Abstract
Background
Live oral rotavirus vaccines have been less immunogenic and efficacious for children of developing countries than for those in middle income and industrialized countries, and the basis for these differences is not fully understood. Recently, we demonstrated that breastmilk from mothers in India had significantly higher IgA and neutralizing activity against rotavirus that could reduce the effective titer of rotavirus vaccines reaching the gut when compared with that from mothers in the United States. We extended our study to understand the specific contribution of those nonanti-body components in breastmilk to the neutralizing activity against rotavirus vaccine we observed.
Methods
Breastmilk samples were collected from mothers of breastfeeding infants aged between 4 and 29 weeks (ie, vaccine eligible age) in India (N = 40), South Africa (N = 50) and the United States (N = 51). We examined breastmilk for lactoferrin, lactadherin, rotavirus-specific IgA and neutralizing activity against 3 rotavirus vaccine strains (Rotarix, RotaTeq G1 and 116E) using enzyme immunoassays, a plaque reduction assay or a microneutralization assay.
Results
We observed higher levels of lactoferrin, lactadherin, IgA and neutralizing activity in breastmilk specimens from Indian and South African women than those from American women. We demonstrated positive associations between levels of lactoferrin or IgA and neutralizing activity in Indian and South African specimens, but not in American specimens. We demonstrated that the inhibitory effect of lactoferrin was dose- or species-dependent, as evidenced by greater reduction in titer of Rotarix and 116E by human lactoferrin. Lactadherin also exhibited inhibitory activity to rotavirus vaccines but appeared to be less effective.
Conclusions
The lower immunogenicity and efficacy of rotavirus vaccines in developing countries could be explained, in part, by synergistic inhibitory effect of high levels of antibody and nonantibody components in breastmilk consumed by infants at the time of immunization. Therefore, there is a need for alternative rotavirus vaccine strategies in breast-feeding populations.
Keywords: rotavirus, breastmilk, lactoferrin, lactadherin, vaccine
Rotavirus is the most common cause of severe diarrhea in children less than 5 years of age. Rotavirus infection causes an estimated 453,000 deaths per year worldwide, with more than 85% of these deaths occurring in resource-limited countries.1 In 2006 and 2009, World Health Organization recommended use of the 2 licensed vaccines, a monovalent attenuated human rotavirus vaccine (Rotarix) and a pentavalent bovine-human rotavirus reassortant vaccine (RotaTeq). Both vaccines showed high efficacy (85%–98%) against severe diarrhea and hospitalization from rotavirus among young children in developed and middle income countries.2–4 However, the same vaccines were less immunogenic and have shown modest efficacy ranging from 18% to 64% in resource-limited countries of Africa, Asia and Latin America.5–10
The mechanisms underlying the lower immunogenicity and efficacy of live oral rotavirus vaccines in developing countries remain poorly understood but are likely to be multifactorial, including the inhibitory effects of immune factors in breastmilk at the time of immunization.11,12 However, results from clinical trials of early candidate and recently licensed vaccines have been controversial. Early studies reported that breast-feeding appeared to reduce serococonversion rates among American and Finnish infants who received RIT 4237 bovine rotavirus vaccine or rhesus rotavirus vaccine (RRV).13–15 Subsequent studies showed similar responses and protection in breastfed and nonbreastfed US infants who received rhesus rotavirus reassortant vaccine (RotaShield) or pentavalent vaccine RotaTeq.16,17 However, a recent study has demonstrated a small reduction in immunogenicity and efficacy of Rotarix in breastfed European infants compared with those who exclusively formula-fed.18 Effects of breastmilk and breast-feeding on efficacy of rotavirus vaccines in infants of the developing world have not been evaluated.
We recently reported significantly higher IgA titer and neutralizing activity and greater inhibitory effect on rotavirus vaccines in breastmilk from women in India than those in the United States.19 In the present study, we extended our study to examine profiles of both rotavirus-specific IgA and nonspecific innate immune factors in breastmilk from mothers of developed and resource-limited countries and investigate their possible associations with neutralizing activity in breastmilk. Innate immune factors such as lactoferrin, lactadherin, mucin and butyrophilin in breastmilk have previously shown to inhibit the replication of animal and human rotaviruses in intestinal epithelial cells.20,21 These factors have also been reported to mediate protection against symptomatic infection in infants.22,23 To date, we know of no studies that have specifically evaluated both IgA and innate immune factors in breastmilk as inhibitors of rotavirus vaccine replication and response.
MATERIALS AND METHODS
Subjects and Specimen Collection
From January 2007 to October 2008, we collected 5 to 10 mL of breastmilk from mothers in India and the United States who were breast-feeding their infants of 4 to 29 weeks, approximately the recommended age for the administration of rotavirus vaccine. South African samples were collected during the period May 2002 to March 2005 from mothers who were breast-feeding similar aged infants. Each specimen was assigned a unique identifier to maintain the anonymity of the donor and data on the ages of the mother and infant and the date of collection were recorded. All specimens were kept frozen at –70°C before being shipped and analyzed in the laboratory at the US Centers for Disease Control and Prevention. Informed consent was obtained from all participants. The protocols were reviewed and approved by the institutional review boards of each of the participating institutions. This research did not require review by the Centers for Disease Control and Prevention institutional review board because the Centers for Disease Control and Prevention tested preexisting, anonymous specimens.
Detection of Rotavirus-specific Antibody in Breastmilk
Rotavirus-specific IgA in breastmilk samples was determined by enzyme-linked immunosorbent assay as previously described.24,25 Briefly, microplate wells were coated with rabbit hyperimmune serum to rhesus rotavirus (RRV) and incubated with diluted RRV or blotto (5% skim milk in phosphate-buffered saline [PBS]); after washing, breastmilk samples that were serially diluted from 1:2 to 1:2048 in diluent buffer (PBS supplemented with 1% skim milk and 0.5 % [v/v] of 10% polyoxyethylene ether W1) were added to the wells, followed by horseradish peroxidase–conjugated goat antihuman IgA antibodies (Sigma). After incubation and washing, the reactions were developed with tetramethyl enzidine (Sigma, St. Louis, MO) and stopped with 1N HCl. Optical density (OD) was determined at 450 nm with an enzyme immunoassay reader (MRX Revelation, Dynex Technologies, Chantilly, VA). IgA titers in breastmilk were calculated as the reciprocal of the highest dilution that gave a mean OD greater than the cutoff value (3 standard deviations above the mean OD of the blotto wells).
Measurement of Nonantibody Components in Breastmilk
Lactoferrin concentrations in breastmilk specimens were determined with an enzyme-linked immunosorbent assay kit specific for human lactoferrin (Kamiya Biomedical Co., Seattle, WA). Samples were diluted 1:50,000 with diluent buffer, and lactoferrin concentrations were measured using standard curves (range: 0–100 ng/mL) according to manufacturer’s instructions. Lactadherin concentrations in breastmilk were determined from standard curves (range: 0–50 µg/mL) using a commercial enzyme-linked immunosorbent assay kit (Cusabio Biotech Co., China).
Measurement of Neutralizing Activity in Breastmilk
Overall rotavirus-specific neutralizing activity in breast-milk was measured by a microneutralization assay as previously described.24 Briefly, breastmilk samples (50 µL) in 2-fold dilutions were mixed with an equal volume of trypsin-activated vaccine virus (Rotarix, RotaTeq G1 [WC3 × WI79] or 116E) in 96-well plates to yield a concentration of 4000 fluorescence focus unit per well and incubated at 37°C for 1 hour. Monolayers of MA104 cells grown in 96-well plates were washed with PBS and incubated with 100 µL of diluted breastmilk and virus mixture. After incubation at 37°C for 1 hour, the plates were washed with PBS and incubated with 100 µL of Iscove’s Modified Dulbecco’s Medium containing 5 µg/ mL trypsin (Gibco, Grand Island, NY). After 20 hours incubation at 37°C, the plates were fixed with 15 µL of 37% formaldehyde at 4°C for 30 minutes. Rotavirus antigen in MA104 cells was detected by incubating plates with a rabbit anti-RRV hyperimmune serum, horseradish peroxidase–labeled antirabbit IgG (KPL, Gaithersburg, MD) and then tetramethyl enzidine. Neutralizing titer in a breast-milk specimen was determined as the reciprocal of the highest dilution that showed a greater than 70% reduction in the absorbance value compared with that in virus-only controls.
Neutralizing activity against rotavirus vaccine strains by lactoferrin and lactadherin was assessed in cell culture by a plaque reduction assay or a microneutralization assay. Human lactoferrin and bovine lactoferrin (both from Sigma) were dissolved in H2O (1 mg/mL), and human lactadherin (R&D systems, Inc., Minneapolis, MN) was reconstituted in PBS (200 µg/mL). Approximately 100 plaque forming units of trypsin-activated vaccine virus (Rotarix, RotaTeq G1 or 116E) in 100 µL of culture medium was incubated with an equal volume of serially diluted lactoferrin and lactadherin (100, 50, 25, 12.5 and 6.25 µg) for 1 hour at 37°C. The mixtures were placed on monolayers of MA104 cells in 6-well plates and after 1 hour incubation, the wells were overlaid with 0.3% ME agarose (Lonza, Rockland, ME) in Iscove’s Modified Dulbecco’s Medium (Gibco) containing 5 µg/mL of trypsin. The plates were then stained with 2% neutral red (Sigma), and rotavirus plaques in triplicate wells were counted. The percent reduction in virus titer was calculated by comparing the numbers of plaques in wells of virus-lactoferrin or virus-lactadherin mixtures to those of virus-only controls. For microneutralization assay, lactoferrin (50 µL) and lactadherin (50 µL) in 2-fold dilutions (100, 50, 25, 12.5, 6.25, 3.13, 1.6, 0.78, 0.39 and 0.19 µg/well) were mixed with an equal volume of trypsin-activated vaccine virus (Rotarix, RotaTeq G1 or 116E) to yield a concentration of 4000 fluorescence focus unit per well and incubated at 37°C for 1 hour. Monolayers of MA104 cells grown in 96-well plates were washed with PBS and incubated with diluted lactoferrin- or lactadherin-virus mixture. After incubation at 37°C for 1 hour, the plates were washed with PBS and incubated with 100 µL of Iscove’s Modified Dulbecco’s Medium (Gibco) containing 5 µg/mL trypsin. After 20 hours incubation at 37°C, the plates were fixed with 15 µL of 37% formaldehyde at 4°C for 30 minutes. Rotavirus antigen in MA104 cells was detected by incubating plates with a rabbit anti-RRV hyperimmune serum, horseradish peroxidase–labeled antirabbit IgG and tetramethyl enzidine and then stopped with 1N HCl. OD was determined at 450 nm with an enzyme immunoassay reader. Inhibitory effect of lactoferrin and lactadherin on rotavirus vaccine strains was determined as the reduction in absorbance value in treated samples compared with that in virus-only controls.
Statistical Analysis
The Kruskal–Wallis test was used to analyze the differences in concentration of nonantibody components among breastmilk specimens from mothers in different countries. The Kolmogorov– Smirnov goodness-of-fit test was used to compare the distributions of antibody titers in breastmilk specimens from each country. Univariate and multivariable linear regression analyses were performed to examine the relationships between neutralizing activity and titer of IgA or concentration of lactoferrin or lactadherin in breastmilk specimens. Log transformed (log2) titers of neutralizing activity and IgA were used in the regression models. Regression models were assessed for violations of assumptions. When the assumptions of the regression model were not met, Spearman rank correlations were used in univariate analyses and multivariate analyses were not performed. A P < 0.05 was considered statistically significant. The calculations were performed with the PASW Statistics 18.0 (IBM, Chicago, IL).
RESULTS
A total of 141 breastmilk specimens were collected from mothers with infants of 4 to 29 weeks of age in India (n = 40), South Africa (n = 50) and the United States (n = 51). The age of American and South African women ranged from 16 to 41 years (median age of 35; range: 16–41 years) versus (median age of 22; range: 16–40 years), respectively. The age information of Indian women was not available. The age of infants from 3 countries ranged from 1.0 to 6.6 months, with a median of 1.5 (range: 1–1.8 months), 2.8 (range: 1.4–6.6 months) and 3.1 (range: 1.2–5.7 months) for South Africa, United States and India, respectively.
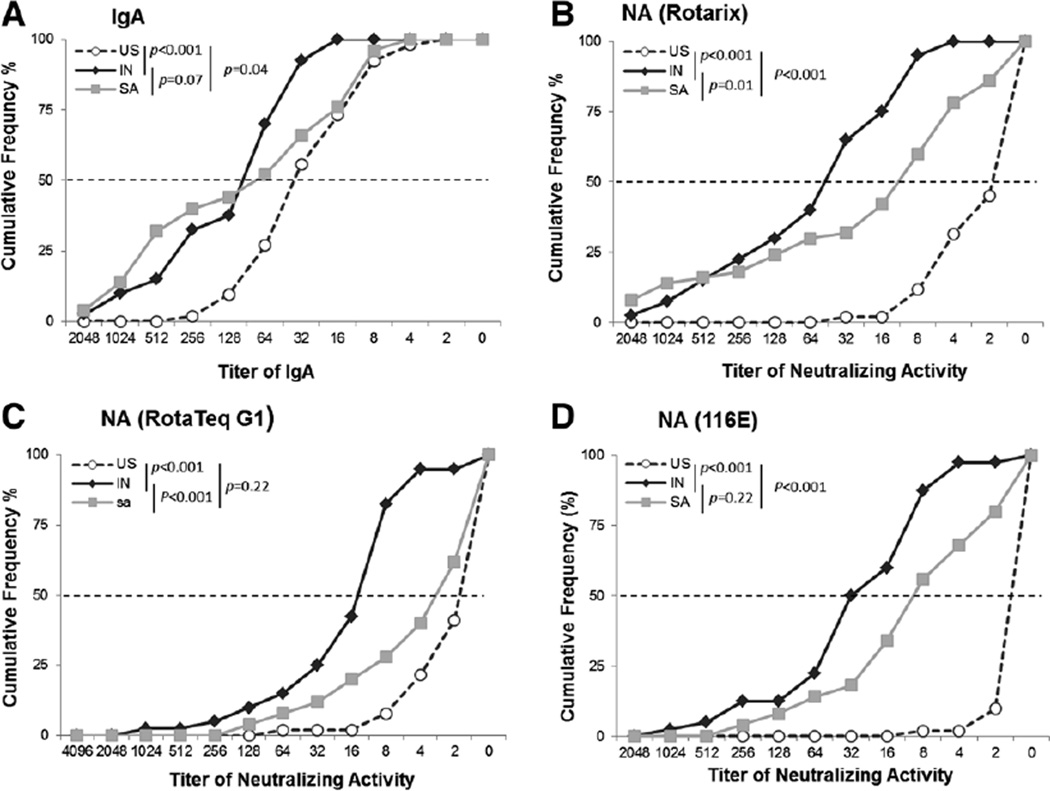
We extended our previous study by examining breastmilk specimens from South African mothers for rotavirus-specific IgA and overall neutralizing activity against 3 rotavirus vaccine strains, Rotarix, RotaTeq G1 and 116E (Fig. 1). Cumulative frequency of IgA titers in breastmilk specimens from South African women fell between those from Indian and American women but was more similar to that of Indian specimens (P = 0.07). IgA titers in Indian and South African samples were as much as 4-fold higher than those from American mothers (P < 0.05). Breastmilk showed gradient cumulative frequencies in neutralizing activity to all 3 vaccine strains, with the highest, intermediate and lowest titers seen in specimens from India, South Africa and the United States, respectively.
FIGURE 1.
Cumulative frequency profiles of rotavirus-specific antibodies in breastmilk specimens from mothers in India (IN), South Africa (SA) and the United States (US). Milk specimens were tested for IgA (A) and neutralizing activity (NA) against vaccine strains Rotarix (B), RotaTeq G1 (C) and 116E (D) as described in the text.
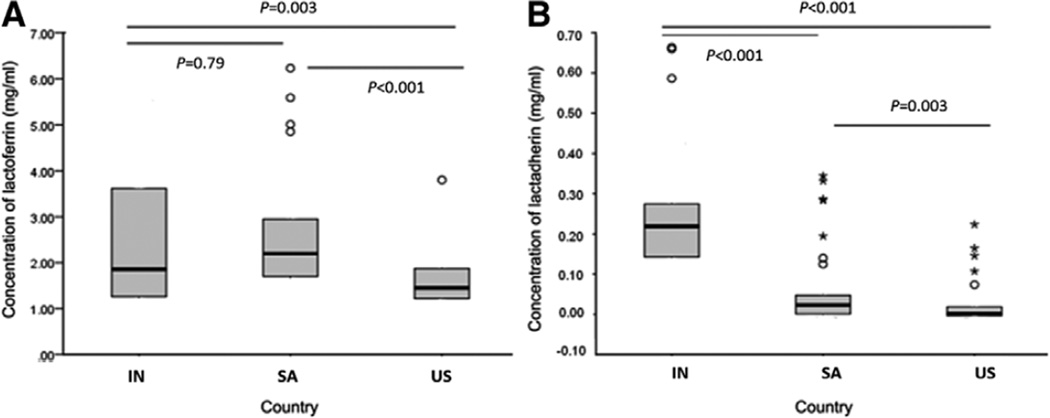
We measured the concentrations of lactoferrin and lactadherin in breastmilk from women in India, South Africa and the United States (Fig. 2). The median concentration of lactoferrin in specimens from Indian, South African and American women was 1.85 mg/mL (range: 0.56–5.52 mg/mL), 2.2 mg/mL (range: 0.82–6.23 mg/mL) and 1.45 mg/mL (range: 0.42–2.67 mg/mL), respectively. The median concentration of lactadherin in specimens from Indian, South African and American mothers was 0.22 mg/ mL (range: 0.06–0.66 mg/mL), 0.02 mg/mL (range: 0–0.33 mg/ mL) and 0.002 mg/mL (range: 0–0.073 mg/mL), respectively. The levels of both lactoferrin and lactadherin in breastmilk of both Indian and South African women were significantly higher than those from women in the United States (P < 0.005). The concentrations of lactoferrin in breastmilk specimens from South African women were not significantly different from those of Indian women (P = 0.79), whereas the concentrations of lactadherin in breastmilk specimens from South African mothers were significantly lower than those from Indian mothers (P < 0.001).
FIGURE 2.
Box plots (median, quartiles) of the concentration quartiles of lactoferrin (A) and lactadherin (B) in breastmilk from mothers in India (IN), South Africa (SA) and the United States (US). Milk specimens were tested for lactoferrin and lactadherin as described in the text.
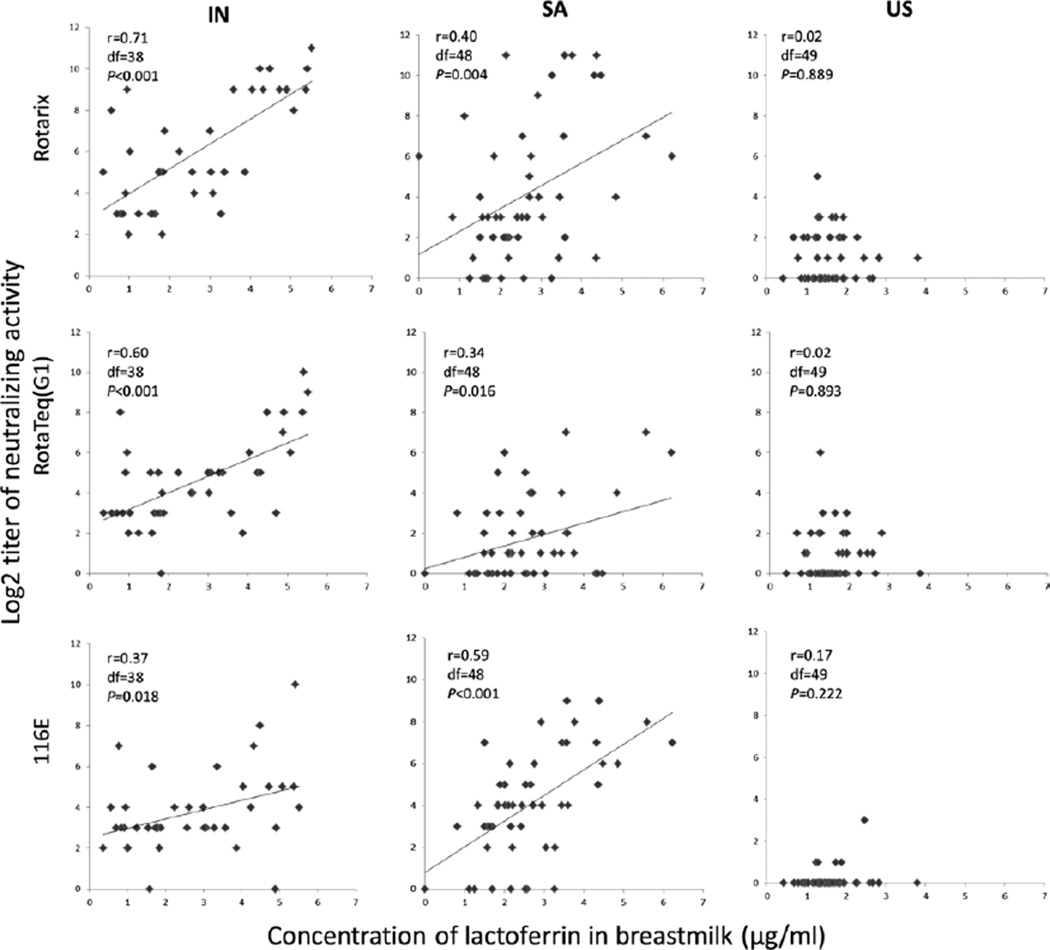
We then analyzed possible associations between the concentrations of lactoferrin or lactadherin and the levels of neutralizing activity in breastmilk against rotavirus vaccine strains (Fig. 3). In univariate analyses, concentrations of lactoferrin in breastmilk from Indian mothers were significantly associated with neutralizing activity against Rotarix (r = 0.71, df = 38, P < 0.001), RotaTeq G1 (r = 0.60, df = 38, P < 0.001) and 116E (r = 0.37, df= 38, P = 0.018). Similarly, the concentrations of lactoferrin in specimens from South African women had a weak but significant association with neutralizing activity against Rotarix (r = 0.40, df = 48, P = 0.004), RotaTeq G1 (r = 0.34, df = 48, P = 0.016) and 116E (r = 0.59, df = 48, P < 0.001) in univariate analyses. By contrast, no associations were observed between the levels of lactoferrin and neutralizing activity against the 3 vaccine strains in breastmilk from American mothers. Additionally, no significant associations were observed between the concentrations of lactadherin in breastmilk from India, South Africa and the United States and neutralizing activity against Rotarix and RotaTeq G1 (results not shown).
FIGURE 3.
Regression analyses of relationships between the concentrations of lactoferrin and neutralizing activity against 3 vaccine strains in breastmilk from mothers in India (IN), South Africa (SA) and the United States (US). The analyses were done as described in the text. The linear regression assumptions were not satisfied for the samples from the US, so Spearman rank correlation was used to examine the relationship between the concentrations of lactoferrin and neutralizing activity against 3 vaccine strains in breastmilk from American mothers.
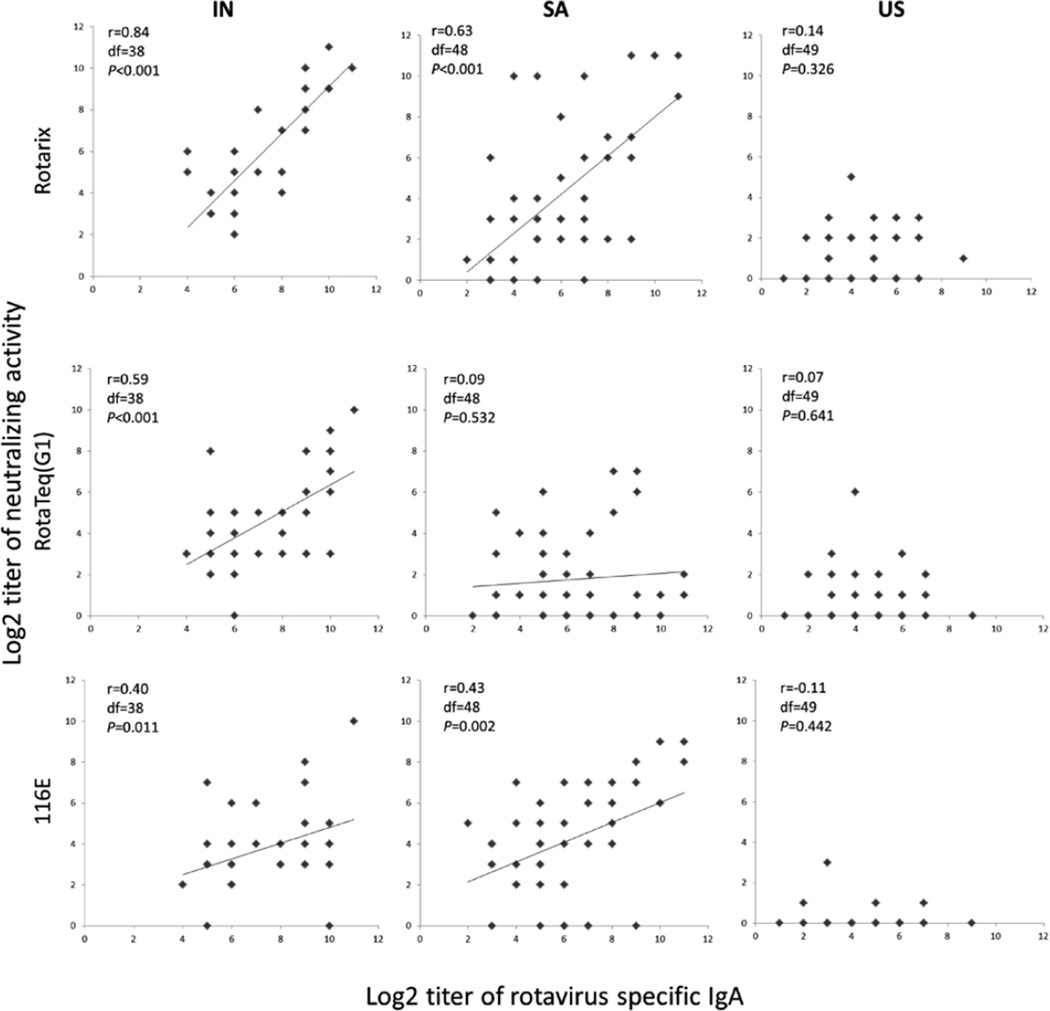
We further observed significant associations between the titers of rotavirus-specific IgA in specimens from Indian women and neutralizing activity against the 3 vaccine strains (Rotarix: r = 0.84, df = 38, P < 0.001; RotaTeq G1: r = 0.59, df = 38, P < 0.001; 116E: r = 0.40, df = 38, P = 0.011) in univariate analyses (Fig. 4). Similar associations were also observed in univariate analyses between IgA titers and neutralizing activity against Rotarix (r = 0.63, df = 48, P < 0.001) and 116E (r = 0.43, df = 48, P = 0.002) but not RotaTeq G1 (r = 0.09, df = 48, P = 0.532) in South African samples. No such associations were seen in specimens from American women.
FIGURE 4.
Regression analyses of relationships between rotavirus-specific IgA titer and neutralizing activity against 3 vaccine strains in breastmilk from mothers in India (IN), South Africa (SA) and the United States (US). The analyses were done as described in the text. The linear regression assumptions were not satisfied for the samples from the US, so Spearman rank correlation was used to examine the relationship between rotavirus-specific IgA titer and neutralizing activity against 3 vaccine strains in breastmilk from American mothers.
In multivariable regression analyses, the IgA titer in Indian women was significantly correlated with neutralizing activity against Rotarix (partial R = 0.67, df = 36, P < 0.001) but not RotaTeq G1 (partial R = 0.25, df = 36, P = 0.22) or 116E (partial R = 0.14, df = 36, P = 0.41) when the concentrations of lactoferrin and lactadherin were held constant. The concentrations of lactoferrin and lactadherin were not correlated with neutralizing activity against any of the 3 vaccine strains in Indian women in the multivariate regression model. IgA titers and concentrations of lactoferrin were highly correlated (r = 0.72, df = 38, P < 0.001) in Indian women. Among women in South Africa, the IgA titer was significantly correlated with the neutralizing activity against Rotarix (partial R = 0.56, df = 46, P < 0.001) and 116E (partial R = 0.29, df = 46, P = 0.04) and not correlated with neutralizing activity against RotaTeq (partial R = 0.02, df = 46, P = 0.88) in multivariable regression analyses when the concentrations of lactoferrin and lactadherin were held constant. The concentration of lactoferrin was correlated with neutralizing activity against 116E (partial R = 0.48, df = 46, P < 0.001) and RotaTeq G1 (partial R = 0.29, df = 46, P = 0.04) but not Rotarix (partial R = 0.27, df = 46, P = 0.07) in South African women in the multivariate regression model. The concentration of lactadherin was not predictive of neutralizing activity against any of the 3 vaccine strains in South African women in the multivariate regression model. In South African women, IgA titers and concentrations of lactoferrin were weakly correlated (r = 0.28, df = 48, P = 0.05).
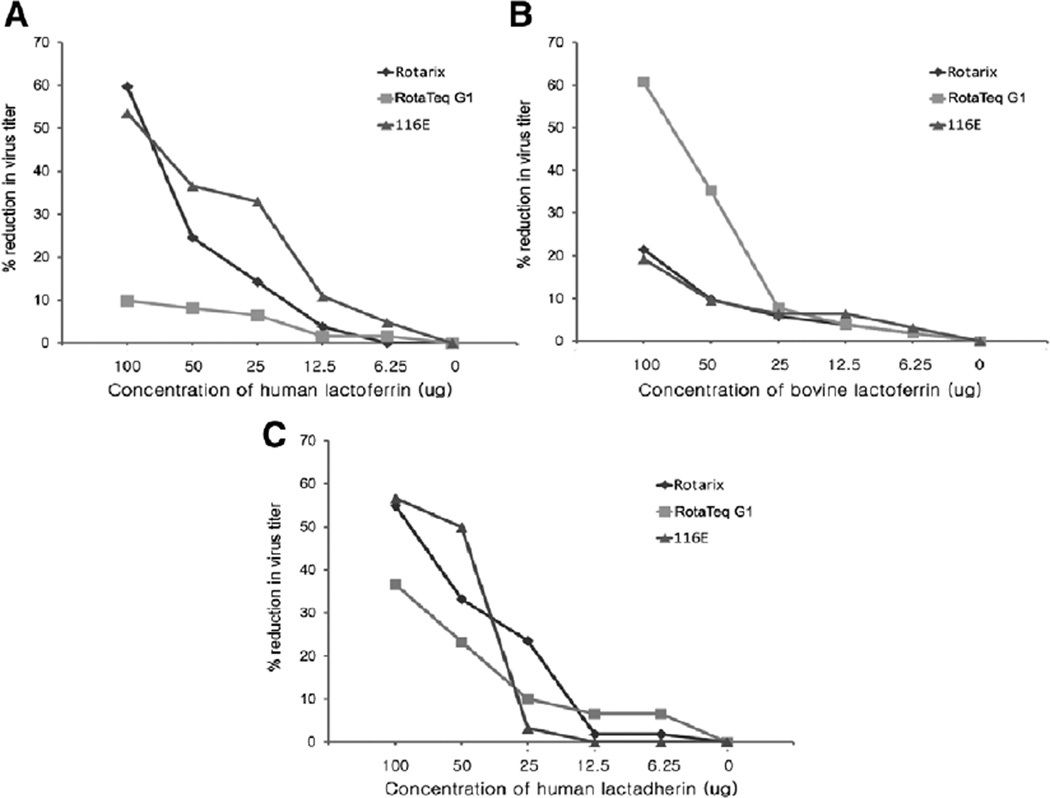
Lastly, we examined whether human lactoferrin and lactadherin could reduce the titer of rotavirus vaccine strains in MA104 cells by using a plaque reduction assay (Fig. 5). The replication of Rotarix and 116E was inhibited by human lactoferrin in a dose-dependent manner. For example, 100 µg of human lactoferrin reduced the titer of Rotarix by 60% and that of 116E by 55%. By contrast, human lactoferrin appeared to have little effect on RotaTeq G1. To test if inhibitory effect of lactoferrin on rotavirus is species-specific, we performed the same assay using bovine lactoferrin. Bovine lactoferrin showed inhibitory activity against RotaTeq G1, but appeared to be less effective against Rotarix and 116E. Human lactadherin reduced titers of all 3 vaccine strains in a dose-dependent manner. Bovine lactadherin was not available for testing.
FIGURE 5.
Inhibitory effect of lactoferrin and lactadherin on rotavirus vaccine strains: species-specific activity by a plaque reduction assay. Human lactoferrin (A), bovine lactoferrin (B) and human lactadherin (C) were assayed and analyzed as described in the text.
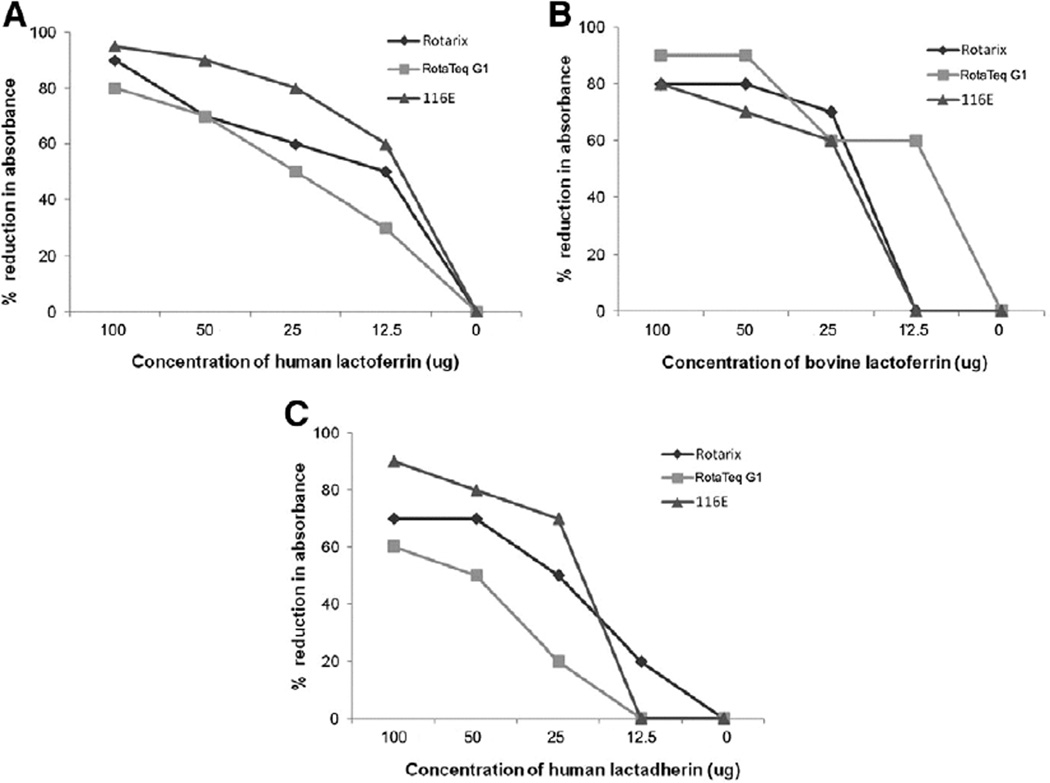
We also examined neutralizing activity of human lactoferrin and lactadherin against the 3 vaccine strains by a microneutralization assay (Fig. 6). Both human lactoferrin and lactadherin reduced the infectivity of Rotarix, RotaTeq G1 and 116E in a dose-dependent manner. However, lactoferrin appeared to be slightly more efficient. Bovine lactoferrin also showed inhibitory effect on the infectivity of all 3 vaccine strains, indicating a lack of apparent species-specific neutralizing activity by this method.
FIGURE 6.
Inhibitory effect of lactoferrin and lactadherin on rotavirus vaccine strains: no apparent species-specific activity by a microneutralization assay. Human lactoferrin (A), bovine lactoferrin (B) and human lactadherin (C) were assayed and analyzed as described in the text.
DISCUSSION
Both antibody and innate immune factors and their neutralizing activity in breastmilk have been shown to inhibit rotavirus replication in vitro and are believed to play a role in the protection against rotavirus gastroenteritis in children.11,22,23 In our previous study, we demonstrated significantly higher titers of rotavirus-specific IgA and neutralizing activity in breastmilk specimens from mothers in India than those from American women.19 That study suggested that higher levels of antibody in breastmilk may have greater reduction in the potency, immunogenicity and efficacy of live oral rotavirus vaccines among infants in resource-limited countries at the very time of vaccine administration. Similarly in this study, we demonstrated significantly higher levels of lactoferrin and lactadherin in breastmilk specimens from mothers in India and South Africa than those from the United States. We further observed significant associations between concentrations of lactoferrin and levels of IgA in breastmilk from Indian mothers. In multivariate regression analyses, levels of IgA were significantly correlated with titers of neutralizing activity against Rotarix strain in specimens from India and against Rotarix and 116E strains in specimens from South Africa. The concentration of lactoferrin was only correlated with neutralizing activity against 116E and RotaTeq G1 strains in specimens from South Africa, and the concentration of lactadherin was not predictive of neutralizing activity against any of the vaccine strains in any of the settings. Our findings show that both IgA and lactoferrin contribute to neutralizing activity against rotavirus in breastmilk of mothers in some developing country settings.
Lactoferrin exhibited inhibitory activity against rotavirus vaccine strains in vitro in a dose-dependent manner. This neutralization and reduction in titer appeared to be assay and species-dependent. By a plaque reduction assay, we found human lactoferrin inhibited the replication of the 2 human vaccine strains Rotarix and 116E and bovine lactoferrin reduced the titer of a reassortant bovine vaccine strain containing a gene encoding human rotavirus VP7. Our results were in agreement with a previous report where human lactoferrin and lactadherin showed significant dose-dependent inhibition against Wa strain, whereas bovine lactoferrin had no significant effect on Wa.21 On the other hand, we observed similar inhibition against vaccine strains with human or bovine lactoferrin by using a microneutralization assay.
The mechanisms of antirotavirus activities by lactoferrin and lactadherrin are complicated and remain poorly defined. Data from plaque reduction assays, which usually measure early events in virus–cell interactions, suggest that lactoferrin could bind to the very same viral receptor (eg, integrins) on the surface of cells in a species-specific manner and repel virus or interfere with virus–cell interactions and thus hinder its subsequent cell penetration. In addition, lactoferrin could inhibit rotavirus infection by directly binding to viral particles.26 Our findings of broad inhibition of human and bovine vaccine strains by human or bovine lactoferrin using a microneutralization assay that measures multiple steps of virus–cell interactions support a notion of indirect antiviral activities by nonantibody components after virus enters the cell. For example, lactoferrin and lactadherin could induce the activation of nature killer cells, macrophages or dendritic cells in the gut,27 thereby mobilizing and alerting the adaptive immune system to produce rotavirus-specific antibodies. Studies are needed to compare and substantiate this potential antirotavirus mechanism in children from low, middle and high income countries.
To our knowledge, this is the first study to report gradient concentrations of nonantibody components in breastmilk of mothers in developing versus developed countries. The findings suggest that innate immune factors, together with antibodies, could act independently or work synergistically to suppress the replication of oral rotavirus vaccines and in part explain the lower immunogenicity and efficacy of the currently licensed Rotarix and RotaTeq and early candidate vaccines among children in resource-limited countries of Africa, Asia and Latin America.6,9,10,28 These findings could have implications for the development of new rotavirus vaccines and ongoing immunization programs using licensed vaccines. For example, recent clinical trials of 116E, a live oral rotavirus vaccine derived from neonatal strain, demonstrated a high seroconversion (89.7%) among vaccinated infants in India.29 This robust immune response may be due to a 30-minute delay of breast-feeding before and after immunization, thus likely reducing neutralizing activity on the vaccine by antibody and nonantibody components in breast-milk.29 Additionally, the presence of bovine rotavirus VP4 in the 116E vaccine may also resist the suppressive effect by immune factors in breastmilk.30 Studies are in progress to assess whether a transient delay of breast-feeding before or after vaccine administration could improve immune response and protective efficacy of Rotarix in several developing countries.
Some limitations should be considered in interpreting our data. First, we only examined nonantibody components and IgA in breastmilk from mothers in a few countries; more specimens from other resource-limited African, Asian and Latin American countries should be examined to fully assess differential profiles and potential impact on the performance of live oral rotavirus vaccines in different settings. Second, although high levels of antibody and associated neutralizing activity in breastmilk of mothers in resource-limited countries are believed to result from frequent exposures to rotavirus, we need to establish whether such associations can be extrapolated to innate immune factors such as lactoferrin and lactadherin. Further studies are needed to investigate the effects of rotavirus infection or exposure and environmental conditions on the innate immune factors among mothers and children in resource-limited countries. Third, lactoferrin or lactadherin and antibody are critical components of innate and adaptive immunity and are known to play an important role in protection against bacterial and viral infections. Although our study shows inhibitory effect of breastmilk on rotavirus vaccines, it is essential that in resource-limited settings, this finding does not fuel a perception that breast-feeding may be harmful. In such settings, it is important to promote exclusive breast-feeding for the first 6 months of the infant’s life as an important infant survival strategy.31 Lastly, to avoid this potential inhibitory effect of breast-feeding on the response to live rotavirus vaccine, it is important that alternative approaches to oral vaccination, such as inactivated vaccines, need to be accelerated as an insurance policy for global immunization against rotavirus.28
ACKNOWLEDGMENTS
We thank Dr. Andi Shane for collaborating in the transport of breastmilk specimens from South Africa and Drs. H. F. Clark, Guillermo M. Ruiz-Palacios and Jon R. Gentsch for providing RotaTeq G1 (WC3–WI79), Rotarix and 116E strains, respectively, used in this study.
Footnotes
The finding and conclusions in this report are those of the authors and do not necessarily represent the views of Centers for Disease Control and Prevention.
S.-S.M. and B.J. contributed to the study design. S.-S.M., J.E.T., R.I.G., U.P. and B.J. contributed to the implementation of the study and the preparation of the article. P.R. was the principal investigator for India. P.H.D. was the principal investigator for United States; D.A. and A.C. were the principal investigators for South Africa. R.B. and M.-L.N. were coinvestigators on the original South African study and contributed to this project with data and selection of samples. All authors reviewed and approved the final version of the report.
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Tate JE, Burton AH, Boschi-Pinto C, et al. WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 2.Vesikari T, Karvonen A, Puustinen L, et al. Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J. 2004;23:937–943. doi: 10.1097/01.inf.0000141722.10130.50. [DOI] [PubMed] [Google Scholar]
- 3.Vesikari T, Matson DO, Dennehy P, et al. Rotavirus Efficacy Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 5.Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 6.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 7.Zaman K, Sack DA, Yunus M, et al. Bangladeshi Rotavirus Vaccine study group. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27:1333–1339. doi: 10.1016/j.vaccine.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 8.Narang A, Bose A, Pandit AN, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin. 2009;5:414–419. doi: 10.4161/hv.5.6.8176. [DOI] [PubMed] [Google Scholar]
- 9.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 10.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 11.Glass RI, Ing DJ, Stoll BJ, et al. Immune response to rotavirus vaccines among breast-fed and nonbreast-fed children. In: Mestecky Jea, editor. Milk Immunology of the Neonate. New York: Plenum Press; 1991. pp. 249–254. [DOI] [PubMed] [Google Scholar]
- 12.Patel M, Shane AL, Parashar UD, et al. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200(suppl 1):S39–S48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vesikari T, Isolauri E, Delem A, et al. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985;107:189–194. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- 14.Pichichero ME, Losonsky GA, Rennels MB, et al. Effect of dose a comparison of measures of vaccine take for oral rhesus rotavirus vaccine. The Maryland Clinical Studies Group. Pediatr Infect Dis J. 1990;9:339–344. doi: 10.1097/00006454-199005000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Pichichero ME. Effect of breast-feeding on oral rhesus rotavirus vaccine seroconversion: a metaanalysis. J Infect Dis. 1990;162:753–755. doi: 10.1093/infdis/162.3.753. [DOI] [PubMed] [Google Scholar]
- 16.Rennels MB, Wasserman SS, Glass RI, et al. Comparison of immunogenicity and efficacy of rhesus rotavirus reassortant vaccines in breastfed and nonbreastfed children. US Rotavirus Vaccine Efficacy Group. Pediatrics. 1995;96:1132–1136. [PubMed] [Google Scholar]
- 17.Goveia MG, DiNubile MJ, Dallas MJ, et al. REST Study Team. Efficacy of pentavalent human-bovine (WC3) reassortant rotavirus vaccine based on breastfeeding frequency. Pediatr Infect Dis J. 2008;27:656–658. doi: 10.1097/INF.0b013e318168d29e. [DOI] [PubMed] [Google Scholar]
- 18.Vesikari T, Prymula R, Schuster V, et al. Efficacy and immunogenicity of live-attenuated human rotavirus vaccine in breast-fed and formula-fed European infants. Pediatr Infect Dis J. 2012;31:509–513. doi: 10.1097/INF.0b013e3182489cac. [DOI] [PubMed] [Google Scholar]
- 19.Moon SS, Wang Y, Shane AL, et al. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J. 2010;29:919–923. doi: 10.1097/INF.0b013e3181e232ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Superti F, Ammendolia MG, Valenti P, et al. Antirotaviral activity of milk proteins: lactoferrin prevents rotavirus infection in the enterocyte-like cell line HT-29. Med Microbiol Immunol. 1997;186:83–91. doi: 10.1007/s004300050049. [DOI] [PubMed] [Google Scholar]
- 21.Kvistgaard AS, Pallesen LT, Arias CF, et al. Inhibitory effects of human and bovine milk constituents on rotavirus infections. J Dairy Sci. 2004;87:4088–4096. doi: 10.3168/jds.S0022-0302(04)73551-1. [DOI] [PubMed] [Google Scholar]
- 22.Newburg DS, Peterson JA, Ruiz-Palacios GM, et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet. 1998;351:1160–1164. doi: 10.1016/s0140-6736(97)10322-1. [DOI] [PubMed] [Google Scholar]
- 23.Yolken RH, Peterson JA, Vonderfecht SL, et al. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. 1992;90:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang B, Estes MK, Barone C, et al. Heterotypic protection from rotavirus infection in mice vaccinated with virus-like particles. Vaccine. 1999;17:1005–1013. doi: 10.1016/s0264-410x(98)00317-x. [DOI] [PubMed] [Google Scholar]
- 25.Jiang B, Wang Y, Saluzzo JF, et al. Immunogenicity of a thermally inactivated rotavirus vaccine in mice. Hum Vaccin. 2008;4:143–147. doi: 10.4161/hv.4.2.5263. [DOI] [PubMed] [Google Scholar]
- 26.Sojar HT, Hamada N, Genco RJ. Structures involved in the interaction of Porphyromonas gingivalis fimbriae and human lactoferrin. FEBS Lett. 1998;422:205–208. doi: 10.1016/s0014-5793(98)00002-7. [DOI] [PubMed] [Google Scholar]
- 27.Zhou YJ, Gao J, Yang HM, et al. The role of the lactadherin in promoting intestinal DCs development in vivo and vitro. Clin Dev Immunol. 2010;2010:357541. doi: 10.1155/2010/357541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine. 2008;26:6754–6758. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Bhandari N, Sharma P, Taneja S, et al. Rotavirus Vaccine Development Group. A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 2009;200:421–429. doi: 10.1086/600104. [DOI] [PubMed] [Google Scholar]
- 30.Angel J, Franco MA, Greenberg HB. Rotavirus vaccines: recent developments and future considerations. Nat Rev Microbiol. 2007;5:529–539. doi: 10.1038/nrmicro1692. [DOI] [PubMed] [Google Scholar]
- 31.Bahl R, Frost C, Kirkwood BR, et al. Infant feeding patterns and risks of death and hospitalization in the first half of infancy: multicentre cohort study. Bull World Health Organ. 2005;83:418–426. [PMC free article] [PubMed] [Google Scholar]