Abstract
Brain arteriovenous malformation (BAVM) is an important risk factor for intracranial hemorrhage, especially in children and young adults. Inflammation has been implicated in BAVM lesion progression. Among various inflammatory components, macrophage is one of the major inflammatory cells present in human ruptured and unruptured BAVM and in the BAVM lesions of animal models. The role of macrophage in BAVM pathogenesis is not fully understood. In this review, we summarize recent studies on macrophages and introduce a non-invasive imaging protocol as a potential tool for detecting macrophage in BAVM and predicting the risk of BAVM rupture.
Keywords: Brain arteriovenous malformation, Pathogenesis, Inflammation, Macrophage
Introduction
Brain arteriovenous malformations (BAVMs) are complexes of tortuous, tangled vessels located in the brain parenchyma, in which a loss of normal capillary bed results in fistulous connections between arteries and veins. Abnormal arteriovenous shunting contributes to high flow in focal vascular structures, especially in the tangled nidus and draining veins. BAVMs have an abnormal wall structure and are at risk of rupture, often resulting in catastrophic hemorrhage.[1] Most patients experience their first hemorrhage between the ages of 10 and 60.[2, 3] BAVMs are prevalent in about 0.01% of the general population,[2, 4] and the majority of those are sporadic. Approximately 5% of BAVMs are familial or associated with other abnormalities, such as hereditary hemorrhagic telangiectasia (HHT) or Sturge-Weber syndrome.[5, 6]
Although BAVMs are thought to be congenital lesions with occasional de novo growth, the etiopathogenesis is still not fully understood. Accumulated studies on the molecular and cellular biology of BAVMs have indicated that inflammation plays an important role in the progression and rupture of BAVM.[7–10] Macrophages have been found in the vascular wall as well as adjacent brain parenchyma of unruptured, previously untreated BAVMs.[10, 11] The expression of macrophage migration inhibitory factor (MIF), a key activation factor of macrophages, is increased in BAVM and is associated with proliferation and apoptosis of vascular cells.[12] Macrophages are thought to promote BAVM progression and rupture through secretion of pro-inflammatory cytokines (including tumor necrosis factor-α [TNF-α], interlukin-6 [IL-6], and vascular endothelial growth factor [VEGF]), that potentiate pathological angiogenesis and abnormal vascular remodeling.[13]
Macrophages and BAVM lesion progression
The development of cerebral vasculature is a result of vasculogenesis and angiogenesis. Current evidence of BAVM etiology focuses primarily on abnormal angiogenesis, which occurs in two phases: 1) vascular endothelial cell proliferation and migration, in which VEGF and matrix metalloproteinase (MMPs) are key mediators; 2) vascular stabilization, during which endothelial cells form capillary tubes, intercellular junctions are strengthened, and pericytes and the precursors of smooth muscle cells are recruited to the newly formed endothelial tubes.[14]
Abnormalities during these two phases of angiogenesis can ultimately lead to the development of BAVM. Although the most well-known function of brain macrophages involves immunity and barrier genesis, the perivascular and blood-derived macrophages could also be involved in angiogenesis.[15] During the development period, macrophages interact with tip cells to chaperone vascular anastomoses.[16] LysM-Cre-mediated deletion of Notch1 in macrophages causes abnormal anastomoses between angiogenic sprouts in the retina.[17] Delta-like 4 (Dll4) positive tip cells interact in close proximity with Notch1-expressing macrophages at vascular branch points.
Previous studies have shown that macrophages are the major bone marrow-derived cells (BMDCs) recruited to the VEGF-induced brain angiogenic focus.[18] Since the number of BM-derived macrophages in VEGF-induced brain angiogenic regions peaks earlier than angiogenesis, the macrophages likely play a role in the activation of angiogenesis. Further studies have revealed that 80% of the infiltrated BM-derived macrophages express MMP-9, indicating that macrophages’ involvement in angiogenesis occurs during vascular endothelial cell migration by activating MMP-9.[18, 19]
Evidence obtained through analyses of surgically-resected BAVM suggests that BAVM is an active angiogenic and inflammatory lesion rather than a static congenital anomaly.[9] Endothelial proliferation increases in BAVM. BAVMs also have higher levels of MMP-9, IL-6, VEGF-A, and angiopoietin-2 (ANG-2).[20–22] Ang-2, the predominant form of angiopoietin in inflamed tissues and a functional antagonist of Tie-2, promotes vascular destabilization.[23] In the presence of VEGF, the destabilized vessels undergo angiogenic changes and sprout to form new vessels.[24] Expression of Tie-2 is thought to be restricted to endothelial cells.[23, 25] However, recent studies reveal that Tie-2-expressing monocytes and macrophages present in human peripheral blood as a response to elevated Ang-2 in inflamed tissues, where they then play an important role in modulating cytokines implicated in angiogenesis and inflammatory processes.[26] Therefore, the macrophages in BAVM are very likely to be associated with vascular destabilization. The role of Tie-2-expressing macrophages in BAVM pathogenesis would be a worthy subject of investigation in future studies.
Current knowledge regarding the development of BAVM comes from animal models that were generated in the adult mouse through conditional deletion of hereditary hemorrhagic telangiectasia (HHT) causative genes, endoglin (Eng) and activin-like kinase 1 (Alk1, ACVLR1), in combination with focal angiogenic stimulation. HHT is an autosome-dominate disorder, and HHT patients have a higher prevalence of BAVM development than the normal population. Adult mice with Eng or Alk1 deletion develop BAVM after brain focal angiogenic stimulation. The BAVM in these mice mimic many phenotypes of human sporadic BAVMs, and therefore are the most useful models for studying BAVM pathogenesis.[27–30]
Recent studies in bAVM animal have revealed that after angiogenic stimulation, similar degrees of cerebrovascular dysplasia developed in Eng+/− mice and wild-type (WT) mice transplanted with Eng+/− bone marrow (BM). In addition, the dysplasia in Eng+/− mice could be partially rescued by transplantation of WT BM.[31] This suggests that Eng deficiency in BM is sufficient to cause cerebrovascular dysplasia in the adult mouse after angiogenic stimulation. Macrophages are the major BM-derived cells detected in the brain angiogenic foci in mice,[32] and in human surgical resected BAVM specimens with or without a history of hemorrhage or previous treatment with embolization or radiosurgery.[9, 10, 18] These findings suggest that macrophages are directly involved in BAVM development. However, deletion of Eng in LysM positive macrophages during the embryonic developmental stage did not cause BAVM formation even after brain focal VEGF stimulation.[30, 33] Therefore, gene deficiency in macrophages alone might not be sufficient for BAVM formation. Macrophages, however, do not constitute a “pure” population, since they can be divided into distinct subgroups based on their functions and gene expression profiles.[34] It is possible that certain macrophage subgroups, rather than all macrophages, contribute significantly to BAVM development.
Macrophages can undergo classical M1 activation or alternative M2 activation.[35] The M1 phenotype is characterized by the expression of high levels of pro-inflammatory cytokines, and reactive nitrogen and oxygen intermediates. M1 macrophages promote Th1 response, and have strong microbicidal and tumoricidal activity. In contrast, M2 macrophages are considered to exhibit anti-inflammatory activity, and play an important role in tissue remodeling and wound repair. Therefore, they may be crucial for tissue homeostasis to be restored.[36]
A study by Hasan et al[37] suggests that an imbalance of M1/M2 macrophages plays a role in cerebral aneurysm rupture. In addition, iron overload induces macrophage polarization toward pro-inflammatory M1.[38] BAVM is an active inflammatory lesion, and about 30% of unruptured BAVMs have microhemorrhage, which increases iron deposition and pro-inflammatory mediators.[7] Future studies should explore the association between macrophage polarization and BAVM progression and hemorrhage.
Macrophage and BAVM hemorrhage
Unfavorable outcomes of BAVM are attributed mostly to hemorrhage; however, not all cases of BAVM hemorrhage are symptomatic and caused by vascular rupture. Silent intra-lesional hemorrhages in BAVMs have been reported.[39, 40] About 14–20% of BAVM patients without hemorrhagic history exhibit signs of prior hemorrhage.[40] Recent studies found that 30% of resected surgical specimens from patients with unruptured BAVMs and without history of hemorrhage contain microscopic evidence of hemosiderin deposition in the vascular wall or intervening stromal tissue.[10, 11] Further analyses suggest a strong association between old silent hemorrhage and the risk of future symptomatic hemorrhage.[10]
The casual relationship between macrophage infiltration and clinically symptomatic hemorrhage as well as silent hemorrhage is still unclear. Silent hemorrhage and other inflammatory cytokines could activate and recruit macrophages into the lesions. Inflammation, including macrophage infiltration, could impair the vascular integrity and consequently induce silent or clinically symptomatic hemorrhage.
The factors that initiate monocyte activation and macrophage infiltration are still unclear. Macrophage infiltration could be initiated during the early development of BAVMs, since CD68+ cells present in unruptured BAVM specimens that have no hemosiderin deposition. However, unruptured BAVMs with silent hemorrhage (iron deposition) tend to have more macrophages than those without it.[11] Histological examination of BAVM in patients and in mouse BAVM models demonstrates that the degree of hemosiderin or iron deposition (hemorrhagic product) correlates positively with the number of macrophages in the lesion.[10, 28] These data suggest that microhemorrhage is one of the factors that induce macrophage infiltration in BAVM.
The BAVM vessels in an Alk1-deficient BAVM model have less mural cell coverage, increased fibrin and iron deposition, and small pockets of extravasated red blood cells in the brain parenchyma. Therefore, impaired vascular integrity could cause erythrocyte exudation and enhance macrophage infiltration in BAVM.
Vascular destabilization induced by inflammation or macrophage infiltration could result in erythrocyte extravasation from vascular walls. The hemoglobin breakdown products from the extraverted erythrocytes will, in turn, attract more macrophages. This process results in chronic inflammation that drives abnormal vascular remodeling, which further impairs vascular integrity.
Potential use of macrophages as a biomarker for BAVM future hemorrhage
Since macrophages are associated with BAVM hemorrhage and rupture, the macrophage load might be used to identify BAVM at risk of rupture. A noninvasive means to detect macrophage infiltration is under development. Ferumoxytol (AMAG Pharmaceuticals, Lexington, MA), a superparamagnetic iron oxide nanoparticle approved for treatment of iron deficiency anemia in patients with chronic renal failure, is used as a contrast agent in MRI to track macrophages.[41] It stays in vessels for up to 72 hours after intravascular delivery, which is cleared by macrophages starting at 24 hours after the delivery. Macrophages containing ferumoxytol can stay in tissues for an extended period and thus allow delayed detection by MRI.[42] Since it is superparamagnetic, ferumoxytol is hypointense on T2*-weighted images and hyperintense on T1-weighted images. These iron particles were first used to detect inflammation in patients with aneurysm and then extrapolated to patients with BAVMs.[42, 43] Pilot studies co-localized T2* gradient echo MR signal loss after ferumoxytol infusion with Prussian blue and CD68+ macrophages in the aneurysm domes and BAVM surgical specimens.[44] Thus, using ferumoxytol-enhanced MRI for assessing BAVM macrophage load is feasible and can be developed as a potential biomarker to assess the risk of BAVM rupture.
A crucial finding regarding this new contrast in aneurysm is that the timing of ferumoxytol uptake in aneurysm walls reflects aneurysm stability and predicts the risk of rupture. Hasan et al[45] showed that all aneurysms exhibit early uptake (24 hours after infusion) of ferumoxytol on MRI ruptured in the next 6 months, whereas none of those with late uptake (72 hours after infusion) ruptured during the follow-up period. Therefore, the early signal change on MRI is thought to be associated with active inflammation and increase of pro-inflammatory macrophages, hence indicating a greater risk of hemorrhage. A further study demonstrated that ferumoxytol-enhanced MRI allows assessment of the effects of anti-inflammatory pharmacological interventions on cerebral aneurysm.[46] Although results are impressive, this method has not been tested in BAVMs.
Imaging subtle changes in BAVM may be challenging because of high-blood volume in the nidus.[47] Residual intravascular ferumoxytol signal interferes with the detection of iron nanoparticles in the vascular wall or intervening stromal tissue. Delayed imaging at 5 days after ferumoxytol infusion might be an optimal protocol for BAVMs because it does not show as much of the intravascular tracer.
Although current application of ferumoxytol-enhanced MRI in BAVM is limited, preliminary data suggest that this new contrast MRI is a promising technique for detecting macrophages and predicting BAVM rupture. Future studies will be needed to develop reliable imaging biomarkers, similar to those in aneurysm studies, in order to identify rupture-prone BAVMs, allowing relatively real-time surveillance of the severity of intralesional inflammation and assessment of the therapy.
Summary
The pathogenesis of BAVMs is complex and currently vague. Evidence obtained from HHT BAVM models and analysis of human BAVM specimens suggests that macrophages play a critical role in vascular integrity and vascular remodeling. Figure 1 summarizes the potential roles and mechanisms of macrophages in BAVM pathogenesis. Tracking macrophages is a promising and innovative method to predict BAVM rupture.
Figure 1. Roles and mechanisms of macrophages in BAVM pathogenesis.
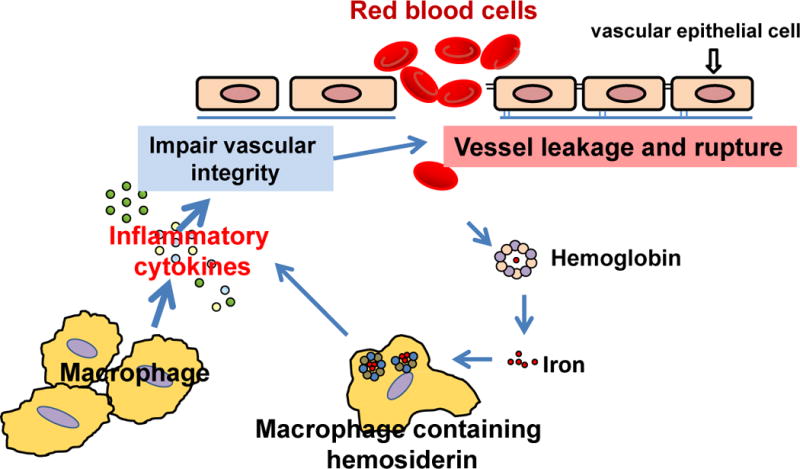
Macrophages infiltrate the brain angiogenic region, which secretes inflammatory cytokines promoting abnormal vascular remodeling and impairing vascular integrity. Impaired vascular integrity increases vascular leakage or causes vessel rupture. Red blood cells get into the brain tissue, break down, and release hemoglobin and iron. Macrophages engulf the iron and process it into hemosiderin. Macrophages containing hemosiderin produce more inflammatory cytokines that further impair vascular integrity and promote lesion progression.
Acknowledgments
This work was supported by grants to Hua Su from the National Institutes of Health (R01 NS027713, R01 HL122774 and R21 NS083788) and from the Michael Ryan Zodda Foundation and UCSF Research Evaluation and Allocation Committee (REAC). Support was also provided by grants from the National Natural Science Foundation of China (81271313) to Yuanli Zhao and Yi Guo, and from the Hebei Provincial Natural Science Foundation of China (H2013201283) to Yi Guo. We thank Voltaire Gungab for assistance with manuscript preparation.
References
- 1.Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356:2704–2712. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 2.ApSimon HT, Reef H, Phadke RV, Popovic EA. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke. 2002;33:2794–2800. doi: 10.1161/01.str.0000043674.99741.9b. [DOI] [PubMed] [Google Scholar]
- 3.Brown RD, Jr, Wiebers DO, Torner JC, O’Fallon WM. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted Country, Minnesota. J Neurosurg. 1996;85:29–32. doi: 10.3171/jns.1996.85.1.0029. [DOI] [PubMed] [Google Scholar]
- 4.Al-Shahi R, Fang JS, Lewis SC, Warlow CP. Prevalence of adults with brain arteriovenous malformations: a community based study in Scotland using capture-recapture analysis. J Neurol Neurosurg Psychiatry. 2002;73:547–551. doi: 10.1136/jnnp.73.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikuchi K, Kowada M, Sasajima H. Vascular malformations of the brain in hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease) Surg Neurol. 1994;41:374–380. doi: 10.1016/0090-3019(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 6.Laufer L, Cohen A. Sturge-Weber syndrome associated with a large left hemispheric arteriovenous malformation. Pediatr Radiol. 1994;24:272–273. doi: 10.1007/BF02015455. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Pawlikowska L, Chen Y, Su H, Yang GY, Young WL. Brain arteriovenous malformation biology relevant to hemorrhage and implication for therapeutic development. Stroke. 2009;40:S95–97. doi: 10.1161/STROKEAHA.108.533216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, Hashimoto T, Zaroff J, Lawton MT, Marchuk DA, Kwok PY, Young WL. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, Hashimoto T, Yang GY, Young WL. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008;62:1340–1349. doi: 10.1227/01.neu.0000333306.64683.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Saunders T, Su H, Kim H, Akkoc D, Saloner DA, Hetts SW, Hess C, Lawton MT, Bollen AW, Pourmohamad T, McCulloch CE, Tihan T, Young WL. Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke. 2012;43:1240–1246. doi: 10.1161/STROKEAHA.111.647263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Tihan T, Lawton MT, Kim H, Young WL, Zhao Y, Su H. Distinctive distribution of lymphocytes in unruptured and previously untreated brain arterviovenous malformation. Neuroimmunol Neuroinflamm. 2014;1:147–152. doi: 10.4103/2347-8659.143674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Zheng M, Shu H, Zhan S, Wang H, Zhou D, Zeng S, Tang K, Feng L. Macrophage migration inhibitory factor reduces apoptosis in cerebral arteriovenous malformations. Neurosci Lett. 2012;508:84–88. doi: 10.1016/j.neulet.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Choi EJ, McDougall CM, Su H. Brain arteriovenous malformation modeling, pathogenesis and novel therapeutic targets. Transl Stroke Res. 2014;5:316–329. doi: 10.1007/s12975-014-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58:313–320. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Han J, Bai HJ, Kim KW. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 2009;276:4622–4635. doi: 10.1111/j.1742-4658.2009.07174.x. [DOI] [PubMed] [Google Scholar]
- 16.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118:3436–3439. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao Q, Liu J, Pappu R, Su H, Rola R, Gabriel RA, Lee CZ, Young WL, Yang GY. Contribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leucocytes and macrophages. Arterioscler Thromb Vasc Biol. 2008;28:2151–2157. doi: 10.1161/ATVBAHA.108.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao Q, Su H, Palmer D, Sun B, Gao P, Yang GY, Young WL. Bone marrow-derived cells contribute to vascular endothelial growth factor-induced angiogenesis in the adult mouse brain by supplying matrix metalloproteinase-9. Stroke. 2011;42:453–458. doi: 10.1161/STROKEAHA.110.596452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto T, Lam T, Boudreau NJ, Bollen AW, Lawton MT, Young WL. Abnormal balance in the angiopoietin-tie2 system in human brain arteriovenous malformations. Circ Res. 2001;89:111–113. doi: 10.1161/hh1401.094281. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto T, Lawton MT, Wen G, Yang GY, Chaly T, Jr, Stewart CL, Dressman HK, Barbaro NM, Marchuk DA, Young WL. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54:410–423. doi: 10.1227/01.neu.0000103421.35266.71. [DOI] [PubMed] [Google Scholar]
- 22.Rothbart D, Awad IA, Lee J, Kim J, Harbaugh R, Criscuolo GR. Expression of angiogenic factors and stuctural proteins in central nervous system vascular malformations. Neurosurgery. 1996;38:915–924. doi: 10.1097/00006123-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 24.Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204:1–10. doi: 10.1002/path.1618. [DOI] [PubMed] [Google Scholar]
- 25.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinase tie-1 and tie2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 26.Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178:7405–7411. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- 27.Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, Lawton MT, Kim H, Chen Y, Chen W, Young WL. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69:954–962. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, Degos V, Lawton MT, Tihan T, Davalos D, Akassoglou K, Nelson J, Pile-Spellman J, Su H, Young WL. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol. 2013;33:305–310. doi: 10.1161/ATVBAHA.112.300485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, Mao L, Arnold T, Young WL, Su H. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke. 2014;45:900–902. doi: 10.1161/STROKEAHA.113.003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One. 2014;9:e88511. doi: 10.1371/journal.pone.0088511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi EJ, Walker EJ, Degos V, Jun K, Kuo R, Su H, Young WL. Endoglin deficiency in bone marrow is sufficient to cause cerebrovascular dysplasia in the adult mouse after vascular endothelial growth factor stimulation. Stroke. 2013;44:795–798. doi: 10.1161/STROKEAHA.112.671974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao Q, Zhu Y, Su H, Shen F, Yang GY, Kim H, Young WL. VEGF induces more severe cerebrovascular dysplasia in Endoglin+/− than in Alk1+/− mice. Transl Stroke Res. 2010;1:197–201. doi: 10.1007/s12975-010-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrido-Martin EM, Nguyen HL, Cunningham TA, Choe SW, Jiang Z, Arthur HM, Lee YJ, Oh SP. Common and distinctive pathogenetic features of arteriovenous malformations in hereditary hemorrhagic telangiectasia 1 and hereditary hemorrhagic telangiectasia 2 animal models–brief report. Arterioscler Thromb Vasc Biol. 2014;34:2232–2236. doi: 10.1161/ATVBAHA.114.303984. [DOI] [PubMed] [Google Scholar]
- 34.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasan D, Chalouhi N, Jabbour P, Hashimoto T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: preliminary results. J Neuroinflammation. 2012;9:222. doi: 10.1186/1742-2094-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yousem DM, Flamm ES, Grossman RI. Comparison of MR imaging with clinical history in the identification of hemorrhage in patients with cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 1989;10:1151–1154. [PMC free article] [PubMed] [Google Scholar]
- 40.Prayer L, Wimberger D, Stiglbauer R, Kramer J, Richling B, Bavinzski G, Czech T, Imhof H. Haemorrhage in intracerebral arteriovenous malformations: detection with MRI and comparison with clinical history. Neuroradiology. 1993;35:424–427. doi: 10.1007/BF00602821. [DOI] [PubMed] [Google Scholar]
- 41.Stabi KL, Bendz LM. Ferumoxytol use as an intravenous contrast agent for magnetic resonance angiography. Ann Pharmacother. 2011;45:1571–1575. doi: 10.1345/aph.1Q431. [DOI] [PubMed] [Google Scholar]
- 42.Chalouhi N, Jabbour P, Magnotta V, Hasan D. The emerging role of ferumoxytol-enhanced MRI in the management of cerebrovascular lesions. Molecules. 2013;18:9670–9683. doi: 10.3390/molecules18089670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dosa E, Tuladhar S, Muldoon LL, Hamilton BE, Rooney WD, Neuwelt EA. MRI using ferumoxytol improves the visualization of central nervous system vascular malformations. Stroke. 2011;42:1581–1588. doi: 10.1161/STROKEAHA.110.607994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasan DM, Mahaney KB, Magnotta VA, Kung DK, Lawton MT, Hashimoto T, Winn HR, Saloner D, Martin A, Gahramanov S, Dosa E, Neuwelt E, Young WL. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler Thromb Vasc Biol. 2012;32:1032–1038. doi: 10.1161/ATVBAHA.111.239871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasan D, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, Young WL, Hashimoto T, Winn HR, Heistad D. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: a pilot study. Stroke. 2012;43:3258–3265. doi: 10.1161/STROKEAHA.112.673400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasan DM, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, Young WL, Hashimoto T, Richard Winn H, Heistad D. Evidence that acetylsalicylic acid attenuates inflammation in the wall of human cerebral aneurysms: preliminary results. J Am Heart Assoc. 2013;2:e000019. doi: 10.1161/JAHA.112.000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasan DM, Amans M, Tihan T, Hess C, Guo Y, Cha S, Su H, Martin AJ, Lawton MT, Neuwelt EA, Saloner DA, Young WL. Ferumoxytol-enhanced MRI to image inflammation within human brain arteriovenous malformations: a pilot investigation. Transl Stroke Res. 2012;3:166–173. doi: 10.1007/s12975-012-0172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


