Abstract
Signaling proteins often sequester complementary functional sites in separate domains. How do the different domains communicate with one another? An attractive system to address this question is the mitotic regulator, human Pin1 (Lu et al., Nature 380:544–547, 1996). Pin-1 consists of two mutually tethered domains: a WW domain for substrate binding and a catalytic domain for peptidyl-prolyl isomerase (PPIase) activity. Pin1 accelerates the cis–trans isomerization of phospho-Ser/Thr-Pro (pS/T-P) motifs within proteins regulating the cell cycle and neuronal development. The early X-ray (Ranganathan et al., Cell 89:875–886, 1997; Verdecia et al., Nat Struct Biol 7:639–643, 2000) and solution NMR studies (Bayer et al., J Biol Chem 278:26183–26193, 2003; Jacobs et al., J Biol Chem 278:26174–26182, 2003) of Pin1 indicated inter- and intradomain motions. We have explored how such motions might affect interdomain communication, using NMR. Our accumulated results indicate substrate binding to Pin1 WW domain changes the intra/interdomain mobility, thereby altering substrate activity in the distal PPIase domain catalytic site. Thus, Pin1 shows evidence of dynamic allostery, in the sense of Cooper and Dryden (Eur J Biochem 11:103–109, 1984). We highlight our results supporting this conclusion and summarize them via a simple speculative model of conformational selection.
Keywords: Pin1, WW domain, PPIase, IDR, Allostery, Protein dynamics, Correlated motion, NMR
Introduction
Biological networks rely on the actions of numerous modular proteins. Often, such proteins consist of flexibly linked domains with active sites that serve complementary functions. This design raises the question of how the different domains’ functional sites communicate with one another.
We have explored this question through studies of the human peptidyl-prolyl isomerase (PPIase), Pin1 (Lu et al. 1996). Pin1 consists of two flexibly linked domains: a WW domain (residues 1–39) for substrate binding and a larger catalytic PPIase domain (residues 50–163) (1PIN, Ranganathan et al. 1997, Fig. 1a). The “WW” refers to two conserved tryptophans (W11 and W34 in Pin1) defining this class of binding domains (Sudol and Hunter 2000). Pin1 targets phosphorylated Ser/Thr-Pro (pS/T-P) motifs (Fig. 1b), accelerating the cis–trans isomerization of the prolyl imide bonds by factors of 103–104 over thermal cis–trans isomerization (k thermalCT ~2.5 × 10−3 s−1 for X-L-Pro peptides; Grathwohl and Wüthrich 1981). These pS/T-P motifs are prevalent in proteins pertinent to cancer and neuronal disease (Landrieu et al. 2011; Lee et al. 2011; Pastorino et al. 2006; Xu and Etzkorn 2009) and often reside in intrinsically disordered regions (IDRs) (van der Lee et al. 2014).
Fig. 1.

a Structure of human Pin1 (PDB id code 1PIN; Ranganathan et al. 1997). Domains: PPIase (aquamarine); WW β-strands (magenta); interdomain linker (green). Functional sites, left to right: PPIase catalytic pocket (yellow); PPIase interdomain surface (red); WW interdomain surface, Loop II (blue); WW substrate binding Loop I (wheat). b Cis–trans isomerase action of Pin1 on pS/T-P motifs. c pCdc25C (EQPLpTPVTDL) complexed with Pin1-WW domain (PDB 18IG; Wintjens et al. 2001)
Both domains of Pin1 have pS/T-P binding sites: for the WW domain, this involves Loop I (residues 16–21); for the PPIase domain, this entails a catalytic loop (residues 64–80) and binding pocket within the PPIase hydrophobic core. The isolated WW and PPIase domains retain binding and isomerase activity, respectively, in vitro. Yet, there is substantial evidence demonstrating the importance of interdomain communication in vivo. Unigenic studies showed that the WW domain is essential for in vivo Pin1 activity (Behrsin et al. 2006). NMR studies of Pin1/tau interactions revealed PPIase activity dependent on the phosphorylation motif bound by the WW domain (Landrieu et al. 2006; Smet et al. 2005). Substitution mutations (Poolman et al. 2013; Sami et al. 2011) or SUMO1 conjugation (Chen et al. 2013) in either domain have compromised Pin1 activity.
While there is evidence for Pin1 interdomain communication, its underlying mechanism is not fully understood. Structural studies have supplied both hints and riddles. X-ray structures have shown significant interdomain contact, partially mediated by an interstitial PEG400 molecule (Ranganathan et al. 1997; Verdecia et al. 2000). Yet, the pS/T-P binding sites of the two domains are far apart (e.g., H59 in the PPIase domain, R17 in the WW domain, Fig. 1a, c), separated by the interdomain interface. Moreover, NMR indicated substantial interdomain motion (Bayer et al. 2003; Jacobs et al. 2003) that was sensitive to substrate binding and substrate sequence (Jacobs et al. 2003).
Stimulated by these findings, we have been investigating the impact of substrate and inhibitor binding on Pin1 side chain and backbone dynamics, using NMR (Morcos et al. 2010; Namanja et al. 2007, 2011; Peng et al. 2007; Wilson et al. 2013). One of our chief insights is that domain–domain communication involves some aspect of dynamic allostery, as proposed by Cooper and Dryden (1984). In particular, our accumulated results suggest allosteric regulation of the PPIase domain due to changes in conformational flexibility upon substrate binding to the WW domain.
This article highlights our NMR studies suggesting this dynamic allostery. These studies involve two established phosphopeptide substrates of Pin1. The first substrate, pCdc25C, is a natural substrate—a ten-residue (EQPLpTPVTDL) peptide containing the pT48-P49 site of Cdc25C, a mitotic phosphatase targeted by Pin1. Full-length Cdc25C has five pS/T-P sites in an IDR within the N-terminal regulatory domain (Stukenberg and Kirschner 2001; Trunnell et al. 2011). The second substrate, FFpSPR, is a variant of Pintide (WFYpSPR), an artificial substrate derived from screening peptide libraries to find minimal sequences with optimal PPIase efficiency (k cat/K M) (Yaffe et al. 1997). We summarize our findings with a simple speculative model for interdomain allostery, based on Monod–Wyman–Changeux (MWC) conformational selection (Monod et al. 1965).
Hints of allostery from binding and isomerase activity
To characterize the Pin1–substrate interactions, we used standard NMR chemical shift mapping methods (Hajduk and Greer 2007; Lepre et al. 2004) to study the substrate-induced chemical shift perturbations (CSPs) of Pin1 amide 15N-1H HSQC cross-peaks. Analysis of the resulting CSP isotherms (CSP versus substrate concentration) using two-state exchange models estimated the binding affinity (equilibrium dissociation constant K d, Table 1).
Table 1.
NMR estimates of binding affinities and cis–trans isomerase activities for Pin1 constructs, at 295 K, pH 6.6, and 16.4 T. K d values (columns 3 and 4) estimated via 2-D 15N-1H NMR titrations of Pin1 constructs. Values are average (rmsd) in micromolar. Cis–trans isomerase rate constants k EXSY (column 5) were fit from series of 1H–1H EXSY spectra, 2 mM substrate in the presence of 50 μM protein, at 295 K
| Protein | Ligand | K d WW (μM) | K d PPIase (μM) | k EXSY (s−1) |
|---|---|---|---|---|
| Substrates | ||||
| Pin1 | pCdc25C | 6(1) | >120 (60)a | 33 (1) |
| Isol-PPIase | pCdc25C | ND | ND | 41 (1) |
| I28A | pCdc25C | 46 (5) | >120 (60)a | 73 (2) |
| Pin1 | FFpSPR | 43 (14) | >80 (60) | 44 (3)b |
| Isol-PPIase | FFpSPR | ND | ND | 91 (9) |
| Inhibitors | ||||
| Pin1 | Cis-locked | NB | 28 (17) | |
| Isol-PPIase | Cis-locked | NB | 7 (4) | |
| Pin1 | Trans-locked | 53 (16) | 37 (13) | |
| Isol-PPIase | Trans-locked | ND | 66 (27) |
ND no data, NB no binding
aITC measurements of isolated PPIase domain indicate K d > 1 mM
bTable value estimated from data using 10 μM isolated PPIase domain
The substrates preferentially bound to Loop I of the WW domain (S16-R21) (Fig. 1a, c). Loop I corresponds to the hyper-variable binding loop present in all WW domains, which modulates binding preference (Sudol and Hunter 2000; Verdecia et al. 2000). Both substrates demonstrated higher binding affinity for the WW domain (K d, PPIase/K d, WW > 10 for pCdc25C and K d, PPIase/K d, WW ~ 2 for FFpSPR); the affinity mismatch was less severe for FFpSPR, consistent with its design for optimal PPIase activity. The CSPs for residues within the PPIase catalytic pocket were typically smaller and noisier than those of the WW domain (Fig. 2), resulting in large uncertainties in the fitted PPIase domain K ds. We also measured K ds for pCdc25C interacting with the separate (isolated) WW and PPIase domains, using isothermal titration calorimetry (ITC) (Bouchard 2014). We found reasonable agreement for the WW domain: K ITCd, isol – WW = 13 ± 2 μM for the isolated domain versus K NMRd, WW = 6 ± 1 μM for the WW domain within full-length Pin1. But we found severe disagreement for the PPIase domain. The isolated PPIase gave a nearly flat ITC isotherm, suggesting K ITCd, PPIase ~1 mM, yet NMR CSPs of the PPIase domain within full-length Pin1 gave K NMRd, PPIase ~120 ± 60 μM. It is tempting to interpret these differences as cooperative binding, as suggested in recent MD studies of Pin1 interacting with FFpSPR (Guo et al. 2015). However, given the noisy PPIase NMR CSPs, we feel the more prudent course is to treat the ITC results as more accurate and repeat the NMR titrations (work in progress).
Fig. 2.
NH CSPs for Pin1 caused by (top) pCdc25C (Namanja et al. 2007) and (bottom) FFpSPR (Namanja et al. 2010) at 16.4 T and 295 K
The WW domain binding preference of pCdc25C and FFpSPR has been echoed by other Pin1 substrates (Jacobs et al. 2003). This preference reflects interactions between the negatively charged pS/T motif and conserved side chains of the WW domain, such as the positively charged guanidine of R17 in Loop I, and the polar W34 εNH (Daum et al. 2006; Verdecia et al. 2000; Wintjens et al. 2001). A parallel explanation involving conformational dynamics emerged from our studies of the isolated WW domain (Peng et al. 2007). Using amide 15N spin relaxation, we showed extensive Loop I mobility on the sub-nanosecond and microsecond–millisecond time scales that decreased upon binding to pCdc25C. We related the enhanced mobility of Loop I to the distinctive “extra” serine residue it contains, when compared to the loop sequences of all other WW domain classes (Verdecia et al. 2000). Specifically, we deleted one Loop I serine (S18-S19 → S18) and kept the residues making specific contacts with pCdc25C (e.g., R17 and W34). The deletion mutant kept the wild-type fold but reduced the intrinsic flexibility of Loop I and its pCdc25C binding affinity (K deletiond, WW/K wild typed, WW ~ 5). We concluded that the Loop I sequence encodes enhanced flexibility necessary for binding, and perhaps also for adapting to different residues flanking the common pS/T-P core, as exemplified by pCdc25C versus FFpSPR (Peng et al. 2007).
An initial sign of allostery came from NMR titrations of Pin1 with conformationally locked inhibitors, designed by Etzkorn and co-workers (Wang et al. 2003, 2004). These analogs replace pS in the FFpSPR substrate with alkene isoteres, resulting in non-isomerizable cis- and trans-locked competitive inhibitors (K i, cis = 1.7 ± 0.1 μM and K i, trans = 40 ± 2 μM; Wang et al. 2004). The trans-locked inhibitor bound both domains, whereas the cis-locked inhibitor bound exclusively to the substrate pocket of the PPIase domain (Namanja et al. 2011). Remarkably, the cis-locked inhibitor showed higher affinity (~4-fold) for the isolated PPIase domain (WW absent) compared to full-length Pin1 (WW present) (Table 1). This indicated allostery; WW domain binding to one side of the PPIase domain could alter the binding properties of the distal catalytic pocket on the other side of the PPIase domain.
Another sign of allostery came from 2-D NMR 1H–1H exchange spectroscopy (EXSY) (Jeener et al. 1979) measurements of cis–trans isomerase activity for the isolated PPIase domain versus full-length Pin1. The derived measure of isomerase activity, the net exchange rate constant, k EXSY, came from fits of the 1H cis–trans exchange cross-peaks (see, e.g., Fig. 3) to two-state exchange models (Namanja et al. 2011; Wilson et al. 2013). Notably, k EXSY reports on both the cis-to-trans and trans-to-cis conversions via k EXSY = {(k cat, c/K M, c) + (k cat,t/K M, t)}E 0/{1 + ([t]/K M, t) + ([c]/K M, c)}. E 0 is the total amount of Pin1, the “c” and “t” subscripts denote cis and trans Michaelis–Menten parameters, and [c] and [t] are the substrate concentrations (Segel 1968). Interestingly, the isolated PPIase domain showed slightly greater activity (larger k EXSY) than full-length Pin1 for both pCdc25C and FFpSPR (Table 1) (Namanja et al. 2011; Wilson et al. 2013). Pin1 NMR studies by Nicholson and co-workers also revealed greater k EXSY for the isolated PPIase domain compared to full-length Pin1 in studies of a mono-phosphorylated peptide substrate based on the amyloid precursor protein (Greenwood et al. 2011). Yet, the earliest Pin1 studies showed the opposite trend—slightly decreased activity of isolated PPIase domain compared to full-length Pin1 (Lu et al. 1999). We are presently unable to explain these opposing trends. One issue may be the different methods used. The NMR 2-D EXSY assays report k EXSY, whereas the early studies reported k cat/K M for the cis-to-trans conversion process, using chromophoric assays involving phosphopeptide p-nitroanilides (Kofron et al. 1991; Ma et al. 2012). A satisfactory explanation remains pending; nevertheless, both assays indicated that interdomain contact could alter PPIase domain activity.
Fig. 3.

2-D 1H–1H EXSY cis–trans exchange cross-peaks. (Left) pCdc25C pThr5-1Hγ methyl proton resonances (Namanja et al. 2007); (right) FFpSPR P4-1Hδ proton resonances (Namanja et al. 2010)
Substrate-induced changes in side-chain dynamics suggest dynamic allostery
The studies above suggested allosteric regulation of the PPIase domain catalytic pocket by interdomain contact with the WW domain. But what inter-residue interactions might support such allostery? Fresh insight came from studies of the side chain dynamics of methyl-bearing residues in 50 % 2D, U-15N/13C protein, in the absence/presence of substrate (Namanja et al. 2007, 2011). These studies involved measurements of methyl deuterium (2D) relaxation rate constants R 1 and R 1ρ for 13C1H2 2D isotopomers (Millet et al. 2002; Muhandiram et al. 1995), accompanied by backbone 15N relaxation measurements to account for overall protein solution tumbling. We analyzed these rates using the popular Lipari–Szabo formalism (Lipari and Szabo 1982a, b; Nicholson et al. 1992) to extract for each methyl an order parameter S 2 axis. S 2 axis measures the amplitude of internal (re-orientational) motion for each methyl carbon–carbon symmetry axis on the sub-nanosecond time scale. S 2 axis is a pure number between 0 and 1. Unrestricted internal motion yields S 2 axis = 0; increased restriction increases S 2 axis. For an internally rigid symmetry axis, S 2 axis = 1.
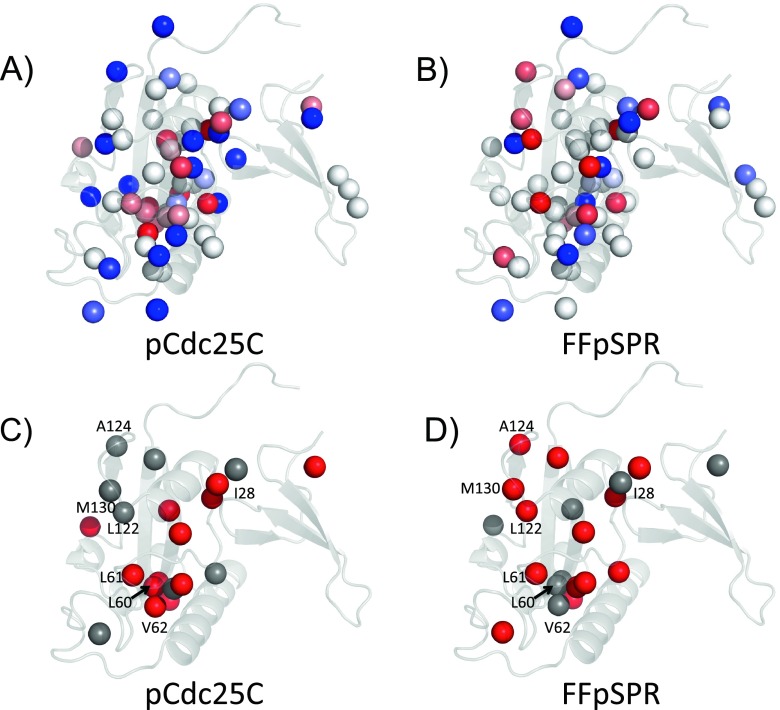
The majority of Pin1 methyls (~80 %) are in the PPIase domain. To assess the substrate-induced changes in methyl side chain flexibility, we mapped ΔS 2 axis = S 2 axis, complex − S 2 axis, apo onto the Pin1 structure (1PIN1) (spheres in Fig. 4). These changes included flexibility losses (ΔS 2 axis > 0) and gains (ΔS 2 axis < 0), relative to the apo state. The magnitudes |ΔS 2 axis| were typically ~0.05–0.1, with the largest changes ~±0.3. Both substrates induced flexibility losses (stiffening) along an internal hydrophobic path, or “conduit,” connecting the PPIase interdomain interface with the PPIase catalytic pocket (red spheres, Fig. 4a, b). Thus, changes in side chain dynamics, rather than stark structural changes, offered a plausible means for allosteric communication within the PPIase domain. The conduit likely reflects correlations among side chain fluctuations due to local steric interactions. Recent Pin1 MD simulations by Zhou and co-workers support this view (Guo et al. 2015), as do other computational studies indicating allosteric communication via correlated side chain dynamics without compulsory large-scale changes in the backbone (Bowman and Geissler 2012; Davis et al. 2006; Dubay et al. 2011; Ho and Agard 2010).
Fig. 4.
Changes in Pin1 methyl symmetry axis order parameters, Δ S 2axis = S 2axis, complex − S 2axis, apo, caused by a pCdc25C (Namanja et al. 2007); b FFpSPR (Namanja et al. 2011). Spheres are methyl carbons. Colors: red = loss of flexibility Δ S 2axis > 0; white = no significant change; blue = gain of flexibility Δ S 2axis < 0. The color intensity scales with the magnitude of |Δ S 2axis|. c, d Spheres (red + gray) denote the conduits of flexibility loss for the pCdc25C and FFpSPR complexes. Red spheres (no intensity scaling) select a particular conduit. c red = Pin1–pCdc25C; d red = Pin1–FFpSPR
The two substrate responses were grossly similar: perturbed side-chain mobility in the PPIase hydrophobic core that included a conduit of flexibility loss (binding-induced increase of S 2 axis). But closer inspection also showed interesting differences. In Fig. 4c, d, the spheres (gray and red together) represent both the Pin1–pCdc25C and Pin1–FFpSPR conduits. The red spheres in Fig. 4c, d identify the conduits specific for pCdc25C and FFpSPR, respectively. The pCdc25C substrate led to greater stiffening of the conserved hydrophobic cluster, L60, L61, and V62 within the catalytic pocket (FFpSPR showed significant stiffening only at L61), whereas FFpSPR showed greater stiffening for residues coordinating the substrate proline (L122δ1, A124β, and M130ε) and in the catalytic loop.
An explanation for the substrate-dependent responses came from our accompanying 15N backbone dynamics studies, which showed that pCdc25C, but not FFpSPR, decreased interdomain contact (Namanja et al. 2007, 2011). The backbone studies recapitulated the findings by Jacobs et al. (2003) on pCdc25C and Pintide. Hence, the differences between pCdc25C and FFpSPR conduits suggest differing perturbations to interdomain contact. We therefore expected the strength of the conduit response (i.e., quantified by number of methyl sites and magnitude of S 2 axis changes; Namanja et al. 2011) to be sensitive to the level of WW domain contact.
We established this sensitivity by comparing the effects of the cis-locked inhibitor on the side chain dynamics studies of both full-length Pin1 versus the isolated PPIase domain (Namanja et al. 2011). The full-length conduit response (WW domain present) was significantly greater than that for the isolated PPIase domain (WW domain absent). As stated, the cis-locked inhibitor exclusively bound the PPIase domain, and so the differing responses reflected the change in interdomain contact.
Substrate binding also increased side chain flexibility (Fig. 4, blue spheres). The increases could not be explained by solvent accessibility, packing, or residual protein aggregation (Namanja et al. 2007). Binding-induced increases in side chain flexibility were reported in the pioneering NMR studies inter-relating side chain with conformational entropy (Lee et al. 2000), and have since appeared for both side chains and the backbone (e.g., Brüschweiler et al. 2013; Igumenova et al. 2006; Iwahara et al. 2005; Jarymowycz and Stone 2006; Zidek et al. 1999). Flexibility increases may mitigate unfavorable conformational entropy costs associated with complex formation (Bruschweiler et al. 2013). Interestingly, our ITC measurements of Pin–pCdc25C indicate favorable enthalpic (negative) and entropic (positive) contributions (Bouchard 2014).
Incidental versus functional
Two other observations suggest functional relevance for the side chain conduit response. First, the conduit was stereo-selective; it was more prominent when the cis-locked (rather than trans-locked) inhibitor occupied the PPIase catalytic pocket (data not shown) (Namanja et al. 2011). This implies a role in substrate/product binding and release. Second, many methyls showing consistent flexibility changes (increases or decreases) across all binding interactions are conserved at greater than 90 % across Pin1 homologs (Namanja et al. 2011). Methyl-bearing residues often make up the hydrophobic cores that stabilize the protein fold; hence, one could dismiss the observed conservation as merely reflecting the preservation of protein stability. But dynamic allostery and protein stability need not be mutually exclusive objectives. Recent theoretical and computational work by England (2011) shows that residue clusters supporting allostery coincide with those optimizing hydrophobic surface burial upon protein folding. Thus, ligand-induced stabilization of a hydrophobic core may “moonlight” as a mechanism for allosteric communication.
Insights from mutagenesis
To further understand the dynamic conduit, we investigated a substitution mutant, I28A. I28 is part of the WW domain side of the interdomain interface (Loop II, residues 27–30), and the dynamic conduit (Fig. 4). We studied I28A interactions with pCdc25C and compared them to wild-type Pin1 (Wilson et al. 2013).
Surprisingly, I28A showed increased isomerase activity (larger k EXSY), even greater than that of the isolated PPIase domain (i.e., k EXSY (I28A) > k EXSY (isolated PPIase) > k EXSY (Pin1), Table 1). The WW domain of I28A also showed a ~7-fold decrease in pCdc25C binding affinity relative to the wild type (Table 1). Far-UV CD temperature scans and 15N-1H HSQC spectra ruled out misfolding and reduced stability. I28 does not directly contact pCdc25C, being part of the interdomain interface on the opposite side of the WW domain from Loop I (Fig. 1c). These facts suggested allosteric coupling between Loop II and I within the WW domain (Wilson et al. 2013); such coupling was supported by cross-correlated motions between these two loops, revealed by a mutual information analysis (McClendon et al. 2009) of a 554-ns explicit solvent MD simulation of the isolated Pin1-WW (Morcos et al. 2010). These correlated motions could enable conformational trauma (ligand binding, mutation) at one loop to perturb the other.
The main effect of I28A was reduced interdomain contact relative to the wild type. I28A showed chemical shift differences at the interdomain interface (Fig. 5, left, dashed rectangle). Comparisons with the isolated PPIase domain and full-length Pin1 attributed these differences to altered WW domain contact. In particular, the I28A A140 and L141 (Fig. 5, right) resonances showed the similar upfield perturbations relative to full-length wild-type Pin1 as demonstrated by the isolated PPIase domain, indicating a substantial reduction of interdomain contact relative to (apo) wild-type Pin1 (unpublished results). Figure 5, right, further confirmed that pCdc25C binding to wild-type Pin1 caused a loss of interdomain contact. By contrast, binding of FFpSPR and the locked inhibitors gave a downfield perturbation of A140 and L141 (data not shown). While these effects are not yet understood, they are consistent with our 15N spin relaxation results, suggesting that FFpSPR enhances interdomain contact (the opposite of pCdc25C).
Fig. 5.
(Left) NH CSPs for Pin1 residues 70–163 caused by (top panel) deletion of WW domain from apo Pin1; (middle panel) the I28A mutation in apo Pin1; (bottom panel) the I28A mutation for the pCdc25C complex (Wilson et al. 2013). (Right) NH chemical shifts of A140 and L141 (PPIase domain interface) report on the extent of interdomain contact (unpublished results)
Another indicator of reduced interdomain contact in I28A was altered conformational dynamics, relative to the wild type. 15N spin relaxation revealed enhanced microsecond–millisecond mobility for I28A Loop II residues (e.g., N26, T29, and N30 in Fig. 6a) that was absent in the wild type, suggesting new conformational freedom for Loop II. Finally, we compared the effects of pCdc25C binding on I28A side chain dynamics to those of the wild type (Fig. 6b–d). Comparison of the red spheres in Fig. 6c (wild-type conduit) with those of Fig. 6d (I28A conduit) shows a clear perturbation that underscores the connection between the dynamic conduit, the extent of interdomain contact, and Pin1 substrate binding.
Fig. 6.
Changes in I28A backbone and side chain dynamics, compared to those of the wild type (Wilson et al. 2013). a Spheres are backbone NHs with enhanced flexibility in apo I28A that are absent in wild-apo Pin1. Color code: red = enhanced microsecond–millisecond dynamics, blue = enhanced sub-nanosecond flexibility at the WW domain N, C termini. b pCdc25C-induced changes in methyl side-chain dynamics for I28A Δ S 2axis = S 2axis, complex − S 2axis, apo (Wilson et al. 2013). Colors: (red) loss of flexibility Δ S 2axis > 0; (white) no significant change; (blue) gain of flexibility Δ S 2axis < 0. The color intensity scales with the magnitude of |Δ S 2axis|. c, d Spheres (red + gray) denote the conduits of flexibility loss of both Pin1–pCdc25C and I28A–pCdc25C complexes. Red spheres (no intensity scaling) denote a particular conduit. c red = Pin1–pCdc25C; d red = I28A–pCdc25C
Interdomain allosteric communication
For our pCdc25C results, we can summarize our findings with the simple speculative model below. Our emphasis on pCdc25C over FFpSPR reflects our more extensive experimental data for the former. A key caveat of the model is its restriction to substrates like pCdc25C that reduce interdomain contact relative to the apo state. It is not appropriate for FFpSPR, which seemed to enhance interdomain contact (Namanja et al. 2011). A plausible model for the Pin1–FFpSPR interaction and allosteric effects has been presented in the recent MD study of Pin1 (Guo et al. 2015). Generally, we expect substrates with different phosphorylation patterns and flanking residues may elicit different modes of Pin1 interaction.
Our Pin1–pCdc25C model is based on conformational selection per Monod, Wyman, and Changeux (MWC) (Monod et al. 1965). A basic MWC premise is a pre-existing equilibrium between distinct apo protein conformations. Evidence for this equilibrium in Pin1 comes from (i) its solution structure determination, which first suggested conformational selection in Pin1 (Bayer et al. 2003); (ii) our findings that loop I of the apo Pin1-WW domain already samples the conformations of the pCdc25C-bound form (Morcos et al. 2010; Peng et al. 2007); and (iii) the recent studies of Pin1 binding to PEG400, a small non-substrate molecule that consistently occupies the interdomain space in the Pin1 X-ray structures (Matena et al. 2013).
Our model is as follows. Guided by our EXSY data (Table 1), we assume that the catalytic pocket of the isolated PPIase domain samples conformations capable of higher isomerase activity (i.e., larger k EXSY) than those sampled by its counterpart in full-length Pin1. We then stipulate that full-length apo Pin1 exchanges between two conformational sub-ensembles, corresponding to the WW and PPIase domains in more compacted versus more extended dispositions (Fig. 7, upper panel). A low activation barrier separates the compact and extended free energy wells, such that inter-well hopping (interdomain association/dissociation) is facile at room temperature. Fast conformational fluctuations among the conduit residues may help promote these slower (less frequent) hops. Critically, the two sub-ensembles have different intrinsic cis–trans isomerase activities. In the extended sub-ensemble, the (apo) PPIase domain adopts conformations resembling the isolated PPIase domain, and is therefore capable of higher isomerase activity (larger k EXSY) (Fig. 7, red star highlighting).
Fig. 7.
Speculative model for Pin1 interdomain allostery based on pCdc25C data. WW domain (purple hatched and solid ovals); PPIase domain (aquamarine hatched and solid rectangles). Apo Pin1 exchanges between compact versus extended sub-ensembles; the extended sub-ensemble is more active (higher cis–trans k EXSY), indicated by the red star on the PPIase domain. Ligand binding (e.g., pCdc25C) stabilizes the extended sub-ensemble
The model further assumes pCdc25C has higher binding affinity for Loop I (WW domain) in the extended sub-ensemble. Hence, when pCdc25C is introduced, it stabilizes the extended conformational sub-ensemble (Fig. 7, lower panel, lower well), shifting the Pin1 conformational populations to those in which Loop II disfavors interdomain contact. The population shift manifests as the PPIase conduit response and CSPs indicating reduced interdomain contact (e.g., Fig. 5). Stabilization of the extended sub-ensemble means the PPIase catalytic pocket adopts conformations resembling that of the isolated PPIase domain. “Resembling” does not mean “matching”; hence, k EXSY(WT-Pin1) < k EXSY(WT-PPIase). Nevertheless, k EXSY(WT-Pin1) exceeds that which would have prevailed had apo-state interdomain contact persisted. This last point needs to be demonstrated and is the subject of our current work.
Our model may explain Pin1’s interactions with substrates like Cdc25C phosphatase, which have multiple Pin1 pS/T-P motifs within IDR regions (Stukenberg and Kirschner 2001). If at least one pS/T-P motif is trans, Pin1 could dock there first via its WW domain (Fig. 8). This would stabilize the extended conformational sub-ensemble and release the PPIase domain to search for other pS/T-P sites. The weaker binding affinity of the PPIase domain would be partially compensated by more frequent encounters with other pS/T-P sites, due to WW-domain anchoring. Indeed, our titrations of the cis- and trans-locked inhibitors indicate the feasibility of the dual binding sketched in Fig. 8; that is, the WW and PPIase domains can simultaneously bind a trans and cis pS/T-P motif, respectively (Namanja et al. 2011).
Fig. 8.
Speculated Pin1 interaction (PDB id code 1NMV; Bayer et al. 2003) with two pS/T-P motifs within an intrinsically disordered protein segment. Colors: yellow = PPIase catalytic pocket; red = PPIase interdomain surface; blue = WW interdomain surface (Loop II); wheat = WW substrate binding Loop I
Concluding remarks
Pin1 interacts with numerous protein substrates (Innes et al. 2013; Landrieu et al. 2006; Liou et al. 2011; Smet-Nocca et al. 2013). Substrates with different numbers of pS/T-P sites with different spacings and/or flanking residues may select for different Pin1 recognition mechanisms. Some of these mechanisms may involve interdomain dynamic allostery, whose rough features coincide with Fig. 7. Yet, other substrate sites may increase interdomain contact, as suggested by FFpSPR and the PEG400 studies of Bayer and co-workers (Matena et al. 2013). To be more definitive, we need a more quantitative analysis (e.g., kinetic and thermodynamic measurements; Marlow et al. 2010) and investigations into the role of the faster sub-nanosecond fluctuations in promoting allosteric transitions.
The modular design of Pin1 is common among signaling proteins (Lim 2010), and so the interdomain communication described here likely occurs in other proteins. So what? Is there a practical benefit? Perhaps there is, for the design of allosteric inhibitors (DeLaBarre et al. 2014; Lewis et al. 2008). Domain interfaces remote from the catalytic sites (as in Pin1) could be useful inhibitor sites. Naively, the required strategies would be similar to those used for targeting protein–protein interactions.
Finally, these studies of dynamic allostery target a more fundamental question. Can we generate molecules from scratch that execute dynamic allosteric communication per a preconceived plan? There are encouraging signs saying “yes,” such as recent work by Teichmann and co-workers (Perica et al. 2014), Kalodimos and co-workers (Tzeng and Kalodimos 2013), and Hardy and co-workers (Dagbay et al. 2014). Yet, “robust” engineering of dynamic allostery would seem to require the ability to quantitatively predict allosteric behavior over specified length and time scales. Until we have this ability, de novo design of dynamic allostery will rely more on serendipity and limitless patience, rather than the application of principles. Further studies to learn how nature has already achieved this, in proteins such as Pin1, are therefore worthy investments.
Acknowledgments
JWP thanks Dr. Andrew T. Namanja, Dr. John S. Zintsmaster, Dr. Xingsheng Wang, Dr. Tao Peng, Dr. Jill J. Bouchard, Dr. Kimberly A. Wilson, Dr. Petra Rovó, Mr. Brendan J. Mahoney, Ms. Meiling Zhang, Dr. Jaroslav Zajicek, Dr. Felicia A. Etzkorn (VA Tech), Cheryl L. Schairer, and Shelby L. Peng for perseverance, inspiration, and useful discussions.
Compliance with Ethical Standards
ᅟ
Funding
This work was funded by the National Institutes of Health (NIH) Grant R01-GM083081 (JWP).
Conflict of interest
J.W. Peng declares no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animal subjects performed by the author.
Footnotes
This article is part of a Special Issue on 'The Role of Protein Dynamics in Allosteric Effects' edited by Gordon Roberts.
References
- Bayer E, Goettsch S, Mueller JW, Griewel B, Guiberman E, Mayr LM, Bayer P. Structural analysis of the mitotic regulator hPin1 in solution: insights into domain architecture and substrate binding. J Biol Chem. 2003;278:26183–26193. doi: 10.1074/jbc.M300721200. [DOI] [PubMed] [Google Scholar]
- Behrsin CD et al (2006) Functionally important residues in the peptidyl-prolyl isomerase Pin1 revealed by unigenic evolution. J Mol Biol [DOI] [PubMed]
- Bouchard J (2014) Ensemble interpretation of domain mobility in modular protein Pin1 by NMR and molecular dynamics. University of Notre Dame
- Bowman GR, Geissler PL. Equilibrium fluctuations of a single folded protein reveal a multitude of potential cryptic allosteric sites. Proc Natl Acad Sci U S A. 2012;109:11681–11686. doi: 10.1073/pnas.1209309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüschweiler S, Konrat R, Tollinger M. Allosteric communication in the KIX domain proceeds through dynamic repacking of the hydrophobic core. ACS Chem Biol. 2013;8:1600–1610. doi: 10.1021/cb4002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, et al. SENP1 deSUMOylates and regulates Pin1 protein activity and cellular function. Cancer Res. 2013;73:3951–3962. doi: 10.1158/0008-5472.CAN-12-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, Dryden DT. Allostery without conformational change. A plausible model. Eur J Biochem. 1984;11:103–109. doi: 10.1007/BF00276625. [DOI] [PubMed] [Google Scholar]
- Dagbay K, et al. A multipronged approach for compiling a global map of allosteric regulation in the apoptotic caspases. Methods Enzymol. 2014;544:215–249. doi: 10.1016/B978-0-12-417158-9.00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum S, Fanghanel J, Wildemann D, Scheine-Fischer C. Thermodynamics of phosphopeptide binding to human peptidyl prolyl cis/trans isomerase Pin1. Biochemistry. 2006;45:1215–12135. doi: 10.1021/bi0608820. [DOI] [PubMed] [Google Scholar]
- Davis IW, Arendall WB, 3rd, Richardson DC, Richardson JS. The backrub motion: how protein backbone shrugs when a sidechain dances. Structure. 2006;14:265–274. doi: 10.1016/j.str.2005.10.007. [DOI] [PubMed] [Google Scholar]
- DeLaBarre B, Hurov J, Cianchetta G, Murray S, Dang L. Action at a distance: allostery and the development of drugs to target cancer cell metabolism. Chem Biol. 2014;21:1143–1161. doi: 10.1016/j.chembiol.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Dubay KH, Bothma JP, Geissler PL. Long-range intra-protein communication can be transmitted by correlated side-chain fluctuations alone. PLoS Comput Biol. 2011;7 doi: 10.1371/journal.pcbi.1002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England JL. Allostery in protein domains reflects a balance of steric and hydrophobic effects. Structure. 2011;19:967–975. doi: 10.1016/j.str.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Grathwohl C, Wüthrich K (1981) Nmr studies of the rates of proline cis-trans isomerization in oligopeptides. Biopolymers 20:2623–2633
- Greenwood AI, Rogals MJ, De S, Lu KP, Kovrigin EL, Nicholson LK. Complete determination of the Pin1 catalytic domain thermodynamic cycle by NMR lineshape analysis. J Biomol NMR. 2011;51:21–34. doi: 10.1007/s10858-011-9538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Pang X, Zhou HX. Two pathways mediate interdomain allosteric regulation in pin1. Structure. 2015;23:237–247. doi: 10.1016/j.str.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduk PJ, Greer J. A decade of fragment-based drug design: strategic advances and lessons learned. Nat Rev Drug Discov. 2007;6:211–219. doi: 10.1038/nrd2220. [DOI] [PubMed] [Google Scholar]
- Ho BK, Agard DA. Conserved tertiary couplings stabilize elements in the PDZ fold, leading to characteristic patterns of domain conformational flexibility. Protein Sci. 2010;19:398–411. doi: 10.1002/pro.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igumenova TI, Frederick KK, Wand AJ. Characterization of the fast dynamics of protein amino acid side chains using NMR relaxation in solution. Chem Rev. 2006;106:1672–1699. doi: 10.1021/cr040422h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes BT, Bailey ML, Brandl CJ, Shilton BH, Litchfield DW. Non-catalytic participation of the Pin1 peptidyl-prolyl isomerase domain in target binding. Front Physiol. 2013;4:18. doi: 10.3389/fphys.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahara J, Peterson RD, Clubb RT. Compensating increases in protein backbone flexibility occur when the Dead ringer AT-rich interaction domain (ARID) binds DNA: a nitrogen-15 relaxation study. Protein Sci. 2005;14:1140–1150. doi: 10.1110/ps.041154405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DM, Saxena K, Vogtherr M, Bernado P, Pons M, Fiebig KM. Peptide binding induces large scale changes in inter-domain mobility in human Pin1. J Biol Chem. 2003;278:26174–26182. doi: 10.1074/jbc.M300796200. [DOI] [PubMed] [Google Scholar]
- Jarymowycz VA, Stone MJ. Fast time scale dynamics of protein backbones: NMR relaxation methods, applications, and functional consequences. Chem Rev. 2006;106:1624–1671. doi: 10.1021/cr040421p. [DOI] [PubMed] [Google Scholar]
- Jeener J, Meier BH, Bachmann P, Ernst RR. Investigation of exchange processes by two-dimensional NMR spectroscopy. J Chem Phys. 1979;71:4546–4553. doi: 10.1063/1.438208. [DOI] [Google Scholar]
- Kofron JL, Kuzmic P, Kishore V, Colon-Bonilla E, Rich DH. Determination of kinetic constants for peptidyl prolyl cis-trans isomerases by an improved spectrophotometric assay. Biochemistry. 1991;30:6127–6134. doi: 10.1021/bi00239a007. [DOI] [PubMed] [Google Scholar]
- Landrieu I, Smet C, Wieruszeski JM, Sambo AV, Wintjens R, Buée L, Lippens G (2006) Exploring the molecular function of PIN1 by nuclear magnetic resonance. Curr Protein Pept Sci 7:179–194 [DOI] [PubMed]
- Landrieu I, et al. Molecular implication of PP2A and Pin1 in the Alzheimer’s disease specific hyperphosphorylation of Tau. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Kinnear SA, Wand AJ. Redistribution and loss of side chain entropy upon formation of a calmodulin-peptide complex. Nat Struct Biol. 2000;7:72–77. doi: 10.1038/71280. [DOI] [PubMed] [Google Scholar]
- Lee TH, Pastorino L, Lu KP. Peptidyl-prolyl cis-trans isomerase Pin1 in ageing, cancer and Alzheimer disease. Expert Rev Mol Med. 2011;13 doi: 10.1017/S1462399411001906. [DOI] [PubMed] [Google Scholar]
- Lepre CA, Moore JM, Peng JW. Theory and applications of NMR-based screening in pharmaceutical research. Chem Rev. 2004;104:3641–3676. doi: 10.1021/cr030409h. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Lebois EP, Lindsley CW. Allosteric modulation of kinases and GPCRs: design principles and structural diversity. Curr Opin Chem Biol. 2008;12:269–280. doi: 10.1016/j.cbpa.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Lim WA. Designing customized cell signalling circuits. Nat Rev Mol Cell Biol. 2010;11:393–403. doi: 10.1038/nrm2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou YC, Zhou XZ, Lu KP. Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem Sci. 2011;36:501–514. doi: 10.1016/j.tibs.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari G, Szabo A. Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J Am Chem Soc. 1982;104:4546–4559. doi: 10.1021/ja00381a009. [DOI] [Google Scholar]
- Lipari G, Szabo A. Model-free approach to the interpretation of nuclear magnetic resonance relaxation of macromolecules. 2. Analysis of experimental results. J Am Chem Soc. 1982;104:4559–4570. doi: 10.1021/ja00381a010. [DOI] [Google Scholar]
- Lu KP, Hanes SD, Hunter T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature. 1996;380:544–547. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Zhou XZ, Shen M, Lu KP. Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science. 1999;283:1325–1328. doi: 10.1126/science.283.5406.1325. [DOI] [PubMed] [Google Scholar]
- Ma SL, Pastorino L, Zhou XZ, Lu KP. Prolyl isomerase Pin1 promotes amyloid precursor protein (APP) turnover by inhibiting glycogen synthase kinase-3beta (GSK3beta) activity: novel mechanism for Pin1 to protect against Alzheimer disease. J Biol Chem. 2012;287:6969–6973. doi: 10.1074/jbc.C111.298596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow MS, Dogan J, Frederick KK, Valentine KG, Wand AJ. The role of conformational entropy in molecular recognition by calmodulin. Nat Chem Biol. 2010;6:352–358. doi: 10.1038/nchembio.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matena A, et al. Transient domain interactions enhance the affinity of the mitotic regulator Pin1 toward phosphorylated peptide ligands. Structure. 2013;21:1769–1777. doi: 10.1016/j.str.2013.07.016. [DOI] [PubMed] [Google Scholar]
- McClendon CL, Friedland G, Mobley DL, Amirkhani H, Jacobson MP. Quantifying correlations between allosteric sites in thermodynamic ensembles. J Chem Theory Comput. 2009;5:2486–2502. doi: 10.1021/ct9001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet O, Muhandiram DR, Skrynnikov NR, Kay LE. Deuterium spin probes of side-chain dynamics in proteins. 1. Measurement of five relaxation rates per deuteron in (13)C-labeled and fractionally (2)H-enriched proteins in solution. J Am Chem Soc. 2002;124:6439–6448. doi: 10.1021/ja012497y. [DOI] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/S0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Morcos F, et al. Modeling conformational ensembles of slow functional motions in Pin1-WW. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhandiram DR, Yamazaki T, Sykes BD, Kay LE. Measurement of 2H T1 and T1r relaxation times in uniformly 13C-labeled and fractionally 2H-labeled proteins in solution. J Am Chem Soc. 1995;117:11536–11544. doi: 10.1021/ja00151a018. [DOI] [Google Scholar]
- Namanja AT, Peng T, Zintsmaster JS, Elson AC, Shakour MG, Peng JW. Substrate recognition reduces side-chain flexibility for conserved hydrophobic residues in human Pin1. Structure. 2007;15:313–327. doi: 10.1016/j.str.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Namanja AT, et al. Toward flexibility-activity relationships by NMR spectroscopy: dynamics of Pin1 ligands. J Am Chem Soc. 2010;132:5607–5609. doi: 10.1021/ja9096779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namanja AT, Wang XJ, Xu B, Mercedes-Camacho AY, Wilson KA, Etzkorn FA, Peng JW. Stereospecific gating of functional motions in Pin1. Proc Natl Acad Sci U S A. 2011;108:12289–12294. doi: 10.1073/pnas.1019382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson LK, Kay LE, Baldisseri DM, Arango J, Young PE, Bax A, Torchia DA. Dynamics of methyl groups in proteins as studied by proton-detected 13C NMR spectroscopy. Application to the leucine residues of staphylococcal nuclease. Biochemistry. 1992;31:5253–5263. doi: 10.1021/bi00138a003. [DOI] [PubMed] [Google Scholar]
- Pastorino L, et al. The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature. 2006;440:528–534. doi: 10.1038/nature04543. [DOI] [PubMed] [Google Scholar]
- Peng T, Zintsmaster JS, Namanja AT, Peng JW. Sequence-specific dynamics modulate recognition specificity in WW domains. Nat Struct Mol Biol. 2007;14:325–331. doi: 10.1038/nsmb1207. [DOI] [PubMed] [Google Scholar]
- Perica T, et al. Evolution of oligomeric state through allosteric pathways that mimic ligand binding. Science. 2014;346:1254346. doi: 10.1126/science.1254346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman TM, Farrow SN, Matthews L, Loudon AS, Ray DW. Pin1 promotes GR transactivation by enhancing recruitment to target genes. Nucleic Acids Res. 2013;41:8515–8525. doi: 10.1093/nar/gkt624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R, Lu KP, Hunter T, Noel JP. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell. 1997;89:875–886. doi: 10.1016/S0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- Sami F, Smet-Nocca C, Khan M, Landrieu I, Lippens G, Brautigan DL. Molecular basis for an ancient partnership between prolyl isomerase Pin1 and phosphatase inhibitor-2. Biochemistry. 2011;50:6567–6578. doi: 10.1021/bi200553e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel I (1968) Biochemical calculations: how to solve mathematical problems in general biochemistry. John Wiley & Sons
- Smet C, Wieruszeski JM, Buée L, Landrieu I, Lippens G (2005) Regulation of Pin1 peptidyl-prolyl cis/trans isomerase activity by its WW binding module on a multi-phosphorylated peptide of Tau protein. FEBS Lett 579:4159–4164. doi:10.1016/j.febslet.2005.06.048 [DOI] [PubMed]
- Smet-Nocca C, Launay H, Wieruszeski JM, Lippens G, Landrieu I. Unraveling a phosphorylation event in a folded protein by NMR spectroscopy: phosphorylation of the Pin1 WW domain by PKA. J Biomol NMR. 2013;55:323–337. doi: 10.1007/s10858-013-9716-z. [DOI] [PubMed] [Google Scholar]
- Stukenberg PT, Kirschner MW. Pin1 acts catalytically to promote a conformational change in Cdc25. Mol Cell. 2001;7:1071–1083. doi: 10.1016/S1097-2765(01)00245-3. [DOI] [PubMed] [Google Scholar]
- Sudol M, Hunter T. NeW wrinkles for an old domain. Cell. 2000;103:1001–1004. doi: 10.1016/S0092-8674(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Trunnell NB, Poon AC, Kim SY, Ferrell JE., Jr Ultrasensitivity in the regulation of Cdc25C by Cdk1. Mol Cell. 2011;41:263–274. doi: 10.1016/j.molcel.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng SR, Kalodimos CG. Allosteric inhibition through suppression of transient conformational states. Nat Chem Biol. 2013;9:462–465. doi: 10.1038/nchembio.1250. [DOI] [PubMed] [Google Scholar]
- van der Lee R, et al. Classification of intrinsically disordered regions and proteins. Chem Rev. 2014;114:6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecia MA, Bowman ME, Lu KP, Hunter T, Noel JP. Structural basis for phosphoserine-proline recognition by group IV WW domains. Nat Struct Biol. 2000;7:639–643. doi: 10.1038/77929. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Hart SA, Xu B, Mason MD, Goodell JR, Etzkorn FA. Serine-cis-proline and serine-trans-proline isosteres: stereoselective synthesis of (Z)- and (E)-alkene mimics by Still-Wittig and Ireland-Claisen rearrangements. J Org Chem. 2003;68:2343–2349. doi: 10.1021/jo026663b. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Xu B, Mullins AB, Neiler FK, Etzkorn FA. Conformationally locked isostere of phosphoSer-cis-Pro inhibits Pin1 23-fold better than phosphoSer-trans-Pro isostere. J Am Chem Soc. 2004;126:15533–15542. doi: 10.1021/ja046396m. [DOI] [PubMed] [Google Scholar]
- Wilson KA, Bouchard JJ, Peng JW. Interdomain interactions support interdomain communication in human Pin1. Biochemistry. 2013 doi: 10.1021/bi401057x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintjens R, Wieruszeski J-M, Drobecq H, Rousselot-Pailley P, Buée L, Lippens G, Landrieu I (2001) 1H NMR study on the binding of Pin1 Trp-Trp domain with phosphothreonine peptides. J Biol Chem 276:25150–25156. Defines intermolecular interactions between Pin1-WW and phosphopeptides in solution, including pCdc25C. [DOI] [PubMed]
- Xu GG, Etzkorn FA. Pin1 as an anticancer drug target. Drug News Perspect. 2009;22:399–407. doi: 10.1358/dnp.2009.22.7.1414594. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- Zidek L, Novotny MV, Stone MJ. Increased protein backbone conformational entropy upon hydrophobic ligand binding. Nat Struct Biol. 1999;6:1118–1121. doi: 10.1038/70057. [DOI] [PubMed] [Google Scholar]