism (RFLP) approach on bisulfite modified template (which did not require large amounts of DNA) by following the methodology used by Dallol et al., who successfully had determined the CpG region methylation status on ROBO1 promoter and identified DF3 region on the promoter (from −964 to +27 bp of the translation start site) as a hotspot for methylation.17 Bisulfate modification along with Taqα1-induced digestion enabled to detect fragmented DNA’s (due to methylation at 340 and 264 bp on agarose gel. Matching controls (undigested DNA) were used for these studies. The methylation analysis of DNA showed that neither Caucasian (RW-NB26) nor African-American (E006AA) primary tumor cells exhibit hypermethylation at the ROBO1 promoter (Fig. 3ai–aii). It is noteworthy that the DNA of African-American metastatic (MDA-PCa2B) cells exhibited methylation of ROBO1 promoter (Fig. 3ai). However, no such methylation was found in Caucasian metastatic (PC-3) cells (Fig. 3aii). We next validated the hypermethylation as a cause of ROBO1 suppression by treating MDA-PCa2B cells with a demethylating agent 5-aza-2’-deoxycytidine 5-Aza-D) for 6 days. The PCR analysis of methylated DNA showed that 5-Aza-D treatment inhibited the methylation of ROBO1 in metastatic cells (Fig. 3aiii). This was further confirmed by qRT-PCR and immunoblot analysis of cells (Fig. 3bi–ii). Notably, the effect of 5-Aza-D treatment on ROBO1 expression was observed more at translational level than at mRNA message in tumor cells (Fig. 3bii).
Figure 3.
Identifying epigenetic and post-translational events which cause loss of ROBO1 and functional relevance of ROBO1 in cell migration (a) Image showing the methylation status of ROBO1 gene in (ai) African-American and (aii) CaP models including cells representing normal, primary/localized and metastatic condition. (bi) Image showing the methylation status of ROBO1 gene promoter in metastatic MDA-PCa-2b cells after treatment with demethylating agent 2-Aza-D. (bii) Histogram showing the ROBO1 mRNA expression in MDA-PCa2b cells after treatment with demethylating agent 2-Aza-D as assessed by qRT-PCR assay. GAPDH was used as an internal control. Fold change 2-Delta Ct is the normalized expression 2-Delta Ct in cells. (biii) Immunoblot image showing the protein levels of ROBO1 in MDA-PCa2b cells after treatment with demethylating agent 2-Aza-D as assessed by immunoblot analysis. (biv) Histogram showing the densitometry analysis of immunoblots. Equal loading of protein was confirmed by stripping the blots and re-probing with β-actin antibody. Each histogram represents mean ± SE of three independent experiments, *p<0.05 (c) immunoblot image showing the protein level of ROBO1 in primary/localized tumor E006AA and metastatic MDA-PCa2b cells after the treatment with proteasome inhibitor MG-132 Equal loading of protein was confirmed by stripping the blots and re-probing with β-actin antibody. (c) Photomicrographs showing the effect on the motility of (ci) primary and (ciii) metastatic cells after ROBO1 suppression (upper panel; ci) and forced expression of ROBO1 (bottom panel; ciii) as assessed by wound healing assay. Histograms show the distance in micrometers covered by cells after (cii) ROBO1-suppression in E006AA cells and (civ) ROBO1 expression in MDA-PCa2b cells. (d) Photomicrographs show the invasiveness of primary (E006AA) and metastatic (PCa-2B) cells after ROBO1-suppression and ROBO1-expression, respectively, as assessed by chemoinvasion assay. (dii) Histograms show the number of cells/field after ROBO1-suppression in E006AA cells and ROBO1 expression in MDA-PCa2b cells as assessed by counting the crystal violet-stained cells. The invaded cells were counted in ten random fields of vision. Values were the number of cells relative to that of control cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We next asked if post-translational event such as proteasome-induced protein degradation plays a role in ROBO1 loss in metastatic cells. For this purpose, primary E006AA, and metastatic MDA-PCa2b cells were treated with MG-132, (5 µg/ml; a proteasome inhibitor) for 24 hr. The immunoblot analysis of MG-132-treated cells showed that inhibiting protein degradation (by using MG132) did not produce significant modulation in the ROBO1 protein levels in cells (Fig. 3biii). These data clearly rule out the protein degradation pathway as a cause for ROBO1 loss in metastatic CaP cells. Taken together these data indicate that ROBO1 expression is more indicative of metastatic process in African-Americans than Caucasians. Therefore, for subsequent studies, we focused our attention on determining the relevance of ROBO1 in African-American CaP.
Functional relevance of the loss of ROBO1 in CaP cells in African-Americans
ROBO1 receptor activated by SLIT ligands causes a repulsive effect on glioma cell movement toward and across the midline.9,10 The pattern of cell movement and migration during organogenesis bears somewhat similarity to the tumor cell movement in the primary tissues. It becomes important to understand underlying mechanism through which organ-confined primary tumor cells escape spread to secondary sites. Keeping in view these similarities, we focused our attention on the possible role of ROBO1 on the migration phenotype of indolent and metastatic CaP cells. We employed two-prong genetic approach to determine the effect of ROBO1 suppression and ROBO1-expression on the motility and migrations of primary and metastatic tumor cells. For this purpose, we selected primary E006AA (exhibiting moderate ROBO1-expression) and metastatic MDA-PCa2b cells (exhibiting very low ROBO1 expression). ROBO1 was suppressed in E006AA cells by the siRNA transfection whilst ROBO1 was expressed in MDA-PCa2b cells by transfection with pcDNA3-ROBO1. Since tumor milieu consists of cells, some of which express receptors while others ligands, we cultured CaP in presence of recombinant SLIT2 protein (natural ligand for ROBO1). ROBO1-suppressed and ROBO1-expressing tumor cells were evaluated for migration potential by employing a scratch-wound assay. We observed that primary tumor cells when rendered ROBO1 suppressed, acquire significantly increased migration potential (Fig. 3ci; p < 0.005). This is evident from measuring the distance covered by ROBO1-suppressed cells (i.e. 2,300 µM) as compared to control cells (i.e., 750 µm) at 36 hr post-transfection (Fig. 3cii). Since metastatic MDA-PCa2b cells exhibit the least ROBO1 expression, we next determined the effect of ROBO1 reactivation on their migration potential and found that forced-expression of ROBO1 significantly decreased the migration of these cells (Fig. 3ciii; p < 0.005). This was evident from comparison of average distances covered by ROBO1-expressed cells 200 µm) and control cells (2,550 µm) at 36 hr post-transfection (Fig. 3civ). We next determined the significance of ROBO1expression on the invasive potential of tumor cells by using chemoinvasion assay. The siRNA-mediated loss of ROBO1 caused an increase in the invasiveness of primary E006A tumor cells (Fig. 3di). However, when ROBO1 was expressed in metastatic MDA-PCa2b cells, their invasiveness significantly reduced (Fig. 3di). Quantitative analysis showed that loss of ROBO1 caused >4-fold increase p < 0.005) in the invasiveness of primary tumor cells while as reactivation of ROBO1 significantly decreased the invasiveness of metastatic cells (Fig. 3dii; p < 0.001). Taken together, the data from wound healing and chemoinvasion assays, suggest that the presence of ROBO1 is critical for CaP cells to remain localized within a tumor and its loss thus is a hallmark for these cells to become invasive phenotype.
Determining the mechanism of action of ROBO1: Interaction with SH2-SH3 protein DOCK1
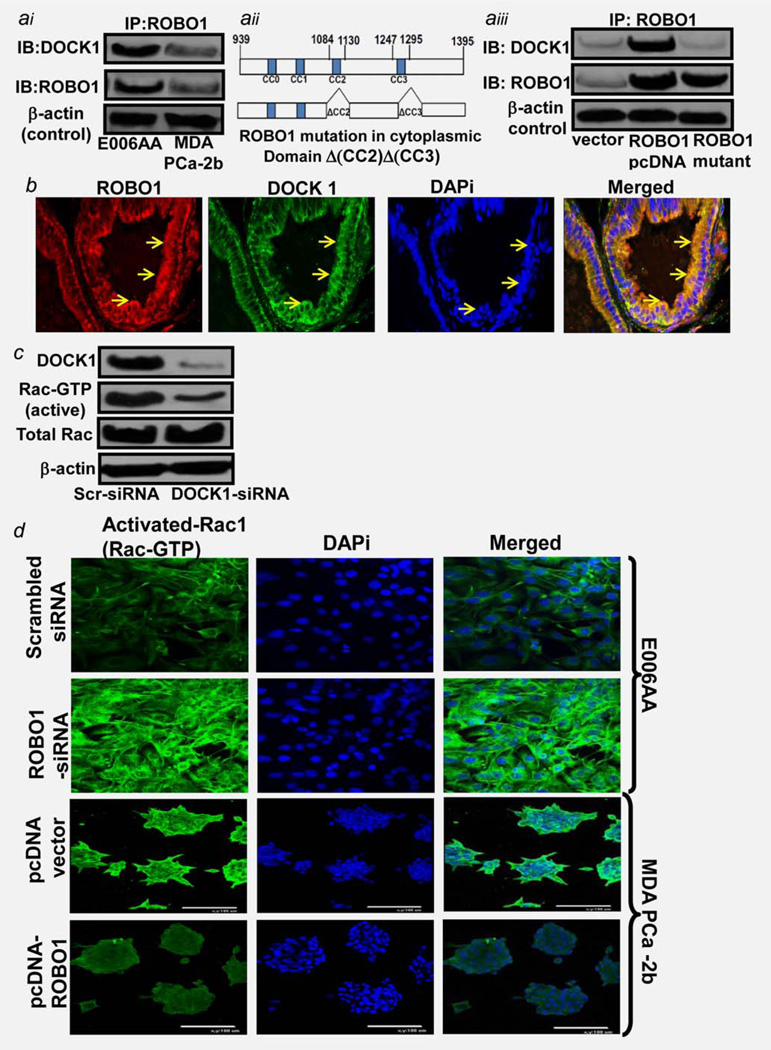
ROBO1 transmembrane receptor gets activated by a ligand SLIT2.7 The ligand-receptor interactions (such as IGF-IGFR1; EGF-EGFR) are known to trigger the activation of a downstream pathway in tumors. Therefore, it is possible that SLIT-ROBO1 interaction might be causing activation of a downstream pathway in tumor cells. Studies from Drosophila, C. elegans and mammalian neuronal cells showed that cytoplasmic domain of ROBO1 receptor interacts with several molecules (which are known to maintain the cytoskeleton integrity) in normal cells during organogenesis.8,9 Previously, Fan et al. showed that ROBO1 during organogenesis interacts with an SH3-SH2 adaptor protein DOCK1 (Dedicator of Cytokinesis-1) to control axonal movement.21 We hypothesized that ROBO1 might be adopting similar approach to control movement of malignant cells within prostatic tumors. We determined if ROBO1 has an interaction with DOCK1 protein in primary E006AA and metastatic MDA-PCa2b cells by performing an immunoprecipitation pull down assay using a human ROBO1-specific antibody. The immunoprecipitate was analyzed for ROBO1 and DOCK1 proteins. ROBO1 protein was found to be in complex with DOCK1 protein in both cell types (Fig. 4ai). Notable, the ROBO1: DOCK1 complex levels were fairly low in metastatic CaP cells and significantly high in primary E006AA cells (Fig. 4ai).
Figure 4.
Determining ROBO1 regulation of signaling required for motility of tumor cells. (ai) Immunoblot image showing the physical interaction (Complex formation) of ROBO1, with DOCK1 (Guanine nucleotide exchange factor) in primary cells E006AA and metastatic cells PCa-2b as assessed by immunoprecipitation (IP) pull-down assay. IP was carried out using anti-ROBO1 antibody as described in material and methods. ROBO1-specific immunoprecipitants from cells were analyzed for ROBO1, and DOCK1 proteins by immunoblot assay. (aii) Image shows the map of intracellular cytoplasmic domain of ROBO1 protein without (Upper lane and with mutations at CC2 and CC3 motifs. (aiii) Immunoblot image shows the protein:protein interaction of ROBO1 and DOCK1 in PCa-2b cells after transfections with ROBO1-expressing (PcDNA-ROBO1 and CC2/CC3-mutant ROBO1 expressing plasmid, as assessed by IP-pull down assay. ROBO1-specific immunoprecipitants from cells were analyzed for ROBO1, and DOCK1 proteins by immunoblot assay. For IP-pull-down assays (ai, aiii) the equal loading of proteins was confirmed by confirming β-actin in 10% cleared lysates. IgG alone was used as negative control. (b) Photomicrograph showing the co-localization of ROBO1 with DOCK1 protein in African-American benign hyperplastic prostatic tissues as assessed by confocal-scanning microscopy. Red color staining indicates the DOCK and green color indicated the ROBO1 protein. DAPi was used as nuclear stain (c). Immunoblot image shows the status of activation of Rac1 protein under DOCK1 +ve, and DOCK1-suppressed conditions in primary E006AA cells. Immunoblot membranes were probed for DOCK1, Rac-GTP, total Rac and β-actin (loading control proteins. Cells were transfected with scrambled-siRNA (Control and DOCK1-siRNA as described in material and methods. (d) Photomicrographs show the activation of Rac protein (in terms of Rac-GTP levels) in primary/localized E006AA after ROBO1-silencing, and metastatic MDA-PCa-2b cells after ectopic expression of ROBO1. Green color staining indicates the ROBO1 protein. DAPi was used as nuclear staining. Merged figures show the cytoplasmic levels of Rac-GTP in cells.
We next determined the regions on the cytoplasmic domain of ROBO1 protein critical for ROBO1:DOCK1 complex and validated if ROBO1 expression is critical to the ROBO1:DOCK1 complex formation in metastatic CaP cells. To achieve this objective, we used ROBO1-expressing plasmid with mutations at CC2 and CC3 motifs of cytoplasmic domain of ROBO1 (Fig. 4aii). We show that DOCK1 directly binds to ROBO1, and binding depends on the CC2 and CC3 motifs on cytoplasmic domain of ROBO1 (Fig. 4aiii). This was evident from data showing that full length ROBO1-expressing plasmid (pcDNA3.1-ROBO1) induced ROBO1 expression in MDA-PCa2b cells; however, transfection of cells with ROBO1-CC2/CC3 motifs mutant plasmid (pcDNA3.1-ROBO1-Δ(CC2)Δ(CC3) did not induce ROBO1 expression (Fig. 4aiii). Furthermore, ROBO1-CC2/CC3 motif-mutant plasmid failed to induce ROBO1:DOCK1 complex in MDA-PCa2b cells (Fig. 4aiii). These data strengthens the hypothesis that CC2 and CC3 motifs on cytoplasmic domain of ROBO1 are required to binding with DOCK1 in CaP cells.
ROBO1:DOCK1 proteins co-localize in African-American prostatic tissues
Immunoprecipitation assays in CaP-cell models showed an interaction between ROBO1 and DOCK1. We next asked if same could be observed in prostatic tissues of African-American men. By conducting immunoflorescence-confocal microscopy of BPH-prostatic tissues, we show that ROBO1 (Green fluorescence) and DOCK1 (Red fluorescence) colocalize (Seen in merged images at the membrane of prostate epithelial cells (Fig. 4b). DAPi-nuclear staining validated that ROBO1:DOCK1 complex is localized to the cytoplasmic region of cells (Fig. 4b).
ROBO1 through its association with DOCK1 controls Rac1 activation in CaP cells
Recent studies have shown that the molecular mechanism underlying actin cytoskeleton reorganization and migration involves a Rho-GTPase protein called Rac1.22 and references therein. Rac1 is a intracellular signal transducer that cycles between an inactive GDP-bound form and an active GTP-bound form under tight regulation and controls actin cytoskeleton organization and regulates cell migration.22 Fan et al. demonstrated that DOCK1, a guanine exchange factor (GEF), has the potential to control Rac1 activity.21 We speculated a possibility of ROBO1 controlling Rac1 activity through DOCK1 and investigated the relevance of DOCK1 on Rac1-activation in primary CaP cells. We show that siRNA-mediated suppression of DOCK1 significantly decreased Rac1 activity (Rac-GTP formation in E006AA cells (Fig. 4c). These data indicate that loss of DOCK1 shifted the balance toward Rac1-GTP formation resulting in increased Rac1 activity.
We next determined if ROBO1 regulates Rac1 activation in CaP cells by employing immunoflorescence-confocal microscopy. To understand the correlation between ROBO1 and Rac1 activation, we determined Rac1-GTP levels in primary and metastatic CaP cells under ROBO1-expression and suppression conditions. As evident from increased Rac1-GTP protein formation (Green fluorescence) at cytoplasmic region of cells, we show that suppression of ROBO1 caused activation of Rac1 (upper panel; Fig. 4d). The DAPi-nuclear staining showed that Rac1-GTP is highly concentrated in the cytoplasm of E006AA cells (Fig. 4d). Notably, forced expression of ROBO1 caused a decrease in Rac1 activation in MDA-PCa2b cells (Bottom panel; Fig. 4d). These data show an inverse correlation of ROBO1 with Rac1 activity in CaP cells.
ROBO1 by regulating DOCK1/ Rac1 tightly maintains E-cadherin/β-catenin in cells
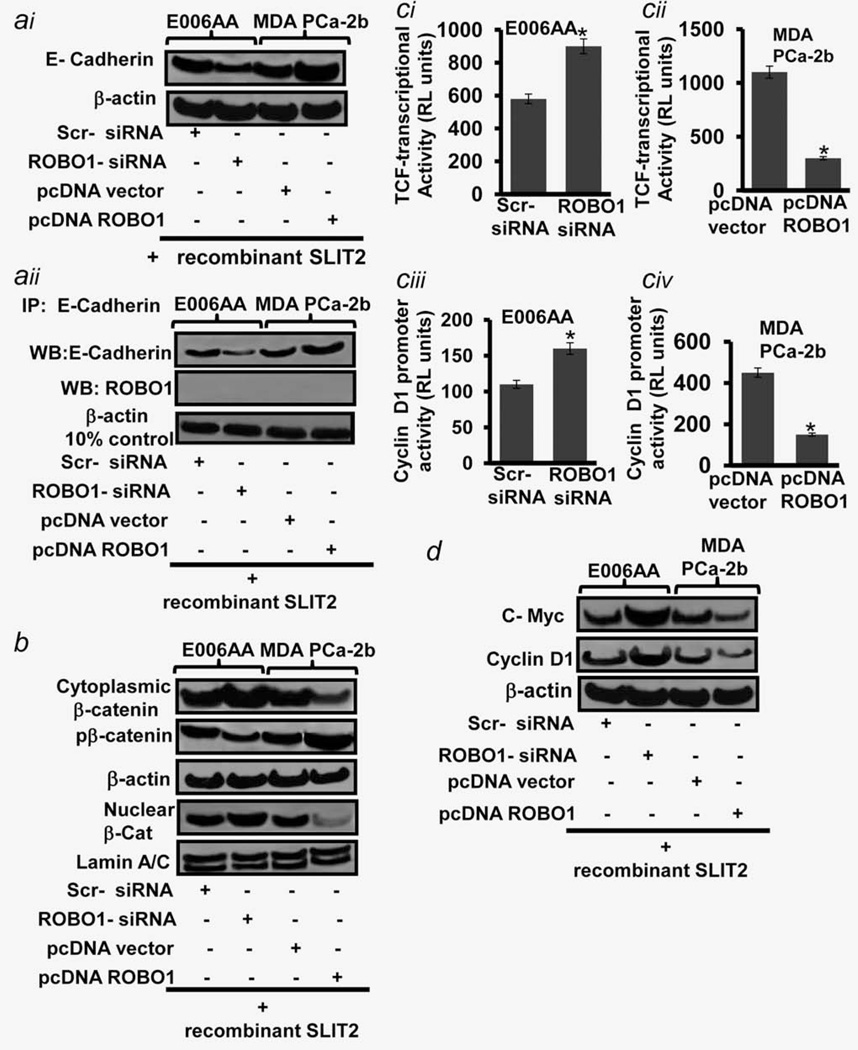
Migration of epithelial cells is characterized by the disruption of cell-cell contact which is based on cadherin-mediated cell– cell adhesion.23 Recent studies showed that cells having reduced expression of E-cadherin (Considered as “Gate Keeper” of the cytoskeleton stability and increased Rac1 activity show increased motility.24 We next determined the correlation between ROBO1 and E-Cadherin in CaP cells. By employing gene-manipulation approach, we show that suppressing of ROBO1 caused a decrease in E-Cadherin levels in E006AA cells whilst forced expression of ROBO1 caused reverse effect in MDA-PCa2b cells (Fig. 5ai). These data suggest a positive correlation of ROBO1 expression with E-Cadherin levels in CaP cells. Similar to ROBO1, E-Cadherin is also recruited to the cell membrane, we therefore determined if there is a physical interaction between the two molecules. The E-cadherin-specific immunoprecipitation assay of ROBO1-expressing and -suppressing CaP cells exhibited a negative outcome thus ruled out possibility if interaction between two membrane proteins (Fig. 5aii).
Figure 5.
Determining the stability of cytoskeleton assembly and downstream signaling regulated by ROBO1 in prostatic tumor cells. (ai) Immunoblot image shows the expression level of E-Cadherin in primary/localized (E006AA) and metastatic (MDA-PCa2B) cells. (aii) Immuno-blot image shows the expression level of E-Cadherin and ROBO1 in immunoprecipitates of E006AA and metastatic MDA-PCa2b cell which were tested for E-Cadherin-specific IP-pull down assay. β-actin in 10% input (Cleared lysates and IgG alone was used as controls. (b) Immunoblot image shows the proteins levels of total cytoplasmic, phosphorylated-cytoplasmic and nuclear β-catenin in primary E006AA and met-astatic MDA-PCa2b cells. (ci–ii) Histograms show the transcriptional activation of TCF-responsive element (β-catenin signaling marker in primary E006AA and metastatic MDA-PCa2b cells. (ciii–iv) Histograms show the transcriptional activation of Cyclin D1 promoter (Downstream target of β-catenin signaling) in primary E006AA and metastatic MDA-PCa2b cells. (d) Immunoblot image shows the proteins levels of C-Myc, Cyclin D1 and β-actin in primary E006AA and metastatic MDA-PCa2b cells. For figures (ai–ii, b, ci–iv and d), Cells were grown in presence of recombinant SLIT2 (ROBO1-ligand) protein and under ROBO1-suppressed or ROBO1-expressing conditions. Equal loading of protein was confirmed by stripping the blots and re-probing with β-actin antibody. ROBO1-expression was achieved by pcDNA-ROBO1 transfection. ROBO1-suppression was achieved by ROBO-siRNA transfections.
We asked if the loss of ROBO1 causes the destabilization of E-Cadherin/β–catenin cytoskeleton interaction. If our speculation is true, then free cytoplasmic β-catenin should be noticeable in such cells. We determined subcellular β-catenin levels in E006AA and MDA-PCa2b cells (With varied ROBO1 expression. ROBO1 suppression was observed to increase the cytoplasmic and nuclear β-catenin levels in E006AA cells (Fig. 5b). However, forced-expression of ROBO1 caused a decrease in cytoplasmic and nuclear β-catenin protein levels in MDA-PCa2b cells (Fig. 5b). Notably, ROBO1-suppression caused a decrease in the phosphorylated forms of β-catenin, and a converse effect was observed in ROBO1-expressing condition (Fig. 5b). A possible reason could be that the loss of ROBO1 causes an accumulation of free β-catenin in the cytoplasm, and all of which may not be undergoing the classical phosphorylation-mediated proteasomal degradation.
We and others showed that free cytoplasmic β-catenin after translocation to nucleus act as a co-activator of TCF-transcriptional factor and induce the expression of metastasis-associated genes in CaP cells.25,26 By measuring the transcriptional activation of TCF-responsive element and expression of downstream targets (Cyclin D1 and Myc), we next determined if loss of ROBO1 or reactivation of ROBO1 has any bearing on the co-activator activity of nuclear β-catenin in CaP cells. As compared to control, the siRNA-mediated ROBO1-suppression caused an induction in TCF-responsive element and Cyclin D1 promoter activities in primary E006AA cells (Fig. 5ci and ciii). Conversely, forced-expression of ROBO1 reduced the TCF-responsive element and Cyclin D1 promoter activities in metastatic MDA-PCa2b cells (Fig. 5cii and civ). These data were validated by immunoblot analysis which showed that ROBO1-suppression caused an increase in Cyclin D1 and C-MYC proteins levels in E006AA cells, while as ROBO1-expression caused a reverse effect in MDA-PCa2b cells (Fig. 5d). Taken together, these data indicate that increased cell motility is a basic requirement for primary prostatic tumor cells to acquire invasive phenotype and ROBO1 by controlling DOCK1/Rac1 keeps undercheck the motility of primary cells.
Discussion
Studies show that a high proportion of men initially presenting with apparently localized CaP-disease have undetectable metastases.1,2 Without treatment nearly one third of stage II CaP patients with localized-disease can be expected to develop distant metastasis.1–3 Clinicians treating CaP diseases face major challenges such as lack of definitive tests and reliable biomarkers which could determine if an individual presenting with localized prostate malignancy will go on to develop metastatic disease . This study shows that ROBO1 transmembrane protein is a molecular switch which when turned-off triggers the migration of primary tumor cells thus could be utilized as a (i) biomarker to discriminate organ-confined CaP from metastatic phenotype, and (ii) molecular target for treating metastatic CaP in African-Americans.
Dickinson et al.27 showed that ROBO system is expressed during sheep fetal ovary development and ROBO1 protein was localized to pregranulosa cells in the primordial follicle.28 ROBO1 has recently found prominence because of its role in organogenesis and developmental diseases8,9 Relevance of presence or deficiency of ROBO1 in normal tissues has been described.8,9 Aberrations in ROBO1 expression are reported to cause defects during lung development in mouse models.28 Not much is known about the role of ROBO1 in CaP except a study by Latil et al. which showed mRNA expression of ROBO1 in primary prostatic tissue.29 Current study provides information about the transcriptional as well as translational level of ROBO1 in ethnic group-based (i) CaP progressive models, and (ii) prostatic tissues. We provide evidence that transcriptional and translational levels of ROBO1 are decreased during CaP development in men belonging to Caucasian and African-American races. However, the important finding of this study is that ROBO1 expression could be specifically highly indicative of metastasis development in African-American CaP patients. This is based on our observation that translational levels of ROBO1 are remarkably different between prostate-localized (primary) and metastatic CaP in African-Americans (Figs. 1 and 2). Our data indicate that ROBO1 has the potential to provide some prediction whether the CaP disease has traits of being invasive or meta-static. There is not much literature about the specific localization of ROBO1 protein within cells. Seki et al. reported that ROBO1 undergoes protease-mediated cleavage and cleaved products localize to various sub-cellular compartments such as nucleus in tumor cells.30 Our study shows that ROBO1 protein is localized to the plasma membrane of CaP cells. The antibodies used in our studies were specific to full-length ROBO1 therefore could not detect nuclear localization of cleaved product, if any. The artificial modulation of ROBO1 expression in CaP cells validated our findings. The mechanism that regulates ROBO1 protein localization to cell membrane is not completely understood though it warrants further investigation that was beyond the scope of this study. Role of ROBO1 in cell migration during organogenesis is well established.8,9 This study showed that loss of ROBO1 confers invasive phenotype to indolent tumor cells and reactivation of this gene reduces migratory potential of metastatic cells. This study suggests that ROBO1 is a natural inhibitor of metastasis which is lost in invasive cells, thus offers an opportunity to develop novel therapies targeting this molecule for treating metastatic CaP.
DOCK1 is reported to bind to surface proteins (Such as Her2) known for their role in cell migration.31 Using a yeast-two hybrid assay and 293T cells, Fan et al. showed a physical interaction of ROBO1 with DOCK1, and demonstrated that SH3-domains of DOCK1 protein are required for ROBO1 interactions.21 Consistent with this report, our finding showed the interaction of ROBO1 with DOCK1 in CaP cells and is the first report in CaP models and prostatic tissues (Fig. 3). Our interpretation of this data is that DOCK1 has the potential to induce migration, and ROBO1 by forming a tight complex restricts the DOCK1 activity in primary cells. We speculate that loss of ROBO1 lifts the restrictions on DOCK1 DOCK1 is reported to be inducer of Rac1 protein which is known to induce motility of cells.22,32 Sequeira et al. showed that Rac1 is required for metastasis of CaP cells.32 Engers et al. showed a statistically significant association between increased Rac1 levels, Gleason score and decreased survival in CaP patients.33 This study provides an insight into the regulation of Rac1 by ROBO1 in CaP cells. Our interpretation of this data is that the loss of ROBO1 lifts restrictions over DOCK1 that in turn activates Rac1. There have been attempts to develop Rac1 inhibitors for cancer treatment; however, homology with RhoGTPase family is a major limitation to develop Rac1-specific drug.34 The clinically significant finding of this study is that suppression of DOCK1 was observed to reduce the activity of Rac1 in indolent CaP cells, therefore identifying DOCK1 suppression and ROBO1-reactivation as future strategies to treat metastatic CaP in African-Americans. E-cadherin is reported to maintain the intercellular junctions and cytoskeleton stability by tight physical interactions with catenin molecules.23 Rac1 activation is reported to drive disassembly of E-Cadherin based intercellular junctions.24 Several studies showed the loss of E-Cadherin as an important event for CaP progression.35 We reported a progressive loss of E-cadherin during developmental stages of CaP in TRAMP mice, an autochthonous model of CaP.36 Current study shows that ROBO1 is a critical regulator of E-Cadherin stability in CaP cells. Although no physical association between ROBO1 and E-Cadherin was found, a strong positive correlation between ROBO1 and E-Cadherin mediated by DOCK1/Rac1 was identified in cells. A noticeable finding was that activation of ROBO1 was observed to increase E-Cadherin levels suggesting that ROBO1 by limiting DOCK1/Rac1 pathway maintains stability of E-cadherin with cytoskeleton molecules. For cytoskelton stability, the E-cadherin binds to actins and catenins.23 Disintegration of E-Cadherin/β-catenin complex leads to the generation of free β-catenin that subsequently translocates to the nucleus as a transcriptional co-activators.24,37 We previously reported that CaP cells exhibit elevated β-catenin signaling.24 This study identified ROBO1 as a suppressor of β-catenin signaling and suggest the maintenance of cytoskeleton stability a major function of ROBO1 in primary CaP cells. Chang et al. also reported an association between ROBO1 and β-catenin in breast cancer patients.38 Our study is a proof of concept that reactivation of ROBO1 could restore E-Cadherin/β-catenin balance, block activation of β-catenin signaling and inhibit invasiveness of metastatic cells. Taken together, we suggest that ROBO1 could be developed (a) as a predictive marker that discriminates between indolent and metastatic CaP, and (b) as a future molecular target for therapeutics to control metastasis in high-risk CaP patients such as African-Americans. Further investigations should be conducted in relevant in vivo models.
Supplementary Material
What’s new?
While African-American prostate-cancer patients are known to have an increased risk of developing aggressive, metastatic disease, the molecular mechanisms are not well understood. In this study, the authors found that ROBO1 acts as a tumor-suppressor gene, and is expressed differently in primary vs metastatic tumors in African-American patients. ROBO1 may thus provide a valuable predictive biomarker for distinguishing between indolent and metastatic prostate cancer in high-risk populations. It may also provide a useful therapeutic target for metastatic CaP in these patients.
Acknowledgements
The authors acknowledge the technical support provided by Alyssa Langfald and Neelofar Jan. They are thankful to Greg J. Bashaw (Department of Neuroscience, University of Pennsylvania) for providing us the ROBO1-mutant plasmids. The major funding for this project was provided by The Hormel Institute (as institutional start-up funds) University of Minnesota (to M.S.) and Prostate Cancer Gift Fund (to B.R.K.). The co-author (S.K.) is supported by PHS grants P30CA016056 and R21CA143589.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate cancer Intervention Versus Observation Trial:VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30:81–87. doi: 10.1016/j.cct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11:14–23. [PMC free article] [PubMed] [Google Scholar]
- 3.Thatai LC, Banerjee M, Lai Z, et al. Racial disparity in clinical course and outcome of metastatic androgen-independent prostate cancer. Urology. 2004;64:738–7343. doi: 10.1016/j.urology.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Reddy S, Shapiro M, Morton R, Jr, et al. Prostate cancer in black and white Americans. Cancer Metastasis Rev. 2003;22:83–86. doi: 10.1023/a:1022216119066. [DOI] [PubMed] [Google Scholar]
- 5.Powell IJ, Bollig-Fischer A. Minireview: the molecular and genomic basis for prostate cancer health disparities. Mol Endocrinol. 2013;27:879–891. doi: 10.1210/me.2013-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24:410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehlen P, Delloye-Bourgeois C, Chedotal A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer. 2011;11:188–196. doi: 10.1038/nrc3005. [DOI] [PubMed] [Google Scholar]
- 8.Gonda Y, Andrews WD, Tabata H, et al. Robo1 regulates the migration and laminar distribution of upper-layer pyramidal neurons of the cerebral cortex. Cereb Cortex. 2013;23:1495–1508. doi: 10.1093/cercor/bhs141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Bendito G, Flames N, Ma L, et al. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci. 2007;27:3395–3406. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khusial PR, Vadla B, Krishnan H, et al. Src activates Abl to augment Robo1 expression in order to promote tumor cell migration. Oncotarget. 2010;1:198–209. doi: 10.18632/oncotarget.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koochekpour S, Maresh GA, Katner A, et al. Establishment and characterization of a primary androgen-responsive African-American prostate cancer cell line, E006AA. Prostate. 2004;60:141–152. doi: 10.1002/pros.20053. [DOI] [PubMed] [Google Scholar]
- 12.Theodore S, Sharp S, Zhou J, et al. Establishment and characterization of a pair of non-malignant and malignant tumor derived cell lines from an African American prostate cancer patient. Int J Oncol. 2010;37:1477–1482. doi: 10.3892/ijo_00000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navone NM, Olive M, Ozen M, et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res. 1997;3:2493–2500. [PubMed] [Google Scholar]
- 14.Siddique HR, Parray A, Tarapore RS, et al. BMI1 polycomb group protein acts as a master switch for growth and death of tumor cells: regulates TCF4-transcriptional factor-induced BCL2 signaling. PLoS One. 2013;85:e60664. doi: 10.1371/journal.pone.0060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddique HR, Adhami VM, Parray A, et al. The S100A4 oncoprotein promotes prostate tumorigenesis in a transgenic mouse model: regulating NFjB through the RAGE receptor. Genes Cancer. 2013;4:224–234. doi: 10.1177/1947601913492420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoud AM, Zhu T, Parray A, et al. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS One. 2013;8:e78479. doi: 10.1371/journal.pone.0078479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dallol A, Forgacs E, Martinez A, et al. Tumour specific promoter region methylation of the human homologue of the Drosophila Roundabout gene DUTT1 (ROBO1 in human cancers. Oncogene. 2002;21:3020–3028. doi: 10.1038/sj.onc.1205421. [DOI] [PubMed] [Google Scholar]
- 18.Saleem M, Adhami VM, Zhong W, et al. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev. 2006;15:217–226. doi: 10.1158/1055-9965.EPI-05-0737. [DOI] [PubMed] [Google Scholar]
- 19.Siddique HR, Parray A, Zhong W, et al. BMI1, stem cell factor acting as novel serum-biomarker for Caucasian and African-American prostate cancer. PLoS One. 2013;8:e52993. doi: 10.1371/journal.pone.0052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkar S, Goldgar S, Byler S, et al. Demethylation and re-expression of epigenetically silenced tumor suppressor genes: sensitization of cancer cells by combination therapy. Epigenomics. 2013;5:87–94. doi: 10.2217/epi.12.68. [DOI] [PubMed] [Google Scholar]
- 21.Fan X, Labrador JP, Hing H, et al. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003;40:113–126. doi: 10.1016/s0896-6273(03)00591-9. [DOI] [PubMed] [Google Scholar]
- 22.Mack NA, Whalley HJ, Castillo-Lluva S, et al. The diverse roles of Rac signaling in tumorigenesis. Cell Cycle. 2011;10:1571–1581. doi: 10.4161/cc.10.10.15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 24.Monaghan-Benson E, Burridge K. Mutant B-RAF regulates a Rac-dependent cadherin switch in melanoma. Oncogene. 2013;32:4836–4844. doi: 10.1038/onc.2012.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleem M, Murtaza I, Tarapore RS, et al. Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis. 2009;30:808–816. doi: 10.1093/carcin/bgp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson DR, Zylstra CR, Williams BO. Wnt signaling and prostate cancer. Curr Drug Targets. 2008;9:571–580. doi: 10.2174/138945008784911831. [DOI] [PubMed] [Google Scholar]
- 27.Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction. 2010;139:697–704. doi: 10.1530/REP-10-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domyan ET, Branchfield K, Gibson DA, et al. Roundabout receptors are critical for foregut separation from the body wall. Dev Cell. 2013;24:52–63. doi: 10.1016/j.devcel.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latil A, Chêne L, Cochant-Priollet B, et al. Quantification of expression of netrins, slits and their receptors in human prostate tumors. Int J Cancer. 2003;103:306–315. doi: 10.1002/ijc.10821. [DOI] [PubMed] [Google Scholar]
- 30.Seki M, Watanabe A, Enomoto S, et al. Human ROBO1 is cleaved by metalloproteinases and gamma-secretase and migrates to the nucleus in cancer cells. FEBS Lett. 2010;584:2909–2915. doi: 10.1016/j.febslet.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Laurin M, Huber J, Pelletier A, et al. Rac-specific guanine nucleotide exchange factor DOCK1 is a critical regulator of HER2-mediated breast cancer metastasis. Proc Natl Acad Sci USA. 2013;110:7434–7439. doi: 10.1073/pnas.1213050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sequeira L, Dubyk CW, Riesenberger TA, et al. Rho GTPases in PC-3 prostate cancer cell morphology, invasion and tumor cell diapedesis. Clin Exp Metastasis. 2008;25:569–579. doi: 10.1007/s10585-008-9173-3. [DOI] [PubMed] [Google Scholar]
- 33.Engers R, Ziegler S, Mueller M, et al. Prognostic relevance of increased Rac GTPase expression in prostate carcinomas. Endocr Relat Cancer. 2007;14:245–256. doi: 10.1677/ERC-06-0036. [DOI] [PubMed] [Google Scholar]
- 34.Bidaud-Meynard A, Arma D, Taouji S, et al. A novel small-molecule screening strategy identifies mitoxantrone as a RhoGTPase inhibitor. Biochem J. 2013;450:55–62. doi: 10.1042/BJ20120572. [DOI] [PubMed] [Google Scholar]
- 35.Mol AJ, Geldof AA, Meijer GA, et al. New experimental markers for early detection of high-risk prostate cancer: role of cell-cell adhesion and cell migration. J Cancer Res Clin Oncol. 2007;133:687–695. doi: 10.1007/s00432-007-0235-8. [DOI] [PubMed] [Google Scholar]
- 36.Saleem M, Adhami VM, Ahmad N, et al. Prognostic significance of metastasis-associated protein S100A4 (Mts1) in prostate cancer progression and chemoprevention regimens in an autochthonous mouse model. Clin Cancer Res. 2005;11:147–153. [PubMed] [Google Scholar]
- 37.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Chang PH, Hwang-Verslues WW, Chang YC, et al. Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/β-catenin pathway. Cancer Res. 2012;72:4652–4661. doi: 10.1158/0008-5472.CAN-12-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.