Abstract
BACKGROUND
The Arizona Alzheimer's Consortium (AAC) created the Arizona Alzheimer's Registry, a screening and referral process for people interested in participating in Alzheimer's disease related research. The goals of the Registry were to increase awareness of Alzheimer's disease research and accelerate enrollment into AAC research studies.
METHODS
Participation was by open invitation to adults 18 and older. Those interested provided consent and completed a written questionnaire. A subset of Registrants underwent an initial telephone cognitive assessment. Referral to AAC sites was based on medical history, telephone cognitive assessment, and research interests.
RESULTS
A total of 1257 people consented and 1182 underwent an initial cognitive screening. Earned media (38.7%) was the most effective recruitment strategy. Participants had a mean age of 68.1 (SD 10.6), 97% were Caucasian, had 15.2 (SD 2.7) mean years of education, and 60% were female. 30% reported a family history of dementia and 70% normal cognition. Inter-rater agreement between self-reported memory status and the initial telephone cognitive assessment had a kappa of 0.31-0.43. 301 were referred to AAC sites.
CONCLUSION
IThe Registry created an infrastructure and process to screen and refer a high volume of eager Registrants. These methods were found to be effective at prescreening individuals for studies, which facilitated AAC research recruitment. The established infrastructure and experiences gained from the Registry have served as the prototype for the web-based Alzheimer's Prevention Registry, a national registry focusing on Alzheimer's disease prevention research.
Keywords: Alzheimer's disease, registry, prevention, recruitment
Background
Alzheimer's disease (AD) poses a significant public health challenge, since age is a major risk factor and the population of those 65 and older is doubling over the next 20 years(1). Current estimates of the prevalence of AD suggest that 4.7 million Americans are living with this disease; by 2050 that number is expected to almost triple(2). The United States government has established a national plan to address this public health challenge with the first goal being to prevent and effectively treat AD by 2025(3). Enrollment into randomized controlled trials to assess the efficacy of both prevention and treatment strategies is a vital goal of the National Plan to Address Alzheimer's Disease (3). There are numerous potential AD therapeutics under investigation(4) and thus large volumes of eligible volunteers are required.
As the number of AD-related research studies continues to increase, there is a need to rapidly communicate with and screen large numbers of potential participants to both inform them and gauge their interest in participation, thereby helping to overcome recruitment challenges (5, 6). Although disease-specific registries have typically been created either to collate medical record data from patients with rare diseases or as a public health instrument, they can also serve as a research pre-enrollment mechanism. For example, the Leon Thal Symposium of 2010 explored the use of registries to aid with recruitment into AD research (7). Community helplines and AD registries provide advantages such as enhancing pre-screening capabilities, assessing site feasibility, and laying the foundation for cohort studies (8, 9).
In, 2006 the Arizona Alzheimer's Consortium (AAC) created the Arizona Alzheimer's Registry (“Registry”) to facilitate enrollment into AD research studies being conducted at AAC sites. This paper describes the design, implementation experience, and outcomes of this Registry.
Methods
Registry
The AAC was the first statewide NIA-funded AD research consortium and includes Arizona State University, Banner Alzheimer's Institute (BAI), Banner Sun Health Research Institute, Barrow Neurological Institute, Mayo Clinic Arizona, Translational Genomics Research Institute (TGen), and University of Arizona. The AAC charged BAI with creating a screening and referral process for people interested in participating in AD related research in their communities, as well as a relational database for all relevant information. This process and database was called the Arizona Alzheimer's Registry and was hosted at BAI. The goals of the Registry were to increase awareness of research in the fields of dementia and AD, expedite enrollment into AD related research studies, and increase research activity within the AAC. The aim of the Registry was to match motivated Registrants into AAC clinical research studies according to interest, location, and eligibility. An early and ongoing element of the process was creating an accurate catalogue of AAC research projects.
Registrants
Anyone age 18 and older was eligible to participant in the Registry, although recruitment activities (disclosed below) targeted ages 50 and older. Interested individuals were required to be able to communicate in English and cooperate with symptom assessment and study procedures. No other criteria were required. Respondents provided written informed consent under guidelines approved by the human subjects committee at Western Institutional Review Board (WIRB). Cognitively impaired individuals provided written assent in addition to written informed consent from a legally authorized representative.
Participants were recruited using a variety of methods including community events such as memory screenings, lectures, seminars, and conferences targeting either professionals or the general public. Registry flyers, brochures, and letters were distributed at public events, displayed in AAC clinics, and through mass-mailings. Public service announcements and paid advertising through print, radio, and television media were utilized. The Registry created and maintained a website (www.AZalz.org) as both a source of information and a registration tool.
Interested individuals contacted the Registry directly by mail, telephone, email, website, or in-person at public events. Trained Registry staff collected the participant's contact information (name, address, and telephone number), provided a brief description of the Registry and offered a welcome packet for those interested in the program. The welcome packet included a letter, brochure, comprehensive questionnaire, and consent form. The questionnaire included a medical history, current medication use, family history of dementia, research interests and availability, and geographic preferences for research site location. After receiving the completed consent and questionnaire, Registry staff contacted the Registrant or authorized representative via telephone for a verbal review of the consent form and telephone assessment. During the telephone consent review, pertinent information provided in the questionnaire was reviewed for accuracy or clarification.
Telephone assessment
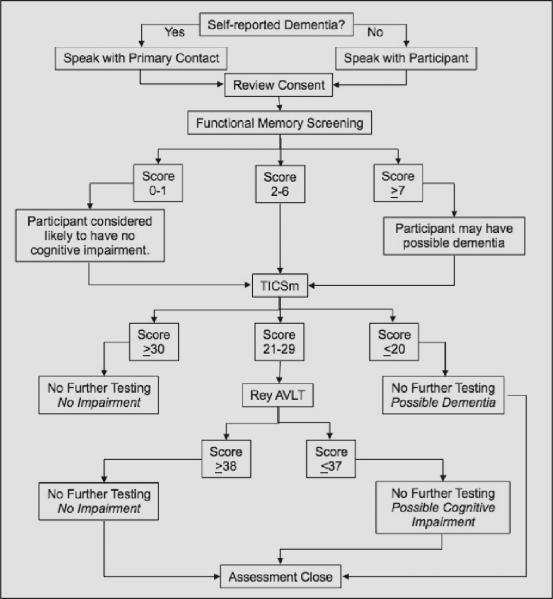
The telephone assessment included some or all of the following: self reported memory status, functional memory assessment (10), Telephone Interview Cognitive Screen, modified (TICSm) (11, 12), Rey Auditory Verbal Learning Test (AVLT) (13), and Mild Cognitive Impairment Screen (MCIS) (11). Figure 1 depicts s the telephone assessment process. Subjective memory status was characterized as “normal for age,” “mild memory loss,” or “significant memory loss.” At the completion of the assessment a Registrant was assigned one of three possible outcomes: no impairment, possible cognitive impairment, or possible dementia. Subsequent follow-up telephone assessments were conducted on a subset of participants as funding permitted. An algorithm was developed to select those whose follow-up assessment could result in a change in diagnostic category and thus potentially initiate a new site referral.
Figure 1.
Sequence of telephone assessment components
Referral
An AAC clinician reviewed the assessment outcome in conjunction with pertinent medical history: this determined the Registrant's referral trajectory. Efforts were made to refer potentially eligible Registrants to existing AAC studies based on research interests and geographic preference. If no such referral could be made, Registrants were retained in the Registry database for possible referral to future studies. In some cases, additional clinical assessment with the Registrant's primary care physician (PCP), referral to a specialist, or to the “Confirm” sub-study was recommended. The Confirm sub-study was added to the Registry process when we learned that a significant minority of Registrants wished to have an evaluation of their cognitive concerns within the context of research; AAC medical providers offered this. Registrants who were referred to AAC research sites were then screened for eligibility and contacted by the AAC site that received the referral. AAC sites that received referrals were requested to provide feedback to the Registry regarding the referral outcome, and assumed responsibility for Registrants enrolled into studies. All data were maintained in a secure electronic database housed at BAI (Filemaker Pro 11.0v3, San Francisco, CA).
Data analysis
Data from July 1, 2006 through June 22, 2011, the period during which funding was available, were included. Analyses and descriptive statistics were calculated using STATA 11.0 (StataCorp, College Station, TX). Self-reported memory status and telephone cognitive assessment measures were calculated for inter-rater agreement using Cohen's Kappa. Three kappa statistics were calculated each using different 3×3 tables for agreement. The first calculation assigned perfect agreement as self reported status of no memory impairment matching the telephone cognitive assessment of no impairment, self-report of mild memory impairment matching the presence of possible cognitive impairment on assessment, and significant memory impairment matching the presence of possible dementia on assessment. The second calculation used a weighted assignment with perfect agreement as in the first calculation and partial agreement of 0.5 for mild memory loss and possible dementia and significant memory loss and possible cognitive impairment. The final calculation accepted perfect agreement as a self-report of no memory impairment matching the assessment of no impairment and any self-report of memory impairment matching any assessment of possible cognitive impairment or possible dementia. Standard interpretation of kappa values was used (14).
Results
Registry
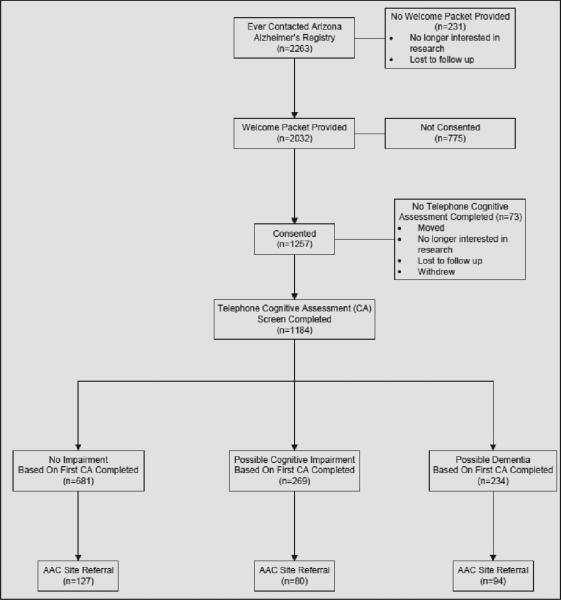
Figure 2 depicts the progression of Registrants. A total of 2263 contacted the Registry, and 2032 were provided a Welcome Packet. Of those that received a packet, 1257 were consented into the Registry and 1182 underwent initial telephone assessment.
Figure 2.
Registry consort diagram depicting the progress of volunteers through stages of participation
Registrants
The demographic characteristics of people who contacted the Registry between 2006 and 2011 are displayed in Table 1. The mean and median age at the time of contact and intake into the registry was 68 years old. Registrants were predominantly white (97.3%), non-Hispanic (94.0%), female (60.3%), and were highly educated with a median of 16 years of education. Most were married and living at home with a spouse or other family member. A total of 681 (30.1%) reported having a family history of dementia. A total of 247 (19.9%) reported having a diagnosis of dementia or cognitive impairment, with AD being the most commonly reported dementia syndrome (60.3%).
Table 1.
Registry participant demographics
| Age | 68.1 ± 10.6 | |
| Education | 15.2 ± 2.7 | |
| Sex | Female | 1168 (60.3) |
| Male | 770 (39.7) | |
| Race | White | 1485 (97.3) |
| Other | 19 (1.2) | |
| Asian | 10 (0.7) | |
| Black or African American | 9 (0.6) | |
| American Indian/ Alaska Native | 4 (0.3) | |
| Ethnic background | Non Hispanic | 855 (94.0) |
| Hispanic | 55 (6.0) | |
| Marital status | Married | 1113 (69.5) |
| Divorced | 218 (13.6) | |
| Widowed | 194 (12.1) | |
| Single | 77 (4.8) | |
| Living situation | Family/Spouse | 955 (75.4) |
| Alone | 294 (23.2) | |
| Other | 18 (1.5) | |
| Self reported family history of dementia | Yes | 681 (30.1) |
| No or Missing | 1582 (69.9) | |
| Self reported memory status | Normal | 1037 (61.2) |
| Mild Memory Loss | 483 (28.5) | |
| Significant Memory Loss | 168 (9.9) | |
| Unknown | 7 (0.4) | |
| Self reported diagnosis | Alzheimer's Disease | 149 (60.3) |
| Dementia, unspecified | 36 (14.6) | |
| Mild Cognitive Impairment | 22 (8.9) | |
| Other Dementia | 40 (16.1) |
Individuals primarily contacted the Registry to enroll themselves (78.0%) or their spouses (12.9%) and the method of contact used most frequently was telephone (62.8%) or email (22.3%). Table 2 provides the results of multiple marketing methods used to recruit potential enrollees. Earned media produced the largest percent of potential enrollees (38.7%), with newspaper articles being the most successful (31.8%).
Table 2.
Referral source as reported by participants
| n (%) | ||
|---|---|---|
| Source | Newspaper article (earned) | 718 (31.8) |
| Direct mail (paid) | 380 (16.8) | |
| Presentation/meeting | 201 (8.9) | |
| Website(s) | 163 (7.2) | |
| Personal Contact | 149 (6.6) | |
| Newsletter/mag article (earned) | 128 (5.7) | |
| Unreported | 95 (4.2) | |
| Internal corporate affiliate | 94 (4.2) | |
| Health professional | 78 (3.5) | |
| Newspaper advertising (paid) | 49 (2.2) | |
| All Other | 205 (9.1) | |
| Source Categories | Earned Media | 875 (38.7) |
| Other | 582 (25.8) | |
| Paid Advertising | 471 (20.8) | |
| Health Related | 237 (10.5) | |
| Unreported | 95 (4.2) |
Cognitive status and assessment
Baseline self-reported memory status was categorized as normal 1037 (61.2%), mild memory loss 483 (28.5%), significant memory loss 168 (9.9%), or unknown 7 (0.4%). Telephone assessment outcomes as of the first assessment completed were no impairment 681 (57.5%), possible cognitive impairment 269 (22.7%), and possible dementia 234 (19.8%). Table 3 shows the inter-rater agreement for the initial round of telephone assessment.
Table 3.
Inter-rater agreement of self reported memory status and telephone assessment
| Test | Agreement | Kappa |
|---|---|---|
| Kappa | 61.75% | 0.31 |
| Kappa Weighted 1 | 67.12% | 0.36 |
| Kappa Weighted 2 | 72.64% | 0.43 |
Referrals
Non-treatment, brain imaging, lifestyle intervention and prevention were the most popular study type preference with 1095 (87.1%), 1045 (83.1%), 980 (78.0%), and 943 (75.0%) respondents respectively. Multiple selections were permissible. When asked if interested in genetic testing 48.3% responded “yes.” Most people 1007 (83.9%) were available to begin participation in a study immediately and 853 (67.9%) were willing to attend study visits at a frequency of once a month.
There was a wide array of study types available, including non-intervention studies for people with and without memory problems and treatment trials for people with a diagnosis of AD or Mild Cognitive Impairment (MCI), and the number of studies enrolling at AAC sites fluctuated over time. A total of 301 Registrants were referred to AAC sites for possible participation in ongoing studies.
Discussion
The feasibility of constructing and executing a statewide AD research registry was demonstrated in this endeavor. An infrastructure and process to screen and refer a high volume of potential research participants was created, awareness about AD research opportunities was increased, a large number of people were enrolled, and hundreds of potential participants were referred to AAC sites. The Registry model was well received by the general public and served as a mechanism for Registrants to assess their own cognitive status while making a contribution to the scientific community. Registrants were generally highly motivated and many reported having a family history of dementia, suggesting that this personal experience may motivate individuals to join a registry of this nature.
The highly individualized consent and screening components required one-on-one engagement. As the Registry evolved and funding fluctuated, it was necessary to shift the emphasis away from recruitment of new enrollees and instead focus on retention. As a result, only a subset of Registrants received follow-up telephone cognitive assessments. We acknowledge that, ideally, regular follow-up (one of the most expensive Registry activities(15-17)) would have been conducted with every Registrant, but resources did not permit this.
Inter-rater agreement between subjective memory status and the telephone cognitive assessment was found to be poor. It is possible that some Registrants were worried well with concerns not discernable with our objective assessment tools; however, our measures may not have the sensitivity required to detect early cognitive changes. It is important to note that a substantial number of Registrants anecdotally noted that they joined the Registry in order to investigate their concerns about their memory and thinking abilities, indicating that perhaps the Registry served as the initial stepping stone in an effort to seek medical advice. A review of research on the relationship between memory complaints and cognitive testing suggests that subjective concerns may be predictive of dementia even when assessment does not reveal impairment(18).
Registrants with ambiguous telephone screening findings presented a challenge when it came to referral to studies. When telephone screening revealed a possible, previously unrecognized, cognitive disorder, Registrants were encouraged to follow up with their physicians or, in some cases, a specialist to assess whether there was an identifiable problem and to clarify diagnosis, which would have helped for accuracy of research referral. However, Registrants rarely followed through on this recommendation. The Confirm sub-study was successful at resolving these ambiguities, increasing referral to appropriate studies, and serving as a retention mechanism, although it significantly expanded the scale of Registry operations.
The quantity of study referrals was considered to be the primary measure of success of the Registry as opposed to the number of successful enrollments into studies. At the time the Registry was developed, AD treatment trials were becoming increasingly more complicated with intricate inclusion and exclusion criteria and lengthy trial durations. At the same time, there were very few studies available to cognitively normal individuals who made up the majority of Registry enrollees. Moreover, many of the cognitively normal participants were primarily interested in prevention studies, which were not widely available. Therefore, while the total number of research referrals was considered satisfactory, referrals were of course limited by the nature of the studies available, and would have been higher, for example, in the current era, when large prevention studies are or will be enrolling volunteers.
There are some limitations to this project. For instance, interested volunteers for the Registry were self-selected and, as anticipated, were not representative of the general population of Arizona. Registrants were mostly white, non-Hispanic, female, and highly educated older adults, similar to what is typically observed in other AD-related cohort studies and clinical trials (1). The Registry model relied on Registrants completing a lengthy written questionnaire, returning it by mail, and undergoing a telephone screening assessment at enrollment. This process may have been viewed as prohibitively burdensome to otherwise interested participants.
Results from the present study may help with future recruitment efforts. For instance, use of an abbreviated enrollment process focused only on pertinent demographic characteristics, rather than lengthy medical history questionnaire and telephone-based cognitive assessments, would be less burdensome to individuals and would likely result in more registry enrollees. Moreover, since inclusion criteria vary from study to study, and medical history can change frequently, this refined approach may optimize registry efficiency. Additionally, a robust mechanism for tracking referral outcomes may provide a greater appreciation of registry efficacy.
Use of the Internet for research registries has significant potential(6), although since there are still many individuals without regular access to the Internet, it is imperative that there still be phone and/or paper-based registry options available. Given the need for increasing the participation of minority and other under-served communities in AD research, future efforts should consider focused recruitment of these individuals(19). A substantial number of cognitively healthy adults joined the Registry expressing interest in prevention research studies. The established infrastructure and experiences gained from this Registry have served as the prototype for the web-based Alzheimer's Prevention Registry (www.endALZnow.org), a registry focusing on raising awareness of AD prevention research and informing its members about study opportunities in their communities.
Acknowledgments
Portions of this study were presented at the American Geriatrics Society 2012 Annual Scientific Meeting, Seattle, WA and the 2012 Arizona Alzheimer's Consortium 14th Annual Conference, Phoenix. Arizona State University, Banner Alzheimer's Institute, Barrow Neurological Institute at St. Joseph's Hospital and Medical Center, Mayo Clinic Arizona, State of Arizona, Banner Sun Health Research Institute, Translational Genomics Research Institute, and University of Arizona.
Funding: NIA ADCC P30 AG19610 and the State of Arizona supported the Registry. Other funding supported by the American Federation for Aging Research - Medical Student Training in Aging Research. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Footnotes
Conflict of interest: Dr. Saunders received research support from the American Federation for Aging Research - Medical Student Training in Aging Research. None of the authors have any financial interests, relationships or affiliations relevant to the subject of this manuscript.
Ethical standards: Participants provided written informed consent under guidelines approved by the human subjects committee at Western Institutional Review Board (WIRB).
References
- 1.National Institute on Aging . 2010 Alzheimer's disease progress report. NIH Publication. National Institutes of Health; 2011. Report No.: 11-7829. [Google Scholar]
- 2.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013 May 7;80(19):1778–83. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services National plan to address Alzheimer's disease: 2013 update. 2013 [Google Scholar]
- 4.Pogacic Kramp V. List of drugs in development for neurodegenerative diseases: Update october 2011. Neurodegener Dis. 2012;9(4):210–83. doi: 10.1159/000335520. [DOI] [PubMed] [Google Scholar]
- 5.Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer's disease clinical trials. Alzheimers Res Ther. 2010 Dec 21;2(6):34. doi: 10.1186/alzrt58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turning to the internet for Alzheimer's trial volunteers [Internet] Biomedical Research Forum, LLC; 2013. [December 4, 2013]. [updated November, 27]. Available from: http://www.alzforum.org/news/conference-coverage/turning-internet-alzheimers-trial-volunteers. [Google Scholar]
- 7.Khachaturian ZS, Petersen RC, Snyder PJ, Khachaturian AS, Aisen P, de Leon M, et al. Developing a global strategy to prevent Alzheimer's disease: Leon thal symposium 2010. Alzheimers Dement. 2011 Mar. 7(2):127–32. doi: 10.1016/j.jalz.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Austrom MG, Bachman J, Altmeyer L, Gao S, Farlow M. A collaborative Alzheimer disease research exchange: Using a community-based helpline as a recruitment tool. Alzheimer Disease & Associated Disorders. 2010;24:S49–53. [PubMed] [Google Scholar]
- 9.Iliffe S, Curry L, Kharicha K, Rait G, Wilcock J, Lowery D, et al. Developing a dementia research registry: A descriptive case study from north thames DeNDRoN and the EVIDEM programme. BMC Med Res Methodol. Jan 27. 2011;11(1):9. doi: 10.1186/1471-2288-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mezey M, Maslow K. Recognition of dementia in hospitalized older adults. Try This: Best Practices in Nursing Care for Hospitalized Older Adults with Dementia. 2007;(D5) doi: 10.1097/01.NAJ.0000304475.80530.a6. [DOI] [PubMed] [Google Scholar]
- 11.Shankle WR, Romney AK, Hara J, Fortier D, Dick MB, Chen JM, et al. Methods to improve the detection of mild cognitive impairment. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4919–24. doi: 10.1073/pnas.0501157102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh K, Breitner J, Magruder-Habib K. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–10. [Google Scholar]
- 13.Rey A. L'examen psychologique dans les cas d'encephalopathie tramatique. Archives de Psychologie. 1941;28:215–85. [Google Scholar]
- 14.Altman DG. Practical Statistics for Medical Research. Chapman & Hall; London: 1991. Inter-rator agreement. pp. 403–9. [Google Scholar]
- 15.Goldberg J, Gelfand HM, Levy PS. Registry evaluation methods: A review and case study. Epidemiol Rev. 1980;2:210–20. doi: 10.1093/oxfordjournals.epirev.a036224. [DOI] [PubMed] [Google Scholar]
- 16.Solomon DJ, Henry RC, Hogan JG, Van Amburg GH, Taylor J. Evaluation and implementation of public health registries. Public Health Rep. Mar-Apr. 1991;106(2):142–50. [PMC free article] [PubMed] [Google Scholar]
- 17.Richesson R, Vehik K. Patient registries: Utility, validity and inference. Adv Exp Med Biol. 2010;686:87–104. doi: 10.1007/978-90-481-9485-8_6. [DOI] [PubMed] [Google Scholar]
- 18.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. Nov. 2000;15(11):983–91. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.A conference devoted to better engaging clinical trial volunteers [Internet] Biomedical Research Forum LLC; 2013. [December 4, 2013]. [updated November 26]. Available from: http://www.alzforum.org/ news/ conference-coverage/conference-devoted-better-engaging-clinical-trial-volunteers?id=3647. [Google Scholar]