Abstract
Skin immunization is effective against a number of infectious diseases, including smallpox and tuberculosis, but is difficult to administer. Here, we assessed the use of an easy-to-administer microneedle (MN) patch for skin vaccination using an inactivated rotavirus vaccine (IRV) in mice. Female inbred BALB/c mice in groups of six were immunized once in the skin using MN coated with 5 µg or 0.5 µg of inactivated rotavirus antigen or by intramuscular (IM) injection with 5 µg or 0.5 µg of the same antigen, bled at 0 and 10 days, and exsanguinated at 28 days. Rotavirus-specific IgG titers increased over time in sera of mice immunized with IRV using MN or IM injection. However, titers of IgG and neutralizing activity were generally higher in MN immunized mice than in IM immunized mice; the titers in mice that received 0.5 µg of antigen with MN were comparable or higher than those that received 5 µg of antigen IM, indicating dose sparing. None of the mice receiving negative-control, antigen-free MN had any IgG titers. In addition, MN immunization was at least as effective as IM administration in inducing a memory response of dendritic cells in the spleen. Our findings demonstrate that MN delivery can reduce the IRV dose needed to mount a robust immune response compared to IM injection and holds promise as a strategy for developing a safer and more effective rotavirus vaccine for use among children throughout the world.
Keywords: Rotavirus, IRV, Microneedle, Skin immunization
1. Introduction
Group A rotavirus is a major cause of severe diarrhea in children less than 5 years of age and is responsible for an estimated 453,000 deaths per year worldwide [1]. Two licensed live oral rotavirus vaccines, RotaTeq® and Rotarix™, have shown high efficacy among infants in developed and middle-income countries [2,3], but are less effective in low income countries of Africa, Asia, and Latin America where a vaccine is needed most [4–8]. Both vaccines are also associated with a small risk of gastroenteritis and intussusception among vaccinated infants [9,10]. These limitations are believed to be due largely to the use of live vaccines administered via the oral route [11]. To address these problems, we are developing an inactivated rotavirus vaccine (IRV) for parenteral immunization as an alternative approach to oral vaccination for infants throughout the world [12,13]. We previously showed that thermally inactivated rotavirus when formulated with aluminum hydroxide was highly immunogenic in mice [14]. We further demonstrated that intramuscular (IM) administration of our candidate IRV, i.e., CDC-9, induced high immunogenicity and protection against infection from oral challenge with a virulent human rotavirus strain in gnotobiotic piglets [15].
While IM injection of IRV has been successful, there are ongoing efforts to further increase the delivery of this parenteral vaccine. Here, we hypothesized that vaccination in the skin could improve the immunogenicity and possible dose sparing of IRV because skin has a special immunologic network [16,17]. The skin is extremely rich in antigen-presenting cells (APCs) that include Langerhans cells (LCs), dermal dendritic cells (DCs), macrophages and monocytes, as well as accessory cells such as keratinocytes [17–19]. These APCs recognize, uptake, and present foreign antigens to T and B cells in the draining lymph nodes to initiate adaptive immune responses. Cutaneous immunization has been effective in preventing infectious diseases, such as smallpox, tuberculosis and rabies [17,20] and has achieved dose sparing for a number of vaccines compared to IM or subcutaneous (SC) injection [21]. In addition, vaccination using hypodermic needles requires trained medical personnel and thus has limitations for mass vaccination. Furthermore, the generation of bio-hazardous sharp wastes and the concern from needle-associated injuries and diseases are ongoing issues that could negatively impact vaccination coverage [22–24].
In this study, we investigated the use of microneedles (MNs) as a simple and reliable means to target IRV to the skin through the use of a patch that avoids the generation of hypodermic needle waste. MNs have been studied for the administration of a number of other vaccines and have shown evidence for dose sparing and increased immunogenicity compared to IM and SC delivery [25,26]. Using the tools of microfabrication, MN patches can be manufactured at low-cost for inexpensive mass production and can be administered painlessly, possibly by patients themselves.
2. Materials and methods
2.1. Preparation of IRV
CDC-9, a human G1P [8] rotavirus strain, was cultivated in Vero cells with roller bottle using Iscove’s Modified Dulbecco’s Medium (IMDM; Invitrogen, Grand Island, NY). Triple-layered particles (TLPs) were purified from culture supernatants by CsCl gradient centrifugation and inactivated by heat at 60 °C for 4 h [14]. Inactivated TLPs were concentrated by ultracentrifugation to a concentration of 3 mg/ml using the Bradford method in Hanks’ balanced salt solution (HBSS) with CaCl2 and MgCl2 (Invitrogen) supplemented with 10% sorbitol (Sigma–Aldrich, St. Louis, MO) and stored at 4 °C before being coated onto MN arrays.
2.2. Fabrication of MN
MNs were fabricated from stainless steel sheets and coated with IRV using a procedure modified from a published study [27]. The MNs were 750 µm long and assembled in rows of 5 MN each. For coating, the vaccine at a concentration of 3 mg/ml in HBSS was diluted 1:1 with a coating buffer consisting of 15% w/v trehalose, 2% w/v carboxymethyl cellulose and 1% w/v Lutrol F68 (all from Sigma–Aldrich) dissolved in deionized water. This solution was used to increase the viscosity and the wettability of the vaccine solution, and the trehalose helped stabilize the vaccine during drying. The MNs were coated using an automated coating station developed at the Georgia Institute of Technology. Each MN was dipped into a well containing the vaccine in coating solution 7 times and then allowed to air-dry for approximately 5 min. The coated MNs were then placed in a sterile container which was sealed and stored overnight in a laminar flow hood. The seal was broken and the arrays were removed in the animal facility at the time of insertion. The needles were then packaged and stored at 25 °C overnight before use. To measure the amount of vaccine coated on each array of MNs, 6 arrays were put in a 0.5 ml tube and dissolved in 200 µl of phosphate-buffered saline (PBS) in triplicate. The protein concentration in the eluate was measured by Micro BCA Protein Assay kit (Pierce Biotechnology). The average amount coated on a five-needle MN array was 1 µg. Five of these arrays (i.e. a total of 25 MNs) were used to administer a 5 µg dose. To administer a 0.5 µg dose, the coating solution of 1.5 mg/ml IRV was used to prepare a single MN array with 0.5 µg IRV.
2.3. Immunization of mice
Female BALB/c mice (6–8 weeks old; Charles River Laboratories, Wilmington, MA) were used for the immunization study. All mice were anesthetized with 110 mg/kg ketamine (Ketathesia™, Butler Schein Animal Health, Dublin, OH) and 11 mg/kg xylazine (Anased®, Lloyd Laboratories, Shenandoah, IA) that were injected IM and were pre-bled from the submandibular vein with an animal lancet (Medipoint International, Mineola, NY). The hair on the back of the mice was shaved using electric sheers and the remaining hair was removed using a depilatory cream (Nair, Church & Dwight, Princeton, NJ). The area was then thoroughly cleaned using sterile water and alcohol swabs. After drying, mice in groups of six were immunized once by inserting IRV-coated MN (5 µg or 0.5 µg) on the back for 10 min or once by injecting IM 5 µg or 0.5 µg of IRV in 100 µl of PBS into the upper quadriceps muscles. For controls, mice in groups of six were immunized once with MN prepared using coating solution without IRV or once IM with 5 µg of IRV reconstituted from MN in 100 µl PBS. Mice were bled on day 10 and exsanguinated on day 28, when spleens were removed and placed in tubes containing RPMI 1640 media.
2.4. Measurement of cellular immune responses
Splenocytes were prepared as a cell suspension by gently pressing organ segments through a 70 µm nylon cell strainer (BD Falcon, Franklin Lakes, NJ) using a plastic pipette and then passed through the mesh. Spleen cell suspensions were depleted of red blood cells using Tris–NH4Cl lysing buffer. The cells were washed twice with PBS plus 2% fetal bovine serum (Invitrogen, Grand Island, NY) and suspended in RPMI containing 20 mM HEPES, 100 U/ml of penicillin, and 100 mg/ml of streptomycin plus 10% fetal bovine serum. The cells were stored at −80 °C before use.
Splenocytes were thawed, counted and used for stimulation assay for dendritic cell responses by flow cytometry [28]. The cells (2 × 106 cells/ml) in IMDM were stimulated with either 200 µl of the supernatants of CDC-9 (7 × 107 fluorescence focus unit/ml)-infected Vero cells or 200 µl of the supernatants of mock-infected Vero cells (negative control). In addition, 10 µl of anti-CD28/anti-CD49d monoclonal antibody cocktail (BD Bio-sciences, San Diego, CA) was added to each sample as co-stimulator. Antigen stimulation was done in 15 ml polystyrene Falcon tubes (BD Falcon) by incubating at 37 °C with 5% CO2 for 5 h, followed by another 5 h incubation with the addition of brefeldin A (10 µg/ml; Sigma–Aldrich), which blocked the secretion of cytokines from the cells. The cells were then washed once with 1× IMag™ buffer (BD Biosciences) and treated with 2 mM EDTA (Sigma–Aldrich) in PBS for 10 min to detach adherent cells. After washing with 1× IMag™ buffer, the cells were fixed with 1% paraformaldehyde (Sigma–Aldrich) for 5 min, washed, and then frozen at −70 °C in 1× IMag™ buffer with 10% dimethyl sulfoxide (Sigma–Aldrich) before use.
Frozen cells were thawed, washed once with 1× IMag™ buffer, and used for the purification of DCs using Dynabeads Mouse DC Enrichment Kit (Invitrogen Dynal AS, Oslo, Norway) according to the manufacturer’s instructions. The DCs (93% pure) were incubated with 1× BD FACS permeabilizing soultion 2 (BD Bio-science) for 10 min, washed with 1× IMag™ buffer, and then stained for 30 min in the dark using a five-color assay with the following combinations of conjugated monoclonal antibodies: (1) MHCII-PE-Cy7/CD11c-Alex Fluor700/CD205-PE/CD11b-APC/CD80-FITC, (2) MHCII-PE-Cy7/CD11c-Alex Fluor700/CD205-PE/CD11b-APC/CD86-FITC, (3) MHCII-PE-Cy7/CD11c-Alex Fluor700/ B220-PE/mPDCA1-APC/CD80-FITC, and (4) MHCII-PE-Cy7/CD11c-Alex Fluor700/B220-PE/mPDCA1-APC/CD86-FITC. Cells were also stained with PE-labeled, isotype-matched control monoclonal anti-bodies. PE-Cy7, Alexa Fluor 700, FITC, APC conjugates were obtained from BD Biosciences; anti-CD205-PE and anti-mPDCA1-APC conjugates were obtained from Mitenyi Biotec (Auburn, CA); and anti-MHCII-PE-Cy7 conjugate was obtained from eBioscience (San Diego, CA). All monoclonal antibodies were titrated and used at optimal concentrations. Stained cells were analyzed using a LSR II (BD Biosciences) and 30,000–50,000 events were counted. Data were analyzed with FACSDiva software (BD Biosciences) [29].
2.5. Measurement of humoral immune responses
Rotavirus-specific IgG in pooled sera on day 0 and individual sera on day 10 and 28 were measured using a modified immunoassay, as described previously [14]. Briefly, 96-well plates (Immulon 2; NalgeNunc, Rochester, NY) were coated with diluted rabbit hyper-immune serum to rotavirus Wa at 4 °C overnight. After washing, the plates were incubated at 37 °C first with blotto (5% skim milk in PBS) as a blocking agent, and then with supernatants of rhesus rotavirus-infected MA104 cell cultures, followed by the addition of serially diluted mouse sera. Plates were further incubated sequentially with HRP-conjugated goat anti-mouse IgG antibody (Southern Biotech, Birmingham, AL). The color of reactions was developed with tetramethyl benzidine (TMB; Aldrich, Milwaukee, WI) substrate and stopped with 1 N HCl. The optical density (OD) at 450 nm was measured with an EIA reader (MRX Revelation, Dynex Technologies, Chantilly, VA). Antibody titer in a serum specimen was defined as the reciprocal of the highest dilution that gave a mean OD greater than the cutoff value (3 standard deviations above the mean OD of the blotto wells). Rotavirus-specific IgG subclasses in pooled sera on day 0, 10 and 28 were measured by EIA in the same manner, except for the use of HRP-conjugated goat anti-mouse IgG subclass antibody (Southern Biotechnology). The concentration of an IgG subclass in serum was calculated relative to the values of a standard curve, which was constructed with purified mouse IgG subclasses (Southern Biotechnology). The ratio of different IgG subclasses was determined on the basis of their concentrations in serum.
Rotavirus-specific neutralizing activity was measured against Wa rotavirus using a modified microneutralization assay [14,30]. Mouse sera were serially diluted two-fold in duplicate wells of 96-well plates and incubated with 600 fluorescence focus units (ffu) of Wa per well for 1 h. MA104 cells in IMDM supplemented with a concentration of 10 µg/ml trypsin (Invitrogen) and 0.5% chicken serum (Invitrogen) were added to each well. After incubation at 37 °C in a CO2 incubator for 3 days, the plates were fixed with formalin. Rotavirus antigens were detected with a rabbit anti- Wa hyperimmune serum, HRP-labeled anti-rabbit IgG and then TMB. Neutralizing antibody titer was defined as the reciprocal of the highest dilution that gave a greater than 70% reduction in the absorbance value compared with that in virus-only controls.
2.6. Statistics
Statistical analysis was performed with PASW Statistics 18 (SPSS Inc., Chicago, IL) using nonparametric tests. Differences in results between different groups of animals were compared with the one way ANOVA test. Significance was established if P was <0.05.
3. Results
3.1. Structural and antigenic integrity of TLPs after coating on MN
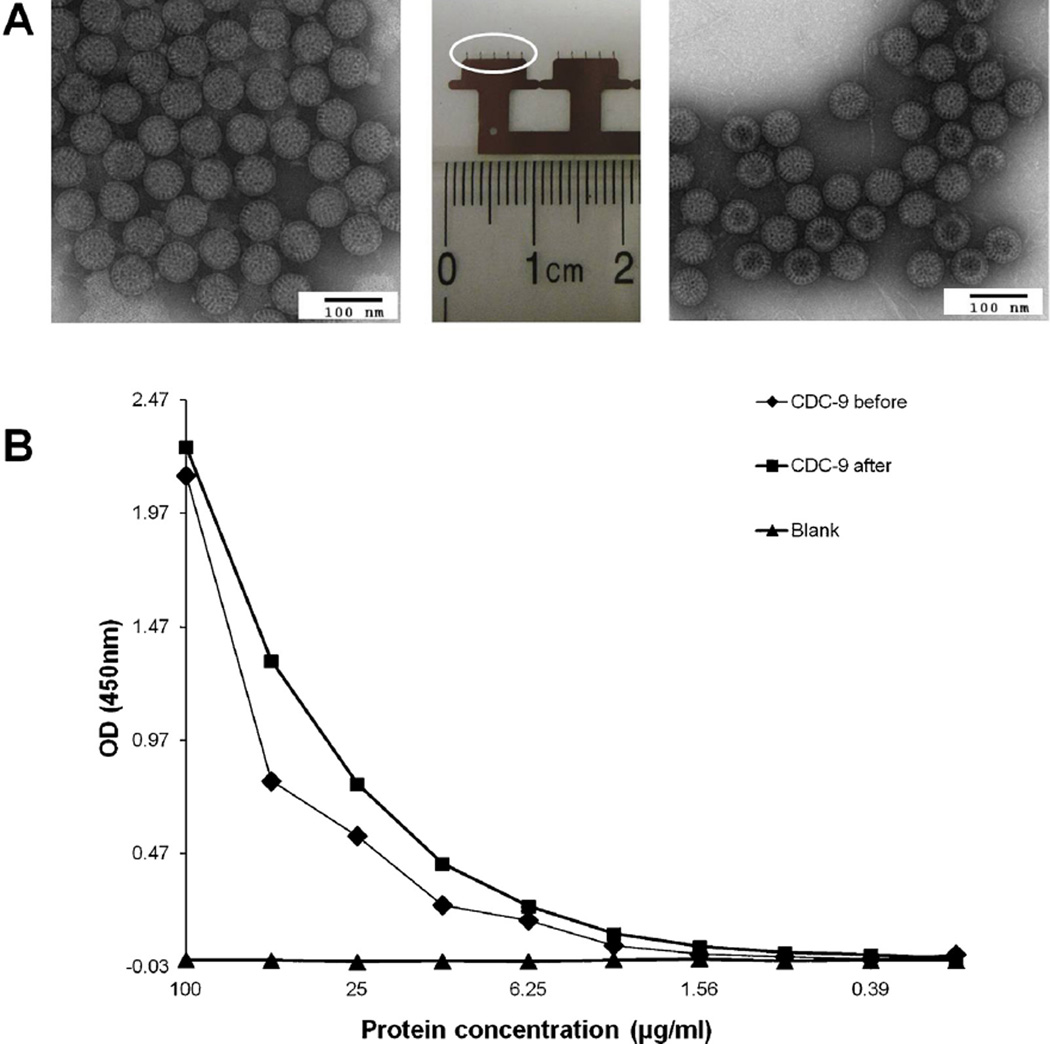
After preparing IRV-coated MN, we assessed the structural integrity of the IRV by electron microscopy after reconstitution, and observed similar intact TLPs in coating buffer before coating onto MN and in PBS after elution from coated MN (Fig. 1A). To examine whether eluted IRV maintained antigenicity, we compared reactivities of TLPs before and after coating by EIA using rabbit hyperimmune serum to the human rotavirus strain Wa and demon-strated similar absorbance values in both preparations (Fig. 1B). These results indicate that inactivated rotavirus TLPs in coating buffer can survive drying on MN and, more importantly, MN coating does not appear to alter structural integrity and antigenic reactivities of our IRV. Our findings agree with those of an experimental influenza vaccine in which a similar formulation was used to coat MN and similar antigen integrity was maintained after coating [27].
Fig. 1.
Stability and antigenicity of inactivated rotavirus particles coated on MN. A: Electron micrographs of inactivated CDC-9 IRV particles before coating (left) and after reconstitution from MN one day after coating (right). Triple-layered CDC-9 particles were stained with phosphotungstic acid and examined with an electron microscope. Bar = 100 nm. The central image shows two MN devices each with a row of five MN (circle shows a five-MN array). B: Levels of rotavirus antigen detected in original and reconstituted preparations by EIA. Similar levels of absorbance in original and reconstituted CDC9 preparations were observed. A blank was tested as a negative control.
3.2. Cellular immune responses after IRV vaccination with MN
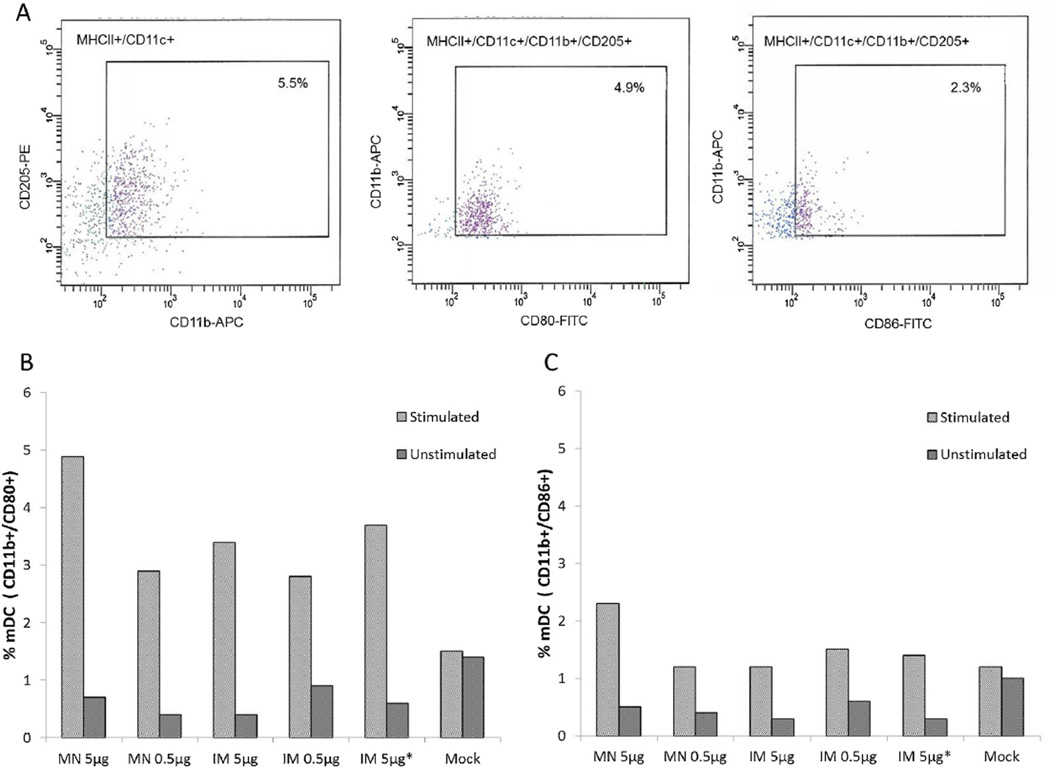
We next vaccinated mice in groups of six and assessed the response of DCs to IRV in spleens of vaccinated mice, as the effector phenotype of DCs is known to influence T and B cell responses to microbial infection and vaccination [31]. We determined the phenotype subset of DCs by measuring the expression of surface markers CD11b and CD205 or B220 and mPDCA and the maturation and activation by detecting the expression of CD11c and the co-stimulatory marker CD80 or CD86 [32,33] in total splenocytes from mice that were exsanguinated 28 days post vaccination, following the stimulation of cells with the CDC-9 strain (Fig. 2). Representative FACS plots are shown for phenotype myeloid DCs (mDC) and matured mDC expressing CD80 or CD86 from splenocytes of mice that received MN vaccination with 5 µg IRV (Fig. 2A). Both MN and IM immunizations with IRV appeared to induce a memory response for the activation of mDCs (Fig. 2B.) IRV of high and low doses administered by MN or IM induced an increase in the proportions of mDCs expressing CD80 compared to that from mock-infected mice. By contrast, except for a slight increase in mice receiving MN vaccination with 5 µg IRV, there was no apparent increase in the percentages of mDCs expressing CD86 in the spleens of mice that received the low-dose (0.5 µg) MN vaccination or any of the IM vaccinations, when compared to that from mock-infected mice (Fig. 2C). These results indicate that IRV vaccination by MN and IM administration appeared to induce a recall response for the activation of mDCs. In contrast, we did not see evidence for the activation of plasmacytoid DCs (pDCs) in spleens of mice (data not shown). We also assessed T-cell proliferation in response to MN or IM vaccination and observed no apparent activation of CD4+ and CD8+ T lymphocytes in spleens of IRV-vaccinated mice compared to those from mock-inoculated mice (data not shown).
Fig. 2.
Phenotype and maturation of DCs in the spleens of mice that received IRV by MN and IM administration. Splenocytes were stimulated with rotavirus, purified for DCs, and examined for the expression of surface and co-stimulatory markers CD11c, CD80 and CD86 as described in the text. A: Representative FACS plots showing the phenotype and maturation expressing CD80 and CD86 of mDCs in the spleens of mice 28 days after receiving 5 µg of IRV using a MN patch, following in vitro stimulation with rotavirus. B and C: Proportions of activated mDCs with CD80 or CD86 expression in stimulated splenocytes of mice 28 days after receiving IRV by MN or IM administration. The character “*” indicates IRV reconstituted from coated MN.
3.3. Humoral immune responses after IRV vaccination with MN
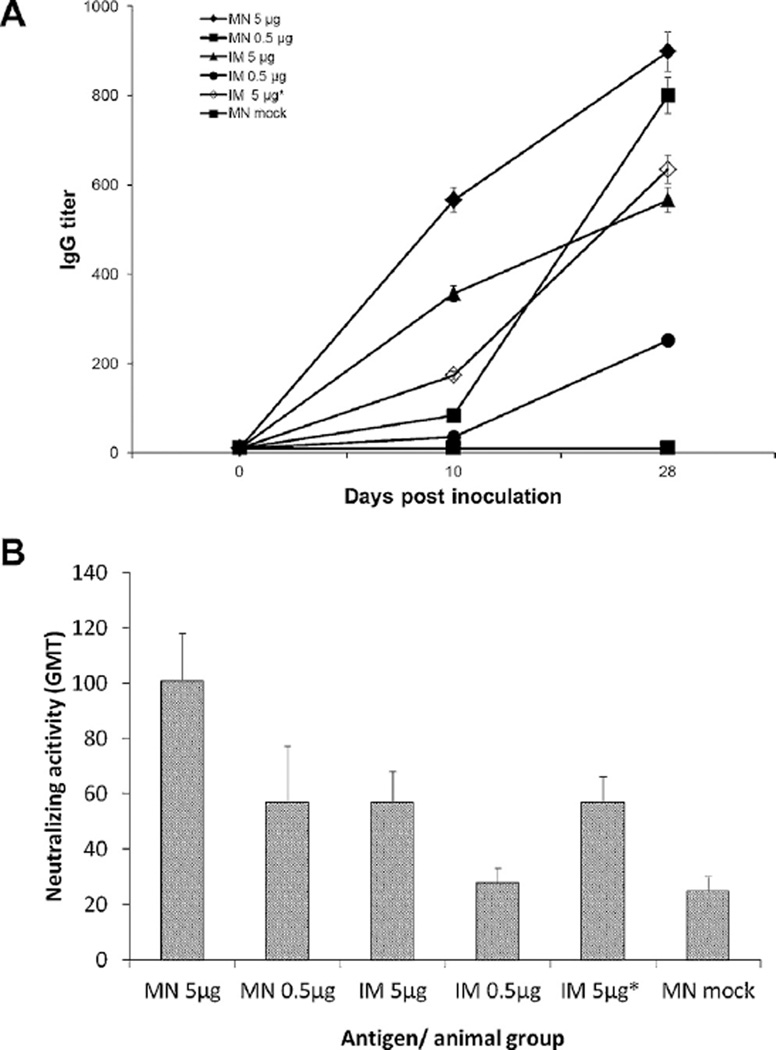
To compare the immunogenicity and assess possible dose-sparing effects of IRV vaccination in the skin using MN and by IM delivery using a hypodermic needle, we measured rotavirus-specific total IgG in serum specimens collected from mice on day 0, 10 and 28 (Fig. 3A). Except for mice that were mock immunized with vaccine-free MN and had no detectable IgG throughout the period of study, all mice in the vaccinated groups developed IgG response that increased in a time- and dose-dependent manner. Mice that received a single high dose of 5 µg IRV with MN had significantly higher levels of IgG than those in groups that received a single dose (IM 5 µg, IM 5 µg reconstituted from MN, MN 0.5 µg and IM 0.5 µg) at day 10 (p < 0.05). On day 28, mice that were vaccinated using MN at antigen dose of 5 µg or by IM route at 5 µg or 5 µg reconstituted from MN had similar levels of IgG. Significant higher IgG titers were seen with MN than IM route at low dose (p < 0.05). Notably, the low-dose MN vaccination generated a similar IgG titer as the high-dose IM vaccination on both days 10 and 28, indicating strong dose sparing associated with MN vaccination in the skin.
Fig. 3.
Rotavirus-specific IgG (A) and neutralizing activity (B) in sera of vaccinated mice. Mice (n = 6 per group) were immunized with MN coated with 5 µg, 0.5 µg, or 0 µg (mock) of IRV or by IM injection with 5 µg or 0.5 µg of IRV, or 5 µg of IRV reconstituted from coated MN. Serum samples collected at days 0, 10 and 28 after vaccination were analyzed for IgG and sera collected on day 28 were examined for neutralizing activity as described in the text. The character “*” indicates IRV reconstituted from coated MN. Error bars show standard deviation.
We measured titers of neutralizing activity in sera of mice at 28 days after immunization (Fig. 3B). Mice vaccinated using MN at high dose of 5 µg had significantly higher levels of neutralizing activity in sera than those that were immunized IM at high or low dose, or using MN at low dose (p < 0.05). Higher levels of neutralizing activity were seen in mice that were vaccinated IM at 5 µg or 5 µg reconstituted from MN than at 0.5 µg, which induced a low level of neutralizing activity similar to that in mock-immunized mice, but the differences were not statistically significant. Again, high-dose MN vaccination generated stronger neutralizing activity than high-dose IM vaccination and low-dose MN vaccination generated equivalent neutralizing activity to high-dose IM vaccination, indicating dose-sparing effects.
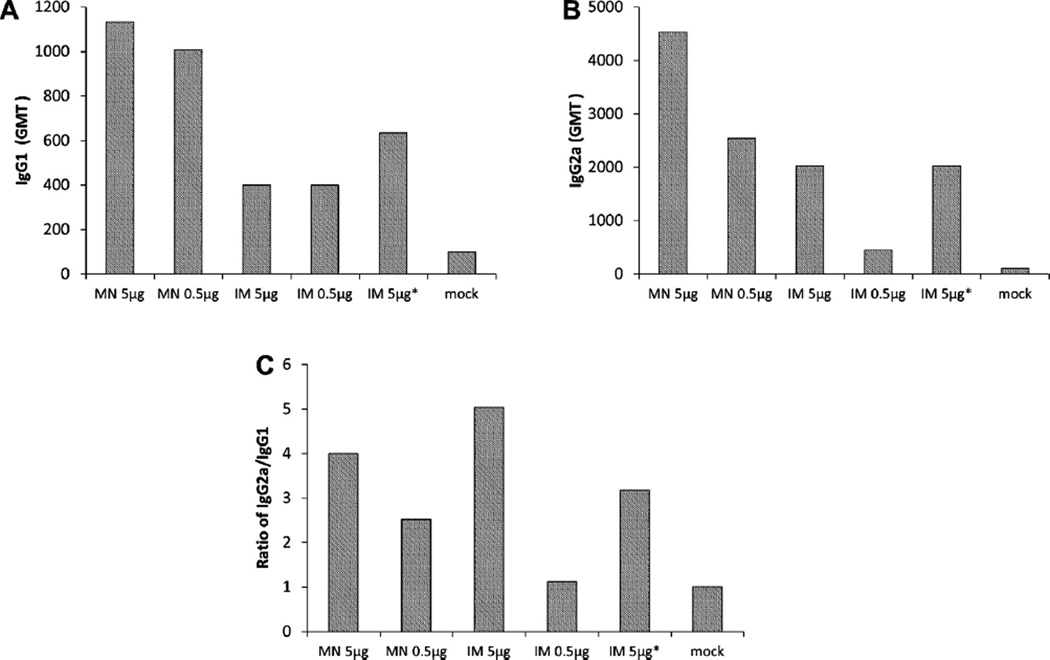
We last examined rotavirus-specific IgG subclasses and the type of Th cell response by determining the levels of IgG1 and IgG2a using EIA. MN-immunized mice had comparable or higher levels of IgG1 and IgG2a than those that received IRV by IM administration (Fig. 4A and B). IgG2a titers were higher than IgG1 titers in all vaccinated mice except those that received low-dose IM vaccination (Fig. 4C). These results indicate that IRV administered by MN and IM elicited both Th1 and Th2 responses and that Th1 cells were generally dominant.
Fig. 4.
IgG subclasses IgG1 (A) and IgG2a (B) in sera of mice that received IRV by MN or IM administration. Pooled sera on day 28 were tested by EIA for IgG1 and IgG2a as described in the text. Data showing the ratio of IgG2a and IgG1 are shown in panel C. The character “*” indicates IRV reconstituted from coated MN.
4. Discussion
This study demonstrated for the first time that IRV administered to skin using a MN patch is effective in inducing robust immune response in mice. This initial finding could pave the way for the development of a low-cost and effective patch-based IRV. Notably, we observed similar immunogenicity of IM administered IRV and IRV reconstituted from coated MN, demonstrating that MN coating did not appear to alter the integrity of viral particles and the antigenicity of IRV. Of interest, a very low dose (0.5 µg) of IRV administered by MN inoculation induced antibody titers at least as strong as those from 5 µg of IRV by IM administration, demonstrating that MN delivery has apparent dose sparing effects. MN immunization also induced higher titers of rotavirus-specific IgG and neutralizing activity than IM immunization at the same dose, demonstrating that MN delivery has the potential to enhance the immunogenicity of IRV. Finally, MN and IM delivery appeared to be equally effective in inducing a recall response in a subset of DCs, suggesting that epidermal Langerhans cells and dermal DCs might uptake the vaccine in the skin and upon stimulation and subsequent activation, migrate as matured mDCs to lymphoid organs such as the lymph nodes and spleen. This type of anamnestic response could help maintain sustainable systemic immunity and protection against rotavirus infection.
We found that both MN and IM administration of IRV in the same formulation induced a generally higher level of IgG2a than IgG1, thus a slightly more Th1 type of response. This differential immune profile may result from virus–host interactions under varying conditions or environments. For example, some DCs when stimulated and activated are more likely to induce Th1 type response, whereas macrophages tend to induce Th2 type response [34]. The type of IgG subclass induced by vaccination has been reported to reflect the quality of immune response and influence the protection against rotavirus and other viral infections in animals [30,35].
In the present study, we compared the immunogenicity of IRV by MN and IM administration in the absence of an adjuvant in mice. Since adjuvant is often used to enhance immune response to IM-administered vaccine and lower the amount of antigen needed, it will be important to test in a future study if MN delivery of IRV without adjuvant can still induce a comparable or better immune response compared to adjuvanted IM vaccine. As we previously reported that at least two immunization regimens are needed to prime the host and boost immune responses [14], we plan to evaluate the number of doses required to augment the titers of antibody, particularly neutralizing activity in serum of vaccinated animals. Studies are also warranted to investigate whether MN and IM immunization of IRV can induce equivalent protection against oral challenge with virulent homotypic and heterotypic human strains in gnotobiotic piglets [15].
In conclusion, as the first study to research skin vaccination with IRV, we demonstrated that a single dose of IRV administered with a MN patch induced robust immune responses in mice. Anti-body titers were generally higher after MN delivery compared to IM injection. Dose sparing was shown, such that low-dose (0.5 µg) MN vaccination generated antibody responses at least as strong as high-dose (5 µg) IM vaccination. MN and IM delivery appeared to be equally effective in inducing a recall response in a proportion of circulating mDCs in the spleen. Overall, these data provide the first proof-of-principle that a MN patch may enable IRV vaccination with increased immunogenicity, dose sparing, simple administration and avoidance of hypodermic needles.
Acknowledgments
We thank Charles Humphrey for performing electron micro-graph analysis of rotavirus particles and John Zhang for helping perform statistical analysis of data. This work was supported in part by the U.S. National Institutes of Health. M.R.P. is an inventor of patents that have been licensed to companies developing MN-based products, is a paid advisor to companies developing MN-based products and is a founder/shareholder of companies developing MN-based products.
Footnotes
The finding and conclusions in this report are those of the authors and do not necessarily represent the views of CDC.
Conflict of interest: S.M., Y.W., C.E., J.R.G and B.J. have no conflict of interest. This potential conflict of interest has been disclosed and is overseen by Georgia Institute of Technology and Emory University.
References
- 1.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 3.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human–bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [see comment] [DOI] [PubMed] [Google Scholar]
- 4.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 5.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 6.Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin. 2009;5:414–419. doi: 10.4161/hv.5.6.8176. [DOI] [PubMed] [Google Scholar]
- 7.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 8.Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 9.Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–2292. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 10.Buttery JP, Danchin MH, Lee KJ, Carlin JB, Mcintyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the national immunization program in Australia. Vaccine. 2011;29:3061–3066. doi: 10.1016/j.vaccine.2011.01.088. [DOI] [PubMed] [Google Scholar]
- 11.Moon SS, Wang Y, Shane AL, Nguyen T, Ray P, Dennehy P, et al. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J. 2010;29:919–923. doi: 10.1097/INF.0b013e3181e232ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine. 2008;26:6754–6758. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Esona MD, Foytich K, Wang Y, Shin G, Wei G, Gentsch JR, et al. Molecular characterization of human rotavirus vaccine strain CDC-9 during sequential passages in Vero cells. Hum Vaccin. 2010;6:247–253. doi: 10.4161/hv.6.3.10409. [DOI] [PubMed] [Google Scholar]
- 14.Jiang B, Wang Y, Saluzzo JF, Bargeron K, Frachette MJ, Glass RI, et al. Immunogenicity of a thermally inactivated rotavirus vaccine in mice. Hum Vaccin. 2008;4:143–147. doi: 10.4161/hv.4.2.5263. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Azevedo M, Saif LJ, Gentsch JR, Glass RI, Jiang B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine. 2010;28:5432–5436. doi: 10.1016/j.vaccine.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 17.Weniger BG, Papania MJ. Alternative vaccine delivery methods. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Philadelphia: Elsevier/Saunders; 2013. pp. 1200–1231. [Google Scholar]
- 18.Stingl G, Steiner G. Immunological host defense of the skin. Curr Probl Dermatol. 1989;18:22–30. doi: 10.1159/000416834. [DOI] [PubMed] [Google Scholar]
- 19.Steinhoff M, Brzoska T, Luger TA. Keratinocytes in epidermal immune responses. Curr Opin Allergy Clin Immunol. 2001;1:469–476. doi: 10.1097/01.all.0000011062.60720.e3. [DOI] [PubMed] [Google Scholar]
- 20.Karande P, Mitragotri S. Transcutaneous immunization: an overview of advantages, disease targets, vaccines, and delivery technologies. Ann Rev Chem Biomol Eng. 2010;1:175–201. doi: 10.1146/annurev-chembioeng-073009-100948. [DOI] [PubMed] [Google Scholar]
- 21.Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304:247–268. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- 22.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19:95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 23.Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003;9:99–103. doi: 10.1038/nm0103-99. [DOI] [PubMed] [Google Scholar]
- 24.Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005;5:905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 25.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201:190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernando GJ, Chen X, Prow TW, Crichton ML, Fairmaid EJ, Roberts MS, et al. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS ONE. 2010;5:e10266. doi: 10.1371/journal.pone.0010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Rel. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaimes MC, Rojas OL, Gonzalez AM, Cajiao I, Charpilienne A, Pothier P, et al. Frequencies of virus-specific CD4(+) and CD8(+) T lymphocytes secreting gamma interferon after acute natural rotavirus infection in children and adults. J Virol. 2002;76:4741–4749. doi: 10.1128/JVI.76.10.4741-4749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon S, Wang Y, Dennehy P, Simonsen KA, Zhang J, Jiang B. Antigenemia, RNAemia, and innate immunity in children with acute rotavirus diarrhea. FEMS Immunol Med Microbiol. 2012;64:382–391. doi: 10.1111/j.1574-695X.2011.00923.x. [DOI] [PubMed] [Google Scholar]
- 30.Jiang B, Estes MK, Barone C, Barniak V, O’Neal CM, Ottaiano A, et al. Heterotypic protection from rotavirus infection in mice vaccinated with virus-like particles. Vaccine. 1999;17:1005–1013. doi: 10.1016/s0264-410x(98)00317-x. [DOI] [PubMed] [Google Scholar]
- 31.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–1526. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 33.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Becker G, Sornasse T, Nabavi N, Bazin H, Tielemans F, Urbain J, et al. Immunoglobulin isotype regulation by antigen-presenting cells in vivo. Eur J Immunol. 1994;24:1523–1528. doi: 10.1002/eji.1830240710. [DOI] [PubMed] [Google Scholar]
- 35.Hancock GE, Speelman DJ, Frenchick PJ, Mineo-Kuhn MM, Baggs RB, Hahn DJ. Formulation of the purified fusion protein of respiratory syncytial virus with the saponin QS-21 induces protective immune responses in Balb/c mice that are similar to those generated by experimental infection. Vaccine. 1995;13:391–400. doi: 10.1016/0264-410x(95)98263-a. [DOI] [PubMed] [Google Scholar]