Abstract
Public health organizations, such as the Centers for Disease Control and Prevention (CDC), are increasingly recommending the use of N95 filtering facepiece respirators (FFRs) in health care settings. For infection control purposes, the usual practice is to discard FFRs after close contact with a patient (“single use”). However, in some situations, such as during contact with tuberculosis patients, limited FFR reuse (i.e., repeated donning and doffing of the same FFR by the same person) is practiced. A related practice, extended use, involves wearing the same FFR for multiple patient encounters without doffing. Extended use and limited FFR reuse have been recommended during infectious disease outbreaks and pandemics to conserve FFR supplies. This commentary examines CDC recommendations related to FFR extended use and limited reuse and analyzes available data from the literature to provide a relative estimate of the risks of these practices compared to single use.
Analysis of the available data and the use of disease transmission models indicate that decisions regarding whether FFR extended use or reuse should be recommended should continue to be pathogen- and event-specific. Factors to be included in developing the recommendations are the potential for the pathogen to spread via contact transmission, the potential that the event could result in or is currently causing a FFR shortage, the protection provided by FFR use, human factors, potential for self-inoculation, the potential for secondary exposures, and government policies and regulations. While recent findings largely support the previous recommendations for extended use and limited reuse in certain situations, some new cautions and limitations should be considered before issuing recommendations in the future. In general, extended use of FFRs is preferred over limited FFR reuse. Limited FFR reuse would allow the user a brief respite from extended wear times, but increases the risk of self-inoculation and preliminary data from one study suggest that some FFR models may begin to lose effectiveness after multiple donnings.
BACKGROUND
The Centers for Disease Control and Prevention (CDC)— including the National Institute for Occupational Safety and Health (NIOSH), as well as the Occupational Safety and Health Administration (OSHA) and the Food and Drug Administration (FDA)—develop regulations and/or recommendations for the use of respiratory protection in health care settings, and each agency plays a different role which impacts the use of them in health care. CDC develops recommendations for the use of respirators to reduce the spread of disease in health care settings. NIOSH certifies respirators and develops recommendations on the use of respiratory protection in health care workplaces to protect workers. OSHA develops and enforces workplace regulations on respiratory protection. FDA clears the sale of certain types of respirators as medical devices.
The most commonly used type of respirator in health care settings are NIOSH certified N95 filtering facepiece respirators (FFRs). These devices are disposable, tight-fitting air-purifying respirators that have a filter efficiency of 95% or greater for a standard test aerosol.(1) FFRs are also used by workers in many industries to reduce the amount of harmful dusts and aerosols they inhale. Workers are expected to wear their FFR during all periods of exposure. However, there are times of non-exposure when workers need to remove their FFR (e.g., take a drink of water, use the restroom, or go on a rest break) or situations during use when their FFR must be replaced.
Employers have several options for FFR usage to handle these situations. During “single use,” users put on (“don”) a new FFR each time they need one and discard their used FFR each time they take it off (“doff”). Another option is commonly referred to as “FFR reuse.” Reuse involves donning and doffing the same FFR more than once until the FFR is discarded. Employers benefit from FFR reuse compared to single use by extending the lifetime of the FFR so that fewer need to be purchased. There is no specific restriction on the number of uses or donnings. Rather, historical guidance is focused on the length of time the FFR can be used and identifying situations when the FFR should be discarded. In general, NIOSH(2) specifies that the service life of all filters on NIOSH-approved respirators is limited by considerations of hygiene, damage, and breathing resistance and that any filter should be replaced if it becomes soiled, damaged, or causes noticeably increased breathing resistance. In workplaces that could produce high cumulative particulate filter loading (i.e., >200 mg), the service time for N95 FFRs should only be extended beyond 8 hr of use (continuous or intermittent) by performing an evaluation that demonstrates that continued use will not reduce the filter efficiency.
FFR Use in Health care
FFRs have been used in industrial settings such as construction, manufacturing, and mining since the 1970s. Starting in the 1990s, these devices found new applications in health care settings.(3) Initially, FFRs were recommended as the minimum level of protection to reduce exposure to infectious aerosols from patients with tuberculosis(4–7) Later, similar recommendations(8) were made for outbreaks and pandemics involving pathogens with potential for aerosol transmission.
FFR use in health care settings has unique challenges and risks. Unlike industrial settings, some models of NIOSH-certified FFRs (commonly called “surgical N95 respirators”) are also cleared for sale by the FDA as medical devices.(9) According to the FDA's 510(k) Premarket Notification Database,(10) the first clearance for a surgical N95 respirator (product code = MSH) occurred in 1996, after FFRs were first recommended by CDC as the minimum level of protection for health care workers (HCWs) treating patients with tuberculosis(4) and NIOSH updated its certification requirements to create the N95 class of filters.(1) Most (22/31 = 71%) of the surgical N95 respirator models in the FDA database were cleared after 2005, which coincides with a period of increased interest in these types of products due to concerns about an infectious disease pandemic.
Because of the concerns that previously used FFRs may be contaminated with infectious material (i.e., act as a fomite), the factors that a health care employer considers in formulating FFR use policies (e.g., single vs. reuse) for its employees are also different from employers in industrial settings. Despite this concern, FFRs are reused under certain conditions in health care.(11) In the health care context, reuse is defined as a HCW donning the same FFR for a series of close patient contacts and doffing it at the end of each of the close patient contacts before it is discarded. Even when FFR reuse is practiced or recommended (discussed in the next section), restrictions are in place (e.g., discard when FFR is contaminated or damaged, becomes difficult to breathe through, and so on) which limits the number of times the same FFR is reused. Thus, FFR reuse is sometimes referred to as “limited FFR reuse.” Options for limited FFR reuse were provided when FFRs were first introduced as the minimum level of respiratory protection for HCWs in close contact with patients with tuberculosis.(4–6)
Another related FFR use practice, termed “extended use,” involves donning a FFR and wearing it for multiple patient encounters without doffing and redonning between patient visits. Thus, the same FFR is worn continuously (for up to several hours) across multiple patient encounters before it is doffed. This practice is only practical when bundled with the practice of cohorting, which involves locating patients with a common diagnosis in the same unit, ward, or zone. Extended use can be implemented separately from reuse (i.e., like single use, discard the FFR once it is doffed) or combined with reuse. Compared to single use and reuse, recommendations for extended use in health care are fairly recent. The first time extended use of FFRs was identified as an option was during the 2009 H1N1 pandemic.(12)
Both extended use and limited reuse of FFRs allow the employer to reduce its consumption of FFRs, prolonging existing supplies during a pandemic or respiratory pathogen outbreak or to save money and reduce waste during day-to-day operations (e.g., close contact with tuberculosis patients) by using fewer FFRs,(13) similar to the benefits found for industrial settings. This commentary examines recommendations related to extended use and limited reuse of FFRs in health care. Key scientific and policy issues are highlighted along with considerations for policy makers to weigh when making decisions on whether to recommend extended use and/or limited reuse of FFRs during routine health care situations and for public health emergencies involving respiratory pathogens that have the potential for aerosol transmission. Finally, key knowledge gaps are discussed to identify additional data needs that could enhance understanding of the risks for transmission of diseases associated with FFR extended use and limited reuse.
CURRENT AND PAST FFR EXTENDED USE AND LIMITED REUSE RECOMMENDATIONS
Table I summarizes past and current recommendations for extended use and limited reuse of FFRs. CDC recommendations were selected for this analysis because of their widespread recognition in health care. In 2007, CDC published general infection control guidance for isolation precautions, which included a list of all pathogens and medical procedures in which respiratory protection was recommended.(14) For certain pathogens affecting defined populations (e.g., TB) or infectious agents of special interest to health care (e.g., epidemiologically important organisms such as severe acute respiratory syndrome (SARS)] and influenza), CDC publishes detailed specialized infection control guidance. For this analysis, we selected all of the respiratory pathogens in which specialized infection control guidance was published as either interim or final recommendations and included the use of respiratory protection (N95 FFR or higher). This strategy provided a diversity of respiratory pathogens for analysis. These situations include two recent outbreaks/pandemics (2004 SARS and 2009 H1N1 flu), two routine situations (TB and seasonal influenza), and two pathogens of concern (Avian Influenza A (H5N1) and Avian Influenza A (H7N9)).
TABLE I. Current and Past CDC Recommendations for Limited Reuse and Extended Use of FFRs in Health Care for Select Respiratory Pathogens.
| Respiratory pathogen | Contact precautions | Possibility of contact transmissionA | Possibility of an FFR shortage | Extended use/Limited reuse recommended |
|---|---|---|---|---|
| TB | No | No | No | Yes |
| SARS | Yes | Yes | Yes | Yes |
| Avian Influenza A (H5N1) | Yes | Yes | No | No |
| 2009 H1N1 Flu | No | Yes | Yes | Yes |
| Seasonal Influenza (AGP Only) | No | Yes | No | No |
| Avian Influenza A(H7N9) | Yes | Yes | No | NoB |
AThe scientific community continues to debate the primary mode(s) of transmission for many respiratory viruses. However, most experts acknowledge that contact transmission cannot be ruled out.(101)BInterim recommendation, subject to change
Cost can be a consideration for adopting extended use and limited reuse practices as it was in adopting the recommendation to allow limited reuse of FFRs when working in close contact with TB patients. However, the CDC recommendations on limited reuse and extended use have primarily considered the specific pathogens involved and the specific characteristics of the event. The first key factor is whether contact transmission is possible for the pathogen. Contact transmission of pathogens occurs through direct or indirect contact with the patient or the patient's environment via blood or body fluids (e.g., respiratory secretions). For pathogens in which contact transmission (e.g., fomites) is not a concern, limited reuse of FFRs has been determined to be a viable option. For TB, the CDC maintains that “a respirator classified as disposable can be reused by the same HCW as long as it remains functional and is used in accordance with local infection control procedures.” (15) Infection control guidelines for TB (14) recommend only airborne precautions; contact isolation precautions are only needed if extrapulmonary lesions are draining, which occurs rarely. Contact transmission of TB is thought to be highly unlikely.(16)
This contrasts with the recommendations for seasonal influenza where contact with contaminated surfaces and objects is considered a possible mode of transmission.(17) In situations where airborne precautions are recommended, and contact precautions are recommended or contact transmission is possible, the second key factor in the CDC recommendations is the likelihood of a localized shortage of the FFRs needed to protect HCWs during high-risk procedures. The use of FFRs for protection of HCWs during routine infectious disease procedures generally does not result in a FFR shortage, as evidenced by CDC's guidance to wear a FFR during aerosol generating procedures (AGPs) on patients diagnosed with seasonal influenza; this does not include an option for FFR extended use or reuse.(17)
CDC recommendations for Avian Influenza A (H7N9)(18) indicate that FFRs should be discarded after leaving the patient room or patient care area (i.e., “single use”). CDC recommendations for Avian Influenza A (H5N1)(19) do not specifically mention single use, extended use, or limited reuse, but instead refer back to the general CDC infection control guidance(14) which specifies single use. These recommendations are consistent with the other four recommendations in Table I based on the potential for contact transmission of these pathogens and that FFR shortages are unlikely in the near-term.
However, during periods of high usage (e.g., public health emergencies such as an influenza pandemic(20) or widespread respiratory pathogen outbreak), supplies of FFRs can quickly become depleted because most hospitals maintain only a small inventory of FFRs. Not surprisingly, shortages were reported at the hospital level during both the 2004 SARS outbreak and the 2009 H1N1 influenza pandemic.(21–23) In a recent evaluation of respiratory protection programs in California hospitals, it was reported that half of the hospital managers interviewed (n = 48) reported shortages of FFRs during the 2009 H1N1 outbreak due to increased demand and supplier lag time in filling orders.(11) During the 2004 SARS and 2009 H1N1 events, recommendations were made allowing the option for extended use and limited reuse, although both recommendations acknowledged situations in which these strategies would not be appropriate.
For SARS, CDC stated in its interim guidance that “health care facilities may consider reuse as long as the device has not been obviously soiled or damaged (e.g., creased or torn)” and “if a sufficient supply of respirators is not available.”(24) The recommendation recognized the importance of preventing contamination through contact with infectious material on the outside of the respirator. CDC also addressed concerns about a shortage of FFRs during the 2009 H1N1 flu pandemic with supply-conserving strategies for hospitals that included the possibility of extended use and limited reuse of FFRs, with extended use preferred over limited reuse.(12,25) Reuse of FFRs was reported to occur quite often in California hospitals during 2009 H1N1 as either a response to shortages or as standard practice; 81% of survey respondents indicated that their hospital had a plan to implement reuse, while only 12.5% indicated plans to apply extended use.(11)
SCIENTIFIC EVIDENCE ON FFR EXTENDED USE AND LIMITED REUSE
As shown in Table I and discussed above, prior and current CDC recommendations made for FFR extended use and reuse were largely based on the type of infection control precautions or transmission mode(s) associated with that pathogen and whether shortages of FFRs were observed or anticipated. Those recommendations were based upon the data available at that time, which often lacked evidence to answer key questions regarding the effectiveness of extended use or limited reuse and the risk of disease transmission from handling potentially contaminated FFRs. In 2006, the Institute of Medicine (IOM) addressed (26) the reusability of facemasks, and summarized the data available to support previous recommendations. The committee agreed with the previous CDC guidance and recommended that “avoiding contamination [of FFRs] will allow for limited reuse.” The IOM also identified key knowledge gaps that served as a catalyst for increasing awareness of the research needs.
Since publication of the IOM report, numerous research groups have attempted to address some of these knowledge gaps. In the following sections, we discuss studies published since 2006 that address key areas of FFR extended use and reuse, including FFR protection, human factors (e.g., physiological/psychological effects), self-inoculation, and secondary exposures (e.g., from particle reaerosolization and co-conta minants). Some earlier studies (pre-2006) are also discussed to provide context where needed. The purpose of this analysis is to improve the scientific basis for future recommendations for employers in health care settings to consider when implementing FFR extended use and/or limited reuse. For each of the issues below, a qualitative assessment of the risks of extended use and limited reuse versus single use is presented (see Table II).
TABLE II. Qualitative Assessment of Increased Risks of FFR Extended Use and Limited Reuse Compared with Single Use.
| Issue | FFR Extended Use | Limited FFR Reuse |
|---|---|---|
| FFR Protection | • Negligible risk of decreased protection | • Minimal risk of decreased protection, butcan be mitigated through limiting thenumber of reuses. |
| Human Factors | • Increased discomfort, but no additionalhealth risk to a medically clearedrespirator user | • No additional health risk to a medicallycleared respirator user |
| Self-inoculation | • Minimal risk for typical patientinteractions, but can be mitigatedthrough training and education • Risks can increase during/after AGP butcan be reduced by limitingcontamination | • Moderate risk for typical patientinteractions but can be mitigatedthrough training and education andlimiting the number of reuses • Risks can increase during/after AGP butcan be reduced by limitingcontamination |
| Secondary Exposures | • Negligible for typical patient interactions • Minimal following AGP but can bereduced by limiting contamination | • Negligible for typical patient interactions • Minimal following AGP but can bereduced by limiting contamination |
FFR Protection
One possible concern with FFR extended use and reuse is that extending the useful life of a FFR could reduce its protective effectiveness (i.e., when worn properly and used in a complete respiratory protection program it provides exposure reduction consistent with the assigned protection factor for this class of respirator). The protection provided by a properly used FFR results primarily from a combination of its ability to filter out (remove) biological aerosols from the inhalation air stream of the wearer and seal tightly to the face (i.e., “fit”). Each of these concerns has been studied (to some extent) or can be assessed using existing data.
Filter Media
Most N95 FFRs contain a polypropylene electret filtering medium within the layers of a FFR (Figure 1). The electret filtering medium has been shown to capture and retain a majority of airborne biological particles compared to the layers next to the face and farthest from the face, although particle size could affect particle deposition location.(27,28) Electrets and other similar types of nonwoven air filter media are not unique to FFRs.(29) They are commonly found in various dust collection systems (e.g., vacuum cleaners, clean rooms, and home heat ventilation and air conditioning (HVAC) systems). Recommended replacement life for electret filters in air cleaning systems is typically 3 months of normal use, as the fundamental mechanisms (diffusion, interception, impaction, electrostatic, and so on) of these types of filters do not readily degrade over time with normal use.
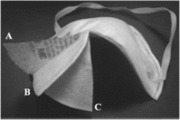
FIGURE 1. Photograph of a NIOSH certified N95 FFR cut open to show the different layers. A, polypropylene material (outermost layer); B, electret filtering medium (typically made from melt-blown or electrospun polypropylene); and C, polypropylene material (innermost layer).
Only a few studies have been done to verify FFR performance in extended use or reuse type scenarios. Moyer and Bergman(30) conducted a laboratory evaluation of the intermittent use (short-term use once per week) of N95 filters over several months. Filtration efficiency was reduced to below 95% for filters from 2 of the 3 manufacturers after 9 and 13 weeks of simulated reuse. Researchers at the Institut de recherché Robert-Sauvé en santé et en sécurité du travail (IRSST) validated the long-term filter performance of a single N95 FFR model. (31) For inert particles below 200 nm, filter efficiency levels remained above 97.3%, even after 5 hr of particle loading (i.e., continuous use). Not surprisingly, another study found that samples from 19 of 21 N95 FFR models stored for up to 10 years had expected levels of filtration performance.(32)
Fit
Fit is a measurement of the efficacy of the seal between the FFR and the face of the wearer. Components of the FFR, such as straps, face seal material, shape, and adjustable nose bands influence FFR fit. Several studies have analyzed strap performance and fit for multiple donnings of FFRs. Roberge et al. measured the restorative forces of straps for five simulated donnings and reported reduction in the strap load for each successive donning with the majority of the reduction occurring after the first donning.(33) However, the FFR model with the lowest restorative strap performance load was still able to pass fit-testing. Bergman et al. examined the effect of FFR reuse on fit by measuring the fit factors of 6 FFR models donned by 10 subjects up to 20 times with wear times of approximately 2 min between each donning.(34) FFR fit gradually decreased over multiple consecutive donnings; however, good fit was observed for some subjects on some models even after 20 donnings. The best levels of fit were observed for the first five donnings, likely because of the relatively little wear on FFR components (e.g., head straps and nosepieces) compared with later donnings.
It was concluded from that study that five donnings could be performed before fit factors started to drop below 100. Catastrophic failure of the FFR (e.g., complete head strap breakage, nosepiece becomes damaged, and so on) should have no effect on risk, if users diligently perform device inspection procedures required during the FFR donning process. Fit of FFRs is also a concern for extended use where the FFR may become wet and deformed due to moist exhaled breath and facial perspiration. Hauge et al. measured real-time fit while HCWs performed three 10-min simulated patient care scenarios. It was determined that initial fit was predictive of fit during the tasks as the five subjects with initial fit factors greater than 200 registered simulated workplace protection factors greater than 400, and the three subjects with initial fit factors less than 200 had simulated workplace protection factors ranging from 132 to 326.(35) Although the tasks were only a combined 30 min, the study design could be considered an extended use scenario covering three patient encounters.
Workplace Protection Factors
Few studies in health care settings measure workplace protection factors (WPF). WPF is a measure of the protection provided by a properly functioning respirator when correctly worn and used in the workplace and is determined as the ratio of the particle concentration outside the respirator over the particle concentration inside the respirator. Infectious bioaerosols are hard to detect and differentiate from non-infectious bioaerosols.(36) Furthermore, assuring compliance during all periods of exposure in the health care setting is challenging.(37) Several studies in other workplaces have assessed protection over extended periods of continuous use by measuring the WPFs: up to 224 min in a steel foundry(38); 172 min in a concrete factory (39); and 60 min on farms.(40) All three studies concluded that the N95 FFRs provided levels of protection consistent with expectations (i.e., protection factors were ≥ the assigned protection factor of 10), with reported geometric mean WPF values ranging from 18 to 223. No evidence of reduced protection as a function of time was noted in these studies. The aerosol challenge encountered at the farm locations consisted of biological aerosols such as endotoxins and fungal spores which are more closely related to the bioaerosols in a hospital than to the dust encountered at the foundry and concrete factory.
Summary
Overall, the scientific studies provide evidence that extended use is unlikely to reduce the protection afforded by a FFR (see Table II) and support the CDC TB infection control guidance which states in the Frequently Asked Questions section, “Disposable respirators can be functional for weeks to months.” However, as noted in Table II, some additional cautions may apply for reuse. Reuse involves multiple repeated uses (donnings) of the same device, and it is possible that some components (straps, nose clips, and so on) could begin to degrade over time and reduce protection. These effects are likely specific to each model of FFR, but the only study published(34) to date on this topic suggests that limiting FFR reuse to no more than five donnings or reuses would provide an adequate safety margin.
Human Factors
One of the consequences of extended use is the need to wear the FFR continuously for up to several hours, compared with single use or reuse in which the FFR would only be worn during the period of close contact with the patient (typically less than 15–20 min). Thus, questions have been raised regarding the safety of long-term FFR use and, if safe, how long HCWs can physiologically and psychologically tolerate extended use.
NIOSH researchers found that FFR use caused no or minimal increases in heart rate, respiratory rate, and transcutaneous carbon dioxide as well as no differences in oxygen saturation on test subjects during 1 hr of low-moderate treadmill exercise when compared with wearing no respirator (control).(41–43) They also reported that 2 hr of continuous FFR use at low-moderate work rate did not cause a change in core body temperature,(42) and there was no significant increase in FFR deadspace heat or humidity after the first hour.(44) Taken together, these studies suggest that FFR use for 1–2 hr should cause minimal physiological stress to individuals medically cleared to wear FFRs.
A study by researchers affiliated with Department of Veterans Affairs reported how long 27 HCWs could tolerate multiple bouts of 2-hr-long extended use periods, interspersed with 15–30 min breaks.(45) Median tolerance times of 6.6 hr and 5.8 hr were reported for the two FFR models without exhalation valves. Only 16 and 18 of the 27 subjects using those two models were able to complete all four 2-hr use periods of continuous use; the most reported reason for stopping use was head and facial discomfort (e.g., heat). In a follow-up analysis of the same data, it was concluded that FFR discomfort negatively affects respirator tolerance over time, but respirator intolerance is not associated with perceived self-reported exertion.(46)
Although the number of participants was small, a recent study reports greater tolerance of extended use of FFRs among HCWs.(47) They reported that 9 of 10 study participants (nurses) were willing to wear FFRs for the entirety of two full 12-hr shifts, stopping only to eat and drink, because it was the end of their shift, or because the FFR was too uncomfortable. The nurses tolerated FFR continuous wear for an average of 223 min on day 1 and 145 min on day 2 and experienced little physiological burden; however, discomfort increased with time, and the nurses reported feeling more short of breath the longer they wore respiratory protection. Transcutaneous carbon dioxide levels increased over time, but were not clinically relevant in that carbon dioxide levels did not reach the requirement for clinically defined hypercapnia.
A study conducted in a teaching hospital in Brazil considered changes in appearance and possible physical damage resulting from FFR reuse.(48) A new N95 FFR was distributed to each nurse once per month and reused as needed until the next new N95 FFR was provided. The researchers found that within 5 days, the majority of the distributed cone-shaped FFRs exhibited visible “wear and tear,” indicating possible physical damage (caused by folding them for storage in a pocket) and visible stains/dirt on the FFR interior and exterior surfaces. Although the performance of the respirators was not assessed, the data suggest that some models may be more suitable for reuse (e.g., those that fold easily) or that hospitals should enforce some restrictions on reuse (e.g., replace every 5 days, rather than every 30 days).
Overall, the available scientific studies provide evidence that HCWs will experience greater discomfort during periods of extended continuous wear of FFRs, but this discomfort will likely be tolerable for most HCWs. Continuous FFR use over extended periods of time up to 12 hr is unlikely to harm workers (see Table II) who have been medically cleared for respirator use. Furthermore, because HCWs need to take occasional breaks during their work shift (e.g., to use the rest room, eat or drink, and so on) FFR extended use of greater than 4 consecutive hours is unlikely in most settings.
Self-Inoculation Hazard
One knowledge gap often cited against allowing FFR extended use and limited reuse is whether a FFR worn during close contact with an infected patient is likely to serve as a fomite. Historically, little data were available to assess the transfer potential of respiratory pathogens from the FFR to the hands of the HCW, resulting in the potential for self-inoculation. Similar to other potential fomites (e.g., surfaces, medical devices, and stethoscopes(49)) assessing the level of risk of self-inoculation associated with touching a used FFR is complex. It is very difficult to trace a specific hospital-acquired infection to a particular object. Thus, while no studies have identified the use of a contaminated FFR as a source of infection, the possibility cannot be ruled out.
Nicas and Sun and Nicas and Jones have provided models for transmission of pathogens, including influenza, in health care settings.(50,51) Nicas and Sun considered fomite hazards of textile and nontextile surfaces and in room air to estimate the expected pathogen dose to a HCW's mucous membranes and respiratory tract.(51) Nicas and Jones modeled four influenza virus exposure pathways including fomite transmission. A similar approach is used below to estimate the potential fomite hazard of used FFRs. Factors that influence the risk of self-inoculation directly associated with handling a contaminated FFR include the quantity of respiratory pathogens deposited on the FFR surface (i.e., contamination levels), viability of the pathogen, transfer efficiency of the pathogen from FFRs to the hands of the wearer, and area of hand contact with the contaminated surface.
FFR Contamination Levels
There are no published studies that quantify the amount of pathogens on FFRs used in clinical settings. However, simple mathematical models can be used to provide some estimates. In one study, influenza aerosol concentration, breathing rate of the wearer, time of patient interaction/FFR use, and particle retention efficiency of the FFR were used as inputs to a linear model to estimate influenza contamination levels inside and on the surface of the FFR (CFFR).(52) Using this model, an increase in any parameter results in higher levels of CFFR (i.e., total number of pathogens on the FFR). For a typical HCW scenario, using model input values estimated from the peer-reviewed literature, the model calculated that CFFR would be approximately 4500 influenza viruses given an influenza aerosol concentration of 12,000 viruses m–3, a breathing rate of 1.140 m3hr–1, a particle retention efficiency of the FFR of 0.991, and a 20-min patient interaction/FFR use time. The values for influenza aerosol concentration and wear time found in the literature varied more than other parameters used in the model. Thus, for extended use which involves longer wear times, the number of pathogens available for transfer to the hands is increased.
This model illustrates the need to take into account HCW procedures (e.g., AGPs) which can increase CFFR by up to 2200% and the potential for administrative controls such as source control of patients (e.g. asking patients to wear facemasks) which can reduce CFFR by up to 71%.(53,54) In addition to administrative controls, the use of engineering controls such as local exhaust ventilation might reduce CFFR. Similarly, previous recommendations issued during the SARS outbreak suggested the use of a surgical mask or faceshield on top of a FFR to reduce CFFR(26); although subsequent work has identified a number of potential concerns, including regulatory compliance with this approach.(55) While developed for influenza, this model could be used to approximate CFFR resulting from any respiratory pathogen if estimates of the concentrations of the pathogen near the breathing zone of the HCW could be obtained.
Pathogen Survival
Given that FFRs can become contaminated with pathogens when used in close contact with infectious patients, the next factor under consideration is how long these pathogens can survive (remain infectious) and, for some types of microorganisms, grow (propagate) on the FFR surface. Some studies in the early 1990s found that under ideal conditions (e.g., humidity >78%), fungi and certain bacteria could grow on filters made of cellulose because they are capable of digesting cellulose.(56,57) However, modern (post-1995) FFRs are made of polypropylene, which cannot serve as a nutrient for bacteria.(58)
Studies confirmed that surrogates for TB were not able to grow on polypropylene-based filter media, even under incubation conditions.(58–61) Although bacteria were found to survive for several days, this was not considered a concern because contact transmission for TB is considered unlikely (see Table I). These studies lent support for the FFR reuse guidance being drafted at that time.
Respiratory viruses have received more recent attention. While growth is not an issue because these pathogens require a host organism to propagate, their persistence or survival on surfaces is a concern. In general, the evidence indicates that viruses are more persistent on nonporous substrates compared with porous materials such as FFRs. Bean et al. reported laboratory-grown influenza A and influenza B survived for 24–48 hr on hard, nonporous surfaces but survived for <8–12 hr on porous substrates.(62) Similarly, another study(63) found that influenza remained viable for 8 hr on FFR samples, but infectivity dropped below detection limits at <24hr.
However, Tiwari et al. examined the persistence of two avian respiratory viruses including influenza H13N7 on various substrates and although they found that both viruses survived longer on nonporous surfaces than on porous ones, the viruses remained active for up to 6 days.(64) In one laboratory study, pH1N1 was detected on FFRs for up to 6 days with an average of 90% reduction (1 log) in viability during this time period.(65) Similar findings were found using MS2 phage as a surrogate for respiratory viruses.(66) A surrogate for SARS coronavirus, transmissible gastroenteritis virus, was shown to remain viable for 24 hr on FFR samples with an estimated 99% (>2 log) reduction in titer.(67)
Another study found that inactivation of Φ6 bacteriophage spiked on a N95 FFR surface was highly sensitive to environmental conditions, with a ∼1 log reduction over 24 hr at 40% relative humidity versus ∼4 log reduction over 24 hr at 60% relative humidity.(68) Although it is difficult to generalize from these conflicting findings, it is clear that for reuse during a work shift with short storage times (<1 hr) most of the trapped pathogen will remain viable. Some reduction in viability might occur for overnight (>12 hr) or weekend (>24 hr) storage depending upon storage conditions (temperature, humidity, light, and so on) and pathogen type and strain.
In many cases where pathogens remain persistent and pose a contact threat, cleaning and disinfection regimens are routinely used. For example, countertops, exam tables, and other surfaces of patient rooms are often cleaned when a patient is discharged. Research has been conducted on cleaning and disinfecting procedures for FFRs for possible reuse by the same HCW. Although the results appear to be promising, the practice is not currently recommended(69–72) and thus is not a viable solution at this time to reduce the fomite potential of a reused FFR.
Recent improvements in antimicrobial chemistries have allowed some manufacturers to begin incorporating these technologies into FFRs. There are now a few surgical N95 FFRs incorporating antimicrobial technologies (product code = ONT) that have been cleared by the FDA as medical devices.(9) Interestingly, one device has been cleared by the FDA with claims of 8 hr of continuous use. Unfortunately, none of these devices has been evaluated in the peer-reviewed literature for claims regarding their ability to reduce fomite potential. However, several studies(73–77) have looked at the ability of prototypes or devices, not cleared by FDA, that incorporate some type of antimicrobial chemistry in them to render trapped pathogens inactive over time (i.e., storage time between uses). These studies suggest that efficacy of antimicrobial FFRs for this application is dependent on the pathogen, antimicrobial agent, storage conditions, and specific test method used which makes generalization of findings difficult.(73,78–81) Although promising, the lack of conclusive evidence suggests that additional work is needed before FFRs incorporating antimicrobial technologies can be factored into FFR reuse recommendations.
Transfer Efficiency
Because FFRs can become contaminated with pathogens likely to remain infectious during typical extended use and reuse scenarios, the next factor to assess is the likelihood of pathogens transferring from the FFR to the hands of HCWs. Unfortunately, no studies exist that quantify the percentage of pathogen transferred from the FFR to the hands of HCWs. However, similar to estimating contamination levels, models can be used where estimates of the key input parameters are available. A simple model for estimating the amount of pathogen transferred to the hands (Chand) of HCWs from contaminated FFRs uses CFFR (the number of pathogens on the FFR as discussed above), transfer efficiency of the pathogen (Et), and contact area of the hands (Ah) and the FFR (AFFR).
Unfortunately, no peer-reviewed sources are available on the transfer efficiency of relevant pathogens from a FFR to skin and others surfaces. However, an unpublished conference presentation reports the transfer efficiency of a bacterium, Bacillus atrophaeus, from FFRs to synthetic skin as 0.005% and 0.05% for touching and rubbing, respectively.(82) Other microbial transfer studies for porous surfaces have shown similar results. For example, Rusin et al. reported transfer efficiencies for a bacterium, Micrococcus luteus, of 0.13% from a 100% cotton substrate and 0.06% from a 50:50 cotton/polyester substrate. Even lower transfer efficiencies (<0.01%) from those surfaces were reported for bacteriophage PRD-1.(83) Another recent study compared the transfer efficiency of bacteria and viruses from several porous and nonporous surfaces to the fingers. (84) In general, the lowest transfer efficiencies were found for porous surfaces under low relative humidity. Isoelectric point and hydrophobicity of the surface were also important factors.
As discussed previously, CFFR can be estimated. For simplicity, we use the influenza values reported above from Fisher et al. as a surrogate for all respiratory pathogens.(52) The contact area of the hands depends upon the action of the HCW (Table III). For extended use, it is likely that only the finger tips are used to touch the FFR surface (e.g., to reposition the FFR). The total surface area of the volar portion of the fingertips has been estimated to be 7.34 cm2.(85) However, when implementing FFR reuse, the proper donning process requires a user seal check step, which requires the user to cover the entire FFR surface by cupping both hands around the filter surface. In this situation, Ah would be very similar to AFFR, which has been estimated to be approximately 175 cm2, but varies among the various FFR models. Assuming uniform deposition of the pathogen over the surface of the FFR, applying input values of 4,500 FFR–1 for CFFR and 0.1% as an approximation for Et to the equation results in an estimated 4.5 pathogens being transferred to the hands of the HCW during the user seal check step and <1 pathogen for each touch involving a fingertip.
TABLE III. Steps in the Donning and Doffing Process Involving Potential Contact with FFR Surface.
| Strategy | Donning | User Seal Check | Doffing |
|---|---|---|---|
| FFR Reuse | Yes | Yes | NoA |
| FFR Extended Use | No | No | NoA |
AHCWs hands should not contact the surface if proper doffing technique is used.
Summary
While the model above indicates that some pathogens from a contaminated FFR could transfer to the hands, other factors also affect the risk of infection. Steps in the fomite pathway such as the transfer of viable pathogens from hands to respiratory tract ports of entry, transport of viable pathogens to the site of infection, and the infectious dose of the pathogen are not unique to extended use and reuse of FFRs, but are common to any potential fomite. A full assessment that takes into account these steps is beyond the scope of this commentary. However, the model developed by Nicas and Sun indicates that each successive step in the fomite pathway further reduces the number of infectious pathogens reaching the site where infection can occur, reducing the risk of self-inoculation from practicing FFR extended use and/or limited reuse.(51)
In theory, extended use should not present a significant self-inoculation hazard, as ideally, the HCW's hands should never come in contact with the contaminated filtering surface when proper doffing protocols are followed.(86) However, the Rebmann study(47) reported that HCWs touched or adjusted their FFR on average 10–20 times per 12 hr. shift. Even with this amount of contact, our analysis, based on the data and the models discussed above, suggests that very few pathogens are likely to make it to the site of infection each time the hand or fingertip comes in contact with the FFR. Thus, extended use is considered minimal risk for typical patient interactions (Table II) when coupled with training and education to reinforce proper use (e.g., don't touch the FFR surface) and adherence to hand hygiene recommendations.
Reusing FFRs provides multiple opportunities for the hands of HCWs to come in contact with any infectious microbes on the respirator surface and thus involves a higher level of risk compared to extended use (Table II). HCWs’ hands would presumably contact the contaminated FFR surface when placing the FFR onto the face, adjusting the position of the FFR and flexible strap across the nasal bridge (if applicable), and when performing the user seal check, a requirement after donning a respirator and after each adjustment to the respirator. Similar to extended use, fomite risks from FFR reuse can be mitigated through training and education to reduce unnecessary touching of the FFR and rigorous adherence to hand hygiene. Steps to limit FFR contamination (e.g., masking patients, use of engineering controls, face shields, and so on) can also limit fomite risks, as Chand is proportional to CFFR.
Risk to Others (secondary exposures)
Concerns have been raised that extended use of FFRs could result in additional opportunities for pathogen transmission to co-workers and patients due to reaerosolization of trapped pathogens to the environment from a sneeze, cough, or through rough handling. Several studies have addressed this issue. Most recently, Fisher et al. examined virus reaerosolization from FFRs and concluded that the risk of virus transfer to the environment from the FFR was negligible, a finding key to extended use and reuse.(87) FFRs were challenged with virus-containing droplet nuclei with a size range of 0.65 to 7.0 μm (with the majority <1.1 μm) and challenged with reversed airflow to simulate a sneeze or cough. The highest reaerosolization of 0.21% occurred with a droplet nuclei challenge while a droplet challenge led to reaerosolization of less than 0.0001%. These findings are consistent with earlier studies that examined reaerosolization of bacteria and inert particles. Qian et al. and Willeke and Qian reported the reaerosolization of less than 0.2% for bacteria deposited on N95 FFRs as aerosols and challenged with a reverse airflow consistent with a violent sneeze or cough.(88,89) Kennedy and Hines found that less than 0.3% of polystyrene latex microspheres reaerosolized from FFRs when dropped from a height of 3 feet(90) while Birkner et al reported the average release of 0.006% polystyrene latex microspheres were released from FFRs dropped from heights up to 1.37 m.(91)
Overall, these data provide evidence that the risks of secondary exposure due to reaerosolization or rough handling associated with FFR extended use or limited reuse can be considered negligible (Table II). Similar to the fomite concerns discussed above, secondary exposure risks could increase as CFFR, the number of pathogens on the FFR, increases (i.e., higher CFFR = higher levels of reaerosolized pathogen), so steps taken to limit FFR contamination (e.g., masking patients, faceshields, local exhaust ventilation systems) should be implemented where possible.
In situations where patients are under contact precautions, such as those co-infected with common health care pathogens with the ability for prolonged environmental survival (e.g., Vancomycin-resistant enterococci, Clostridium difficile, and norovirus), it may be prudent to have HCWs discard FFRs after close contact because these pathogens could be transferred to other patients via the unclean hands of the HCW.
Sharing FFRs among HCWs could also result in a secondary risk if at least one of the users is infectious (symptomatic or asymptomatic). For example, a specialized face mask containing electret filter media (similar to those found in N95 FFRs) was worn in one study (92) to successfully collect infectious virus from the exhaled breath of symptomatic test subjects. Because of respirators’ ability to trap respiratory pathogens, sharing a contaminated FFR could result in disease transmission. However, proper labeling, training, and education can be effective at limiting any inadvertent sharing of FFRs during reuse.
OTHER REGULATORY AND POLICY CONSIDERATIONS
We also conducted an Internet search and reviewed FFR extended use and reuse recommendations issued by other United States agencies (e.g., FDA and OSHA) and professional organizations (e.g., Association for Professionals Infection Control and Epidemiology).(93,94) In terms of FFR extended use and limited reuse, we identified no major discrepancies among the recommendations from the Association for Professionals in Infection Control and Epidemiology (APIC), OSHA, and the CDC recommendations in(Table I). For example, OSHA TB guidance(7,95) indicates that disposable respirators (i.e., FFRs) can be reused by the same HCW, as long as the functional and structural integrity of the respirator is maintained and the outside of the filter is inspected before each use for signs of physical damage or soiling, and discarded if signs are present.
While OSHA is responsible for regulating employers to provide a safe workplace for their employees and CDC makes public health recommendations that are often adopted by hospitals, FDA has a different role in health care settings. The FDA regulates the manufacture and labeling of medical devices.(96) Medical devices are cleared by the FDA under the Food, Drug, and Cosmetic Act based upon data submitted by the manufacturer to support the claimed intended use of the product. Under 21 CFR 878.4040, FDA classifies surgical N95 respirators as a type of surgical apparel, intended to be worn by operating room personnel during surgical procedures to protect both the surgical patient and the operating room personnel from transfer of microorganisms, body fluids, and particulate material. As part of the labeling requirement, FDA recommends that manufacturers state whether a device is intended to be a reusable device or a single-use disposable device.(9)
Some surgical N95 respirator models are cleared by the FDA with claims of being a single-use device, while other manufacturers do not make such claims.(10) For surgical N95 respirators labeled as “single use only,” extended use or limited reuse could be considered as an “off label” use of these products. FDA has specific requirements for reuse (“reprocessing”) of single-use medical devices.(97) Unfortunately, as discussed earlier in this manuscript, some hospital use practices for these types of medical devices such as limited FFR reuse were first recommended(4–7) and put into practice prior to FDA's involvement. There is also a general lack of awareness among infection control professionals and safety/employee health administrators in understanding FDA's role in regulating surgical N95 respirators.(98) These factors contribute to the prevalence of “industrial N95 FFRs” used in health care settings. These industrial N95 FFRs are NIOSH-certified FFRs, but have not been cleared by the FDA as medical devices. Several of these industrial N95 FFRs were stockpiled by the CDC in the Strategic National Stockpile.(99)
In the future the different regulatory and policy perspectives will need to be factored into FFR extended use and limited reuse recommendations. For example, recommendations for operating rooms, where soiling and potential contamination from blood borne pathogens will likely occur, might be different. In those situations, limited reuse should only be considered after consultation with the surgical N95 respirator manufacturer and local hospital infection professionals.
KNOWLEDGE GAPS
While significant progress has been made since 2006, some knowledge gaps remain to be filled further enhancing an understanding of the risks involved with FFR extended use and limited reuse. Various models related to fomite transfer were presented where little experimental data are available for use as inputs. In particular, data on actual FFR contamination levels from various health care situations and transfer efficiency of pathogens from FFRs to the hands are limited. While several papers have been published on survivability of various respiratory pathogens on FFRs and the effectiveness of antimicrobial technologies, it is not known how generalizable the results are, which makes it difficult to fully assess risk. Well-designed and carefully controlled studies carried out using consistent test methods appropriate to FFR reuse might reduce some of these uncertainties.
Moreover, the infectious dose of various pathogens for the various transmission routes is not well understood, an issue further complicated by newly emerging pathogens and strains. Research and development efforts such as Project BREATHE (Better Respiratory Equipment using Advanced Technology for Health care Employees)(3) that promote the development of better respirators for health care workers are needed to identify novel technologies and designs (e.g., launderabilty, a “no touch” user seal check, and so on) to address some of the additional concerns posed by extended use and reuse. The paucity of data on many of the practical aspects of FFR extended use and reuse also suggests that additional studies are needed to validate preliminary findings regarding the acceptable number of donnings and to develop best practices for storage, labeling, and education/training. Surveillance data on FFR usage, including extended use and reuse, during routine operations and public health emergencies are needed to better understand the possible benefits (e.g., cost savings, ability to extend existing supplies, reducing the “burn rate,” and so on) of FFR extended use and limited reuse.
LIMITATIONS
The primary purpose of this article is to assess recent scientific findings to assist policy makers when making decisions on whether to recommend that employers in health care settings permit FFR extended use and/or limited reuse during routine operations and for future public health emergencies. The authors acknowledge that the evidence discussed above is not always as sufficient as desired to develop evidence-based policy decisions. However, decisions on how to protect exposed workers must be made in the present and cannot wait until additional evidence is available. In the interim the available evidence can be useful for policy-based and pragmatic public health decision ideologies.(100) As discussed by Rosella and coauthors, (100) emerging public health situations require a balance between various factors. Both evidential and policy considerations are important. Policy makers need to use the best evidence available to them, even when it has substantial limitations, acknowledge the uncertainties, and account for them in as practical a way as possible.
CONCLUSION
For recommending FFR extended use and/or limited reuse for routine events, policy makers should weigh the increased risks for disease transmission from FFR extended use and limited reuse against the inconvenience, cost, and waste of single use. In public health emergencies, policies on FFR extended use and limited reuse should weigh the risks for disease transmission against the risk of disease transmission associated with sacrificing because of FFR shortages (e.g., foregoing respiratory protection or using surgical masks for pathogens or activities where N95 FFRs are recommended). Decisions regarding whether FFR extended use or limited reuse should be recommended need to continue to be pathogen- and event-specific. The two most important factors driving this decision should be whether the pathogen is likely to spread (in part) via contact transmission and whether the event could result in or is currently causing a FFR shortage.
This analysis of recent research (post-2006) generally supports CDC guidance issued since 2004 for FFR extended use and limited reuse for routine events such as TB and seasonal influenza (during AGP) as well as the public health emergencies such as the 2004 SARS and 2009 H1N1 flu pandemics. While recent findings largely support these CDC recommendations, some new cautions and limitations should be considered in recommendations issued in the future as discussed subsequently.
Extended use offers a lower risk of self-inoculation compared to limited reuse given that the HCWs hands should ideally rarely contact the contaminated FFR surface. Training and education should be stressed to reinforce the need for strict adherence to guidance to minimize unnecessary contact with the FFR surface and strict adherence to hand hygiene practices. Extended use poses no additional health risk to a medically cleared respirator user and despite the additional discomfort should be tolerable for most HCWs. For these reasons, extended use should be preferred over limited reuse, even though FFR reuse requires the least change to current practices.
Limited FFR reuse would allow the HCW to doff the FFR to provide a brief respite from the psychological and physiological factors that decrease FFR comfort, but increases the potential for contact transfer when donning the used FFR and performing the user seal check. However, fomite transfer models indicate that the potential for transfer of pathogens from FFRs to the hands of the wearer is small suggesting that limited FFR reuse can be employed with minimal additional risk in most cases. An exception is reuse of FFRs after AGPs, where higher FFR contamination levels are likely to occur. Education and training should be emphasized to reinforce the need for proper hand hygiene when redonning the FFR, including inspection of the device for physical damage and performing a user seal check. Strict adherence to these steps should further reduce the potential to transfer virus from the hands to the points of entry of infection.
While limited FFR reuse remains a viable option for reducing usage rates and for situations involving a pathogen that does not spread via contact transmission, data suggest that FFR protection can begin to be reduced for some models after multiple donnings or uses. Guidance should emphasize the need for the employer to consult with the respirator manufacturer regarding the maximum number of donnings or uses suggested for the FFR models used in that location or to presumptively limit the number of reuses to no more than five to ensure an adequate safety margin, in the absence of new information to the contrary.
ACKNOWLEDGMENTS
The authors wish to express our sincere gratitude to members of the NPPTL H7N9 working group as well as Jeff Hageman (CDC's Division of Health Care Quality Promotion), Lisa Koonin (CDC's Influenza Coordination Unit), John Noti (NIOSH's Health Effects Laboratory Division), Teresa Seitz (NIOSH's Division of Surveillance, Hazard Evaluations, and Field Studies), and Caroline Ylitalo (3M Company) for their helpful suggestions and contributions. The findings and conclusions in this manuscript have not been formally disseminated by NIOSH and should not be construed to represent any agency determination or policy.
REFERENCES
- “Respiratory Protective Devices,” Code of Federal Regulations Title 42, Part 84 1995. pp. 30336–30404. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) : “NIOSH Guide to the Selection and Use of Particulate Respirators.” . Available at http://www.cdc.gov/niosh/docs/96–101/ (accessed January 28, 2013). [Google Scholar]
- Gosch M.E., Shaffer R.E., Eagan A.E., Roberge R.J., Davey V.J., and Radonovich L.J., Jr: B95: A new respirator for health care personnel. Am J. Infect. Control 41(12):1224–1230 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) : Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Facilities, 1994. MMWR Recomm. Rep. 43(RR-13):1–132 (1994). [PubMed] [Google Scholar]
- Harber P., Barnhart S., Boehlecke B.A., et al. : Respiratory Protection Guidelines, American Thoracic Society March 1996. Am. J. Respir. Crit. Care Med. 154(4 Pt 1):1153–1165 [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) : “TB Respiratory Protection Program In Health Care Facilities - Administrator's Guide.” . Available at http://www.cdc.gov/niosh/docs/99–143/ (accessed December 4, 2013). [Google Scholar]
- Occupational Safety and Health Administration (OSHA) : “Enforcement Procedures and Scheduling for Occupational Exposure to Tuberculosis CPL2–00–106.” . Available at https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=DIRECTIVES&p_id=1586 (accessed December 5, 2013). [Google Scholar]
- Larson E.L., and Liverman C.T.: Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Workers: Update 2010. Washington, D.C.: National Academies Press, 2011. [PubMed] [Google Scholar]
- Food and Drug Administration (FDA) : “Guidance for Industry and FDA Staff: Surgical Masks—Premarket Notification [510(k)] Submissions.” . Available at http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072549.htm (access-ed December 4, 2013). [Google Scholar]
- Food and Drug Administration (FDA) : “510(k) Premarket Notification.” . Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm (accessed December 5, 2013). [Google Scholar]
- Beckman S., Materna B., Goldmacher S., et al. : Evaluation of respiratory protection programs and practices in California hospitals during the 2009–2010 H1N1 influenza pandemic. Am. J. Infect. Control 41(11):1024–1031 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) : “Questions and Answers Regarding Respiratory Protection for Preventing 2009 H1N1 Influenza Among Healthcare Personnel.” . Available at http://www.cdc.gov/h1n1flu/guidelines_infection_control_qa.htm (accessed July 17, 2013). [Google Scholar]
- Rivera P., Louther J., Mohr J., Campbell A., DeHovitz J., and Sepkowitz K.A.: Does a cheaper mask save money? The cost of implementing a respiratory personal protective equipment program. Infect. Control Hosp. Epidemiol. 18(1):24–27 (1997). [DOI] [PubMed] [Google Scholar]
- Siegel J.D., Rhinehart E., Jackson M., and Chiarello L.: 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J. Infect. Control 35(10 Suppl 2):S65–164 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P.A., Lambert L.A., Iademarco M.F., and Ridzon R.: “Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, 2005.” . Available at http://www.cdc.gov/MMWR/PREVIEW/mmwrhtml/rr5417a1.htm (accessed July 19, 2013). [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) : “Core Curriculum on Tuberculosis: What the Clinician Should Know.” . Available at http://www.cdc.gov/tb/education/corecurr/ (accessed July 19,2013). [Google Scholar]
- Centers for Disease Control and Prevention (CDC) : “Prevention Strategies for Seasonal Influenza in Healthcare Settings Guidelines and Recommendations.” . Available at http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm (accessed July 2, 2013). [Google Scholar]
- Centers for Disease Control and Prevention (CDC) : “Interim Guidance for Infection Control Within Healthcare Settings When Caring for Patients with Confirmed, Probable, or Cases Under Investigation of Avian Influenza A(H7N9) Virus Infection “ . Available at http://www.cdc.gov/flu/avianflu/h7n9-infection-control.htm (accessed July 2, 2013). [Google Scholar]
- Centers for Disease Control and Prevention (CDC) : “Interim Recommendations for Infection Control in Health-Care Facilities Caring for Patients with Known or Suspected Avian Influenza.” . Available at http://www.cdc.gov/flu/avian/professional/infect-control.htm (accessed January 28, 2014). [Google Scholar]
- Murray M., Grant J., Bryce E., Chilton P., and Forrester L.: Facial protective equipment, personnel, and pandemics: Impact of the pandemic (H1N1) 2009 virus on personnel and use of facial protective equipment. Infect. Control Hosp. Epidemiol. 31(10):1011–1016 (2010). [DOI] [PubMed] [Google Scholar]
- Commins J.: “Nurses Protest H1N1 Respirator Mask Shortage.” . Available at http://www.healthleadersmedia.com/content/NRS-241429/Nurses-Protest-H1N1-Respirator-Mask-Shortage.html (accessed July 19, 2013). [Google Scholar]
- Mascarenhas R.: “Swine-flu Worries Spur Shortage of Hand Sanitizer, Respirator Supplies in N.J.” . Available at http://www.nj.com/news/index.ssf/2009/11/swine_flu_concerns_cause_short.html (accessed July 2, 2013). [Google Scholar]
- Srinivasan A., Jernign D.B., Liedtke L., and Strausbaugh L.: Hospital preparedness for severe acute respiratory syndrome in the United States: Views from a national survey of infectious diseases consultants. Clin. Infect. Dis. 39(2):272–274 (2004). [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) : “Interim Domestic Guidance on the Use of Respirator to Prevent the Transmission of SARS.” . Available at http://www.cdc.gov/sars/clinical/respirators.html (accessed July 19, 2013). [Google Scholar]
- Centers for Disease Control and Prevention (CDC) : “Interim Guidance on Infection Control Measures for 2009 H1N1 Influenza in Healthcare Settings, Including Protection of Healthcare Personnel.” . Available at http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm (accessed July 19, 2013). [PubMed] [Google Scholar]
- National Research Council (NRC) : Reusability of Facemasks During an Influenza Pandemic: Facing the Flu. Washington, D.C.: National Academies Press, 2006. [Google Scholar]
- Fisher E., Rengasamy S., Viscusi D., Vo E., and Shaffer R.: Development of a test system to apply virus containing particles to filterins facepiece respirators for the evaluation of decontamination procedures. Appl. Environ. Microbiol. 75(6):1500–1507 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noti J.D., Lindsley W.G., Blachere F.M., et al. : Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clin. Infect. Dis. 54(11):1569–1577 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten I.M.: Handbook of Nonwoven Filter Media. . Oxford: Elsevier, 2007. pp. 325–367. [Google Scholar]
- Moyer E.S., and Bergman M.S.: Electrostatic N-95 respirator filter media efficiency degradation resulting from intermittent sodium chloride aerosol exposure. Appl. Occup. Environ. Hyg. 15(8):600–608 (2000). [DOI] [PubMed] [Google Scholar]
- Haghighat F., Bahloul A., Lara J., Mostofi R., and Mahdavi A.: “Development of a Procedure to Measure the Effectiveness of N95 Respirator Filters Against Nanoparticles Report R-754.” . Available at http://www.irsst.qc.ca/media/documents/PubIRSST/R-754.pdf (accessed July 2, 2013). [Google Scholar]
- Viscusi D.J., Bergman M., Sinkule E., and Shaffer R.E.: Evaluation of the filtration performance of 21 N95 filtering face piece respirators after prolonged storage. Am J. Infect. Control 37(5):381–386 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge R., Niezgoda G., and Benson S.: Analysis of forces generated by N95 filtering facepiece respirator tethering devices: A pilot study. J. Occup. Environ. Hyg. 9(8):517–523 (2012). [DOI] [PubMed] [Google Scholar]
- Bergman M.S., Viscusi D.J., Zhuang Z., Palmiero A.J., Powell J.B., and Shaffer R.E.: Impact of multiple consecutive donnings on filtering facepiece respirator fit. Am J. Infect. Control 40(4):375–380 (2012). [DOI] [PubMed] [Google Scholar]
- Hauge J., Roe M., Brosseau L.M., and Colton C.: Real-time fit of a respirator during simulated health care tasks. J. Occup. Environ. Hyg. 9(10):563–571 (2012). [DOI] [PubMed] [Google Scholar]
- Janssen L., Zhuang Z., and Shaffer R.: Principles for the collection of useful respirator performance data in the workplace. J. Occup. Environ. Hyg. 11(4):218–226 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L., Ettinger H., Graham S., Shaffer R., and Zhuang Z.: The use of respirators to reduce inhalation of airborne biological agents. J. Occup. Environ. Hyg. 10(8):D97–D103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L.L., Nelson T.J., and Cuta K.T.: Workplace protection factors for an N95 filtering facepiece respirator. J. Occup. Environ. Hyg. 4(9):698–707 (2007). [DOI] [PubMed] [Google Scholar]
- Bidwell J., and Janssen L.: Workplace performance of an N95 respirator in a concrete block manufacturing plant. J. Int. Soc. Resp. Prot. 21(Fall/Winter):94–102 (2004). [Google Scholar]
- Cho K.J., Jones S., Jones G., et al. : Effect of particle size on respiratory protection provided by two types of N95 respirators used in agricultural settings. J. Occup. Environ. Hyg. 7(11):622–627 (2010). [DOI] [PubMed] [Google Scholar]
- Kim J.H., Benson S.M., and Roberge R.J.: Pulmonary and heart rate responses to wearing N95 filtering facepiece respirators. Am J. Infect. Control 41(1):24–27 (2013). [DOI] [PubMed] [Google Scholar]
- Roberge R., Benson S., and Kim J.H.: Thermal burden of N95 filtering facepiece respirators. Ann. Occup. Hyg. 56(7):808–814 (2012). [DOI] [PubMed] [Google Scholar]
- Roberge R.J., Coca A., Williams W.J., Powell J.B., and Palmiero A.J.: Physiological impact of the N95 filtering facepiece respirator on healthcare workers. Respir. Care 55(5):569–577 (2010). [PubMed] [Google Scholar]
- Roberge R.J., Kim J.H., and Benson S.: N95 filtering facepiece respirator deadspace temperature and humidity. J. Occup. Environ. Hyg. 9(3):166–171 (2012). [DOI] [PubMed] [Google Scholar]
- Radonovich L.J., Jr., Cheng J., Shenal B.V., Hodgson M., and Bender B.S.: Respirator tolerance in health care workers. JAMA 301(1):36–38 (2009). [DOI] [PubMed] [Google Scholar]
- Shenal B.V., Radonovich L.J., Jr., Cheng J., Hodgson M., and Bender B.S.: Discomfort and exertion associated with prolonged wear of respiratory protection in a health care setting. J. Occup. Environ. Hyg. 9(1):59–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebmann T., Carrico R., and Wang J.: Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nurses. Am J. Infect. Control 41(12):1218–1223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte L.R.P., Miola C.E., Cavalcante N.J.F., and Bammann R.H.: Maintenance status of N95 respirator masks after use in a health care setting. Rev. Esc Enferm. USP 44(4):1007–1012 (2010). [DOI] [PubMed] [Google Scholar]
- Campos-Murguía A., León-Lara X., Muñoz J.M., Macías A.E., and Álvarez J.A.: Stethoscopes as potential intrahospital carriers of pathogenic microorganisms. Am J. Infect. Control 42(1):82–83 (2013). [DOI] [PubMed] [Google Scholar]
- Nicas M., and Jones R.M.: Relative contributions of four exposure pathways to influenza infection risk. Risk Anal. 29(9):1292–1303 (2009). [DOI] [PubMed] [Google Scholar]
- Nicas M., and Sun G.: An integrated model of infection risk in a health-care environment. Risk Anal. 26(4):1085–1096 (2006). [DOI] [PubMed] [Google Scholar]
- Fisher E.M., Noti J.D., Lindsley W.G., Blachere F.M., and Shaffer R.E.: Validation and application of models to predict facemask influenza contamination in healthcare settings. Risk Anal. Published online March 4, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D.K., Fabian M.P., Cowling B.J., Grantham M.L., and McDevitt J.J.: Influenza virus aerosols in human exhaled breath: Particle size, culturability, and effect of surgical masks. PLoS Pathog. 9(3):e1003205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K.A., Pappachan J.V., Bennett A.M., et al. : Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic—The risk of aerosol generation during medical procedures. PLoS ONE 8(2):e56278 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge R.J.: Effect of surgical masks worn concurrently over N95 filtering facepiece respirators: Extended service life versus increased user burden. J. Pub. Health Manag. Pract. 14(2):E19–26 (2008). [DOI] [PubMed] [Google Scholar]
- Pasanen A.L., Nikulin M., Berg S., and Hintikka E.L.: Stachybotrys-Atra Corda may produce mycotoxins in respirator filters in humid environments. Am. Indus. Hyg. Assoc. J. 55(1):62–65 (1994). [Google Scholar]
- Pasanen A.L., Keinanen J., Kalliokoski P., Martikainen P.I., and Ruuskanen J.: Microbial growth on respirator filters from improper storage. Scand. J. Work Environ. Health 19(6):421–425 (1993). [DOI] [PubMed] [Google Scholar]
- Wang Z., Reponen T., Willeke K., and Grinshpun S.A.: Survival of bacteria on respirator filters. Aerosol Sci. Tech. 30(3):300–308 (1999). [Google Scholar]
- Reponen T.A., Wang Z., Willeke K., and Grinshpun S.A.: Survival of mycobacteria on N95 personal respirators. Infect. Control Hosp. Epidemiol. 20(4):237–241 (1999). [DOI] [PubMed] [Google Scholar]
- Johnson B., Winters D.R., Shreeve T.R., and Coffey C.C.: Respirator filter re-use test using the laboratory simulant mycobacterium tuberculosis (H37RA strain). J. Am. Biol. Saf. Assoc. 3:105–116 (1998). [Google Scholar]
- Brosseau L.M., NV M.C, and Vesley D.: Bacterial survival on respirator filters and surgical masks. J. Am. Biol. Saf. Assoc. 2(3):32–43 (1997). [Google Scholar]
- Bean B., Moore B.M., Sterner B., Peterson L.R., Gerding D.N., and Balfour H.H., Jr: Survival of influenza viruses on environmental surfaces. J. Infect. Dis. 146(1):47–51 (1982). [DOI] [PubMed] [Google Scholar]
- Sakaguchi H., Wada K., Kajioka J., et al. : Maintenance of influenza virus infectivity on the surfaces of personal protective equipment and clothing used in healthcare settings. Environ. Health Prev. Med. 15(6):344–349 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Patnayak D.P., Chander Y., Parsad M., and Goyal S.M.: Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 50(2):284–287 (2006). [DOI] [PubMed] [Google Scholar]
- Coulliette A.D., Perry K.A., Edwards J.R., and Noble-Wang J.A.: Persistence of the 2009 pandemic influenza A (H1N1) virus on N95 respirators. Appl. Environ. Microbiol. 79(7):2148–2155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E.M., and Shaffer R.E.: Survival of bacteriophage MS2 on filtering facepiece respirator coupons. Appl. Biosaf 15(2):71 (2010). [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., and Sobsey M.D.: Coronavirus survival on healthcare personal protective equipment. Infect. Control Hosp. Epidemiol. 31(5):560–561 (2010). [DOI] [PubMed] [Google Scholar]
- Casanova L.M., and Waka B.: Survival of a surrogate virus on N95 respirator material. Infect. Control Hosp. Epidemiol. 34(12):1334–1335 (2013). [DOI] [PubMed] [Google Scholar]
- Fisher E.M., Williams J.L., and Shaffer R.E.: Evaluation of microwave steam bags for the decontamination of filtering facepiece respirators. PLoS ONE 6(4):e18585 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbuch B.K., Wallace W.H., Kinney K., et al. : A pandemic influenza preparedness study: Use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am. J. Infect. Control 39(1):E1–E9 (2011). [DOI] [PubMed] [Google Scholar]
- Lore M.B., Heimbuch B.K., Brown T.L., Wander J.D., and Hinrichs S.H.: Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann. Occup. Hyg. 56(1):92–101 (2012). [DOI] [PubMed] [Google Scholar]
- Viscusi D.J., Bergman M.S., Novak D.A., et al. : Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J. Occup. Environ. Hyg. 8(7):426–436 (2011). [DOI] [PubMed] [Google Scholar]
- Rengasamy S., Fisher E., and Shaffer R.E.: Evaluation of the survivability of MS2 viral aerosols deposited on filtering face piece respirator samples incorporating antimicrobial technologies. Am. J. Infect. Control 38(1):9–17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkow G., Zhou S.S., Page T., and Gabbay J.: A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 5(6):e11295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Wu C.Y., Lee C.N., et al. : Assessment of iodine-treated filter media for removal and inactivation of MS2 bacteriophage aerosols. J. Appl. Microbiol. 107(6):1912–1923 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Wu C.Y., Wysocki K.M., Farrah S., and Wander J.: Efficacy of iodine-treated biocidal filter media against bacterial spore aerosols. J. Appl. Microbiol. 105(5):1318–1326 (2008). [DOI] [PubMed] [Google Scholar]
- Li Y., Leung P., Yao L., Song Q.W., and Newton E.: Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 62(1):58–63 (2006). [DOI] [PubMed] [Google Scholar]
- Zuo Z., de Abin M., Chander Y., Kuehn T.H., Goyal S.M., and Pui D.Y.: Comparison of spike and aerosol challenge tests for the recovery of viable influenza virus from non-woven fabrics. Influenza Other Respir. Viruses 7(5):637–644 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe K.M., Turner J., and Hernandez M.T.: A method for assessing the disinfection response of microbial bioaerosols retained in antimicrobial filter materials and textiles. J. Microbiol.Meth. 92(2013):11–13 (2012). [DOI] [PubMed] [Google Scholar]
- Majchrzycka K., Gutarowska B., and Brochocka A.: Aspects of tests and assessment of filtering materials used for respiratory protection against bioaerosols. Part II: Sweat in the environment, microorganisms in the form of a bioaerosol. Intern. J. Occup. Safe. Ergon. 16(2):275–280 (2010). [DOI] [PubMed] [Google Scholar]
- Majchrzycka K., Gutarowska B., and Brochocka A.: Aspects of tests and assessment of filtering materials used for respiratory protection against bioaerosols. Part I: Type of active substance, contact time, microorganism species. Intern. J. Occup. Safe. Ergon. 16(2):263–273 (2010). [DOI] [PubMed] [Google Scholar]
- Fisher E.M., Ylitalo C.M., Stepanova N., and Shaffer R.E.: Assessing Filtering Facepiece Respirator Contamination During Patient Care in Flu Season: Experimental and Modeling Approaches. In ISRP—Sixteenth International Conference: A Global View on Respiratory Protection. Boston, . September 23–27, 2012. [Google Scholar]
- Rusin P., Maxwell S., and Gerba C.: Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J. Appl. Microbiol. 93(4):585–592 (2002). [DOI] [PubMed] [Google Scholar]
- Lopez G.U., Gerba C.P., Tamimi A.H., Kitajima M., Maxwell S.L., and Rose J.B.: Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl. Environ. Microbiol. 79(18):5728–5734 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai M., Lau H.K., Pereira B.P., and Pho R.W.: A cadaver study on volume and surface area of the fingertip. J. Hand. Surg. Am. 22(5):935–941 (1997). [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) : “Sequence for Donning Personal Protective Equipment PPE/Sequence for Removing Personal Protective Equipment.” . Available at http://www.cdc.gov/HAI/pdfs/ppe/ppeposter148.pdf (accessed July 19, 2013). [Google Scholar]
- Fisher E.M., Richardson A.W., Harpest S.D., Hofacre K.C., and Shaffer R.E.: Reaerosolization of MS2 bacteriophage from an N95 filtering facepiece respirator by simulated coughing. Ann. Occup. Hyg. 56(3):315–325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Willeke K., Grinshpun S.A., and Donnelly J.: Performance of N95 respirators: Reaerosolization of bacteria and solid particles. Am. Ind. Hyg. Assoc. J. 58(12):876–880 (1997). [DOI] [PubMed] [Google Scholar]
- Willeke K., and Qian Y.: Tuberculosis control through respirator wear: Performance of National Institute for Occupational Safety and Health-regulated respirators. Am. J. Infect. Control 26(2):139–142 (1998). [DOI] [PubMed] [Google Scholar]
- Kennedy N.J., and Hinds W.C.: Release of simulated anthrax particles from disposable respirators. J. Occup. Environ. Hyg. 1(1):7–10 (2004). [DOI] [PubMed] [Google Scholar]
- Birkner J.S., Fung D., Hinds W.C., and Kennedy N.J.: Particle release from respirators, part I: Determination of the effect of particle size, drop height, and load. J. Occup. Environ. Hyg. 8(1):1–9 (2011). [DOI] [PubMed] [Google Scholar]
- Huynh K.N., Oliver B.G., Stelzer S., Rawlinson W.D., and Tovey E.R.: A new method for sampling and detection of exhaled respiratory virus aerosols. Clin. Infect. Dis. 46(1):93–95 (2008). [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration (OSHA) : “Pandemic Influenza Preparedness and Response Guidance for Healthcare Workers and Healthcare Employers.” . Available at https://www.osha.gov/Publications/3328–05–2007-English.html (accessed July 19, 2013). [Google Scholar]
- Rebmann T., Alexander S., Cain T., Citarella B., Cloughessy M., and Coll B.: “APIC Position Paper: Extending the Use and/or Reusing Respiratory Protection in Healthcare Settings During Disasters.” . Available at http://www.apic.org/Resource_/TinyMceFileManager/Advocacy-PDFs/APIC_Position_Ext_the_Use_and_or_Reus_Resp_Prot_in_Hlthcare_Settin-gs1209l.pdf (accessed July 19, 2013). [Google Scholar]
- Occupational Safety and Health Administration (OSHA) : “Healthcare Wide Hazards Tuberculosis.” . Available at https://www.osha.gov/SLTC/etools/hospital/hazards/tb/tb.html#RespiratoryProtection) (accessed August 12, 2013). [Google Scholar]
- Food and Drug Administration (FDA) : “FDA's Role in Regulat-ing PPE.” . Available at http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/GeneralHospitalDevicesandSupplies/PersonalProtectiveEquipment/ucm056084.htm (accessed January 28, 2014). [Google Scholar]
- Food and Drug Administration (FDA) : “Reprocessing of Single-Use Devices.” . Available at http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofSingle-UseDevices/default.htm (accessed December 5, 2013). [Google Scholar]
- Figueroa M.: Respiratory Protection Programs and Healthcare Professionals. In School of Public Health, p. 382 New Brunswick, N.J.: University of Medicine and Dentistry of New Jersey and Graduate School-New Brunswick Rutgers, 2013. [Google Scholar]
- Food and Drug Administration (FDA) : “Summary Fact Sheet for Disposable Respirators for Use During the Swine Flu Emergency.” . Available at http://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161612.htm (accessed December 4, 2013). [Google Scholar]
- Rosella L.C., Wilson K., Crowcroft N.S., Chu A., Upshur R., Willison D., et al. : Pandemic H1N1 in Canada and the use of evidence in developing public health policies—A policy analysis. Soc. Sci. Med. 83:1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C.J., and Milton D.K.: Airborne transmission of communicable infection—The elusive pathway. N. Engl. J. Med. 350(17):1710–1712 (2004). [DOI] [PubMed] [Google Scholar]


